Abstract
Gene therapy trials in human breast, ovarian, and head and neck tumors indicate that adenovirus E1A can sensitize cancer cells to the cytotoxic effects of paclitaxel in vitro and in vivo. Resistance to paclitaxel has been reported to occur in cells expressing low levels of the Forkhead transcription factor FOXO3a. Here we report that FOXO3a is critical for E1A-mediated chemosensitization to paclitaxel. RNAi-mediated knockdown of FOXO3a abolished E1A-induced sensitivity to paclitaxel. Mechanistic investigations indicated that E1A indirectly stabilized FOXO3a by acting at an intermediate step to inhibit a ubiquitin-dependent proteolysis pathway involving the E3 ligase βTrCP and the FOXO3a inhibitory kinase IKKβ. E1A derepressed this inhibitory pathway by stimulating expression of the PP2A/C protein phosphatases, which by binding to the TGFβ-activated kinase TAK1 inhibited its ability to activate IKKβ and thereby to suppress βTrCP-mediated degradation of FOXO3a. In this manner, by stimulating PP2A/C expression, E1A triggers a signaling cascade that stabilizes FOXO3a and mediates chemosensitization. Our findings provide a leap forward in understanding paclitaxel chemosensization by E1A, and offer a mechanistic rational to apply E1A gene therapy as an adjuvant for improving therapeutic outcomes in patients receiving paclitaxel treatment.
Keywords: E1A, FOXO3a, breast cancer, PP2A/C, Chemosensitization
INTRODUCTION
Adenovirus type 5 E1A (E1A) was originally recognized as an oncogene that could facilitate oncogenic transformation by other viral and cellular oncogenes. However, E1A has not been associated with human malignancies despite extensive efforts to identify such a link (1). Instead, E1A was shown to have anti-tumor activities by reversing the transformed phenotype, inhibiting metastasis, and inducing apoptosis in multiple transformed rodent cells and human cancer cell lines (2–6). In addition to the tumor suppressor activities, expression of the E1A gene in stably transfected normal fibroblasts and human cancer cells has also been shown to induce sensitization among different categories of anticancer drugs in vitro, including etoposide, cisplatin, doxorubicin, gemcitabine, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), histone deacetylase (HDAC) inhibitors and paclitaxel in normal fibroblasts, sarcoma, non-small cell lung, hepatocellular, ovarian and breast cancer cells (7–12). Animal studies also showed that the combination of systemic E1A gene therapy with paclitaxel significantly enhanced paclitaxel-induced apoptosis and prolonged survival rates in the animal orthotopic model in vivo (11, 13). Therefore, E1A is now considered a tumor suppressor gene and has been tested in multiple clinical trials in a gene therapy setting for patients with breast (14, 15), ovarian (2, 14), and head and neck cancers (15, 16). A clinical study using E1A gene therapy combined with paclitaxel has been initiated for ovarian cancer. Thus, it is critical and timely to understand the detailed molecular mechanisms that associate with E1A-mediated chemosensitization, and future clinical trials using the combination of chemotherapy with E1A gene therapy can be further improved.
One of the molecular mechanisms by which E1A induces chemosensitization is down-regulation of Her-2/neu overexpression (8, 11). Recently, inhibition of Akt and activation of p38 was reported to provide a general cellular mechanism for E1A-mediated chemosensitization (9, 17). Regulation of some critical tumor suppressors was also proposed as being involved in E1A-induced chemosensitization, such as p53 and p19ARF (18), the proapoptotic protein Bax, caspase 9, and a yet-unidentified inhibitor that ordinarily provides protection against cell death (11, 19–22).
Forkhead box O–class (FOXO) transcription factors include FOXO1 (Forkhead in rhabdomyosarcoma, FKHR), FOXO3a (FKHR-like 1, FKHRL1), and FOXO4 (acute lymphocytic leukemia–fused gene from chromosome X, AFX). The FOXOs activate and/or repress transcription of genes involved in metabolism, apoptosis, DNA damage repair, and cell cycle progression (23). For example, FOXO3a has been shown to elevate p27kip expression and induce cell cycle arrest (24). FOXO3a and FOXO4 have also been shown to inhibit the cell cycle through down-regulation of cyclin D by a p27kip-independent mechanism (25, 26). In breast cancer, FOXO3a has been shown to up-regulate Bim, a pro-apoptotic BH3 only protein (25, 27). The activity of the FOXOs can be inhibited by activating the phosphoinositide 3'-kinase (PI3K)/Akt pathway. FOXO3a can be phosphorylated by Akt at three conserved serine/threonine residues (Thr32, Ser253, and Ser315), and it subsequently translocates from the nucleus to the cytoplasm, where it is retained by binding to the 14-3-3 protein (28). FOXO3a activity can also be inhibited by the IκB kinase (IKK) signaling pathway. IKK physically interacts with and phosphorylates FOXO3a independently of Akt, which causes nuclear exclusion of FOXO3a and subsequently proteolysis of FOXO3a via the βTrCP-mediated ubiquitin (Ub)-dependent proteasome pathway (29). Recently, Erk was also known to phosphorylate FOXO3a at Ser294, Ser344 and Ser 425 sites which enhance interaction with the E3 Ub ligase MDM2, resulting in FOXO3a degradation (30). However, the biological function and detailed molecular mechanism of FOXO3a proteolysis in E1A-mediated chemosensitization are still unclear.
In an attempt to understand the molecular mechanism of E1A-mediated chemosensitization, we found that FOXO3a is critical to that process. E1A stabilizes FOXO3a by preventing βTrCP-mediated Ub-dependent proteolysis through inhibiting the phosphorylation of FOXO3a at Ser644 by IKKβ. E1A induces the expression of PP2A (a protein phosphatase involved in multiple cellular functions, including chemosensitization), which inhibits transforming growth factor β–activated kinase 1 (TAK1)-activated IKK signaling, therefore stabilizing FOXO3a and inducing chemosensitization.
MATERIALS AND METHODS
Cell Lines, DNA Constructs, and Antibodies
Cell lines MDA-MB-231, HeLa and MDA-157 cells were purchased from ATCC and grown in Dulbecco’s modified Eagle’s medium (DMEM)/F12 supplemented with 10% fetal bovine serum. The human breast cancer cell line MDA-MB-231 and its E1A/vector-stable transfectants have been described previously (31). The transfectants were grown under the same conditions as the controls, except that G418 was added to the culture medium. Cell lines have been characterized using DNA analysis by STR fingerprinting (HeLa, March 2009; MDA-MB-231, December 2010, MDA-157, ongoing). Cell lines have been frozen after receipt from ATCC and have not been passed for more than 6 months when performing experiments.
Plasmids E1A (2), IKKβ (29), βTrCP siRNA plasmids (kindly provide by Dr. Serge Y. Fuchs, University of Pennsylvania), PP2A/A, PP2A/C (17), and TAK1-HA (32) were described previously. FOXO3a siRNA plasmids were kindly provided by Dr. Alex Toker.
The monoclonal antibody used against the E1A protein was M58 (Pharmingen). The following were obtained as indicated: HA (11666606001; Roche), FOXO3a (SC-11351; Santa Cruz Biotechnology), IKKβ (2684; Cell Signaling Technology, or SC-7607; Santa Cruz Biotechnology), and pIKKβ (S181) (2681; Cell Signaling Technology). Rabbit anti-human PP2A/A and PP2A/C antibodies were purchased from CalBiochem. We also purchased the following from the suppliers indicated: ubiquitin (3936; Cell Signaling Technology), βTrCP (37–3400; Zymed, or SC-15354; Santa Cruz Biotechnology), TAK1 (SC-7967; Santa Cruz Biotechnology), pTAK1 (4531S; Cell Signaling Technology), and α-tubulin (T-5168; Sigma). Recombinant human TNFα was purchased from Roche. MG132 was purchased from Sigma.
Immunoprecipitation and Western Blotting
Cells were washed twice with PBS, scraped into 500 µl of lysis buffer, and incubated on ice for 20 min. After centrifugation at 14,000 × g for 10 min, 1.5 mg of each supernatant was preincubated with 2 µg of immunoglobulin G and 50 µl of protein G for 1 h at 4°C. Immunoprecipitation was performed overnight with 2 µg antibody and 50 µl of protein G. The immunocomplex was washed five times with lysis buffer, dissolved in loading buffer, subjected to SDS-PAGE, and transferred onto nitrocellulose membranes. The membranes were blocked with 5% nonfat dry milk in PBS containing 0.05% Tween 20 and incubated with primary antibodies, followed by secondary antibodies (Jackson ImmunoResearch Laboratories). The immunoblots were visualized with enhanced chemiluminescence (Amersham).
Paclitaxel-Induced Cell Death
Cells were treated with 20 nM paclitaxel and incubated for 24 h. Aliquots of 1 × 106 cells were collected and washed once with ice-cold PBS and then fixed with ice-cold 70% ethanol overnight. After fixation, cells were washed with PBS to remove residual ethanol, pelleted, and resuspended in PBS containing 50 µg/ml of propidium iodide (Sigma). Staining was performed at 4°C for at least 30 min, and samples were analyzed using an Epics Profile flow cytometer (Coulter) in the Core Facility at The University of Texas MD Anderson Cancer Center.
Orthotopic Breast Tumor Growth Assay
Six-week-old female SCID mice were orthotopically inoculated with tumor cells into the mammary fat pad and treated with vehicle or paclitaxel as described previously (9). Tumor development was followed in individual animals (eight per group) by measuring tumor length (L) and width (W) with calipers every 3 days. Tumor volume was calculated with the formula LW2/2. All animal work and care was performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of China Medical University.
RESULTS
FOXO3a is critical for E1A-mediated chemosensitization
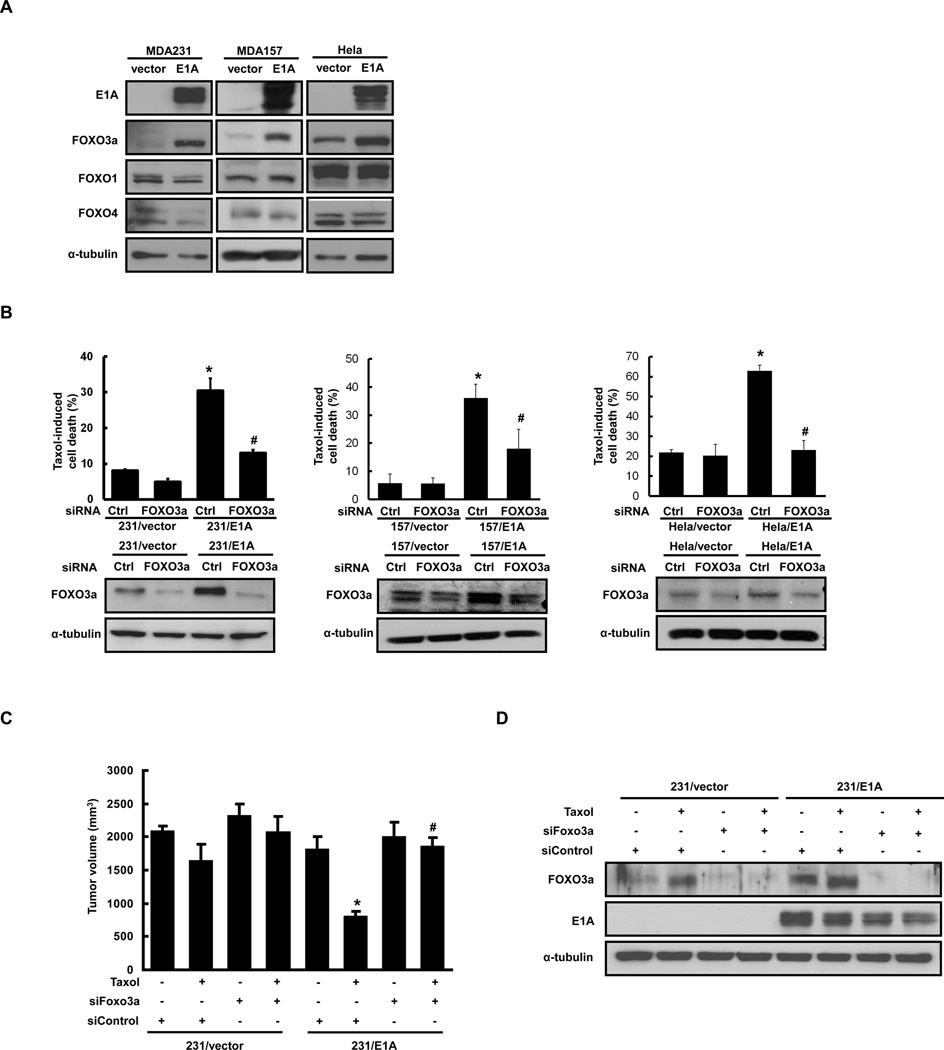
E1A gene therapy has been shown to induce chemosensitization among different chemotherapeutic agents, including paclitaxel in breast and ovarian cancers (33). It has been shown that resistance to paclitaxel occurs in cells expressing low level of FOXO3a (34). We therefore asked whether FOXO3a may contribute to E1A-mediated chemosensitization. To this end, we examined the effects of E1A on FOXO3a expression in various types of cancer cells including MDA-MB-231, HeLa and MDA-MB-157 and found that expression of FOXO3a was significantly increased in E1A-transfected cells (Fig. 1A). We found that FOXO3a-regulated apoptotic genes, such as FasL and p27, were increased in E1A-transfected cells and decreased by knockdown FOXO3a (Supplementary Figure S1A) and involved in E1A-mediated Chemosensitization (Supplementary Figure S1B). More importantly, E1A-induced chemosensitization of paclitaxel was abolished by knockdown of FOXO3a expression using FOXO3a specific small interfering RNA (siFOXO3a) in E1A transfected MDA-MB-231, HeLa and MDA-MB-157 cancer cell lines (Fig. 1B). E1A- induced chemosensitization of doxorubicin and cisplatin were also reduced by knock down of FOXO3a (Supplementary Figure S1C). Using the established stable transfectants, we further investigated the effects of FOXO3a on E1A-mediated chemosensitization in a xenograft tumor model in which mice were injected orthotopically with stably transfected cell clones. The results indicated that E1A induces the chemosensitization of paclitaxel in vivo, in that the tumor volume in 231/E1A-bearing mice treated with paclitaxel was significantly less than that in 231/vector-bearing mice treated with paclitaxel (810.7 ± 73.2 mm3 versus 1648.7 ± 237.4 mm3; Fig. 1C, lane 6 versus lane 2). E1A-induced chemosensitization to paclitaxel was abolished by knockdown the expression of FOXO3a by stable expressing siFOXO3a in 231/E1A cells (810.7 ± 73.2 mm3 versus 1855.1 ± 135.8 mm3; Fig. 1C, lane 6 versus lane 8). Increased tumor volumes by siFOXO3a treatment in 231/E1A correlated well with reduced FOXO3a expression in the tumors (Figure 1D). We therefore concluded that FOXO3a is required for the E1A-mediated chemosensitization to paclitaxel.
Figure 1. FOXO3a Is Critical for E1A-Mediated Chemosensitization.
(A) E1A-expressing vector (E1A) or control vector (vector) was transfected into different types of cells, followed by analysis of E1A, FOXO1, FOXO4 and FOXO3a protein expression using Western blot analysis. α-Tubulin was used as the internal protein loading control. (B) E1A-induced FOXO3a expression was required for E1A-mediated chemosensitization. Upper panel: Chemosensitization of E1A–expressing cells or vector control cells transfected with siFOXO3a or control siRNA as analyzed by the DNA flow cytometry assay. Each type of transfected cell was treated with 20 nM paclitaxel (Taxol) for 24 h. The columns are the mean values from the three independent experiments. Bars indicated means ± SE. Asterisks denote a statistically significant difference compared with values of column 1 (*p < 0.05, two-tailed Student’s t test). E1A-dependent chemosensitization was overturned by siFOXO3a to a significant degree, as indicated by the # symbol. Lower panel: Expression of FOXO3a was analyzed by Western blotting. (C) Tumor volume of orthotopic xenograft tumors formed by MDA-MB-231/vector cells or MDA-MB-231/E1A cells stably transfected with either control siRNA (siControl) or FOXO3a siRNA (siFOXO3a). Each column represents the mean ± SD of eight primary tumors. *p < 0.05 versus column 2 values, by two-tailed Student’s t test. E1A-dependent chemosensitization was overturned by siFOXO3a to a significant degree, as indicated by the # symbol. (D) Expression of FOXO3a protein and E1A was examined by immunoblotting assays using MDA-MB-231/vector tumors or MDA-MB-231/E1A tumors stably transfected with either control siRNA (siControl) or FOXO3a siRNA (siFOXO3a) in combination with paclitaxel treatment.
E1A prevents Ubiquitin-dependent proteolysis of FOXO3a
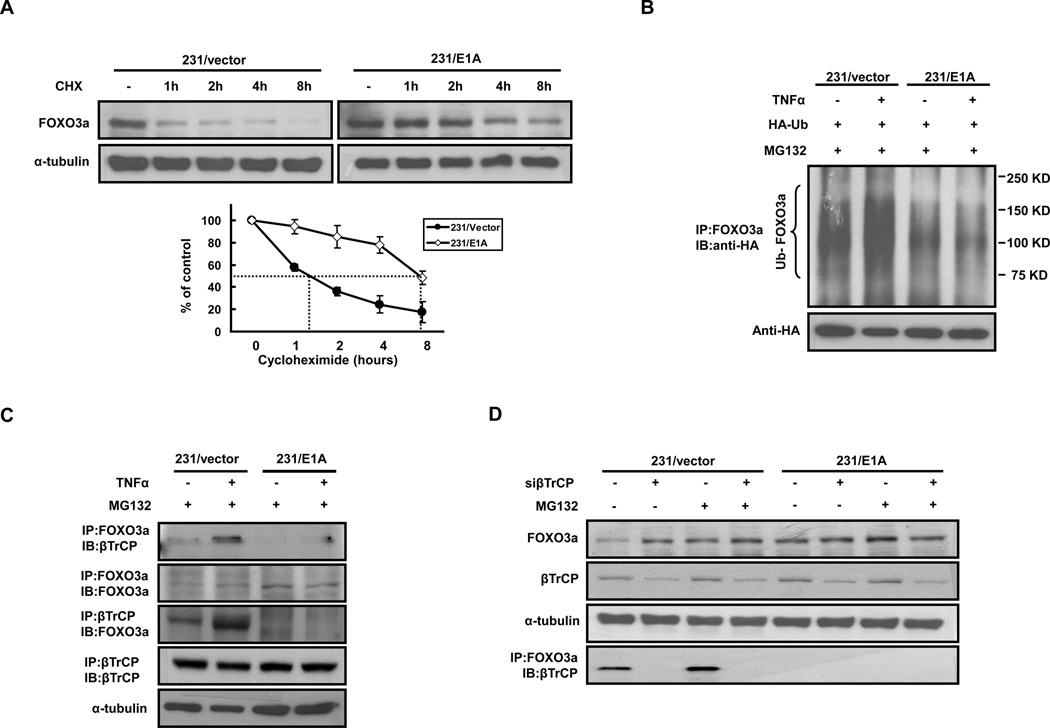
Posttranslational modification and regulation of FOXO3a protein stability are critical for FOXO3a activity (29). Therefore, we attempted to determine the stability of FOXO3a protein in response to E1A in breast cancer cells. For this analysis, we treated control vector and E1A expression vector stable transfectants (231/vector and 231/E1A) with cycloheximide for various times to block de novo protein synthesis and found that the half-life of FOXO3a protein was more than 7 hours for E1A-transfected cells but less than 1.5 hours for control cells by Western blot analysis (Fig. 2A). TNFα-mediated FOXO3a polyubiquitination (29) was significantly decreased in 231/E1A cells compared with that in 231/vector cells (Fig. 2B). These results suggest that E1A increases FOXO3a protein expression by preventing Ub-dependent proteolysis of FOXO3a.
Figure 2. E1A Prevents βTrCP-mediated Ubiquitin-Dependent Proteolysis of FOXO3a.
(A) Determination of the protein stability of FOXO3a in MDA-MB-231/vector cells and MDA-MB-231/E1A cells. The 231/vector cells and 231/E1A cells were treated with 100 µg/ml cycloheximide (CHX) for the indicated times. Total protein was isolated, and expression of FOXO3a was analyzed by Western blotting assay and quantified (bottom). The results are representative of at least three independent experiments. Error bars, SD. (B) Upper panel: 231/vector or 231/E1A cells transfected with HA-ubiquitin (HA-Ub) were treated with the proteasome inhibitor MG132 with or without TNFα (50 ng/ml) and the lysates of these cells were analyzed using immunoprecipitation/immunoblotting. Lower panel: Lysates of 231/vector or 231/E1A cells transfected with HA-ubiquitin were subjected to Western blotting. (C) 231/vector or 231/E1A cells were treated with the proteasome inhibitor MG132 with or without TNFα (50 ng/ml) and the lysates of these cells were analyzed by IP/IB. (D) Knockdown of βTrCP expression by βTrCP-specific siRNAs increased FOXO3a expression and disrupted the interaction between βTrCP and FOXO3a. The 231/vector cells and 231/E1A cells were transfected with siβTrCP or control siRNA. 48 h after transfection, total proteins were isolated, and expression of FOXO3a and βTrCP was analyzed by Western blotting. Lysates of 231/vector or 231/E1A cells transfected with siβTrCP or control siRNA in the presence of MG132 (5 µM) were analyzed by IP/IB (anti-FOXO3a/anti-βTrCP).
βTrCP is involved in E1A-induced FOXO3a induction
βTrCP oncogenic ubiquitin E3-ligase interacts with FOXO3 and induces its ubiquitin-dependent degradation in an IκB kinase-β (IKKβ) phosphorylation dependent manner (27). Thus, we asked whether βTrCP was involved in E1A-mediated FOXO3a protein stabilization. To this end, we first asked whether βTrCP physically interacted with FOXO3a. We analyzed proteosome inhibitor MG132-treated 231/vector and 231/E1A cell lysates by reciprocal co-immunoprecipitation (IP) followed by immunoblotting (IB) using antibodies against FOXO3a and βTrCP. Our results showed that endogenous FOXO3a was associated with endogenous βTrCP in vivo in 231/vector cells and this interaction was stimulated by TNFα treatment. Interestingly, TNFα-induced binding between FOXO3a and βTrCP was significantly reduced in E1A-expressing cells (Fig. 2C). In addition, βTrCP was shown to be required for maintenance of low FOXO3a expression by using siRNA of βTrCP. Transfection with siβTrCP increased FOXO3a expression in 231/vector but not in 231/E1A cells (Fig. 2D). Also, knockdown of βTrCP abolished the association between FOXO3a and βTrCP (Fig. 2D). Taken together, these results suggest that E1A inhibits interaction of FOXO3a and βTrCP which may prevent from FOXO3a degradation.
Inhibition of TAK1-IKK signaling is required for E1A-mediated prevention of βTrCP/FOXO3a interaction and chemosensitization
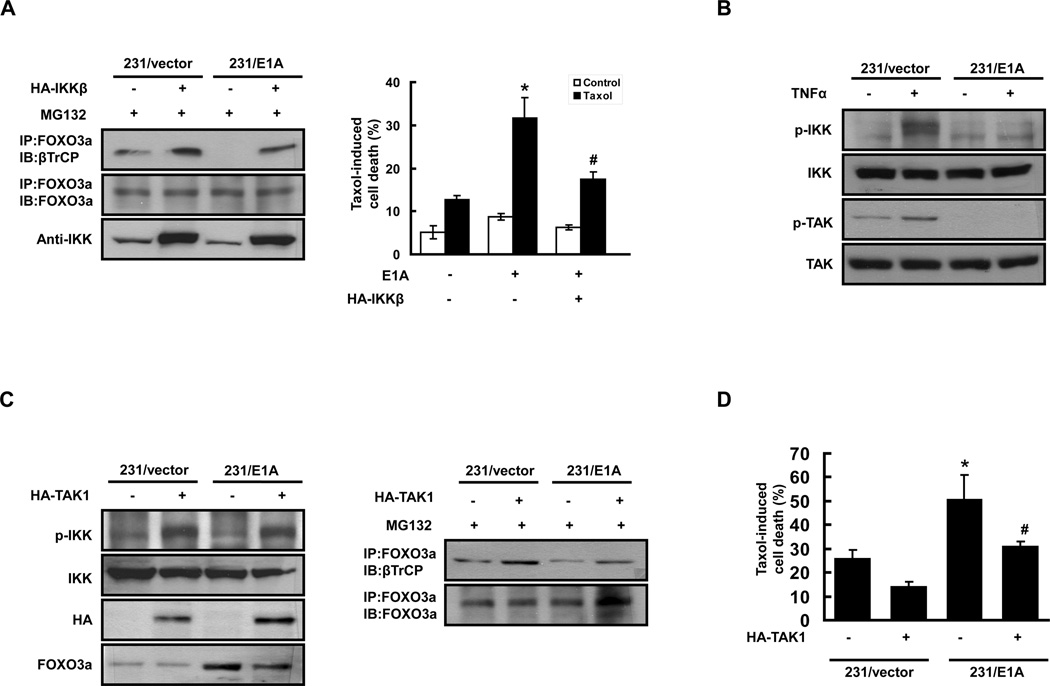
It is known that βTrCP interacts with FOXO3 and induces its ubiquitin-dependent degradation in an IKKβ phosphorylation dependent manner (29). To define whether E1A-mediated FOXO3a stabilization is due to prevention of FOXO3a phosphorylation by IKKβ and subsequent recognition by βTrCP, we investigated the association between FOXO3a and βTrCP in 231/vector and 231/E1A cells transfected with IKKβ-expression plasmid or control vector. Notably, transfection with the IKKβ expression vector reestablished the association between FOXO3a and βTrCP in 231/E1A cells (Fig. 3A, left panel). To further investigate whether inactivation of IKKβ by E1A is required for E1A-induced chemosensitization, we transiently transfected E1A expression vector with or without IKKβ expression vector into MDA-MB-231 cells and determined the effects of paclitaxel-induced cell death. E1A-induced chemosensitization was strikingly suppressed by transfection with IKKβ expression vector (Fig. 3A, right panel), supporting the notion that E1A-repressed IKKβ activity is required for E1A-mediated paclitaxel chemosensitization. To determine whether the Ser644 phosphorylated FOXO3a is able to reestablished the association between FOXO3a and βTrCP in 231/E1A cells, we transfected the GFP-tagged Ser644 phosphorylation-mimic mutant FOXO3a, GFP-FOXO3a-S644E, into 231/vector and 231/E1A cells. Expression of GFP-FOXO3a-S644E reestablished the association between FOXO3a and βTrCP in 231/E1A cells (Supplementary Figure S2). The above data indicated that phosphorylation of FOXO3a at Ser 644 is critical for the association between FOXO3a and βTrCP. To further define whether Akt and ERK signaling pathways are involved in E1A-mediated FOXO3a expression and paclitaxel chemosensitization, we have modulated these two kinases by a specific inhibitor or genetic modulation. E1A-induced FOXO3a expression and chemosensitization were suppressed by transfection with constitutively activated Akt (Myr-Akt) expression vector (Supplementary Figure S3). On the other hand, we found that treatment with MEK inhibitor, U0126, slightly increased FOXO3a expression and paclitaxel chemosensitization in 231/vector cells but not 231/E1A cells (Supplementary Figure S4). The above data suggests that Akt but not ERK signaling pathway may also be involved in E1A-mediated FOXO3a regulation and paclitaxel chemosensitization.
Figure 3. Inhibition of TAK1-IKKβ signaling is Required for E1A-Mediated Prevention of βTrCP/FOXO3a interaction and Chemosensitization.
(A) IKKβ was required for E1A-Mediated Prevention of interaction between βTrCP and FOXO3a and Chemosensitization. Left panel: Lysates of 231/vector cells and 231/E1A cells transfected with the HA-IKKβ expression vector in the presence of MG132 (5 µM) were analyzed by IP/IB (anti-FOXO3a/anti-βTrCP, anti-FOXO3a). Right panel: Chemosensitization of 231/E1A and 231/vector cells transfected with HA-IKKβ expression plasmid or control vector as analyzed by the DNA flow cytometry assay. Each type of transfected cell was treated with 20 nM paclitaxel (Taxol) for 24 h. The columns are the mean values from the three independent experiments. Bars indicated means ± SE. Asterisks denote a statistically significant difference compared with values of group 1 (*p < 0.05, two-tailed Student’s t test). E1A-dependent chemosensitization was overturned by HA-IKKβ to a significant degree, as indicated by the # symbol. (B) Lysates of MDA-MB-231/vector and MDA-MB-231/E1A cells left untreated or treated with TNFα (50 ng/ml) were subjected to Western blotting to analyze the phosphorylation of IKK and TAK1. (C) Lysates of 231/vector and 231/E1A cells transfected with or without HA-TAK1 expression plasmid were subjected to Western blotting to analyze the expression of FOXO3a and phosphorylated IKK protein (Left panel). The interaction between βTrCP and FOXO3a was determined by IP and Western blotting (Right panel). (D) 231/E1A and 231/vector cells were transfected with HA-TAK1 expression plasmid or control vector and then analyzed paclitaxel-induced cell death by DNA flow cytometry. Each type of transfected cells was treated with 20 nM paclitaxel for 24 h. The columns are the mean values from the three independent experiments. Bars indicated means ± SE. Asterisks denote a statistically significant difference compared with values of column 1 (*p < 0.05, two-tailed Student’s t test). E1A-dependent chemosensitization was overturned to a significant degree by overexpression of HA-TAK1, as indicated by the # symbol.
To explore the mechanism(s) through which inhibition of IKK activity participates in the cellular responses to E1A, we determined the phosphorylation of IKK in 231/vector and 231/E1A cells. Consistent to the previous report (35), treatment with TNFα increased the phosphorylation of IKK in 231/vector cells, but this activation was abolished in 231/E1A cells (Fig. 3B). These data suggested that E1A-induced inhibition of IKK signaling may target the upstream kinase of IKK. Recent evidence indicates that TAK1 is essential for the activation of IKK in multiple signaling pathways (26). Therefore, we investigated the possible involvement of TAK1 in E1A-mediated inhibition of IKK signalling, FOXO3a stabilization, and chemosensitization. We found that treatment with TNFα increased the phosphorylation of TAK1 in 231/vector cells, and this TNFα-induced phosphorylation was diminished by expression of E1A (Fig. 3B). Furthermore, transfection with the HA-TAK1 expression vector significantly increased phosphorylation of IKK and the E1A-mediated downregulation of p-IKK was overcome by the forced expression of HA-TAK1 (Fig. 3C, left panel). Experiments were also performed to ascertain whether TAK1 is involved in E1A-mediated FOXO3a interaction with βTrCP and chemosensitization. Forced expression of HA-TAK1 significantly increased the interaction between FOXO3a and βTrCP in 231/vector cells and the E1A-mediated inhibition effect was also recovered by exogenous expression of HA-TAK1 (Fig. 3C, right panel). Consistently, E1A-mediated chemosensitization to paclitaxel was also significantly impaired by expression of TAK1 (Fig. 3D). Taken together, these data indicate that TAK1, the upstream kinase of IKK, is a critical regulator for E1A-repressed FOXO3a interaction with βTrCP and is required for E1A-mediated chemosensitization.
E1A-induced PP2A expression is required for regulation of TAK1-IKK signaling, βTrCP/FOXO3a interaction and chemosensitization
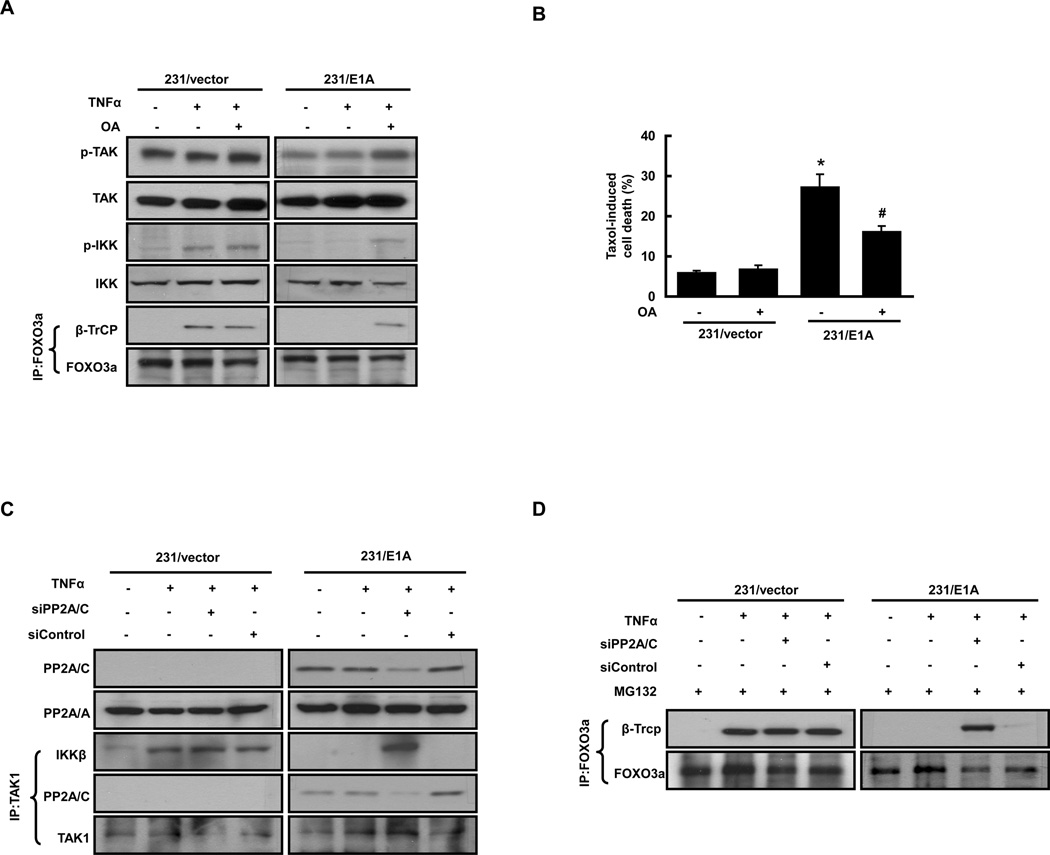
Phosphorylation of protein kinases are tightly regulated by related protein phosphatases, and it has been reported that E1A increases the expression of PP2A/C, the catalytic subunit of PP2A (14). Since E1A inhibits TAK1 phosphorylation, we asked whether PP2A might be involved in E1A-mediated dephosphorylation of TAK1 and IKK and FOXO3a stabilization. To this end, 231/vector and 231/E1A cells were treated with the phosphatase inhibitor okadaic acid (OA). We found that E1A-mediated inhibition of TNFα-induced TAK1 phosphorylation was restored by OA treatment, as were E1A-mediated inhibition of IKK phosphorylation and the interaction between FOXO3a and βTrCP (Fig. 4A). Consistently, E1A-induced chemosensitization was also decreased in 231/E1A cells by treatment with OA (Fig. 4B). We next examined whether PP2A binds to TAK1. As shown in Fig. 4C, treatment with TNFα notably increased the interaction between TAK1 and IKK in 231/vector cells but not in 231/E1A cells. Moreover, PP2A/C formed a complex with TAK1 in 231/E1A cells and severely impaired the TNFα-induced interaction between TAK1 and IKK (Fig. 4C).
Figure 4. E1A-Induced PP2A Expression Is Required for Regulation of TAK1-IKK Signaling, βTrCP/FOXO3a interaction, and Chemosensitization.
(A) Western blot analyses of the phosphorylation of TAK1 and IKK and the interaction between FOXO3a and βTrCP in MDA-MB-231/vector and MDA-MB-231/E1A cells left untreated or treated with TNFα (50 ng/ml) and 10 nM okadaic acid (OA). Equal amounts of cell lysates were subjected to Western blotting with specific anti-phosphorylated TAK1 and IKK antibodies and anti-TAK1 and IKK antibodies. The results are representative of at least three independent experiments. The cell lysates were also subjected to immunoprecipitation/immunoblotting IP/IB (anti-FOXO3a/anti-βTrCP) analysis. (B) 231/E1A and 231/vector cells were untreated or treated with OA (10 nM) combined with 20 nM paclitaxel (Taxol) for 24 h and then analyzed for chemosensitization by DNA flow cytometry. The columns are the means of three independent experiments. Bars indicated means ± SE. Asterisks denote a statistically significant difference compared with values of column 1 (*p < 0.05, two-tailed Student’s t test). E1A-dependent chemosensitization was overturned to a significant degree by treatment with OA, as indicated by the # symbol. (C) E1A-induced PP2A/C expression was required for E1A-mediated signaling. Lysates of 231/vector and 231/E1A cells transfected with siPP2A/C or control siRNA were subjected to IP/IB (anti-TAK1/anti-IKK and anti-PP2A/C) analysis and Western blotting (PP2A/C and PP2A/A). (D) Lysates of 231/vector and 231/E1A cells transfected with siPP2A/C or control siRNA were subjected to IP/IB (anti-FOXO3a/anti-FOXO3a and anti-βTrCP) analysis.
To confirm this novel binding between PP2A and TAK1, we transfected 231/vector and 231/E1A cells with siRNA for catalytic subunit of PP2A, PP2A/C, (siPP2A/C) to target knockdown of PP2A/C protein and then measured the binding preference of TAK1. Transfection with siPP2A/C, but not with control siRNA, decreased E1A-induced PP2A/C expression (Fig. 4C), and accordingly the binding complex of TAK1 and PP2A/C in 231/E1A cells. Interestingly, the formation of TAK1/IKKβ complex was significantly increased by siPP2A/C (Lane 7, Fig. 4C). E1A-mediated inhibition of the interaction between FOXO3a and βTrCP were also restored by knockdown of siPP2A/C (Lane 7, Fig. 4D). These findings indicated that E1A-induced PP2A/C expression is required for regulation of TAK1/IKK signaling, βTrCP/FOXO3a interaction, and chemosensitization.
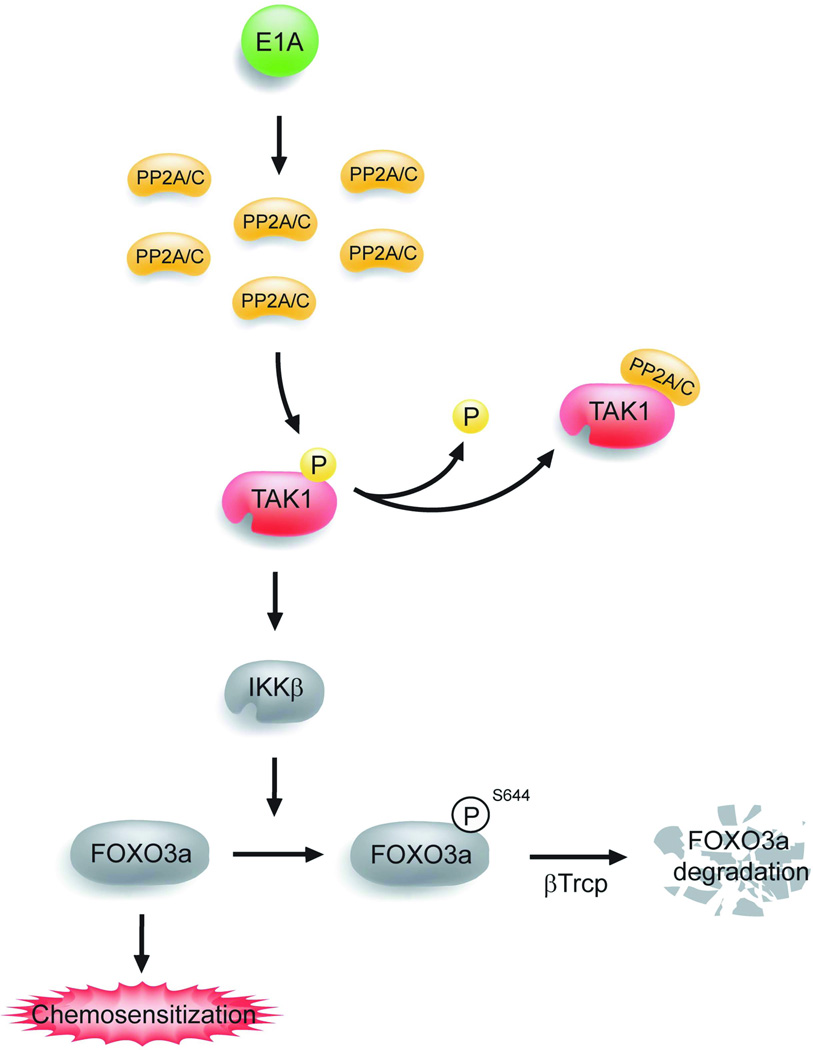
In summary, we found that FOXO3a is critical for E1A-mediated chemosensitization. E1A stabilizes FOXO3a by inducing the expression of PP2A/C and results in enhanced PP2A phosphatase activity. The enhanced PP2A/C interacts and dephosphorylates TAK1 to inactivate TAK1, which also renders inactivation of IKKβ, therefore inhibiting IKKβ-mediated interaction between βTrCP and FOXO3a and preventing βTrCP-induced FOXO3a degradation (Fig. 5).
Figure 5. A model of molecular mechanisms involved in E1A-mediated chemosensitization.
A model in which E1A stabilizes FOXO3a by inducing the expression of PP2A/C, which inhibits the activation of IKKβ through binding and inactivation of TAK1, therefore inhibiting IKKβ-mediated FOXO3a phosphorylation at Ser644 and preventing βTrCP-induced FOXO3a degradation, and thus inducing chemosensitization.
DISCUSSION
E1A is associated with many anti-tumor activities and has been tested in multiple clinical trials. Studies showed that although E1A gene therapy is safe and well tolerated, the tumor response to it is only modest (14–16). However, E1A has been shown to induce sensitization to apoptosis induced by different categories of anticancer drugs; therefore, one improvement that might render E1A more useful as an anticancer therapy is the combination of E1A gene therapy with conventional chemotherapy. Paclitaxel is a front-line chemotherapeutic agent for the treatment of human breast and ovarian cancer. One of the mechanisms that paclitaxel induces apoptosis in cancer cells is through increasing Bim (pro-apoptotic BH3-only protein) expression by activated FOXO3a activity (34). In the current study, we found that E1A can stabilize FOXO3a, therefore sensitizing MDA-MB-231 breast cancer cells to paclitaxel-induced apoptosis both in vitro and in vivo. This result provides a molecular mechanism for stronger anti-tumor strategy by combination of E1A gene therapy and paclitaxel chemotherapy (11, 13, 33). It should be mentioned that the identified molecular mechanism for E1A-induced FOXO3a expression was not observed in the MDA-MB-468 cell line, suggesting that this mechanism might be cell type specific.
We found that E1A can protect FOXO3a from degradation by inhibiting its ubiquitination. Although previous studies showed that FOXO3a can be targeted by the proteasome pathway after being phosphorylated by Akt or IKK (28, 29). It is worthwhile to mention that FOXO3a was also shown to be phosphorylated by Erk at different sites and the phospho-FOXO3a by Erk can be degraded by an E3 ubiquitin ligase MDM2 (30). Our data suggest that the ERK signaling pathway may not be involved in E1A-mediated FOXO3a regulation and paclitaxel chemosensitization. Although the mechanism is not yet clear, a possible reason that the ERK signaling may not be involved could be due to the fact that ERK-mediated degradation of Foxo3a, unlike AKT and IKK, occurs through MDM2 (30) and E1A can regulate the MDM2 family. For instance, it is known that E1A can bind to MDM4 to inhibit MDM2-induced degradation of p53 (36). Recently, a study has indicated that βTrCP1 oncogenic ubiquitin E3-ligase interacts with FOXO3 and induces its ubiquitin-dependent degradation in an IKKβ phosphorylation dependent manner (37). In our study, we found that βTrCP can physically bind to FOXO3a and mediate its degradation and that E1A stabilizes FOXO3a by inhibiting the binding of FOXO3a to βTrCP. βTrCP is the substrate recognition subunits of the Skp1 Cullin1 F-box protein E3 ubiquitin protein ligases that can recognize specifically phosphorylated substrates and confer their ubiquitination. βTrCP plays a key role in the NF-κB signaling pathway by recognizing IKK-phosphorylated IκB and mediating its degradation (38). Our findings revealed a new substrate of βTrCP that requires phosphorylation by IKK. Previous studies showed that IKK can phosphorylate FOXO3a at serine 644 and cause FOXO3a nuclear exclusion (29). Consistent with the other group’s study (37), we found that this Ser644 phosphorylation mediated by IKK is also required for FOXO3a binding to βTrCP and for the further degradation induced through βTrCP. E1A prevents the binding of βTrCP to FOXO3a by inhibiting the IKK-mediated FOXO3a phosphorylation at Ser644.
IKK activation requires its phosphorylation by upstream kinases, including TAK1 (39), and phosphorylation plays a significant role in TAK1 activation (40). We found in this study that E1A inhibits FOXO3a binding to βTrCP by preventing TAK1 activation and its effect on IKK activation. It was previously shown that TRAF6 and RIP1 can activate TAK1 and lead to IKK phosphorylation and activation (41, 42). However, overexpression TRAF6 or RIP1 in E1A-stable cell lines could not restore TAK1 activation and mediate FOXO3a degradation (data not shown), suggesting that prevention of TAK1 activation by E1A is not mediated by those two upstream activators. It is known that PP2A phosphatase activity is enhanced in E1A-expressing cells through E1A-mediated up-regulation of PP2A/C expression, which results in repression of Akt activation (17). A previous study indicates that PP2A functions as a negative regulator in TGF-β1-induced TAK1 activation (43). Therefore, E1A-mediated up-regulation of PP2A/C is involved in TAK1 inactivation and inhibits the binding of TAK1 to IKK, which abolishes IKK’s function in phosphorylating FOXO3a, resulting in the stabilization of FOXO3a.
The activities of protein kinases are finely regulated by phosphorylation and dephosphorylation, however, little is known about the dephosphorylation and respective protein phosphatase involved in the regulation of TAK1. Protein phosphatase 2A (PP2A) is a ubiquitously expressed protein serine/threonine phosphatase accounts for the tumor suppression activity in eukaryotic cells. Mutation of PP2A was found in human breast, colon, and lung cancers and melanoma (44). In addition, a variety of mechanisms for inactivating PP2A were found to be involved in transformed cells. PP2A can be inhibited by the small T antigen of the DNA tumor virus SV40 (45), or by upregulation of the c-Myc-specific inhibitor CIP2A (46), or through the upregulation of SET protein by BCR/ABL oncogene (47). It was previously shown that PP2A can suppress Akt (17) and RalA (48) activation, therefore, inhibit both PI3K/Akt and ERK signaling pathways (49). We found in this paper that TAK1 is a target of PP2A/C in another important signaling pathway—IKK pathway (50). Therefore, PP2A may inhibit the three major oncogenic kinase pathways, PI3K/Akt, ERK and IKK, to exert its tumor suppressor activity. E1A, through upregulation of PP2A/C to stimulate PP2A phosphatase activity, may share the same pathways to suppress tumor development.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. X. Lin, M. Karin, A. Toker, and S.Y. Fuchs for providing reagents and Dr. E.W. McIntush from Bethyl Laboratory for generating antibodies. This work was partially supported by NIH grants R01 CA109311, and P01 CA099031 and by the Kadoorie Charitable Foundations and the National Breast Cancer Foundation, Inc., to M.-C. H.; by NIH SPORE grants in Ovarian Cancer CA83639 (R. C. B Jr. & M.-C. H.) and Breast Cancer CA116199 (G. H. & M.-C. H.); the University of Texas MD Anderson/China Medical University and Hospital Sister Institution Fund, DOH-TD-C-111-005,, by Cancer Center Support Grant CA16672, and a Breast Cancer Research Foundation grant to M.-C. H.; by NSC-2632-B-001-MY3 (to M.-C. H. and J.-L. S.) by National Science Council grant NSC 96-2320-B-004-MY2, NSC 97-2320-B-039-039-MY3, NSC 98-2815-C-039-082-B; by National Health Research Institutes grant from Taiwan (NHRI-EX98-9712BC, NHRI-EX99-9712BC, NHRI-EX100-9712BC); by Department of Health, Executive Yuan grant from Taiwan (DOH99-TD-G111-011); by grants from China Medical University (CMU96-220, CMU97-077, CMU99-TC-22 and CMU97-277) and an Odyssey Scholarship from M. D. Anderson Cancer Center to J.-L.S. This study is supported in part by Taiwan Department of Health Clinical Trial and Research Center of Excellence (DOH100-TD-B-111-004)
REFERENCES
- 1.Yu D, Hung MC. The erbB2 gene as a cancer therapeutic target and the tumor- and metastasis-suppressing function of E1A. Cancer Metastasis Rev. 1998;17:195–202. doi: 10.1023/a:1006054421970. [DOI] [PubMed] [Google Scholar]
- 2.Ueno NT, Yu D, Hung MC. E1A: tumor suppressor or oncogene? Preclinical and clinical investigations of E1A gene therapy. Breast Cancer. 2001;8:285–293. doi: 10.1007/BF02967526. [DOI] [PubMed] [Google Scholar]
- 3.Yu D, Hamada J, Zhang H, Nicolson GL, Hung MC. Mechanisms of c-erbB2/neu oncogene-induced metastasis and repression of metastatic properties by adenovirus 5 E1A gene products. Oncogene. 1992;7:2263–2270. [PubMed] [Google Scholar]
- 4.Deng J, Xia W, Hung MC. Adenovirus 5 E1A-mediated tumor suppression associated with E1A-mediated apoptosis in vivo. Oncogene. 1998;17:2167–2175. doi: 10.1038/sj.onc.1202148. [DOI] [PubMed] [Google Scholar]
- 5.Hung MC, Hortobagyi GN, Ueno NT. Development of clinical trial of E1A gene therapy targeting HER-2/neu-overexpressing breast and ovarian cancer. Adv Exp Med Biol. 2000;465:171–180. doi: 10.1007/0-306-46817-4_16. [DOI] [PubMed] [Google Scholar]
- 6.Frisch SM, Mymryk JS. Adenovirus-5 E1A: paradox and paradigm. Nat Rev Mol Cell Biol. 2002;3:441–452. doi: 10.1038/nrm827. [DOI] [PubMed] [Google Scholar]
- 7.Frisch SM, Dolter KE. Adenovirus E1a-mediated tumor suppression by a c-erbB-2/neu-independent mechanism. Cancer Res. 1995;55:5551–5555. [PubMed] [Google Scholar]
- 8.Brader KR, Wolf JK, Hung MC, Yu D, Crispens MA, van Golen KL, et al. Adenovirus E1A expression enhances the sensitivity of an ovarian cancer cell line to multiple cytotoxic agents through an apoptotic mechanism. Clin Cancer Res. 1997;3:2017–2024. [PubMed] [Google Scholar]
- 9.Liao Y, Hung MC. Regulation of the activity of p38 mitogen-activated protein kinase by Akt in cancer and adenoviral protein E1A-mediated sensitization to apoptosis. Mol Cell Biol. 2003;23:6836–6848. doi: 10.1128/MCB.23.19.6836-6848.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee WP, Tai DI, Tsai SL, Yeh CT, Chao Y, Lee SD, et al. Adenovirus type 5 E1A sensitizes hepatocellular carcinoma cells to gemcitabine. Cancer Res. 2003;63:6229–6236. [PubMed] [Google Scholar]
- 11.Ueno NT, Bartholomeusz C, Herrmann JL, Estrov Z, Shao R, Andreeff M, et al. E1A-mediated paclitaxel sensitization in HER-2/neu-overexpressing ovarian cancer SKOV3.ip1 through apoptosis involving the caspase-3 pathway. Clin Cancer Res. 2000;6:250–259. [PubMed] [Google Scholar]
- 12.Ueno NT, Yu D, Hung MC. Chemosensitization of HER-2/neu-overexpressing human breast cancer cells to paclitaxel (Taxol) by adenovirus type 5 E1A. Oncogene. 1997;15:953–960. doi: 10.1038/sj.onc.1201250. [DOI] [PubMed] [Google Scholar]
- 13.Liao Y, Zou YY, Xia WY, Hung MC. Enhanced paclitaxel cytotoxicity and prolonged animal survival rate by a nonviral-mediated systemic delivery of E1A gene in orthotopic xenograft human breast cancer. Cancer Gene Ther. 2004;11:594–602. doi: 10.1038/sj.cgt.7700743. [DOI] [PubMed] [Google Scholar]
- 14.Hortobagyi GN, Hung MC, Lopez-Berestein G. A Phase I multicenter study of E1A gene therapy for patients with metastatic breast cancer and epithelial ovarian cancer that overexpresses HER-2/neu or epithelial ovarian cancer. Hum Gene Ther. 1998;9:1775–1798. doi: 10.1089/hum.1998.9.12-1775. [DOI] [PubMed] [Google Scholar]
- 15.Yoo GH, Hung MC, Lopez-Berestein G, LaFollette S, Ensley JF, Carey M, et al. Phase I trial of intratumoral liposome E1A gene therapy in patients with recurrent breast and head and neck cancer. Clin Cancer Res. 2001;7:1237–1245. [PubMed] [Google Scholar]
- 16.Villaret D, Glisson B, Kenady D, Hanna E, Carey M, Gleich L, et al. A multicenter phase II study of tgDCC-E1A for the intratumoral treatment of patients with recurrent head and neck squamous cell carcinoma. Head Neck. 2002;24:661–669. doi: 10.1002/hed.10107. [DOI] [PubMed] [Google Scholar]
- 17.Liao Y, Hung MC. A new role of protein phosphatase 2a in adenoviral E1A protein-mediated sensitization to anticancer drug-induced apoptosis in human breast cancer cells. Cancer Res. 2004;64:5938–5942. doi: 10.1158/0008-5472.CAN-04-1533. [DOI] [PubMed] [Google Scholar]
- 18.de Stanchina E, McCurrach ME, Zindy F, Shieh SY, Ferbeyre G, Samuelson AV, et al. E1A signaling to p53 involves the p19(ARF) tumor suppressor. Genes Dev. 1998;12:2434–2442. doi: 10.1101/gad.12.15.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duelli DM, Lazebnik YA. Primary cells suppress oncogene-dependent apoptosis. Nat Cell Biol. 2000;2:859–862. doi: 10.1038/35041112. [DOI] [PubMed] [Google Scholar]
- 20.McCurrach ME, Connor TM, Knudson CM, Korsmeyer SJ, Lowe SW. bax-deficiency promotes drug resistance and oncogenic transformation by attenuating p53-dependent apoptosis. Proc Natl Acad Sci U S A. 1997;94:2345–2349. doi: 10.1073/pnas.94.6.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Putzer BM, Stiewe T, Parssanedjad K, Rega S, Esche H. E1A is sufficient by itself to induce apoptosis independent of p53 and other adenoviral gene products. Cell Death Differ. 2000;7:177–188. doi: 10.1038/sj.cdd.4400618. [DOI] [PubMed] [Google Scholar]
- 22.Teodoro JG, Shore GC, Branton PE. Adenovirus E1A proteins induce apoptosis by both p53-dependent and p53-independent mechanisms. Oncogene. 1995;11:467–474. [PubMed] [Google Scholar]
- 23.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 24.Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–787. doi: 10.1038/35008115. [DOI] [PubMed] [Google Scholar]
- 25.Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000;10:1201–1204. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt M, Fernandez de Mattos S, van der Horst A, Klompmaker R, Kops GJ, Lam EW, et al. Cell cycle inhibition by FoxO forkhead transcription factors involves downregulation of cyclin D. Mol Cell Biol. 2002;22:7842–7852. doi: 10.1128/MCB.22.22.7842-7852.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilley J, Coffer PJ, Ham J. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J Cell Biol. 2003;162:613–622. doi: 10.1083/jcb.200303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 29.Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, et al. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–237. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- 30.Yang JY, Zong CS, Xia W, Yamaguchi H, Ding Q, Xie X, et al. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol. 2008;10:138–148. doi: 10.1038/ncb1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu D, Wolf JK, Scanlon M, Price JE, Hung MC. Enhanced c-erbB-2/neu expression in human ovarian cancer cells correlates with more severe malignancy that can be suppressed by E1A. Cancer Res. 1993;53:891–898. [PubMed] [Google Scholar]
- 32.Blonska M, Shambharkar PB, Kobayashi M, Zhang D, Sakurai H, Su B, et al. TAK1 is recruited to the tumor necrosis factor-alpha (TNF-alpha) receptor 1 complex in a receptor-interacting protein (RIP)-dependent manner and cooperates with MEKK3 leading to NF-kappaB activation. J Biol Chem. 2005;280:43056–43063. doi: 10.1074/jbc.M507807200. [DOI] [PubMed] [Google Scholar]
- 33.Liao Y, Yu D, Hung MC. Novel approaches for chemosensitization of breast cancer cells: the E1A story. Adv Exp Med Biol. 2007;608:144–169. doi: 10.1007/978-0-387-74039-3_11. [DOI] [PubMed] [Google Scholar]
- 34.Sunters A, Fernandez de Mattos S, Stahl M, Brosens JJ, Zoumpoulidou G, Saunders CA, et al. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem. 2003;278:49795–49805. doi: 10.1074/jbc.M309523200. [DOI] [PubMed] [Google Scholar]
- 35.Shao R, Hu MC, Zhou BP, Lin SY, Chiao PJ, von Lindern RH, et al. E1A sensitizes cells to tumor necrosis factor-induced apoptosis through inhibition of IkappaB kinases and nuclear factor kappaB activities. J Biol Chem. 1999;274:21495–21498. doi: 10.1074/jbc.274.31.21495. [DOI] [PubMed] [Google Scholar]
- 36.Li Z, Day CP, Yang JY, Tsai WB, Lozano G, Shih HM, et al. Adenoviral E1A targets Mdm4 to stabilize tumor suppressor p53. Cancer research. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.] 2004;64:9080–9085. doi: 10.1158/0008-5472.CAN-04-2419. [DOI] [PubMed] [Google Scholar]
- 37.Tsai WBCY, Zou Y, Park SH, Xu Z, Nakayama K, Lin SH, Hu MC. Inhibition of FOXO3 tumor suppressor function by betaTrCP1 through ubiquitin-mediated degradation in a tumor mouse model. PLoS One. 2010;5:e11171. doi: 10.1371/journal.pone.0011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuchs SY, Spiegelman VS, Kumar KG. The many faces of beta-TrCP E3 ubiquitin ligases: reflections in the magic mirror of cancer. Oncogene. 2004;23:2028–2036. doi: 10.1038/sj.onc.1207389. [DOI] [PubMed] [Google Scholar]
- 39.Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 40.Singhirunnusorn P, Suzuki S, Kawasaki N, Saiki I, Sakurai H. Critical roles of threonine 187 phosphorylation in cellular stress-induced rapid and transient activation of transforming growth factor-beta-activated kinase 1 (TAK1) in a signaling complex containing TAK1-binding protein TAB1 and TAB2. J Biol Chem. 2005;280:7359–7368. doi: 10.1074/jbc.M407537200. [DOI] [PubMed] [Google Scholar]
- 41.Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 42.Ting AT, Pimentel-Muinos FX, Seed B. RIP mediates tumor necrosis factor receptor 1 activation of NF-kappaB but not Fas/APO-1-initiated apoptosis. Embo J. 1996;15:6189–6196. [PMC free article] [PubMed] [Google Scholar]
- 43.Kim SIK, Wang JH, Choi L, M E. Protein phosphatase 2A is a negative regulator of transforming growth factor-β1–induced TAK1 activation in mesangial cells. The Journal of biological chemistry. 2008;283:10753–10763. doi: 10.1074/jbc.M801263200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schonthal AH. Role of serine/threonine protein phosphatase 2A in cancer. Cancer Lett. 2001;170:1–13. doi: 10.1016/s0304-3835(01)00561-4. [DOI] [PubMed] [Google Scholar]
- 45.Arroyo JD, Hahn WC. Involvement of PP2A in viral and cellular transformation. Oncogene. 2005;24:7746–7755. doi: 10.1038/sj.onc.1209038. [DOI] [PubMed] [Google Scholar]
- 46.Junttila MR, Puustinen P, Niemela M, Ahola R, Arnold H, Bottzauw T, et al. CIP2A inhibits PP2A in human malignancies. Cell. 2007;130:51–62. doi: 10.1016/j.cell.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 47.Neviani P, Santhanam R, Trotta R, Notari M, Blaser BW, Liu S, et al. The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABL-regulated SET protein. Cancer Cell. 2005;8:355–368. doi: 10.1016/j.ccr.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 48.Sablina AA, Chen W, Arroyo JD, Corral L, Hector M, Bulmer SE, et al. The tumor suppressor PP2A Abeta regulates the RalA GTPase. Cell. 2007;129:969–982. doi: 10.1016/j.cell.2007.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mumby M. PP2A: unveiling a reluctant tumor suppressor. Cell. 2007;130:21–24. doi: 10.1016/j.cell.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 50.Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wei Y, et al. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–455. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.