Abstract
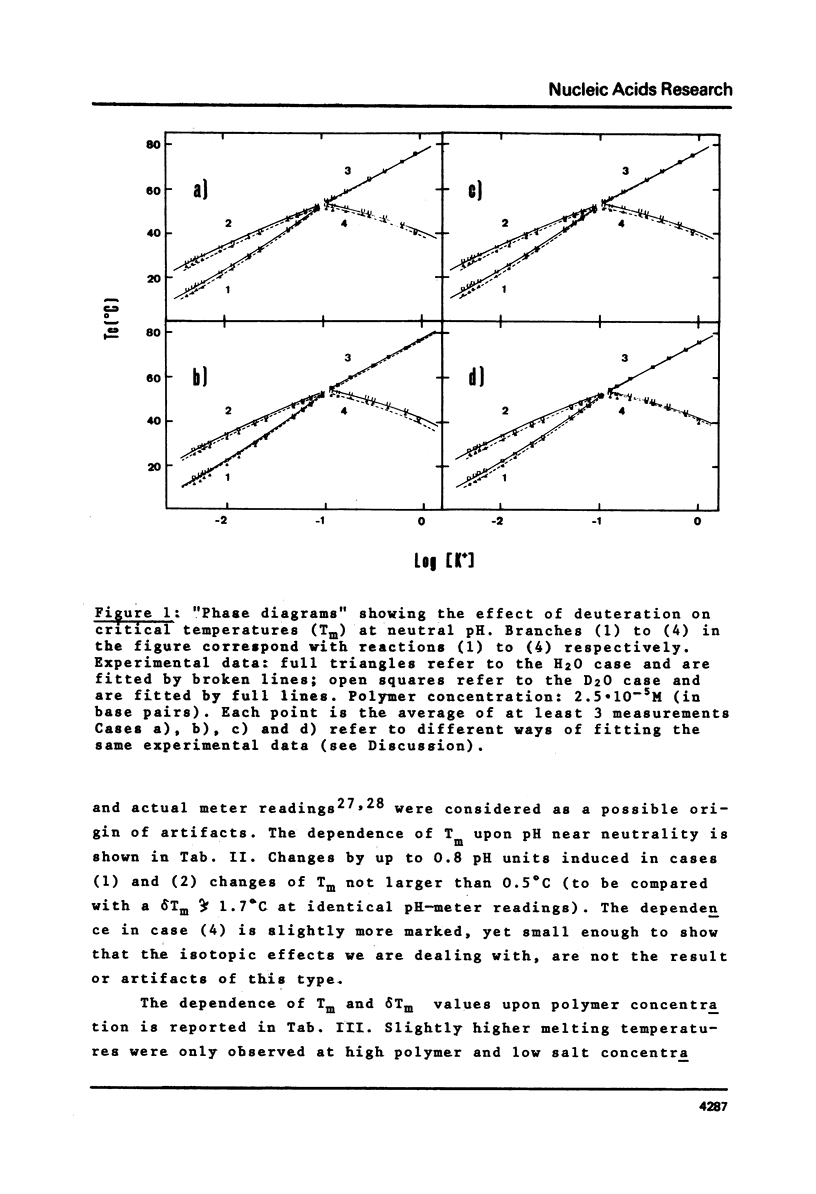
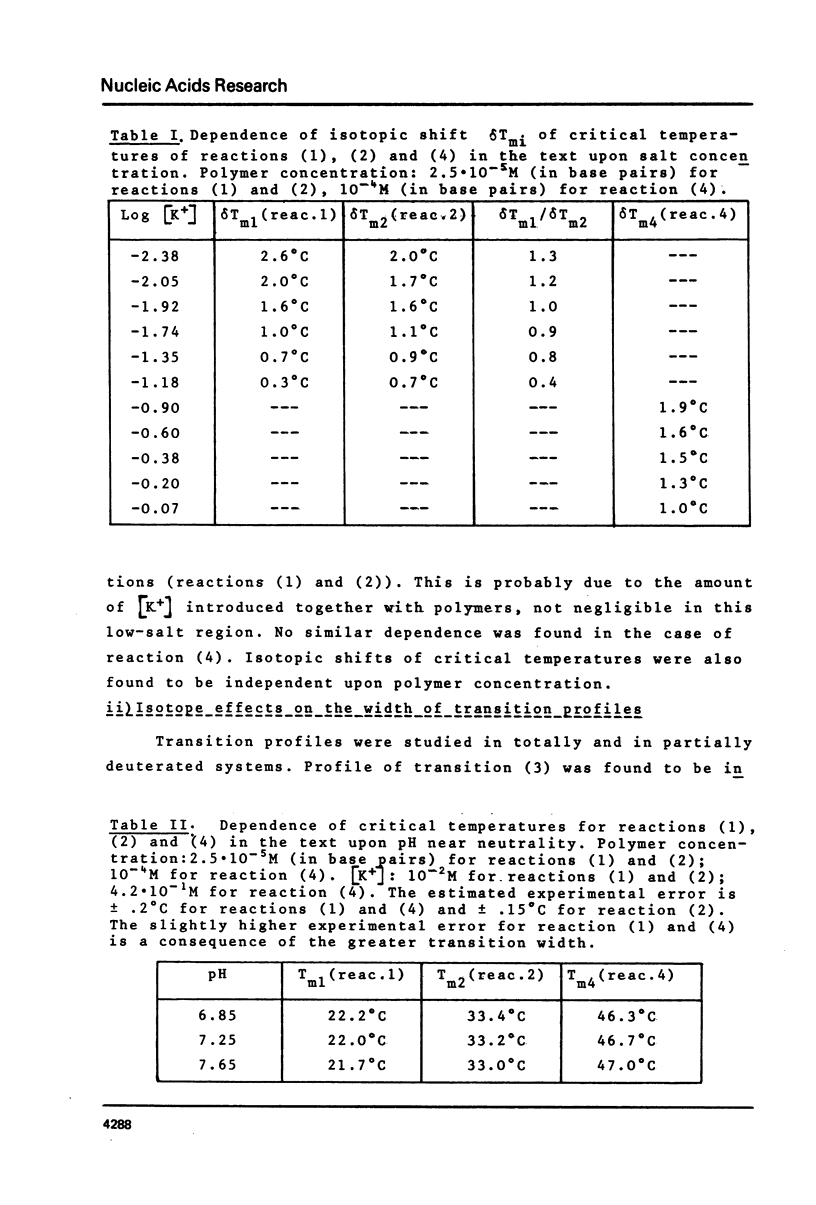
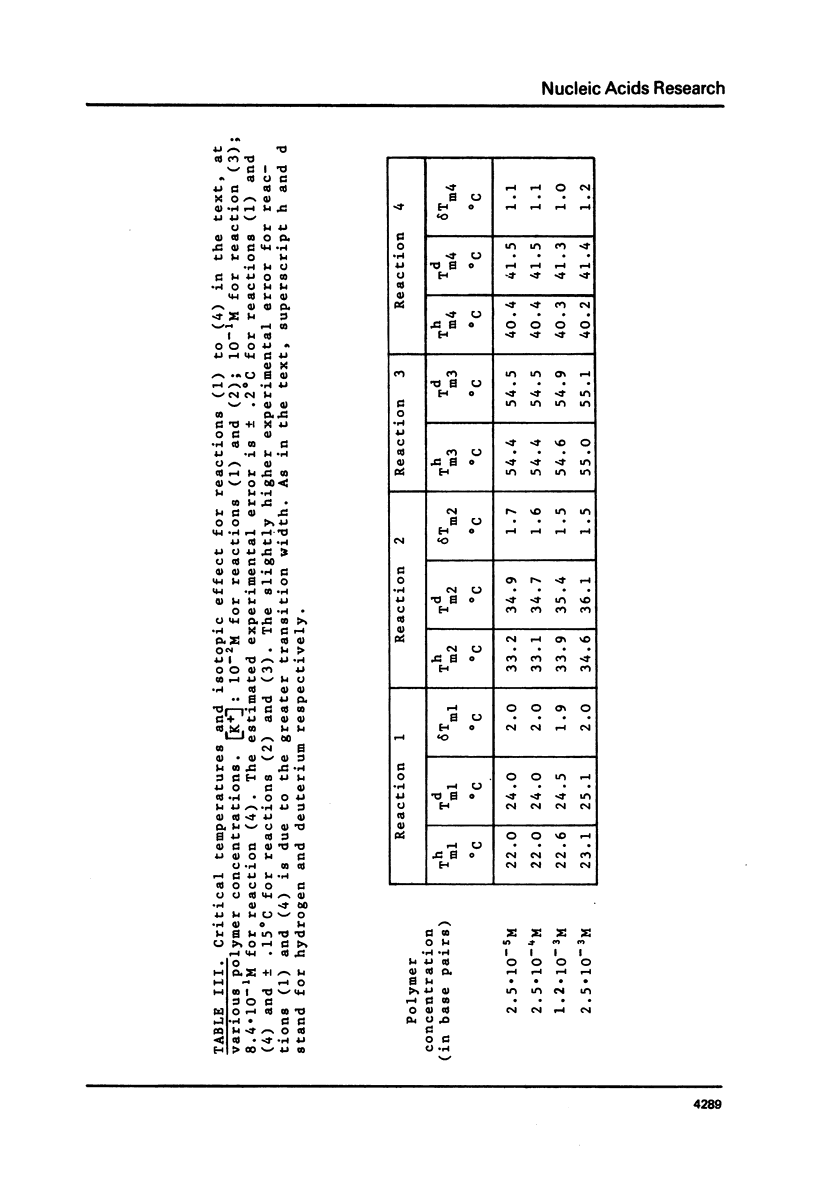
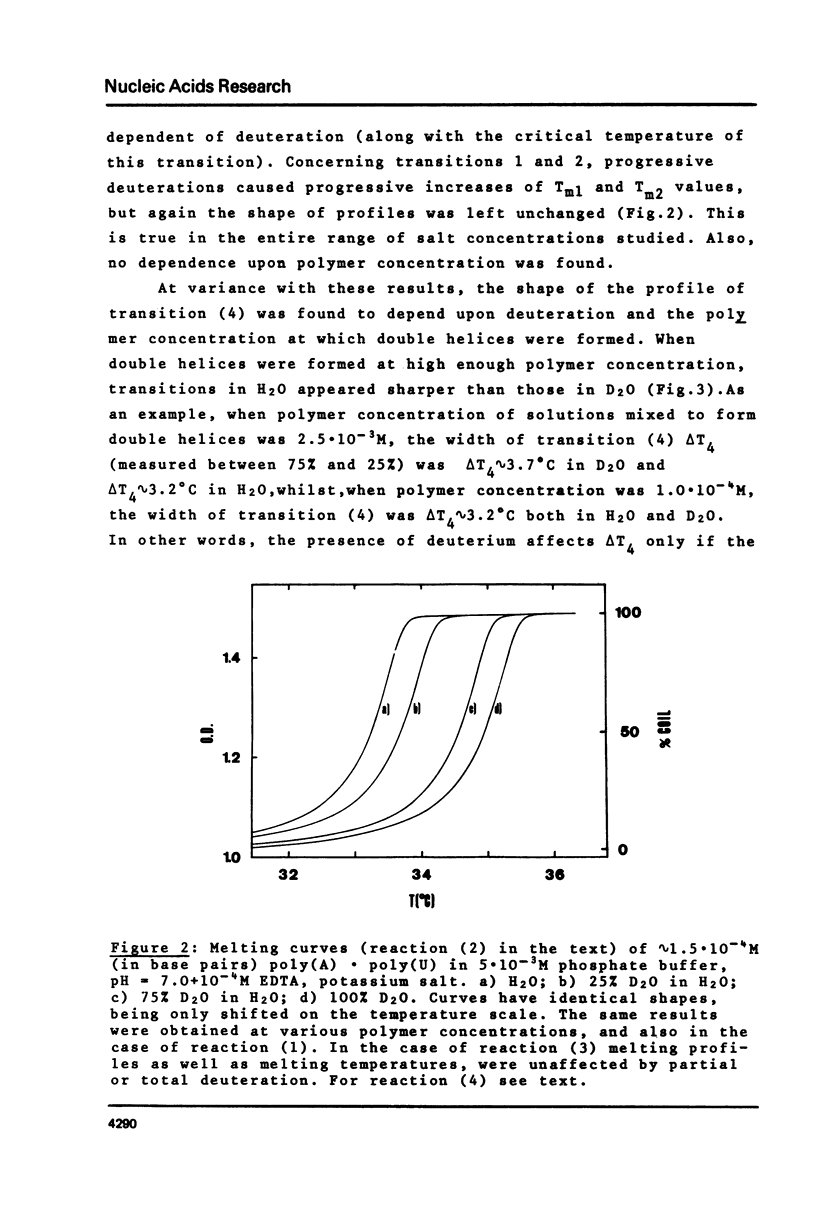
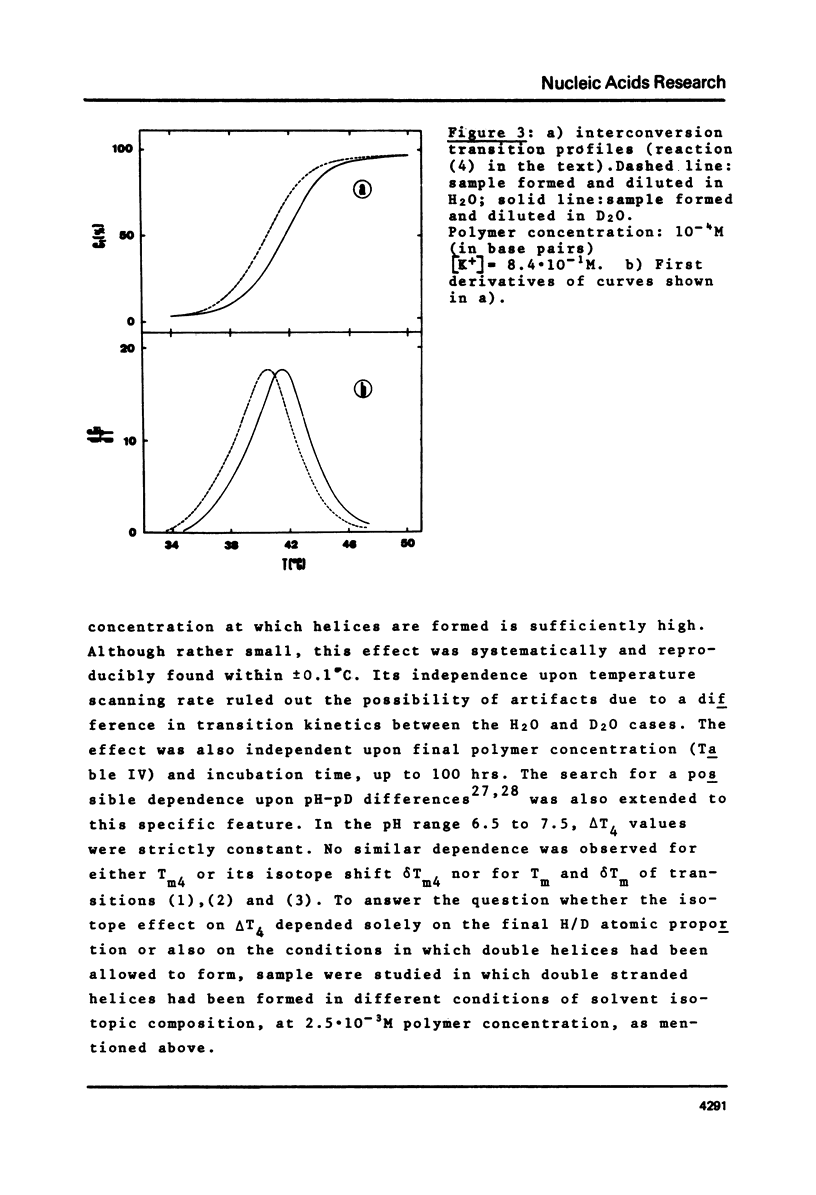
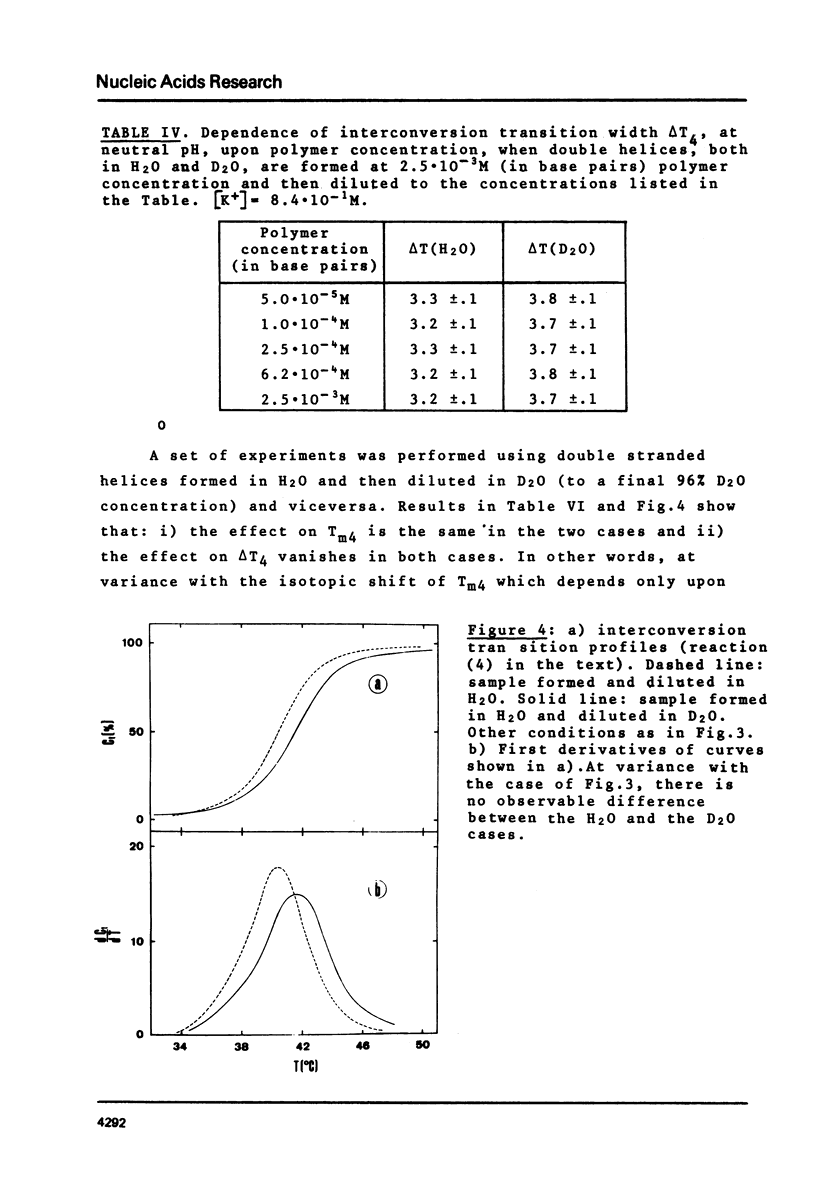
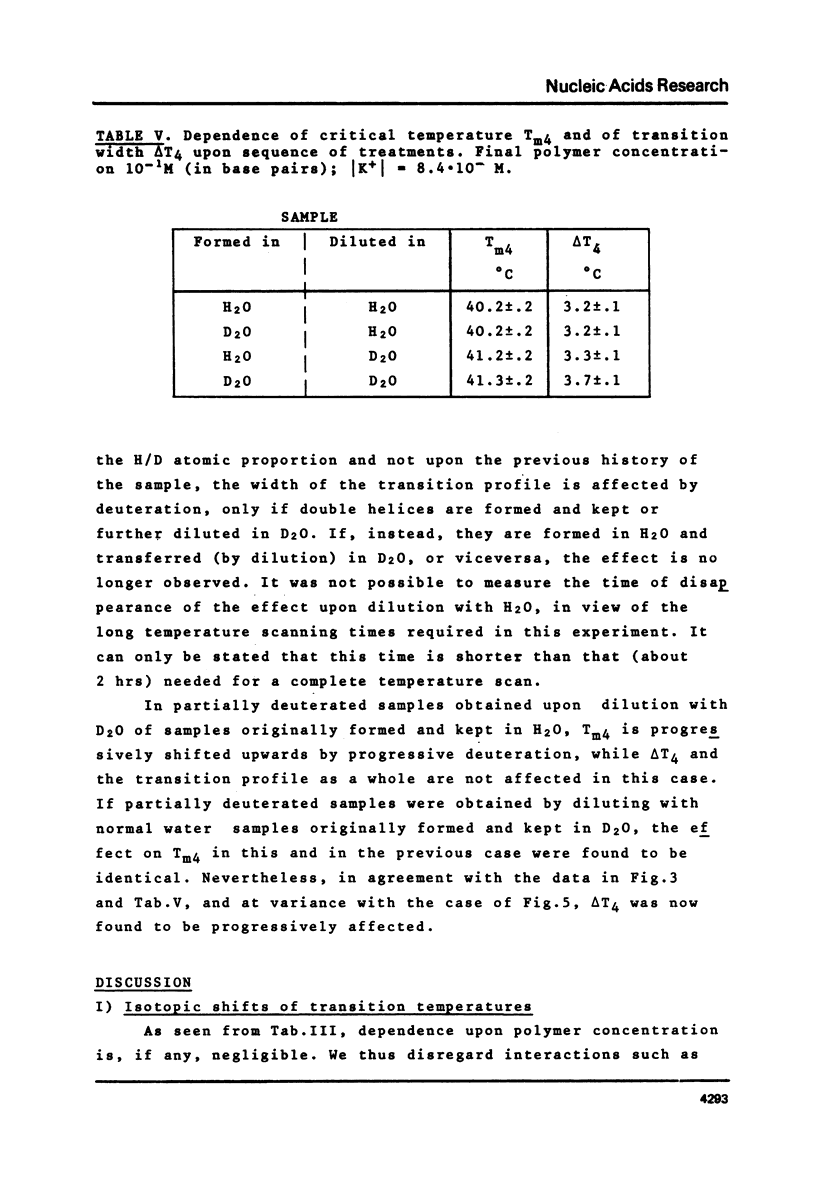
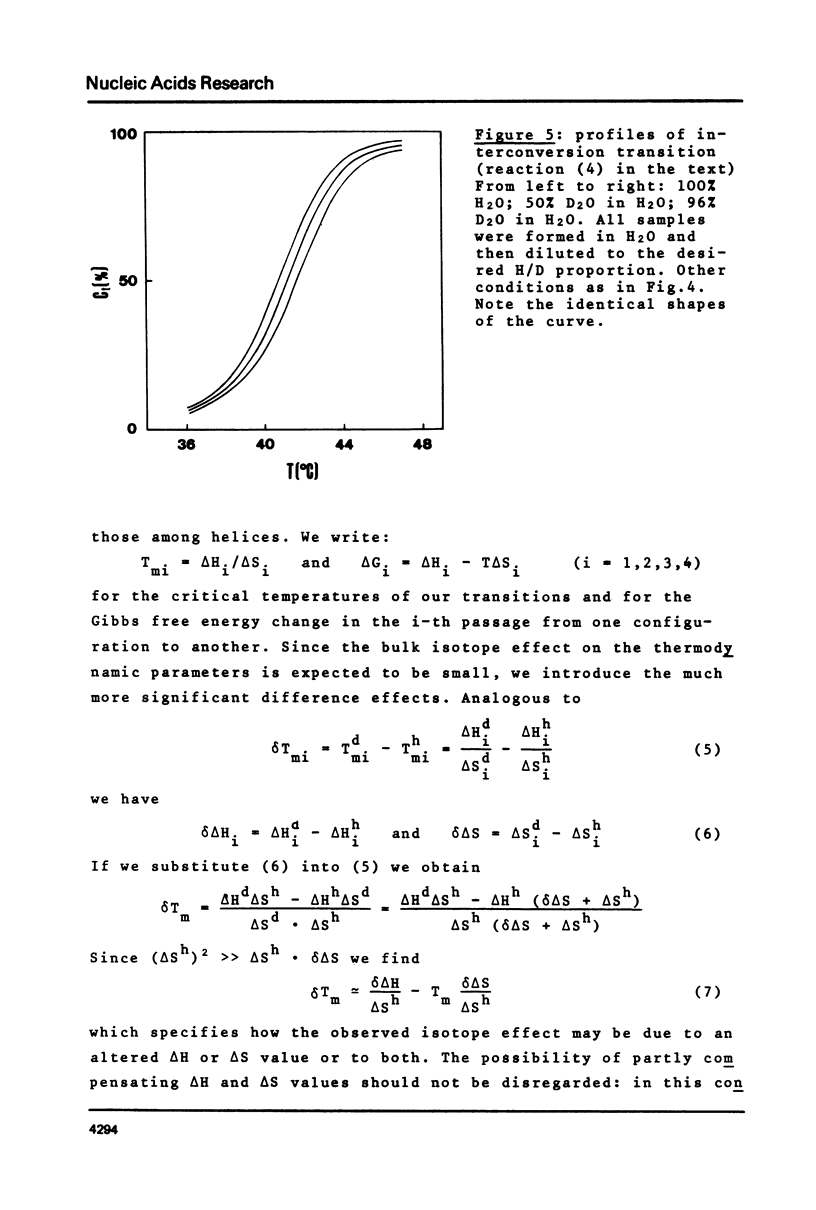
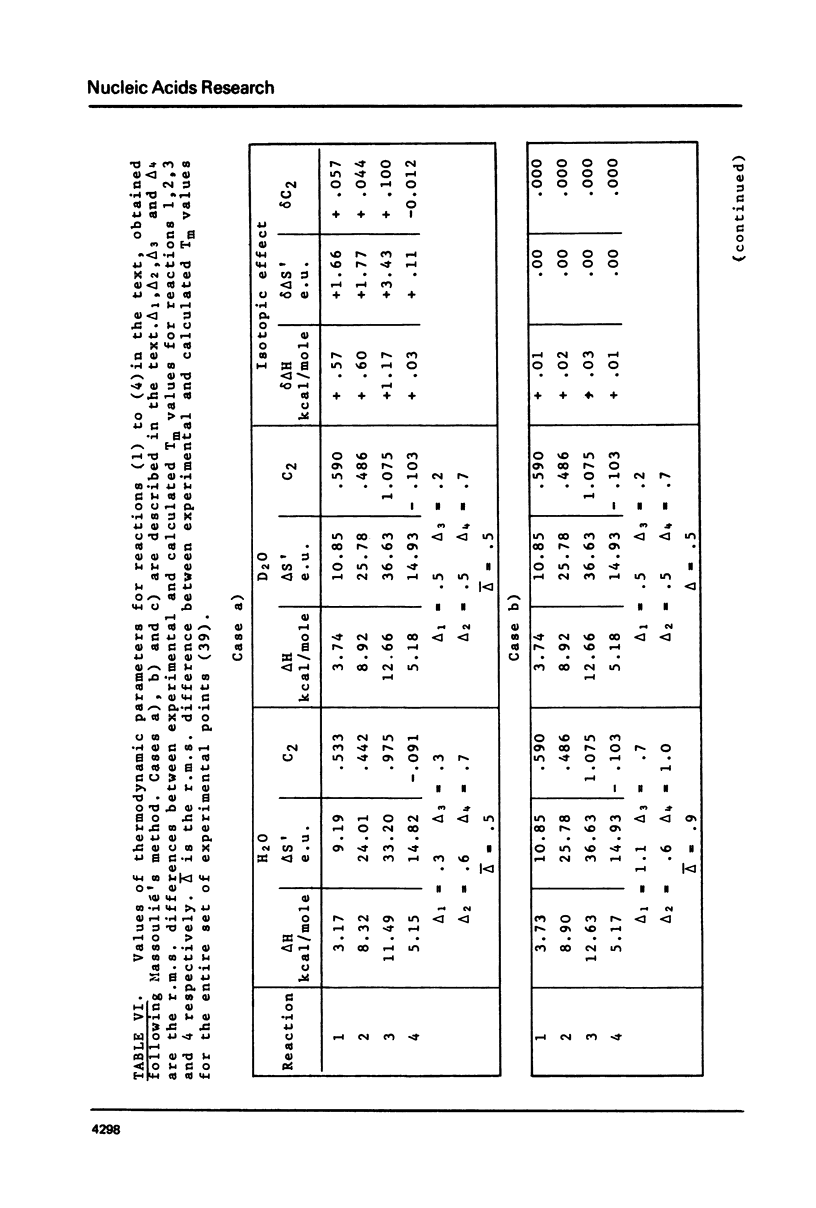
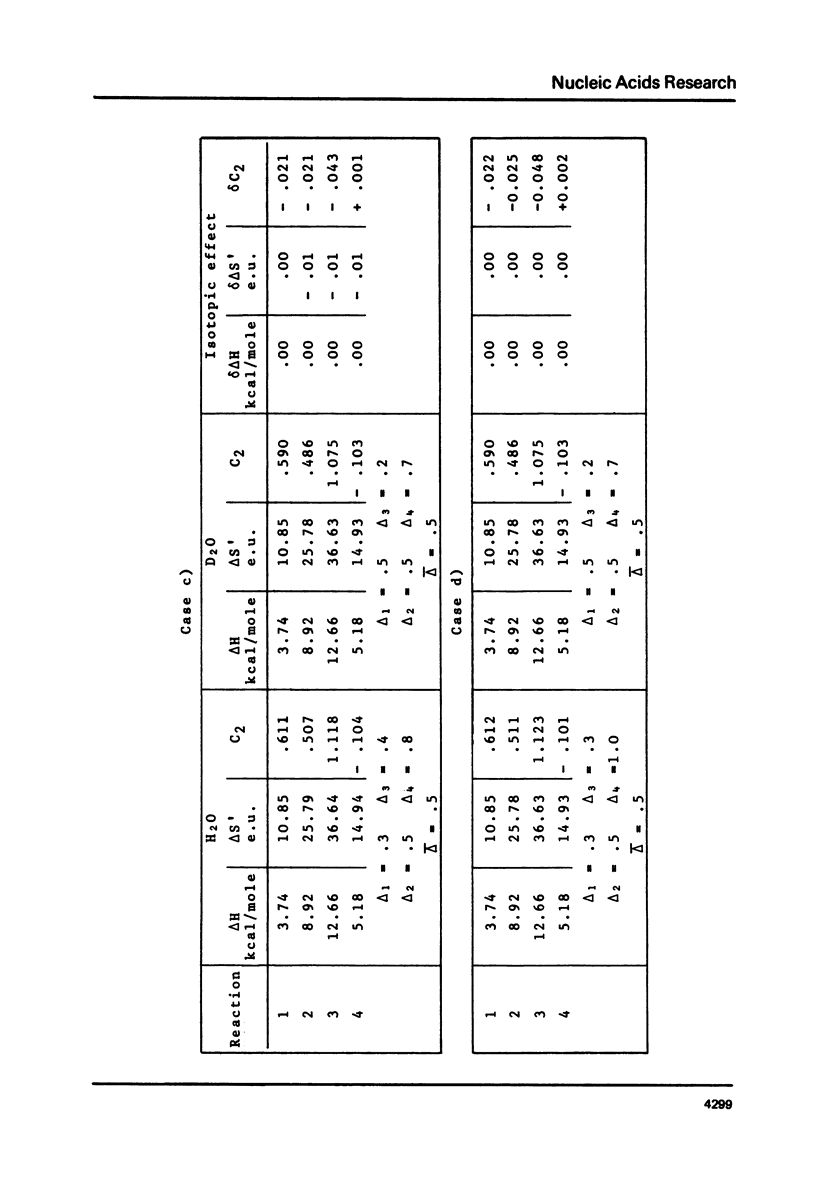
This is a study of the effect of total and partial deuteration of solvent on critical temperatures and profiles of all four reactions occurring in poly(A) x n poly(U) (n = 1 or 2) aqueous systems. The study was done at observational times not longer than hydrogen exchange times at base pairs in helically ordered structures, and it was extended to a wide range of salt concentrations at neutral pH. The dependence of stability of polymer helical order on hydrogen mass does not appear to be merely attributable to the stronger intrahelical deuterium bonding. Substituting Deuterium for Hydrogen implies a probably predominant modulation of the entrophy term of polymer-solvent interactions. Effects of deuteration on the width of the 2(poly(A) x poly (U)) leads to poly(A) x 2poly(U)+poly(A) interconversion reaction were also observed. They bear on the role of polymer-solvent interaction on pattern recognition leading to formation of ordered structures. They also bear on the role of the same interaction on the "breathing" of ordered structures of this type.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez J., Biltonen R. Nucleic acid-solvent interactions: temperature dependence of the heat of solution of thymine in water and ethanol. Biopolymers. 1973;12(8):1815–1828. doi: 10.1002/bip.1973.360120809. [DOI] [PubMed] [Google Scholar]
- Arnott S., Fulmer A., Scott W. E., Dea I. C., Moorhouse R., Rees D. A. The agarose double helix and its function in agarose gel structure. J Mol Biol. 1974 Dec 5;90(2):269–284. doi: 10.1016/0022-2836(74)90372-6. [DOI] [PubMed] [Google Scholar]
- Blake R. D., Fresco J. R. Polynucleotides. VII. Spectrophotometric study of the kinetics of formation of the two-stranded helical complex resulting from the interaction of polyriboadenylate and polyribouridylate. J Mol Biol. 1966 Aug;19(1):145–160. doi: 10.1016/s0022-2836(66)80057-8. [DOI] [PubMed] [Google Scholar]
- Blake R. D., Klotz L. C., Fresco J. R. Polynucleotides. IX. Temperature dependence of kinetics of complex formation in equimolar mixtures of polyriboadenylate and polyribouridylate. J Am Chem Soc. 1968 Jun 19;90(13):3556–3562. doi: 10.1021/ja01015a047. [DOI] [PubMed] [Google Scholar]
- Blake R. D., Massoulié J., Fresco J. R. Polynucleotides. 8. A spectral approach to the equilibria between polyriboadenylate and polyribouridylate and their complexes. J Mol Biol. 1967 Dec 14;30(2):291–308. [PubMed] [Google Scholar]
- Brahms J., Michelson A. M., Van Holde K. E. Adenylate oligomers in single- and double-strand conformation. J Mol Biol. 1966 Feb;15(2):467–488. doi: 10.1016/s0022-2836(66)80122-5. [DOI] [PubMed] [Google Scholar]
- Day R. O., Seeman N. C., Rosenberg J. M., Rich A. A crystalline fragment of the double helix: the structure of the dinucleoside phosphate guanylyl-3',5'-cytidine. Proc Natl Acad Sci U S A. 1973 Mar;70(3):849–853. doi: 10.1073/pnas.70.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dea I. C., McKinnon A. A., Rees D. A. Tertiary and quaternary structure in aqueous polysaccharide systems which model cell wall cohesion: reversible changes in conformation and association of agarose, carrageenan and galactomannans. J Mol Biol. 1972 Jul 14;68(1):153–172. doi: 10.1016/0022-2836(72)90270-7. [DOI] [PubMed] [Google Scholar]
- Eigen M., Pörschke D. Co-operative non-enzymic base recognition. I. Thermodynamics of the helix-coil transition of oligoriboadenylic acids at ACIDIC PH. J Mol Biol. 1970 Oct 14;53(1):123–141. doi: 10.1016/0022-2836(70)90049-5. [DOI] [PubMed] [Google Scholar]
- HERSKOVITS T. T., SINGER S. J., GEIDUSCHEK E. P. Nonaqueous solutions of DNA. Denaturation in methanol and ethanol. Arch Biochem Biophys. 1961 Jul;94:99–114. doi: 10.1016/0003-9861(61)90016-9. [DOI] [PubMed] [Google Scholar]
- KAUZMANN W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- Klotz I. M., Langerman N. R., Darnall D. W. Quaternary structure of proteins. Annu Rev Biochem. 1970;39:25–62. doi: 10.1146/annurev.bi.39.070170.000325. [DOI] [PubMed] [Google Scholar]
- Krakauer H., Sturtevant J. M. Heats of the helix-coil transitions of the poly A-poly U complexes. Biopolymers. 1968 Apr;6(4):491–512. doi: 10.1002/bip.1968.360060406. [DOI] [PubMed] [Google Scholar]
- Kresheck G. C., Schneider H., Scheraga H. A. The effect of D2-O on the thermal stability of proteins. Thermodynamic parameters for the transfer of model compounds from H2-O to D2-O. J Phys Chem. 1965 Sep;69(9):3132–3144. doi: 10.1021/j100893a054. [DOI] [PubMed] [Google Scholar]
- McConnell B., von Hippel P. H. Hydrogen exchange as a probe of the dynamic structure of DNA. I. General acid-base catalysis. J Mol Biol. 1970 Jun 14;50(2):297–316. doi: 10.1016/0022-2836(70)90194-4. [DOI] [PubMed] [Google Scholar]
- Rosenberg J. M., Seeman N. C., Kim J. J., Suddath F. L., Nicholas H. B., Rich A. Double helix at atomic resolution. Nature. 1973 May 18;243(5403):150–154. doi: 10.1038/243150a0. [DOI] [PubMed] [Google Scholar]
- Spodheim M., Neumann E. Kinetic analysis of the annealing period in the formation of the Poly (A)-2Poly (U) triple helix. Biopolymers. 1977 Feb;16(2):289–298. doi: 10.1002/bip.1977.360160206. [DOI] [PubMed] [Google Scholar]
- Teitelbaum H., Englander S. W. Open states in native polynucleotides. I. Hydrogen-exchange study of adenine-containing double helices. J Mol Biol. 1975 Feb 15;92(1):55–78. doi: 10.1016/0022-2836(75)90091-1. [DOI] [PubMed] [Google Scholar]
- Thiele D., Marck C., Schneider C., Guschlbauer W. Protonated polynucleotides structures - 23. The acid-base hysteresis of poly(dG).poly(dC). Nucleic Acids Res. 1978 Jun;5(6):1997–2012. doi: 10.1093/nar/5.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilescu D., Rix M. A. Mesure directe de l'éjection de cations Na+ hors des sites phosphates lors de la dénaturaction thermique du DNA. Biochim Biophys Acta. 1970 Feb 18;199(2):553–555. [PubMed] [Google Scholar]



