Abstract
Background
Mortality between stage I and II palliation for hypoplastic left heart syndrome (HLHS) has been associated with arrhythmias. The stage-related proportion, associations, and clinical impact of arrhythmias in patients with HLHS have not been evaluated. Also, arrhythmia subtypes have not been described in this patient group.
Methods
We performed a retrospective analysis of all patients at Duke University Medical Center who received one or more palliative stages for hypoplastic left heart syndrome from September 2000 to October 2008.
Results
Overall, 49/86 (57%) patients had 63 arrhythmias. The majority of arrhythmias occurred between stage I and II with 44/86 (51%) patients manifesting a new arrhythmia. Arrhythmias occurring in this interval tended to be associated with a higher mortality compared to arrhythmias occurring after stage II, OR = 3.2 [95%CI 0.84, 12.0] (p=0.09). Overall, mortality was similar in patients with and without arrhythmias (p=0.99). Supraventricular tachycardia was the most common arrhythmia (16/63; 25%) but persistent bradycardias (sinus node dysfunction or high grade atrioventricular block) had the worst clinical outcome with 73% mortality (8/11). There was no association between arrhythmia occurrence and degree of tricuspid regurgitation, left ventricular hypertension, genetic syndrome, type of stage I operation, or need for extracorporeal membrane oxygenation (ECMO).
Conclusions
A large proportion of patients with HLHS experience serious arrhythmias requiring therapy, especially between stages I and II. Persistent bradycardia following stage I is associated with a high mortality rate. Considering all arrhythmia patients, overall mortality was not different compared to the arrhythmia free group.
Introduction
Hypoplastic left heart syndrome (HLHS) is characterized by severe underdevelopment of left sided heart structures, causing the left ventricle to be incapable of supporting the systemic circulation. Current management of HLHS patients involves surgical palliations with three stages culminating in a diversion of all systemic venous return directly to the pulmonary arterial circulation (Fontan). Over the last several years, the operative and hospital mortality rates for these procedures have decreased. However, there remains a significant risk for morbidity and adverse events. Significant arrhythmias have been reported in this vulnerable period and may contribute to this high risk1–3.
While arrhythmia potential in the older completed Fontan patient is well characterized4, arrhythmia data following the earlier stages in HLHS patients is lacking. Specifically, the overall frequency, associations, and clinical impact of arrhythmias in patients with HLHS have not been formally investigated. In this single center observational study, we characterize the arrhythmias in all patients who had undergone any of the surgical stages for HLHS. We also analyze the associations of arrhythmia occurrence and arrhythmia type with surgical stage, type of shunt, hemodynamic effects, and outcome.
Methods
We conducted a retrospective review of all patients who underwent stage I palliation for HLHS between September, 2000, and October, 2008, at Duke Children’s Hospital (approved IRB Pro00016015). Patients with double outlet right ventricle and atrioventricular canal defects were also included in the study whenever the right ventricle was dominant and a single ventricle palliation strategy was followed. Patients with atrioventricular discordance were excluded, since this condition is inherently associated with arrhythmias5. Stage I palliation was carried out in 84 patients, and two patients had hybrid stage I palliations with placement of bilateral pulmonary artery bands and stenting of the patent ductus arteriosus. In all patients, Stage II palliation was direct cavopulmonary anastamosis and stage III Fontan was an extracardiac conduit. Arrhythmia diagnoses were characterized by two pediatric cardiologists based on review of all available electrocardiograms (ECGs) and rhythm strips. Arrhythmias in the peri-operative period were identified through routine telemetry surveillance. Following hospital discharge, arrhythmias were identified from review of ECGs and rhythm strips performed in out-patient clinics or emergency departments when patients appeared for routine follow-up or for the occurrence of new symptoms. Outpatients were seen every 1–2 weeks between stages I and II but less regularly between stages II and III and following stage III. Patient follow-up was based on the last available clinic note. One patient each underwent heart transplantation after stage I and after stage II. For these patients, only the data prior to transplant was included.
Data collected included patient gender, birth weight, age at surgery for each stage, date of last follow-up, associated genetic syndromes, other congenital defects, degree of tricuspid regurgitation, requirement for unplanned reoperation after stage I, requirement for extracorporeal membrane oxygenation (ECMO), and presence of a hypertensive left ventricle at diagnosis. Hypertensive left ventricle was defined as the anatomic combination of aortic atresia with mitral valve patency. Unplanned reoperations included revision of shunt type after stage I operation and ligation (or transcatheter closure) of veno-venous collaterals after stage II. Tricuspid regurgitation was assessed from all available echocardiograms and scored by an echocardiographer as none, trivial, mild, moderate, or severe. The evaluator was unaware of the patient’s arrhythmia status. When an arrhythmia was identified, we recorded the age of the patient at the time of the arrhythmia, duration from last surgical stage, concomitant metabolic abnormality, coincident proarrhythmic medications, clinical need for arrhythmia treatment, type of therapy, success of therapy, and mortality or morbidities related to the arrhythmia. Metabolic abnormalities were defined as hypokalemia (K+ < 3.2 mg/dL), hyperkalemia (K+ > 5.5 mg/dL), hypocalcemia (ionized calcium < 1.0 mmol/L), acidosis (pH < 7.30), or alkalosis (pH > 7.50). We considered concomitant medications with potential proarrhythmic side effects to include milrinone, dopamine, epinephrine, dobutamine, digoxin, or any class I or class IIII antiarrhythmic drug. Mortality was deemed directly related to the arrhythmia if the arrhythmia was the first presenting clinical perturbation prior to hemodynamic compromise and subsequent death. A priori morbidities potentially related to the arrhythmia included ECMO, prolonged intensive care unit stay, pleural effusions, need for anticoagulation, or decreased ventricular function.
Arrhythmia Definition
Clinically important arrhythmias included sinoatrial node dysfunction (SAND), atrioventricular (AV) block greater than first degree (“high grade AV block”), atrial ectopic tachycardia (AET), junctional ectopic tachycardia (JET), supraventricular tachycardia (SVT), ventricular tachycardia, ventricular fibrillation, premature ventricular complexes, premature atrial complexes, atrial flutter, and atrial fibrillation. Premature atrial and ventricular complexes were considered as clinically important only if they occurred in a bigeminal pattern or more frequent than normal sinus beats. JET was defined as narrow complex QRS tachycardia with AV dissociation and a ventricular rate exceeding the atrial. AET was defined as a persistent long RP tachycardia exhibiting warm-up and cool-down periods and overdrive suppression with sinus tachycardia or atrial pacing. SAND was defined as sinus or junctional bradycardia with a persistent heart rate <3rd percentile for age. The other arrhythmias were categorized according to standard definitions.
Statistical Analysis
Descriptive data are reported as median value and 95% confidence interval (CI). Comparisons between discrete variables were made with Fisher’s exact test. Univariate logistic regression was used to assess the risk factors associated with arrhythmias. Analysis of age at first arrhythmia was performed using Kaplan-Meier methods with log-rank comparison. Statistical analysis was performed using STATA 10 (College Station, TX).
Dr. Smith received support from NIH 1K23HD060040-01. Dr. Trivedi was supported by a Medtronic fellowship training grant. No other extramural funding was used to support this work. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Results
Study Population and Arrhythmia Associations
Eighty-six patients were included in the study. Pathological bradycardias or tachyarrhythmias occurred in patients before stage I and between each surgical stage. Overall, 49 patients (57%) had 63 total arrhythmias. The median follow-up period was from birth to 166 days (range 4 days of life to 7.2 years). Demographic, anatomic, and surgical factors associated with arrhythmias are shown in Table I. Although not statistically significant, infants with arrhythmias had a lower birth weight and higher rate of unplanned reoperation after stage I. There was no association between arrhythmia occurrence and hypertensive left ventricle, genetic syndrome, other congenital organ system dysfunction, type of shunt used, or need for ECMO. Electrolyte abnormalities were associated with 48/63 (76%) of arrhythmias. Of the electrolyte abnormalities examined, hypokalemia was the most common. Fifty-nine of 63 (94%) of arrhythmias occurred while the patient was receiving a proarrhythmic drug, usually a positive inotropic agent.
Table I.
Demographic, anatomic, and surgical characteristics of patients with and without arrhythmia.
| Characteristic | Arrhythmia | No Arrhythmia | p | |
|---|---|---|---|---|
| Sex | 0.82 | |||
| Male | 30/49 (61%) | 21/37 (57%) | ||
| Female | 19/49 (39%) | 16/37 (43%) | ||
| Birth weight (kg)* | 3.1 [2.7, 3.4] | 3.3 [3.0, 3.6] | 0.05 | |
| Age at surgical stage (days)* | ||||
| Stage I | 4 [3, 6] | 5 [3, 8] | 0.36 | |
| Stage II | 151 [137, 182] | 141 [104, 169] | 0.23 | |
| Stage III | 1231 [751, 1448] | 770 [769, 1054] | 0.46 | |
| Anatomic | ||||
| Moderate – Severe TR† | 8/49 (16%) | 5/37 (13%) | 0.77 | |
| Hypertensive left ventricle | 19/49 (39%) | 15/37 (40%) | 0.99 | |
| Genetic Syndrome | 5/49 (10%) | 7/37 (19%) | 0.20 | |
| Other organ system disease | 14/49 (29%) | 13/37 (35%) | 0.64 | |
| Surgical | ||||
| Type of stage I | 0.99 | |||
| MBT shunt | 17/48 (35%) | 12/36 (33%) | ||
| RVPA shunt | 31/48 (65%) | 24/36 (67%) | ||
| Need for ECMO | 13/49 (27%) | 5/37 (14%) | 0.14 | |
| Unplanned Reoperation | 17/49 (35%) | 6/37 (16%) | 0.08 | |
Median [IQR = interquartile range)];
tricuspid regurgitation
When regards to tricuspid regurgitation, there was no association with arrhythmia when considering initial grade of regurgitation (p=0.8; table I) or the maximum degree of tricuspid regurgitation ever observed within each patient (p=0.8). For all arrhythmia patients, the degree of tricuspid regurgitation from echocardiogram temporally closest in time to first arrhythmia onset was actually less than the maximum ever observed before or after arrhythmia onset in that patient (p=0.001). The temporally nearest echocardiogram had been performed, on average, 0.3 days prior to arrhythmia onset (median: 0 days; range: 10 days prior to 6 days following arrhythmia onset). The degree of arrhythmia-coincident tricuspid regurgitation was also less than the maximum degree of tricuspid regurgitation ever observed in the non-arrhythmia group (p=0.001).
Arrhythmia Occurrence and Subtypes
Table II shows a breakdown of arrhythmias at all stages by arrhythmia subtype. Forty-four of the 63 (70%) arrhythmias occurred between stage I and stage II, and arrhythmia events were maximal at that stage with 44/86 (51%) patients manifesting a new arrhythmia (p < 0.001 versus pre-stage I and p = 0.02 versus stage II–III). All arrhythmia subtypes occurred more frequently between stage I–II except for JET, which occurred more frequently after stage II operation (4/9, 44% of JET cases) than after stage I (3/9, 33% of JET cases). SVT was the most common arrhythmia, accounting for 32% of arrhythmias between stage I–II and 25% of all arrhythmias at any stage.
Table II.
Arrhythmia incidence by subtype and surgical stage.
| Arrhythmia | Pre-Stage I (n=86) |
Stage I – II (n=86) |
Stage II–III (n=45) |
After Stage III (n=10) |
Total (% of total arrhythmias) |
|---|---|---|---|---|---|
| SAND | 1 | 2 | 1 | 0 | 4 (6%) |
| High grade AV block | 0 | 5 | 2 | 0 | 7 (11%) |
| AET | 0 | 7 | 0 | 1 | 8 (13%) |
| JET | 0 | 3 | 4 | 2 | 9 (14%) |
| SVT | 0 | 14 | 2 | 0 | 16 (25%) |
| VT††/VF** | 2 | 3 | 2 | 0 | 7 (11%) |
| Other (PACs¶, PVCs#, A fib§) | 0 | 10 | 1 | 1 | 12 (19%) |
| Total (% new arrhythmia incidence) | 3 (4%) | 44 (51%)*† | 12 (27%)* | 4 (40%)* | 63 |
p<0.001 vs pre-stage I.
p = 0.02 vs. stage II–III.
atrial fibrillation;
premature atrial complexes;
premature ventricular complexes;
ventricular fibrillation;
ventricular tachycardia
Most arrhythmias occurred within the first 10 days after surgery (Figure 1). A large number occurred within the first 24 hours of surgery. The Kaplan-Meier curve depicting freedom from arrhythmia demonstrates that most arrhythmias occurred in the first 2 months of life (Figure 2).
Figure 1.
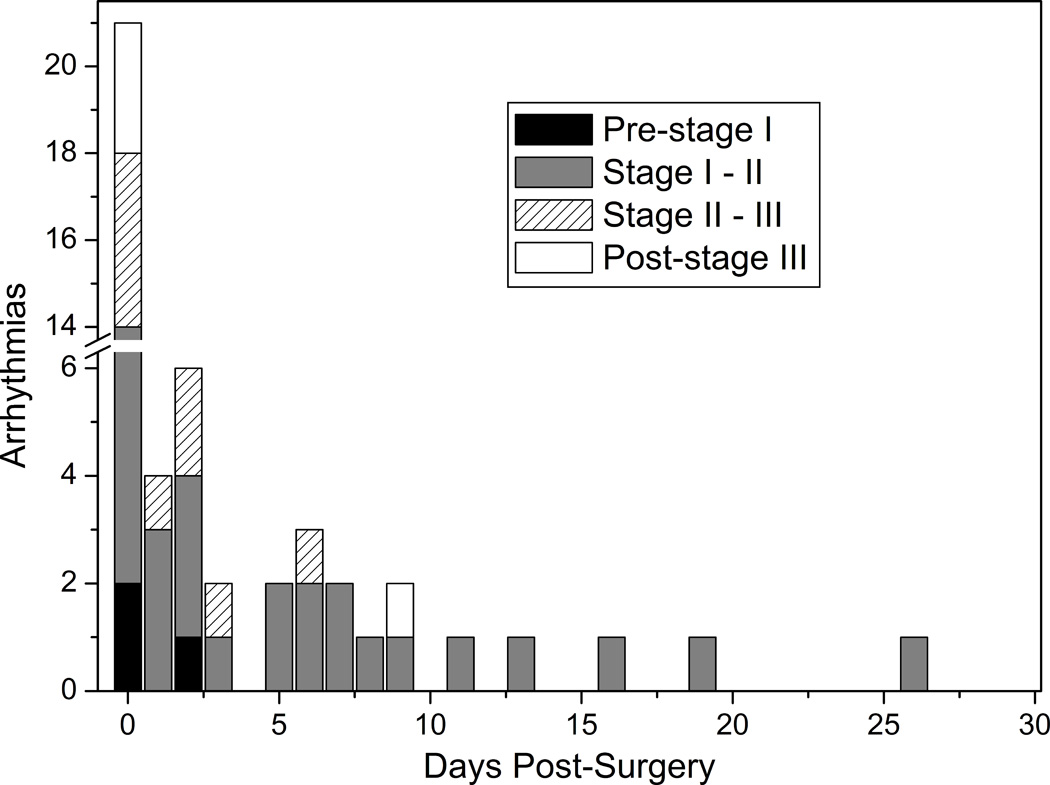
Onset of arrhythmia. Bar graph depicting the onset of arrhythmia up to 30 days following surgery at days post each surgical stage or post-birth (for pre-stage I).
Figure 2.
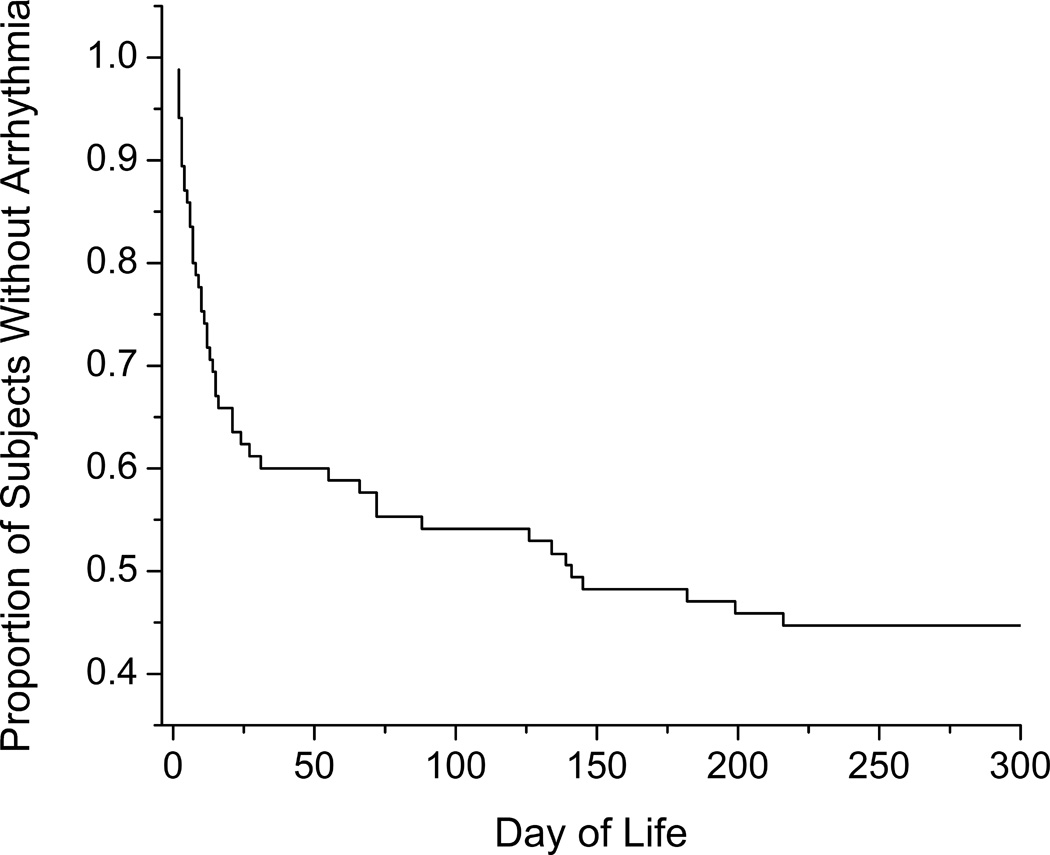
Freedom from arrhythmia. Kaplan-Meier relationship of freedom from arrhythmia up to 300 days of life. Beyond the 300 days, only 2 of the 86 patients manifested their first arrhythmia at day of life 636 and 1146.
Arrhythmia Outcomes
Of the 63 arrhythmias, 52 (83%) required therapy because of hemodynamic instability. Treatment was successful in 51/52 (98%) of patients including appropriate recognition and pacing for bradycardias. Four of 63 arrhythmias (6%) were not treated, because they occurred while on ECMO and resolved spontaneously prior to successful weaning from ECMO. In seven patients, it was elected to not treat the arrhythmia due to brief duration and spontaneous resolution.
Of patients having arrhythmias, 29/49 (59%) also had significant additional morbidity: four required mechanical support with ECMO for severe hemodynamic instability, five had large pleural effusions requiring chest tube placement, 20 required prolonged intensive care unit stay, and 25 had decreased ventricular function. One patient died from unsuccessful resuscitation efforts during an arrhythmia.
Arrhythmias occurring in the interval between stage I and II tended to be associated with a higher overall mortality compared to arrhythmias occurring after stage II, OR = 3.2 [95%CI 0.84, 12.0] (p = 0.09). Among patients with persistent bradycardia (SAND or high grade AV block), 8/11 (73%) died despite appropriate recognition and management with permanent pacing. All of these deaths occurred between stage I and stage II. Of these bradycardic patients, 5/5 (100%) patients with modified Blalock-Taussig (MBT) shunt died compared to 3/6 (50%) with right ventricle–pulmonary artery (RVPA) shunt palliation (p = 0.18).
The 30 day post-operative mortality rate following stage I palliation for HLHS is higher than it is for any other major neonatal heart operation6. Considering all the stages, the 30 day mortality rate in our series was 18.6% (16/86 patients) with 15.1% of these occurring after stage I palliation (13/86 patients). Overall, mortality was similar in patients with and without arrhythmias (p = 0.99). Although not statistically significant, children having arrhythmias within 5 days of any cardiac surgery may be at increased risk of mortality compared to arrhythmias occurring at any other time, OR = 2.06 [CI 0.64, 6.6] or when compared to children having no arrhythmia, OR = 1.62 [CI 0.49, 5.34]. Arrhythmia subtype was not statistically associated with mortality.
Discussion
To our knowledge, this is the first report of arrhythmia associations, arrhythmia outcomes, and description of arrhythmia subtypes in the immediate and short-term post-operative HLHS population. The high proportion of arrhythmias in patients with HLHS has been clinically suspected and supported by previous studies that primarily examined interstage mortality. Our study found a higher proportion of arrhythmias in HLHS patients compared to other types of neonatal heart surgery. For example, patients undergoing arterial switch repair for d-transposition of the great arteries have a reported arrhythmia proportion of 5–21%7–9 and neonatal patients undergoing Blalock-Taussig shunt procedures for defects other than HLHS have a reported arrhythmia occurrence of 5%9.
Potential Arrhythmia Substrates
In our series of infants having HLHS, arrhythmias were common regardless of type of stage I palliation and other anatomic or surgical factors. This finding has implications for arrhythmia substrate in HLHS. Although hypertensive left ventricle has been reported to be associated with increased mortality and possibly more arrhythmias10, we did not find a higher proportion of arrhythmias in that subcategory of patients. Likewise, greater magnitude of tricuspid regurgitation did not correlate with arrhythmia. Thus, the younger patients in this study do not appear to have the same elevated risk of atrial arrhythmias observed in older children with large right atria related to long standing tricuspid valve regurgitation.
We did not find a correlation between genetic syndrome or other organ system abnormality with arrhythmias. In our series, patients with lower birth weight may be at higher risk of arrhythmias, but this finding may also be related to other influences such as early gestational age and severity of illness. One might predict that the right ventriculotomy necessary for the RVPA shunt may predispose to important arrhythmias, but there was no difference in arrhythmias of any type including ventricular arrhythmias in patients with MBT shunt or RVPA shunt. The RVPA shunt is relatively new compared to the MBT shunt, however, and as these patients become older it will be important to reevaluate the incidence of ventricular arrhythmias. In summary, none of the anatomic, constitutional, or surgical factors predisposed to a greater risk of arrhythmia suggesting that the high frequency of arrhythmias in HLHS may be related to an intrinsic substrate.
The factors that were most associated with arrhythmias were related to post-operative low cardiac output and need for hemodynamic support. Most arrhythmias occurred while in the intensive care unit, while receiving positive inotropic drugs, during metabolic derangements, and within 0 – 10 days after surgery. Also, patients requiring unplanned reoperation after stage I tended to have more arrhythmias. These unplanned surgeries included revision of shunt type after stage I operation and ligation of veno-venous collaterals after stage II. These patients had in common some combination of progressive cyanosis, chamber dilatation, and increased wall stress, all of which could have contributed to arrhythmia susceptibility11, 12.
Tachyarrhythmias, especially SVT and AET, were the most common arrhythmias. Focal atrial tachycardia foci have been reported to be mapped to the edges of putative suture lines in post-operative patients who had undergone the Mustard operation for d-transposition of the great arteries13. Similarly, in the presence of elevated wall stress, the substrate for AET may exist at the edges of the atriotomy scar or the septectomy edges. The high proportion of SVT is more difficult to explain. Atrioventricular nodal reentry tachycardia is a reasonable candidate in at least some instances. This tachycardia is probably more common in infants than was once thought14, and it is almost certainly over-represented in older congenital heart disease patients who have right atrial dilatation15 or surgical scar in the subtricuspid isthmus13. Two patients did have the Wolff-Parkinson-White pattern on their electrocardiogram, suggesting that accessory atrioventricular pathways (and their associated SVT) may be over-represented in HLHS patients. Our patients did not routinely undergo electrophysiological testing, so the SVT mechanism was not determined in most.
Bradycardias were not as frequent as tachyarrhythmias but clearly had worse outcomes among the 11 patients with SAND or high grade AV block. The etiology of bradycardia is not clear, and in all but one of these patients, the bradycardia developed after stage I palliation. Congenital SAND is not known to be associated with HLHS. As with other types of congenital heart operations, SAND in these patients may be related to SA node injury from the atriotomy incision, venous cannulation, acquired coronary arterial insufficiency to the SA node, or right atrial stretch. SAND has been associated with the stage II palliation known as the “hemi-Fontan operation”16, 17. This procedure was not performed in any patients in our series, including the single patient who developed SAND following stage II. Congenital AV block is not associated with HLHS. We hypothesize that AV nodal damage could have occurred during the atrial septectomy or secondary to interruption of AV nodal arterial supply. High grade AV block was not more common in MBT shunt patients (in which coronary perfusion is generally more compromised due to diastolic runoff) compared to RVPA shunt patients, suggesting that coronary hypoperfusion is an unlikely explanation. As with the tachyarrhythmias, the possibility of an underlying anatomic substrate or functional predisposition to bradycardia in patients having HLHS is unproven.
Arrhythmia Outcomes in HLHS
Patients with HLHS have a 10 – 46% reported interstage mortality between stage I and stage II and several studies have considered the association between interstage mortality and arrhythmias1–3. Simsic et al. reported that patients having had post-operative arrhythmias had an increased risk of interstage death after Stage I palliation2. Similarly impressive is the 5% incidence of in-hospital sudden death from presumed arrhythmias in a series of 122 cases by Bartram et al3. Considering out-of-hospital deaths after stage I, Hehir et al. found that postoperative arrhythmia was a significant risk factor1. However, in that report, multivariate analysis showed that intact atrial septum and age at operation greater than 7 days emerged as predictors of interstage death, suggesting to the authors that the propensity for arrhythmia may be related to degree of illness at presentation or to other intrinsic factors.
Our findings indicate a worse outcome in patients with bradycardias. We speculate that the tachyarrhythmias did not carry the same dire outcomes, since they were more easily treatable and became quiescent as post-operative hemodynamics improved. Bradycardias including SA node dysfunction and high grade AV block appear to have worse outcome despite appropriate prompt recognition and traditional permanent pacing therapy. In one patient who died, it was clear that the onset of complete heart block coincided with worsening ventricular function that did not improve with pacing. In that instance, AV block may have been the consequence of extreme stretch of the peri-nodal region or of a Bezold-Jarisch reflex response to increased ventricular wall tension. In another patient, complete heart block developed during the stage I procedure, and the patient could not be weaned from cardiopulmonary bypass. This patient eventually died after several days on ECMO. Other than the infant whose ventricular function antedated the occurrence of AV block, it is not clear why appropriately treated bradycardias were associated with greater mortality. Possibilities include atrial dyssynchrony resulting in altered ventricular filling in patients having SAND, impaired chronotropic responsiveness to autonomically mediated cardiac output demands in patients having SAND, and deterioration in ventricular filling and stroke volume due to intraventricular dyssynchrony in patients requiring ventricular pacing.
Clinical Implications
For in-patients having HLHS, aggressive therapy for clinically important tachyarrhythmias appears to be beneficial. However, traditional pacing for persistent bradyarrhythmias may be insufficient to prevent hemodynamic deterioration. Synchronized atrial activation by pacing near Bachmann’s bundle in infants having SAND, and tissue Doppler-based dual site ventricular pacing in infants having AV block are strategies that may help optimize diastolic filling and stroke volume.
Unfortunately, our experience does not provide substantive new insight into the troublesome interstage mortality in outpatients with HLHS. Given the tenuous nature of the interstage period, Ghanayem et al. have demonstrated improved interstage survival after employing home monitoring for all HLHS patients18. Specifically, they monitored daily pulse oximetry and patient weights. If paroxysmal arrhythmias contribute to sudden death in these infants, some arrhythmias may result in desaturation prompting medical attention. As more data on the consequences of arrhythmias in HLHS become apparent, recently available, long distance, continuous rhythm monitoring devices may become valuable. Additionally, invasive electrophysiologic testing may be useful for diagnosis and therapy in those patients who had any episode of reentrant tachycardia after stage I, particularly considering that SVT was the most common tachyarrhythmia observed. At our center, for patients with any history of sustained supraventricular or ventricular reentrant tachyarrhythmia, invasive electrophysiologic testing has recently become routine at the time of their pre-Stage II or pre-Fontan catheterization.
Tachyarrhythmias were most common in this patient population and 76% of the arrhythmias were associated with electrolyte imbalance, most commonly hypokalemia. A greater attention to electrolyte imbalance during post-surgical recovery may help prevent many of these transient arrhythmia episodes.
Considering the high prevalence of tachyarrhythmias in the early postoperative period and especially after stage I palliation, there could be consideration of providing a prophylactic antiarrhythmic medication. However,, most tachyarrhythmias were brief, had spontaneous resolution, or were easily managed. The ultimate outcome of most tachyarrhythmias was that they were not clinically detrimental. The potential negative impact of the uniform use of medications such as beta blockers or amiodarone would need to be ruled out first, in our opinion, before prophylactic treatment is considered.
From this experience, we have evolved a systematic therapeutic algorithm for infants having HLHS and tachyarrhythmias. This approach is speculative but has worked well in our hands. Those having AET routinely receive amiodarone. Those infants having paroxysmal SVT receive digoxin (except in the presence of a preexcited QRS), followed, in order, by beta-adrenergic blocking agents, and either propafenone or amiodarone. Post-operative JET is managed by very modest hypothermia and amiodarone, which is administered very slowly19, 20. Other than those having had JET, these infants always undergo electrophysiological testing prior to the Fontan operation in the drug-free state with the intention of catheter ablation of any persistent arrhythmia substrate.
Limitations
There are several limitations in this single-center retrospective study. Once in-patients were transferred from intensive care settings, arrhythmia recognition was based upon automated alarms, potentiating under-recognition of transient, albeit clinically less important, arrhythmias. Once patients were discharged from the hospital, there was no standardized Holter surveillance. Thus, asymptomatic patients with arrhythmias may not have been identified and, as a result, the number of arrhythmias may be underestimated. We identified only a small number of subjects beyond stage III, as many subjects had not yet undergone stage III palliation. Although the longest follow-up in our study was >7 years, most patients were followed for shorter periods of time. There may be an association of increased risk of mortality and arrhythmias occurring within the first 5 post-surgical days but due to limited numbers this study was not powered to detect these and other specific arrhythmia associations.
Conclusion
Arrhythmias are common in HLHS patients. Most of these arrhythmias occurred between stage I and II, and in the first postoperative week. Tachyarrhythmias were most common, but patients with bradyarrhythmias such as high grade AV block had a higher mortality. Overall mortality was the same in patients with our without arrhythmia. Further long-term, prospective studies will be needed to elucidate the full clinical impact of arrhythmias in HLHS patients.
Acknowledgments
PBS has research funding from Pfizer, Cadence Pharmaceuticals, and Astellas Pharma US.
BT had fellowship training grant funding from Medtronic.
Footnotes
Disclosures
All other authors – No disclosures.
References
- 1.Hehir DA, Dominguez TE, Ballweg JA, et al. Risk factors for interstage death after stage 1 reconstruction of hypoplastic left heart syndrome and variants. Journal of Thoracic and Cardiovascular Surgery. 2008;136(1) doi: 10.1016/j.jtcvs.2007.12.012. 94-U78. [DOI] [PubMed] [Google Scholar]
- 2.Simsic JM, Bradley SM, Stroud MR, et al. Risk factors for interstage death after the Norwood procedure. Pediatric Cardiology. 2005;26(4):400–403. doi: 10.1007/s00246-004-0776-4. [DOI] [PubMed] [Google Scholar]
- 3.Bartram U, Grunenfelder J, Van Praagh R. Causes of death after the modified Norwood procedure: A study of 122 postmortem cases. Annals of Thoracic Surgery. 1997;64(6):1795–1802. doi: 10.1016/s0003-4975(97)01041-2. [DOI] [PubMed] [Google Scholar]
- 4.Walsh EP, Cecchin F. Arrhythmias in adult patients with congenital heart disease. Circulation. 2007;115(4):534–545. doi: 10.1161/CIRCULATIONAHA.105.592410. [DOI] [PubMed] [Google Scholar]
- 5.Huhta JC, Maloney JD, Ritter DG, et al. Complete atrioventricular block in patients with atrioventricular discordance. Circulation. 1983;67(6):1374–1377. doi: 10.1161/01.cir.67.6.1374. [DOI] [PubMed] [Google Scholar]
- 6.Lacour-Gayet F, Maruszewski B, Mavroudis C, et al. Presentation of the International Nomenclature for Congenital Heart Surgery. The long way from nomenclature to collection of validated data at the EACTS. Eur J Cardiothorac Surg. 2000;18(2):128–135. doi: 10.1016/s1010-7940(00)00463-2. [DOI] [PubMed] [Google Scholar]
- 7.Rhodes LA, Wernovsky G, Keane JF, et al. Arrhythmias and intracardiac conduction after the arterial switch operation. J Thorac Cardiovasc Surg. 1995;109(2):303–310. doi: 10.1016/S0022-5223(95)70392-6. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi G, Kurosaki K, Echigo S, et al. Prevalence of arrhythmias and their risk factors mid- and long-term after the arterial switch operation. Pediatr Cardiol. 2006;27(6):689–694. doi: 10.1007/s00246-005-1226-7. [DOI] [PubMed] [Google Scholar]
- 9.Rekawek J, Kansy A, Miszczak-Knecht M, et al. Risk factors for cardiac arrhythmias in children with congenital heart disease after surgical intervention in the early postoperative period. Journal of Thoracic and Cardiovascular Surgery. 2007;133(4):900–904. doi: 10.1016/j.jtcvs.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Glatz JA, Fedderly RT, Ghanayem NS, et al. Impact of mitral stenosis and aortic atresia on survival in hypoplastic left heart syndrome. Annals of Thoracic Surgery. 2008;85(6):2057–2062. doi: 10.1016/j.athoracsur.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 11.Bush HL, Jr, Gelband H, Hoffman BF, et al. Electrophysiological basis for supraventricular arrhythmias: following surgical procedures for aortic stenosis. Arch Surg. 1971;103(5):620–625. doi: 10.1001/archsurg.1971.01350110122020. [DOI] [PubMed] [Google Scholar]
- 12.Garson A, Jr, Gillette PC, Gutgesell HP, et al. Stress-induced ventricular arrhythmia after repair of tetralogy of Fallot. Am J Cardiol. 1980;46(6):1006–1012. doi: 10.1016/0002-9149(80)90359-8. [DOI] [PubMed] [Google Scholar]
- 13.Kanter RJ, Papagiannis J, Carboni MP, et al. Radiofrequency catheter ablation of supraventricular tachycardia substrates after mustard and senning operations for d-transposition of the great arteries. J Am Coll Cardiol. 2000;35(2):428–441. doi: 10.1016/s0735-1097(99)00557-4. [DOI] [PubMed] [Google Scholar]
- 14.Crosson JE, Hesslein PS, Thilenius OG, et al. AV node reentry tachycardia in infants. Pacing Clin Electrophysiol. 1995;18(12 Pt 1):2144–2149. doi: 10.1111/j.1540-8159.1995.tb04639.x. [DOI] [PubMed] [Google Scholar]
- 15.Reich JD, Auld D, Hulse E, et al. The Pediatric Radiofrequency Ablation Registry's experience with Ebstein's anomaly. Pediatric Electrophysiology Society. J Cardiovasc Electrophysiol. 1998;9(12):1370–1377. doi: 10.1111/j.1540-8167.1998.tb00113.x. [DOI] [PubMed] [Google Scholar]
- 16.Cohen MI, Bridges ND, Gaynor JW, et al. Modifications to the cavopulmonary anastomosis do not eliminate early sinus node dysfunction. J Thorac Cardiovasc Surg. 2000;120(5):891–900. doi: 10.1067/mtc.2000.109708. [DOI] [PubMed] [Google Scholar]
- 17.Cohen MI, Wernovsky G, Vetter VL, et al. Sinus node function after a systematically staged Fontan procedure. Circulation. 1998;98(19) Suppl:II352–II358. discussion II358-9. [PubMed] [Google Scholar]
- 18.Ghanayem NS, Hoffman GM, Mussatto KA, et al. Home surveillance program prevents interstage mortality after the Norwood procedure. J Thorac Cardiovasc Surg. 2003;126(5):1367–1377. doi: 10.1016/s0022-5223(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman TM, Bush DM, Wernovsky G, et al. Postoperative junctional ectopic tachycardia in children: incidence, risk factors, and treatment. Ann Thorac Surg. 2002;74(5):1607–1611. doi: 10.1016/s0003-4975(02)04014-6. [DOI] [PubMed] [Google Scholar]
- 20.Laird WP, Snyder CS, Kertesz NJ, et al. Use of intravenous amiodarone for postoperative junctional ectopic tachycardia in children. Pediatr Cardiol. 2003;24(2):133–137. doi: 10.1007/s00246-002-0276-3. [DOI] [PubMed] [Google Scholar]


