Abstract
Corticotropin-releasing factor (CRF), the stress-related neuropeptide, acts as a neurotransmitter in the brain norepinephrine nucleus, locus coeruleus (LC), to activate this system during stress. CRF shifts the mode of LC discharge from a phasic to a high tonic state that is thought to promote behavioral flexibility. To investigate this, the effects of CRF administered either intracerebroventricularly (30–300 ng, i.c.v.) or directly into the LC (intra-LC; 2–20 ng) were examined in a rat model of attentional set shifting. CRF differentially affected components of the task depending on dose and route of administration. Intracerebroventricular CRF impaired intradimensional set shifting, reversal learning, and extradimensional set shifting (EDS) at different doses. In contrast, intra-LC CRF did not impair any aspect of the task. The highest dose of CRF (20 ng) facilitated reversal learning and the lowest dose (2 ng) improved EDS. The dose–response relationship for CRF on EDS performance resembled an inverted U-shaped curve with the highest dose having no effect. Intra-LC CRF also elicited c-fos expression in prefrontal cortical neurons with an inverted U-shaped dose–response relationship. The number of c-fos profiles was positively correlated with EDS performance. Given that CRF excites LC neurons, the ability of intra-LC CRF to activate prefrontal cortical neurons and facilitate EDS is consistent with findings implicating LC-norepinephrine projections to medial prefrontal cortex in this process. Importantly, the results suggest that CRF release in the LC during stress facilitates shifting of attention between diverse stimuli in a dynamic environment so that the organism can adapt an optimal strategy for coping with the challenge.
Keywords: stress, set shifting, behavioral flexibility, prefrontal cortex, reversal learning, norepinephrine
INTRODUCTION
Stress is generally thought to impair cognitive function (Arnsten, 2009; Holmes and Wellman, 2009; Marin et al, 2011). However, there is also evidence that stress enhances cognitive performance and it has been suggested that there is an inverted U-shaped relationship between stress intensity and cognitive performance (Beylin and Shors, 1998; de Kloet et al, 1999; Faraji et al, 2011; Luine et al, 1996). Although the effects of stress on cognition have been attributed to corticosteroids (de Kloet et al, 1999; McEwen, 2001; Sapolsky, 2000), they may also be mediated by corticotropin-releasing factor (CRF), the neuropeptide that orchestrates many aspects of the stress response (Bale and Vale, 2004). CRF acts as a neurohormone to initiate the cascade of pituitary adrenocorticotropin release and the subsequent release of adrenal corticosteroids that is the hallmark of stress (Vale et al, 1981). Additionally, extrahypophysial CRF acts as a neurotransmitter to promote autonomic and behavioral aspects of the stress response (Owens and Nemeroff, 1991; Valentino and Van Bockstaele, 2002). CRF may regulate cognitive processes by its modulation of the forebrain-projecting monoamine systems that are integral to these processes.
The major brain norepinephrine nucleus, locus coeruleus (LC), is one target of CRF neurotransmission (Valentino and Van Bockstaele, 2002, 2008; Van Bockstaele et al, 1996) that is thought to be important in cognition through its extensive hippocampal and cortical projections (Loughlin et al, 1986; Swanson and Hartman, 1976). LC neuronal discharge rate is positively correlated with arousal state (Aston-Jones and Bloom, 1981b; Berridge and Foote, 1991; Berridge et al, 1993). Additionally, LC neurons are phasically activated by salient stimuli and this activation often precedes orientation toward the stimulus (Aston-Jones and Bloom, 1981a; Foote et al, 1980). LC neuronal recordings in monkeys performing operant tasks have suggested that different patterns of LC discharge are associated with different cognitive processes (Aston-Jones and Cohen, 2005; Aston-Jones et al, 1999). Phasic LC discharge characterized by synchronously firing LC neurons that are responsive to discrete sensory stimuli is associated with focused attention and maintaining ongoing behavior with a known outcome. In contrast, a high tonic mode of activity with elevated spontaneous discharge rates, decreased synchrony, and diminished phasic responses to specific sensory stimuli is associated with hyperarousal, labile attention, and going off-task or changing behavior to seek an alternate outcome.
CRF increases LC neuronal firing rate and decreases the signal-to-noise ratio of the sensory response, biasing the mode of LC activity toward a high tonic state that would favor behavioral flexibility (Curtis et al, 1997; Valentino and Foote, 1987, 1988). Stress mimics these neuronal effects and this can be blocked by intra-LC administration of a CRF antagonist (Curtis et al, 2001; Valentino and Wehby, 1988; Valentino et al, 1991). The shift produced by CRF toward a high tonic mode of LC discharge and enhanced behavioral flexibility would be adaptive in a dynamic challenging environment.
The present study was designed to examine the effects of CRF in a rodent-based model for assessing cognitive flexibility, the attentional set shifting task (AST; Birrell and Brown, 2000; Lapiz and Morilak, 2006). The effects of different CRF doses administered intracerebroventricularly (i.c.v.) or directly into the LC (intra-LC) were examined. Because norepinephrine actions in the medial prefrontal cortex have been implicated in certain aspects of set shifting behavior, expression of the immediate early gene, c-fos, and the phosphorylated extracellular signal-regulated kinase (p44/42ERK) were quantified here as indices of neuronal activation and correlated with task performance (Bondi et al, 2010; Lapiz and Morilak, 2006; McGaughy et al, 2008; Roberts et al, 1994; Tait et al, 2007).
MATERIALS AND METHODS
Animals
Adult male Sprague Dawley rats (220–250 g; Charles River Laboratories, Wilmington, MA) were housed individually on a 12 h light/dark cycle with lights on at 0700 hours. Rats acclimated to the colony for a minimum of 5 days before surgery. Animal use and care was approved by the institutional animal care and use committee of the Children's Hospital of Philadelphia.
Experimental Design
After 5 days of acclimation, rats underwent surgery for stereotaxic implantation of cannula guides. They began a phase of food restriction 4 days after surgery and the training for the attentional set shifting procedure began after 5 days of food restriction, with a day of habituation, a day of training, and a day of testing as described below. Rats were transcardially perfused 15 min after completion of the last task.
Surgery
Rats were implanted with a cannula guide into lateral ventricle or bilateral cannula guides into the LC. Rats were anesthetized with isofluorane (2%) and positioned in a stereotaxic instrument with the head tilted at a 15° angle to the horizontal plane (nose down). A guide cannula (22 gauge) was implanted into the lateral ventricle as previously described (Valentino and Foote, 1988). For intra-LC injections, double guide cannulae (26 gauge, C/C dist. 2.2 mm, Plastics One, Roanoke, VA) were implanted with the following coordinates relative to lambda: AP −3.4 mm, ML ±1.1 mm, and DV 5.1 mm below the brain surface. Guide cannulae were affixed to skull and skull screws with cranioplastic cement. An obdurator was inserted into guide cannulae to prevent occlusion. Following 4 days of postsurgical recovery, rats were restricted to 10–15 g food per day, with 85% of free-feeding weight as a guideline, for the remainder of the experiment. Water remained available ad libitum.
Attentional Set Shifting Task
Procedures for the AST were similar to previous studies (Birrell and Brown, 2000; Lapiz-Bluhm et al, 2008; Liston et al, 2006). The testing apparatus was a custom-built white rectangular Plexiglas arena (inner dimensions: 75 × 40 × 30 cm) (Lapiz-Bluhm et al, 2008). Two ceramic pots (internal rim diameter 10 cm; depth 10 cm) were placed at one end of the arena. Each pot was distinguished by a pair of cues along two stimulus dimensions: (1) the medium contained within the pot and (2) an odor applied to the pot (Supplementary Table S1). Food reward (1/4 peanut butter chip) was placed at the bottom of one of the pots and buried with the digging medium. Beginning after 5 days of food restriction, the behavioral procedure was conducted over 3 days for each rat as follows:
Day 1: habituation. Rats were trained to dig reliably for food reward in the pots. Two unscented pots were placed in the home cage and baited, with the reward covered with increasing amounts of sawdust. Rats were required to dig for food within 5 min in order to move on to the next step. After rats learned to reliably retrieve the food from fully baited pots, they were transferred to the testing arena and given three consecutive trials to retrieve the reward from both sawdust-filled pots.
Day 2: training. Rats were trained to complete a series of simple discrimination tasks to a criterion of six consecutive correct trials, in which food was associated with one of two odors (eg, citronella vs lavender) and then one of the two digging mediums (green paper pellets vs Alpha-Dri bedding). All rats were trained using the same stimulus exemplars and in the same order. The positive and negative cues for each rat were randomly determined and equally represented.
Day 3: testing. Rats were tested on a series of five discriminations (Supplementary Table S1). The criterion to proceed to the next stage was the completion of six consecutive correct trials. Stage 1 was a simple discrimination (SD), in which the rat was required to discriminate between two digging media, only one of which predicted the food reward, in unscented pots. Stage 2 was a compound discrimination (CD) for which the same discrimination was required as in the SD, but irrelevant stimuli from a new dimension (odor) were introduced. Stage 3 was an intradimensional attentional shift (IDS), in which two new exemplars from each dimension were introduced, but the task-relevant dimension (medium) was unchanged. Stage 4 tested reversal learning where the reinforcement was associated with the alternate medium as in the previous IDS stage. Stage 5 involved an extradimensional attentional shift (EDS), in which two new exemplars from each dimension were introduced and the relevant dimension was also changed from medium to odor. The assignment of each exemplar in a pair as being positive or negative in a given stage, as well as the left–right positioning of the pots in the arena on each trial, were determined randomly in advance.
CRF Microinjection
Aliquots (10 μg) of ovine CRF (American Peptide Company, Sunnyvale, CA) were kept at −20 °C until use. On the day of the experiment, CRF was dissolved in artificial cerebrospinal fluid (ACSF) and ACSF or CRF were injected 10 min before beginning the AST. Microinjections were performed by lowering a stainless steel injector cannula (28 gauge for i.c.v. and 33 gauge for LC) with a length of 1 mm longer than the guide cannulae into the lateral ventricle or LC region. Animals received i.c.v. injections of ACSF (3 μl) or CRF (30, 100, and 300 ng in 3 μl ACSF) and bilateral intra-LC injections of ACSF (200 nl) or CRF (2, 6, or 20 ng in 200 ACSF). The i.c.v. doses of CRF are comparable to those used in other behavioral studies (Howard et al, 2008; Spina et al, 2002; Sutton et al, 1982). The intra-LC CRF doses are on the linear part of the CRF dose–response curve for increasing LC neuronal discharge and norepinephrine release in forebrain targets (Curtis et al, 1997; Page and Abercrombie, 1999). CRF or vehicle was infused over a 1-min period using a syringe pump and cannulae were left in place for an additional 60 s to minimize the backflow into the injection track. After 10 min, the rats were placed in the testing arena.
Histology
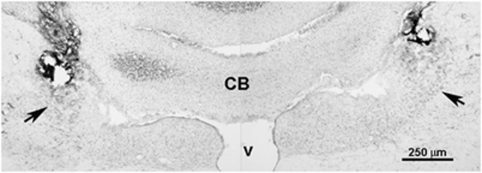
After completing the EDS component (15 min), rats were anesthetized with isofluorane and pontamine sky blue dye was injected through the i.c.v. (3 μl) or LC (200 nl) cannulae to verify placement. Rats were transcardially perfused with heparinized saline followed by 4% paraformaldehyde. Brains were removed, postfixed overnight, and placed in 30% sucrose with 0.1% sodium azide for at least 48 h. Frozen serial 30 μm coronal sections through the LC were cut on a cryostat and stained with neutral red to visualize cannulae placements. Animals were accepted for behavioral analysis and further cortical c-fos and p44/42ERK determination only when one or both injection needle placements were located within the LC (Figure 1).
Figure 1.
Brightfield photomicrograph of a section through the LC showing histological verification of the bilateral injection sites. The figure is a montage of right and left images of the same section. The section is counterstained with Neutral red. Arrows point to the LC. CB, cerebellum; V, ventricle.
C-fos and p44/42ERK Immunohistochemistry
Frozen serial 30 μm coronal sections through the frontal cortex were cut on a cryostat, collected into four wells, and stored at −20 °C in cryoprotectant until all of the brains were obtained so that sections could be processed for immunohistochemistry at the same time. Sections were rinsed to remove cryoprotectant and incubated in 0.75% H2O2 in phosphate buffer for 30 min. Sections were processed to visualize c-fos immunoreactivity as previously described (Carr et al, 2010), with the exception that the rabbit antibody directed against c-fos was obtained from Dr Paul Sawchenko (The Salk Institute, San Diego, CA) and used in a concentration of 1 : 20 000. Immunohistochemical visualization of p44/42ERK was performed on different sections from the same rats using the rabbit monoclonal antibody raised against p44/42ERK1/2 (1 : 1000, Cell Signaling no. 4370). This antibody specifically recognizes activated ERK, but it is not selective for the two isoenzymes, ERK1 and ERK2. The reaction was identical to that described above for c-fos with the exception that nickel was omitted from the DAB solution.
Data Analysis
Trials to reach criterion during each stage were recorded for each rat. The effects of different doses were analyzed using a two-way repeated measures ANOVA with stage as the within factor. The Student–Neuman–Keuls method was used post hoc to determine statistically significant differences between dose groups for a particular stage. Additionally, a comparison between stages within the ACSF group was done to verify differences between IDS and EDS stages.
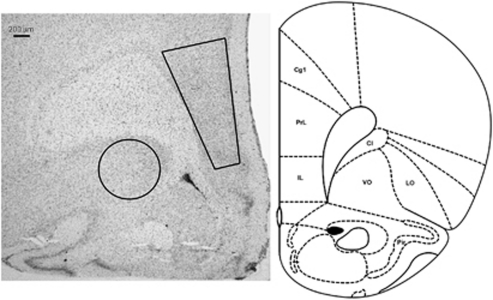
Sections were visualized on a Zeiss Axiovert 25 and digital images were obtained using a Leica DFC 480 camera and imaging software by an individual blinded to the treatment group. Immunoreactive profiles were sampled in the same area of medial prefrontal cortex or orbitofrontal cortex of each section by creating a region-of-interest shape that was superimposed on all other sections in the same region (Figure 2). The c-fos profiles were counted within these areas using Image J. Immunoreactive p44/42ERK profiles were counted manually. At least two sections per animal were used to count immunoreactive profiles and the number of profiles per section was averaged for each subject and the group mean determined from these values. Group data were compared using a one-way factorial ANOVA with t-test for post hoc analysis.
Figure 2.
Region of prefrontal cortex in which immunoreactive profiles were quantified. The brightfield photomicrograph on the left shows a representative section through the frontal cortex at the level of the areas of prefrontal cortex in which immunoreactive cells were quantified. The region of interest in which cells were counted in the medial prefrontal cortex is drawn as a polygon that covers the prelimbic and infralimbic cortex. The region of interest in which cells were counted in the orbitofrontal cortex is drawn as a circle. The photomicrograph is juxtaposed to the representative section from the Rat Brain Atlas (Swanson, 1992). CG1, cingulate cortex; Cl, claustrum; IL, infralimbic cortex; LO, lateral orbitofrontal cortex; Pir, piriform cortex; PrL, prelimbic cortex; VO, ventral orbitofrontal cortex.
RESULTS
Effects of Intracerebroventricular CRF on Attentional Set Shifting
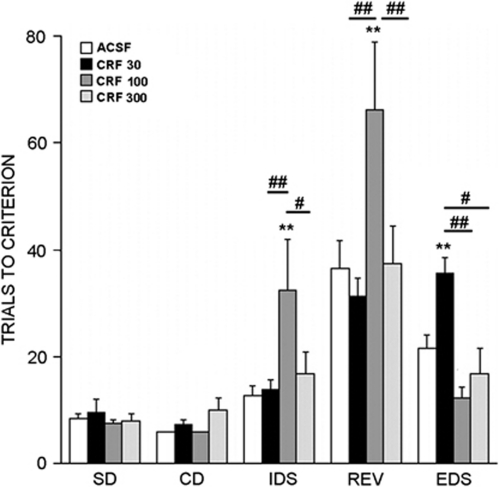
A total of 27 rats that were implanted with i.c.v cannula completed all stages of the AST. Rats administered 1000 ng CRF (i.c.v.) were unable to perform the task from the beginning stages, and hence the highest dose administered was 300 ng. The overall two-way repeated measures ANOVA indicated a trend for an effect of dose (F(3, 23)=2.8, p=0.06), an effect of stage (F(4, 92)=53.4, p<0.001), and a dose × stage interaction (F(12, 92)=6.1, p<0.001). Analysis of only ACSF rats indicated that the mean number of trials to reach criterion was greater for the EDS compared with the IDS stage (p<0.05, Student–Newman–Keuls method).
Figure 3 shows that CRF administered i.c.v. impaired different components of the task depending on the dose. CRF (100 ng, i.c.v.) impaired IDS (p=0.002) and reversal learning (p<0.001) and this effect diminished with a higher dose. Impairment of EDS was produced by the lowest dose of CRF (30 ng) but was not seen with higher doses (p<0.005).
Figure 3.
Intracerebroventricularly administered CRF (ng dose) impairs different components of the AST. The bars indicate the mean number of trials necessary to reach the criterion for simple discrimination (SD), compound discrimination (CD), intradimensional shift (IDS), reversal (REV), and extradimensional shift (EDS) components of the task. Bars are the mean of 4–10 rats for group. Vertical lines represent SEM. **p<0.005, compared with ACSF; #p<0.05, ##p<0.005 compared with other CRF doses.
Effects of Intra-LC CRF on Attentional Set Shifting
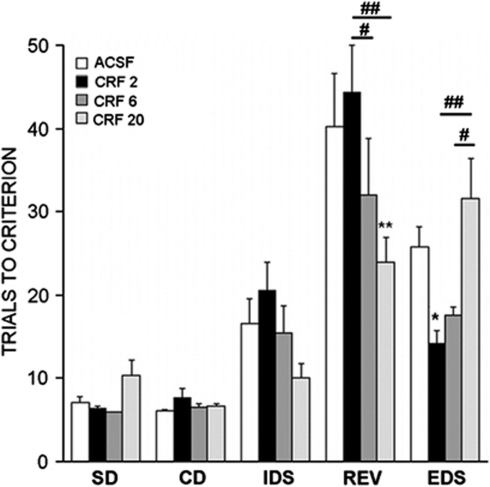
A total of 25 rats implanted with intra-LC cannula completed all stages of the task. The overall two-way repeated measures ANOVA indicated no effect of dose (F(3, 21)=1.3), an effect of stage (F(4, 84)=51.6, p<0.001), and a dose × stage interaction (F(12, 84)=3.2, p<0.001). Analysis of only ACSF rats indicated that the mean number of trials to reach criterion was greater for the EDS compared with the IDS stage (p<0.05, Student–Newman–Keuls method).
The effects of CRF administered into the LC were markedly different from those administered i.c.v. (Figure 4). Particularly, no dose of CRF impaired performance in any of the stages. The highest dose of CRF (20 ng) improved reversal learning (p=0.002). There was an inverted U-shaped dose–response relationship for CRF effects on EDS performance. The lowest dose (2 ng) improved performance (p<0.05) and there was a trend for enhanced EDS performance after 6 ng CRF (p<0.07). However, these improvements reversed as the dose was increased to 20 ng.
Figure 4.
Intra-LC-administered CRF (ng dose) has differential effects on components of the AST. The bars indicate the mean number of trials necessary to reach the criterion for simple discrimination (SD), compound discrimination (CD), intradimensional shift (IDS), reversal (REV), and extradimensional shift (EDS) components of the task. Bars are the mean of 5–8 rats for group. Vertical lines represent SEM. *p<0.05, **p<0.005, compared with ACSF; #p<0.05, ##p<0.005 compared with other CRF doses.
Each CRF dose group had a number of misplaced injections. For the 2 and 6 ng doses, there were four cases each in which the bilateral cannula assembly was shifted such that one cannula was lateral and the other was medial to the LC. For the 20 ng dose, there was one case in which the cannula assembly was shifted as described above and three injections were placed into the nearby dorsal raphe nucleus. These injections outside of the LC gave a very different pattern of responses and dose–response relationship compared with injections within the LC (Supplementary Figure S1).
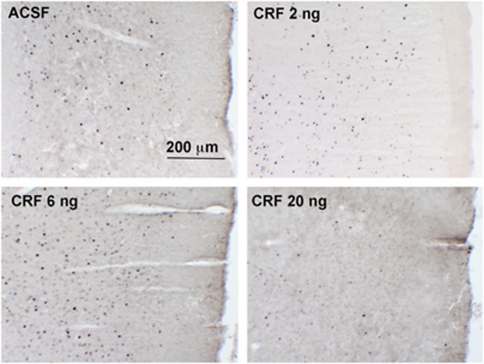
Effects of Intra-LC CRF on C-Fos and p44/42ERK Profiles in Medial Prefrontal Cortex
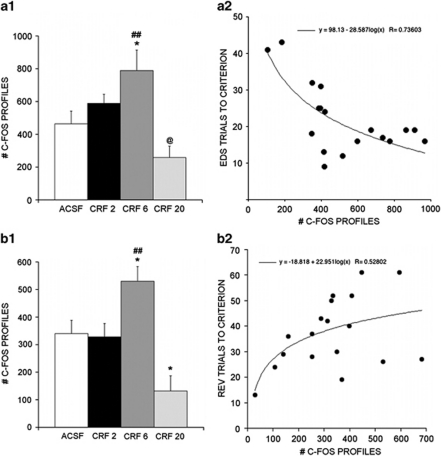
Figure 5 shows c-fos profiles in the medial prefrontal cortex in representative sections from rats administered ACSF or different CRF doses into the LC. There was a significant effect of intra-LC CRF dose on the number of c-fos-immunoreactive profiles in the medial prefrontal cortex (F(3, 14)=6.4, p<0.01). Similar to the effect of CRF on EDS performance, the dose–response relationship for inducing c-fos expression resembled an inverted U-shaped curve with the 6 ng dose producing effects that were significantly different than ACSF (p<0.05) and 20 ng CRF (p<0.001; Figure 6a1). Although the 2.0 ng CRF dose effectively improved EDS performance, it did not produce a statistically significant increase in the number of c-fos profiles in the medial prefrontal cortex. Nonetheless, the number of c-fos profiles in medial prefrontal cortex was negatively correlated with the number of EDS trials to criterion as determined by both linear (F(1, 16)=9.3, p<0.01) and log (F(1, 16)=18.9, p=0.0005) transformation, consistent with a positive association between cellular activation in this region and EDS performance (Figure 6a2).
Figure 5.
Effects of intra-LC CRF (ng dose) on c-fos expression in the medial prefrontal cortex. Photomicrographs of c-fos-immunoreactive profiles in medial prefrontal cortex of rats administered ACSF or different doses of CRF. Top is dorsal and right is medial.
Figure 6.
Quantification of c-fos in the medial prefrontal cortex and orbitofrontal cortex. (a1) Bars represent the mean number of c-fos profiles in the medial prefrontal cortex after injection of ACSF or different doses (ng) of CRF into the LC (n=4–5 rats). *p<0.05 compared with ACSF, ##p<0.005, compared with CRF 20 ng, @p<0.05 compared with CRF 2 ng. (a2) Each point in the scatterplot represents the number of c-fos profiles in medial prefrontal cortex and trials to criterion during extradimensional set shifting for an individual rat regardless of treatment. The line represents the equation describing the relationship based on log transformation of the number of c-fos profiles. There was a significant negative relationship between number of c-fos profiles and trials to criterion indicating a positive relationship with performance on the task (F(1, 16)=18.9, p<0.0005). (b1) Bars represent the mean number of c-fos profiles in the orbitofrontal cortex after injection of ACSF or different doses (ng) of CRF into the LC (n=4–5 rats). *p<0.05 compared with ACSF, ##p<0.005, compared with CRF 20 ng. (b2) Each point in the scatterplot represents the number of c-fos profiles in the orbitofrontal cortex and trials to criterion during reversal learning for an individual rat regardless of treatment. The line represents the equation describing the relationship based on log transformation of the number of c-fos profiles. There was a significant positive relationship between number of c-fos profiles and trials to criterion indicating a negative relationship with performance on the task (F(1, 16)=9.1, p<0.05).
The CRF dose–response relationship for c-fos in the orbitofrontal cortex resembled that for the medial prefrontal cortex (Figure 6b1). There was a significant effect of intra-LC CRF dose (F(3, 14)=9.1, p<0.005) with the 6 ng dose being associated with an increase in c-fos (p<0.05) and the 20 ng dose associated with a decrease (p<0.05) compared with ACSF-treated rats. The number of c-fos profiles in the orbitofrontal cortex was not linearly correlated with trials to criterion for reversal learning (F(1, 16)=3.1, p=0.1) but there was a significant positive correlation between these end points upon log transformation of the data (F(1, 16)=6.2, p<0.05) indicative of a negative association with performance (Figure 6b2). Interestingly, the CRF dose that improved reversal learning (20 ng) was associated with the least number of c-fos profiles in orbitofrontal cortex and a dose that had no effect on reversal learning was associated with increased c-fos expression in the orbitofrontal cortex.
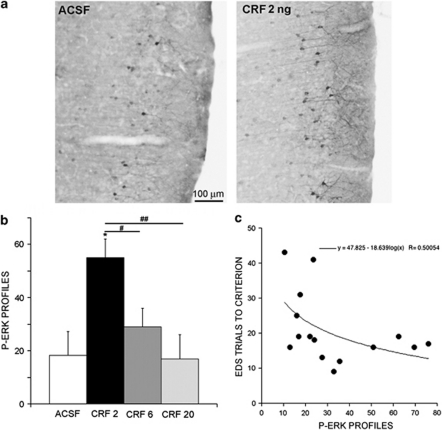
Figure 7a shows representative sections of p44/42ERK-expressing neurons in medial prefrontal cortex of rats administered ACSF or CRF (2 ng) intra-LC. CRF (2 ng) increased the number of p44/42ERK-expressing neurons in the medial prefrontal cortex (F(3, 11)=6.1, p=0.01). There was a trend for the number of p44/42ERK profiles to be negatively correlated with EDS trials to criterion (F(1, 13)=4.3, p=0.057; Figure 7b).
Figure 7.
Expression of p44/42ERK in medial prefrontal cortex induced by CRF injections into the LC. (a) Photomicrograph of p44/42ERK-expressing cells in medial prefrontal cortex of rats administered either ACSF or CRF 2 ng. Top is dorsal and right is medial. (b) Bars indicate the mean number of p44/42ERK profiles in the medial prefrontal cortex of rats administered ACSF or different doses (ng) of CRF into the LC (n=3–5 rats). *p<0.05 compared with ACSF, #p<0.05, ##p<0.005 compared with different doses of CRF. (c) The line represents the equation describing the relationship based on log transformation of the number of c-fos profiles. There was a negative relationship between number of p44/42ERK profiles and trials to criterion indicating a trend of a positive relationship with performance on the task. F(1, 13)=4.3, p=0.057.
Because ERK is upstream from c-fos (Monje et al, 2005; Runyan et al, 2004), a correlation between the two end points was tested (Supplementary Figure S2). When all cases were considered, there was no correlation between the two measures (r2=0.12; F(1, 13)=1.8). However, omission of four cases with the highest number of fos profiles resulted in a highly correlated relationship between p44/42ERK and c-fos expression (r2=0.73; F(1, 9)=24, p<0.001).
DISCUSSION
This is the first report of the effects of the stress neuropeptide, CRF, on attentional set shifting behavior, an animal model of cognitive flexibility. CRF had qualitatively different effects depending on its route of administration. When administered into the lateral ventricle such that it could affect multiple brain regions, CRF generally disrupted different aspects of AST performance with an inverted U-shaped dose–response relationship. In contrast, when administered into the LC, CRF improved reversal learning and EDS performance. Given that the intra-LC doses of CRF also increase LC neuronal discharge rate and norepinephrine release in terminal fields (Curtis et al, 1997; Page and Abercrombie, 1999), these findings are consistent with other evidence for a role of norepinephrine in the medial prefrontal cortex in EDS (Lapiz and Morilak, 2006). Although a causal relationship between c-fos in the medial prefrontal cortex and EDS performance has not been established, the correlation between CRF effects on EDS performance and c-fos-immunoreactive profiles suggests that norepinephrine-elicited activation of prefrontal cortex neurons facilitates EDS performance. The inverted U-shaped dose–response relationship for CRF effects on both EDS behavior and c-fos expression may reflect the similar dose–response relationship for norepinephrine effects on cortical neuronal activity, where moderate concentrations facilitate transmission and high concentrations are inhibitory (Berridge and Waterhouse, 2003; Devilbiss and Waterhouse, 2000; Waterhouse et al, 1998). Together, the results suggest a model whereby low levels of CRF released in the LC during acute stress facilitate cognitive flexibility through a moderate activation of the LC-norepinephrine system. This would be adaptive in a life-threatening dynamic environment. On the contrary, excessive CRF, as may occur in pathological states, could have opposing effects by eliciting levels of norepinephrine that inhibit prefrontal cortex activity.
Effects of Intracerebroventricular CRF on Behavior
Intracerebroventricular CRF elicits active behaviors including increased locomotor activity in a familiar environment, grooming, burying, and aggressive behaviors (Eaves et al, 1985; Howard et al, 2008; Koob et al, 1984; Sutton et al, 1982; Tazi et al, 1987). In certain rodent models, CRF has anxiogenic effects expressed as effects in the elevated plus maze, enhanced conditioned freezing, decreased activity in open field, potentiated startle, and decreased punished responding (Britton et al, 1985; Cole and Koob, 1988; De Boer et al, 1992; Liang et al, 1992). In contrast, studies of the effects of CRF on cognitive processes are lacking. CRF has been reported to increase accuracy in the five-choice serial reaction time test (Ohmura et al, 2009). In the present study, the highest CRF dose that affected AST performance (100 ng, i.c.v.) is somewhat lower than doses that have previously been reported to produce behavioral effects (300–1000 ng, i.c.v.) (Spina et al, 2002) and rats administered 1000 ng CRF were unable to perform the task in the current study.
The lack of a monotonic dose–response relationship for CRF at any stage of the AST may reflect its actions at diverse sites that are accessed by i.c.v. CRF. For example, CRF facilitates conditioned learning when administered into the hippocampus but causes deficits in learning when administered into the lateral septum, the two sites that it would be likely to access via the lateral ventricle (Radulovic et al, 1999). CRF (100 ng, i.c.v.) directly inhibits the dorsal raphe-serotonin system, which would be detrimental to reversal learning (Kirby et al, 2000; Price et al, 1998). However, higher doses (300 ng, i.c.v.) increase LC activity, which may counter some of these effects (see below).
Effects of Intra-LC CRF on Behavior
In contrast to the numerous studies on the behavioral effects of CRF administered i.c.v., studies on the behavioral consequences of intra-LC CRF are scant. One study reported increased activity by 100 ng CRF, both in a cage and in response to swim stress (Butler et al, 1990). All CRF doses used in the present study (2–20 ng) increase LC firing rate and extracellular norepinephrine levels in forebrain regions and are on the linear part of the CRF dose–response curve (Curtis et al, 1997; Page and Abercrombie, 1999). At the same time that CRF increases tonic LC firing rate, it decreases sensory-evoked phasic discharge (Valentino and Foote, 1987, 1988). A shift from phasic to high tonic LC activity is associated with increased arousal and a shift from the maintenance of ongoing behaviors that have known outcomes to going off-task in a search for alternate outcomes (Aston-Jones and Cohen, 2005). This should be expressed as an increase in behavioral flexibility and enhanced EDS performance in the AST. Consistent with this, idazoxan, which activates the LC-norepinephrine system by antagonizing α2-adrenergic receptors, facilitated attentional shifts (Devauges and Sara, 1990). In the present study, CRF, which activates the LC, also improved EDS performance. However, the CRF effect exhibited an inverted U-shaped dose response and was completely absent at a dose (20 ng) that remains effective at increasing tonic LC discharge rate and releasing norepinephrine in forebrain targets (Curtis et al, 1997; Page and Abercrombie, 1999). This suggests complex relationships between norepinephrine and target neurons involved in EDS.
The medial prefrontal cortex is a target region of the LC that is integral to behavioral flexibility and optimal EDS performance (Dias et al, 1996a, 1996b; Milner, 1963). Prefrontal cortical networks generate and maintain representations of rules to guide behavior via the activity of recurrent networks that encode information about stimuli in their absence (Goldman-Rakic, 1995). Norepinephrine, derived solely from LC neurons, acts in the medial prefrontal cortex to strengthen connections between neurons with shared inputs (Wang et al, 2007). Antidepressants that increase norepinephrine levels improve EDS performance and, conversely, lesions of the LC-norepinephrine system impair performance (Bondi et al, 2010; Bondi et al, 2007; Lapiz et al, 2007; McGaughy et al, 2008; Roberts et al, 1994). Similar to the behavioral effects of intra-LC CRF in the present study, the relationships between norepinephrine concentration and activity and functionality of prefrontal cortical neurons resemble an inverted U-shaped curve (Arnsten, 2009; Berridge and Waterhouse, 2003). This is thought to be due, in part, to the existence of multiple noradrenergic receptor subtypes with differential affinities for norepinephrine. For example, it has been proposed that activation of high-affinity α2-adrenergic receptors by moderate levels of norepinephrine is associated with optimal performance in prefrontal cortical-dependent working memory tasks because of enhanced activity and strengthened connections among task-relevant prefrontal cortex networks (Wang et al, 2007). Conversely, activation of low-affinity α1-adrenergic receptors by high norepinephrine levels has been associated with impaired performance in working memory tasks (Birnbaum et al, 1999). On the other hand, evidence for an involvement of α2-adrenergic receptors in stress-induced impairments in EDS performance and for α1-adrenergic receptors in the beneficial effects of norepinephrine reuptake inhibitors emphasizes that the role of various adrenergic receptors in specific cognitive functions is not clearcut (Bondi et al, 2010). Regardless of our knowledge of the adrenergic receptors involved, the biphasic (inverted U-shape) dose–response relationship for norepinephrine effects on forebrain neuronal activity is well documented (Berridge and Waterhouse, 2003). Because the CRF doses tested in this study are on the linear portion of the dose–response curve for LC activation and norepinephrine release (Curtis et al, 1997), a biphasic dose–response relationship for CRF effects on EDS performance must reflect the postsynaptic dose response to norepinephrine.
Effects of Intra-LC CRF on c-fos and p44/42ERK
The CRF dose–response curves for c-fos and p44/42ERK expression in the medial prefrontal cortex resembled that for facilitation of EDS behavior in being biphasic. The correlation between expression of these molecules with EDS performance implicates norepinephrine-induced activation of the medial prefrontal cortical neurons in the behavior. The relationship between the signaling molecules and EDS performance was best fit by a log transformation of the data underscoring the complexity of the relationship and suggesting that within a certain range, minimal increases in neuronal activation may have a large effect on performance. Although causality between prefrontal cortical neuronal activation as indicated by c-fos or ERK expression and improvement in EDS performance was not established here, others have demonstrated that pharmacological improvements in attentional set shifting in rats with medial prefrontal cortical lesions is associated with increased c-fos expression in spared neurons (Tait et al, 2009).
Although these experiments were not designed to elucidate the cellular signaling underlying the ability of the medial prefrontal cortex to facilitate EDS, the results suggest the potential involvement and interactions between p44/42ERK and c-fos. A role for c-fos is supported by the high correlation between c-fos expression and EDS performance. On the other hand, the most behaviorally effective dose (2 ng) was the only one to increase p44/42ERK expression. The ERK pathway in the prefrontal cortex has been implicated in consolidation and recall of recent memory (Leon et al, 2010). Evidence from trace fear conditioning studies also support a role for ERK in the prefrontal cortex in memory retention and memory for the relevancy of the training condition (Runyan et al, 2004). Given that p44/42ERK is upstream of c-fos (Kim and Cochran, 2000; Monje et al, 2005), we speculate that norepinephrine in the prefrontal cortex engages a signaling cascade where the sequential expression of these molecules underlies the ability to optimize EDS performance. The strong correlation between p44/42ERK and low-to-moderate levels of c-fos expression is consistent with this, and loss of this correlation with high c-fos expression may be explained as feedback inhibition of the ERK pathway by c-fos.
The finding that the highest CRF dose improved reversal learning is consistent with the concept that high tonic activity would promote going off-task and reduce perseverance. Supporting this notion, a previous study in monkeys found that high, but not low, doses of an α2-adrenergic agonist improved reversal learning in a visual discrimination task (Steere and Arnsten, 1997). Nonetheless, this finding was unexpected because performance in reversal learning is often attributed to serotonergic effects in the orbitofrontal cortex. It is possible that the enhanced reversal learning with this high dose of CRF was the indirect result of LC activation of the dorsal raphe-serotonin system. The dorsal raphe-serotonin system is thought to be under tonic activation by α1-adrenergic receptors (Baraban and Aghajanian, 1980; Bortolozzi and Artigas, 2003). Unlike the correlation between c-fos in the medial prefrontal cortex and EDS performance, c-fos in the orbitofrontal cortex was not positively correlated with reversal learning, and the effective CRF dose resulted in the least amount of c-fos expression in this region, whereas an ineffective dose was associated with increased fos expression. This suggests that alternate signaling cascades are involved in modulation of reversal learning by the orbitofrontal cortex.
CRF Modulation of LC Activity and Cognition During Stress
The present findings argue against the general idea that acute stress impairs cognition, at least through its effects on the LC-norepinephrine system. The levels of LC activation produced by CRF doses that improved EDS performance (2–6 ng) range from 25 to 60% above baseline (Curtis et al, 1997). In comparison, hypotensive stress, which increases LC discharge through CRF release in the LC, produces a similar magnitude of LC activation (Curtis et al, 2001; Page et al, 1993; Valentino et al, 1991). Similarly, exposure to predator odor increases LC discharge rate by 30–50% through a CRF-dependent mechanism (Curtis and Valentino, 2008). Both of these stressors also bias LC discharge toward a high tonic state. The present results suggest that a function of acute stress-elicited levels of CRF in the LC is to shift the mode of discharge toward a high tonic state in an effort to promote behavioral flexibility through its projections and impact on cells in the medial prefrontal cortex. Excessive CRF, which may be released with particularly severe stressors or in pathological states where CRF is hypersecreted, would not improve, and could potentially impair, cognitive flexibility, possibly as a result of the inhibitory effects of norepinephrine on prefrontal cortical neurons.
Acknowledgments
This research was supported by PHS Grant MH40008, DA09082, MH14654 (KS), ARO 58077-LS-DRP (S Bhatnagar), National Basic Research Program of China (2007CB512306), Institute of Psychology Beijing (09CX133013), and the Chinese Academy of Sciences (KSCX2-EW-J-8). We acknowledge the assistance of Rosemary Trumbull and statistical consultation with Nayla Chaijale.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci. 1981a;1:887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981b;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry. 1999;46:1309–1320. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Baraban JM, Aghajanian GK. Suppression of firing activity of 5-HT neurons in the dorsal raphe by alpha-adrenoceptor antagonists. Neuropharmacology. 1980;19:355–363. doi: 10.1016/0028-3908(80)90187-2. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Foote SL. Effects of locus coeruleus activation on electroencephalographic activity in the neocortex and hippocampus. J Neurosci. 1991;11:3135–3145. doi: 10.1523/JNEUROSCI.11-10-03135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Page ME, Valentino RJ, Foote SL. Effects of locus coeruleus inactivation on electroencephalographic activity in neocortex and hippocampus. Neuroscience. 1993;55:381–383. doi: 10.1016/0306-4522(93)90507-c. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Beylin AV, Shors TJ. Stress enhances excitatory trace eyeblink conditioning and opposes acquisition of inhibitory conditioning. Behav Neurosci. 1998;112:1327–1338. doi: 10.1037//0735-7044.112.6.1327. [DOI] [PubMed] [Google Scholar]
- Birnbaum S, Gobeske KT, Auerbach J, Taylor JR, Arnsten AF. A role for norepinephrine in stress-induced cognitive deficits: alpha-1-adrenoceptor mediation in the prefrontal cortex. Biol Psychiatry. 1999;46:1266–1274. doi: 10.1016/s0006-3223(99)00138-9. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Barrera G, Lapiz MD, Bedard T, Mahan A, Morilak DA. Noradrenergic facilitation of shock-probe defensive burying in lateral septum of rats, and modulation by chronic treatment with desipramine. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:482–495. doi: 10.1016/j.pnpbp.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Bondi CO, Jett JD, Morilak DA. Beneficial effects of desipramine on cognitive function of chronically stressed rats are mediated by alpha1-adrenergic receptors in medial prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:913–923. doi: 10.1016/j.pnpbp.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolozzi A, Artigas F. Control of 5-hydroxytryptamine release in the dorsal raphe nucleus by the noradrenergic system in rat brain. Role of alpha-adrenoceptors. Neuropsychopharmacology. 2003;28:421–434. doi: 10.1038/sj.npp.1300061. [DOI] [PubMed] [Google Scholar]
- Britton K, Britton K, Morgan J, Rivier J, Vale W, Koob G. Chlordiazepoxide attenuates CRF-induced response suppression in the conflict test. Psychopharmacology. 1985;86:170–174. doi: 10.1007/BF00431704. [DOI] [PubMed] [Google Scholar]
- Butler PD, Weiss JM, Stout JC, Nemeroff CB. Corticotropin-releasing factor produces fear-enhancing and behavioral activating effects following infusion into the LC. J Neurosci. 1990;10:176–183. doi: 10.1523/JNEUROSCI.10-01-00176.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr GV, Bangasser DA, Bethea T, Young M, Valentino RJ, Lucki I. Antidepressant-like effects of kappa-opioid receptor antagonists in Wistar Kyoto rats. Neuropsychopharmacology. 2010;35:752–763. doi: 10.1038/npp.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole BJ, Koob GF. Propranolol antagonizes the enhanced conditioned fear produced by corticotropin-releasing factor. J Pharmacol Exp Ther. 1988;247:902–910. [PubMed] [Google Scholar]
- Curtis AL, Bello NT, Valentino RJ. Endogenous opioids in the locus coeruleus function to limit the noradrenergic response to stress. J Neurosci. 2001;21:RC152. doi: 10.1523/JNEUROSCI.21-13-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AL, Florin-Lechner SM, Pavcovich LA, Valentino RJ. Activation of the locus coeruleus noradrenergic system by intracoerulear microinfusion of corticotropin-releasing factor: effects on discharge rate, cortical norepinephrine levels and cortical electroencephalographic activity. J Pharmacol Exp Ther. 1997;281:163–172. [PubMed] [Google Scholar]
- Curtis AL, Valentino RJ.2008Evidence for regulation of locus coeruleus neuronal activity by endogenous corticotropin-releasing factor during exposure to predator odor in unanesthetized ratsProgram No. 187.14.2008. Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience, 2008 (online).
- De Boer SF, Katz JL, Valentino RJ. Common mechanisms underlying the proconflict effects of corticotropin-releasing factor, a benzodiazepine inverse agonist and electric footshock. J Pharmacol Exp Ther. 1992;262:335–342. [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joels M. Stress and cognition: are corticosteroids good or bad guys. Trends Neurosci. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- Devauges V, Sara SJ. Activation of the noradrenergic system facilitates an attentional shift in the rat. Behav Brain Res. 1990;39:19–28. doi: 10.1016/0166-4328(90)90118-x. [DOI] [PubMed] [Google Scholar]
- Devilbiss DM, Waterhouse BD. Norepinephrine exhibits two distinct profiles of action on sensory cortical neuron responses to excitatory synaptic stimuli. Synapse. 2000;37:273–282. doi: 10.1002/1098-2396(20000915)37:4<273::AID-SYN4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996a;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci. 1996b;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Eaves M, Thatcher-Britton K, Rivier J, Vale W, Koob GF. Effects of corticotropin-releasing factor on locomotor activity in hypophysectomized rats. Peptides. 1985;6:923–926. doi: 10.1016/0196-9781(85)90323-7. [DOI] [PubMed] [Google Scholar]
- Faraji J, Metz GA, Sutherland RJ. Stress after hippocampal stroke enhances spatial performance in rats. Physiol Behav. 2011;102:389–399. doi: 10.1016/j.physbeh.2010.11.032. [DOI] [PubMed] [Google Scholar]
- Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci USA. 1980;77:3033–3037. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Architecture of the prefrontal cortex and the central executive. Ann NY Acad Sci. 1995;769:71–83. doi: 10.1111/j.1749-6632.1995.tb38132.x. [DOI] [PubMed] [Google Scholar]
- Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard O, Carr GV, Hill TE, Valentino RJ, Lucki I. Differential blockade of CRF-evoked behaviors by depletion of norepinephrine and serotonin in rats. Psychopharmacology (Berl) 2008;199:569–582. doi: 10.1007/s00213-008-1179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DW, Cochran BH. Extracellular signal-regulated kinase binds to TFII-I and regulates its activation of the c-fos promoter. Mol Cell Biol. 2000;20:1140–1148. doi: 10.1128/mcb.20.4.1140-1148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Rice K, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Koob GF, Swerdlow N, Selligson M, Eaves M, Sutton R, Rivier J, et al. Effects of alpha-flupenthixol and naloxone on CRF-induced locomotor activation. Neuroendocrinology. 1984;39:459–464. doi: 10.1159/000124021. [DOI] [PubMed] [Google Scholar]
- Lapiz MD, Bondi CO, Morilak DA. Chronic treatment with desipramine improves cognitive performance of rats in an attentional set-shifting test. Neuropsychopharmacology. 2007;32:1000–1010. doi: 10.1038/sj.npp.1301235. [DOI] [PubMed] [Google Scholar]
- Lapiz MD, Morilak DA. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience. 2006;137:1039–1049. doi: 10.1016/j.neuroscience.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Lapiz-Bluhm MD, Bondi CO, Doyen J, Rodriguez GA, Bedard-Arana T, Morilak DA. Behavioural assays to model cognitive and affective dimensions of depression and anxiety in rats. J Neuroendocrinol. 2008;20:1115–1137. doi: 10.1111/j.1365-2826.2008.01772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon WC, Bruno MA, Allard S, Nader K, Cuello AC. Engagement of the PFC in consolidation and recall of recent spatial memory. Learn Mem. 2010;17:297–305. doi: 10.1101/lm.1804410. [DOI] [PubMed] [Google Scholar]
- Liang KC, Melia KR, Miserendino MJD, Falls WA, Campeau S, Davis M. Corticotropin-releasing factor: long-lasting facilitation of the acoustic startle reflex. J Neurosci. 1992;12:2303–2312. doi: 10.1523/JNEUROSCI.12-06-02303.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughlin SE, Foote SL, Grzanna R. Efferent projections of nucleus locus coeruleus: morphologic subpopulations have different efferent targets. Neuroscience. 1986;18:307–319. doi: 10.1016/0306-4522(86)90156-9. [DOI] [PubMed] [Google Scholar]
- Luine V, Martinez C, Villegas M, Magarinos AM, McEwen BS. Restraint stress reversibly enhances spatial memory performance. Physiol Behav. 1996;59:27–32. doi: 10.1016/0031-9384(95)02016-0. [DOI] [PubMed] [Google Scholar]
- Marin MF, Lord C, Andrews J, Juster RP, Sindi S, Arsenault-Lapierre G, et al. 2011Chronic stress, cognitive functioning and mental health Neurobiol Learn Memin press). [DOI] [PubMed]
- McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann NY Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Ross RS, Eichenbaum H. Noradrenergic, but not cholinergic, deafferentation of prefrontal cortex impairs attentional set-shifting. Neuroscience. 2008;153:63–71. doi: 10.1016/j.neuroscience.2008.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B. Effects of different brain lesions on card sorting. Arch Neurol. 1963;9:100–110. [Google Scholar]
- Monje P, Hernandez-Losa J, Lyons RJ, Castellone MD, Gutkind JS. Regulation of the transcriptional activity of c-Fos by ERK. A novel role for the prolyl isomerase PIN1. J Biol Chem. 2005;280:35081–35084. doi: 10.1074/jbc.C500353200. [DOI] [PubMed] [Google Scholar]
- Ohmura Y, Yamaguchi T, Futami Y, Togashi H, Izumi T, Matsumoto M, et al. Corticotropin releasing factor enhances attentional function as assessed by the five-choice serial reaction time task in rats. Behav Brain Res. 2009;198:429–433. doi: 10.1016/j.bbr.2008.11.025. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Revs. 1991;43:425–474. [PubMed] [Google Scholar]
- Page ME, Abercrombie ED. Discrete local application of corticotropin-releasing factor increases locus coeruleus discharge and extracellular norepinephrine in rat hippocampus. Synapse. 1999;33:304–313. doi: 10.1002/(SICI)1098-2396(19990915)33:4<304::AID-SYN7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Page ME, Berridge CW, Foote SL, Valentino RJ. Corticotropin-releasing factor in the locus coeruleus mediates EEG activation associated with hypotensive stress. Neurosci Lett. 1993;164:81–84. doi: 10.1016/0304-3940(93)90862-f. [DOI] [PubMed] [Google Scholar]
- Price ML, Curtis AL, Kirby LG, Valentino RJ, Lucki I. Effects of corticotropin-releasing factor on brain serotonergic activity. Neuropsychopharmacology. 1998;18:492–502. doi: 10.1016/S0893-133X(97)00197-8. [DOI] [PubMed] [Google Scholar]
- Radulovic J, Ruhmann A, Liepold T, Spiess J. Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2. J Neurosci. 1999;19:5016–5025. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AC, De Salvia MA, Wilkinson LS, Collins P, Muir JL, Everitt BJ, et al. 6-Hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analog of the Wisconsin Card Sort Test: possible interactions with subcortical dopamine. J Neurosci. 1994;14:2531–2544. doi: 10.1523/JNEUROSCI.14-05-02531.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyan JD, Moore AN, Dash PK. A role for prefrontal cortex in memory storage for trace fear conditioning. J Neurosci. 2004;24:1288–1295. doi: 10.1523/JNEUROSCI.4880-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Spina MG, Merlo-Pich E, Akwa Y, Balducci C, Basso AM, Zorrilla EP, et al. Time-dependent induction of anxiogenic-like effects after central infusion of urocortin or corticotropin-releasing factor in the rat. Psychopharmacology (Berl) 2002;160:113–121. doi: 10.1007/s00213-001-0940-y. [DOI] [PubMed] [Google Scholar]
- Steere JC, Arnsten AF. The alpha-2A noradrenergic receptor agonist guanfacine improves visual object discrimination reversal performance in aged rhesus monkeys. Behav Neurosci. 1997;111:883–891. doi: 10.1037//0735-7044.111.5.883. [DOI] [PubMed] [Google Scholar]
- Sutton RE, Koob GF, LeMoal M, Rivier J, Vale W. Corticotropin-releasing factor produces behavioral activation in rats. Nature. 1982;297:331–333. doi: 10.1038/297331a0. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps Structure of the Rat Brain. Elsevier: Amsterdam; 1992. [Google Scholar]
- Swanson LW, Hartman BK. The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat using dopamine-B-hydroxylase as a marker. J Comp Neurol. 1976;163:467–506. doi: 10.1002/cne.901630406. [DOI] [PubMed] [Google Scholar]
- Tait DS, Brown VJ, Farovik A, Theobald DE, Dalley JW, Robbins TW. Lesions of the dorsal noradrenergic bundle impair attentional set-shifting in the rat. Eur J Neurosci. 2007;25:3719–3724. doi: 10.1111/j.1460-9568.2007.05612.x. [DOI] [PubMed] [Google Scholar]
- Tait DS, Marston HM, Shahid M, Brown VJ. Asenapine restores cognitive flexibility in rats with medial prefrontal cortex lesions. Psychopharmacology (Berl) 2009;202:295–306. doi: 10.1007/s00213-008-1364-8. [DOI] [PubMed] [Google Scholar]
- Tazi A, Dantzer R, LeMoal M, Rivier J, Vale W, Koob GF. Corticotropin-releasing factor antagonist blocks stress-induced fighting in rats. Reg Peptides. 1987;18:37–42. doi: 10.1016/0167-0115(87)90048-6. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL. Corticotropin-releasing factor disrupts sensory responses of brain noradrenergic neurons. Neuroendocrinology. 1987;45:28–36. doi: 10.1159/000124700. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL. Corticotropin-releasing factor increases tonic but not sensory-evoked activity of noradrenergic locus coeruleus neurons in unanesthetized rats. J Neurosci. 1988;8:1016–1025. doi: 10.1523/JNEUROSCI.08-03-01016.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Page ME, Curtis AL. Activation of noradrenergic locus coeruleus neurons by hemodynamic stress is due to local release of corticotropin-releasing factor. Brain Res. 1991;555:25–34. doi: 10.1016/0006-8993(91)90855-p. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele EJ.2002Corticotropin-releasing factor: putative neurotransmitter actions of a neurohormoneIn: Pfaff D, Arnold A, Etgen A, Fahrbach S, Moss R, Rubin R (eds).Hormones, Brain and Behavior Vol 4Academic Press: San Diego; 81–102. [Google Scholar]
- Valentino RJ, Wehby RG. Corticotropin-releasing factor: evidence for a neurotransmitter role in the locus coeruleus during hemodynamic stress. Neuroendocrinology. 1988;48:674–677. doi: 10.1159/000125081. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EE, Valentino RJ. Corticotropin-releasing factor-containing axon terminals synapse onto catecholamine dendrites and may presynaptically modulate other afferents in the rostral pole of the nucleus locus coeruleus in the rat brain. J Comp Neurol. 1996;364:523–534. doi: 10.1002/(SICI)1096-9861(19960115)364:3<523::AID-CNE10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Moises HC, Woodward DJ. Phasic activation of the locus coeruleus enhances responses of primary sensory cortical neurons to peripheral receptive field stimulation. Brain Res. 1998;790:33–44. doi: 10.1016/s0006-8993(98)00117-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.