Abstract
The early phase of preconditioning (PC) lasts 2 to 3 hours and protects against myocardial infarction, but not against stunning. In contrast, the late phase of PC lasts for 3 to 4 days and protects against both myocardial stunning and infarction, making this phenomenon more clinically relevant. Late PC is a genetic reprogramming of the heart that involves the activation of several stress-responsive genes, which ultimately results in the development of a cardioprotective phenotype. Sublethal ischemic insults release chemical signals (nitric oxide [NO], adenosine, and reactive oxygen species) that trigger a series of signaling events (eg, activation of protein kinase C, Src protein tyrosine kinases, Janus kinases 1/2, and nuclear factor-κB) and culminates in increased synthesis of inducible NO synthase, cyclooxygenase-2, heme oxygenase-1, aldose reductase, Mn superoxide dismutase, and probably other cardioprotective proteins. In addition to ischemia, heat stress, exercise, and cytokines can also induce a similar series of events. Perhaps most importantly, many pharmacologic agents (eg, NO donors, adenosine receptor agonists, endotoxin derivatives, or opioid receptor agonists) can mimic the effects of ischemia in inducing the late phase of PC, suggesting that this phenomenon might be exploited therapeutically. The purpose of this review is to summarize the mechanisms that underlie the late phase of ischemic PC.
Keywords: ischemic preconditioning, myocardial ischemia, myocardial reperfusion, nitric oxide
Originally described as an immediate adaptation of the heart to brief sublethal ischemia,1 it is now recognized that ischemic PC consists of 2 chronologically and pathophysiologically distinct phases: an early phase and a late phase of protection. The early phase occurs immediately after the PC stimulus and induces robust protection. Unfortunately, early PC is short-lived (1 to 2 hours), which limits its clinical relevance. In contrast, the late phase of ischemic PC develops 12 to 24 hours after the initial stimulus and lasts 3 to 4 days, although the magnitude of protection may be somewhat less than in the early phase.2–4 Unlike the early phase, the late phase of ischemic PC protects not only against myocardial infarction but also against reversible postischemic cardiac dysfunction (myocardial stunning).5 Because of its 30- to 50-fold longer duration and the broader protection it provides, considerable interest has been focused on the late phase of PC and its clinical exploitation.6
The recognition that the heart shifts to a preconditioned (defensive) phenotype on exposure to stress has undoubtedly been one of the major advances in the field of myocardial ischemia. In the past few years, much has been learned regarding the intricate signaling pathways and genetic changes that underlie this protective adaptation. Because this topic has been reviewed in detail elsewhere,6 the purpose of the present review is to succinctly summarize current information regarding late PC with an emphasis on the molecular events that underlie it.
Stimuli That Elicit Late PC
The phenomenon of late PC can be induced by a wide variety of stimuli, which can be broadly classified as nonpharmacological and pharmacological.6 Nonpharmacological stimuli include ischemia, heat stress, rapid ventricular pacing, exercise, and hypoxia.6 Pharmacological stimuli consist of naturally occurring and often noxious agents, including endotoxin, interleukin-1, tumor necrosis factor-α (TNF-α), TNF-β, leukemia inhibitory factor, and reactive oxygen species (ROS),7 and of clinically applicable drugs including NO-releasing agents,8 adenosine receptor (AR) agonists,9 endotoxin derivatives such as monophosphoryl lipid A (MLA) and its analog RC-552, the ATP-sensitive potassium (KATP) channel opener diazoxide, α1-adrenoceptor agonists, and opioid receptor agonists.10–15
Components of the Mechanism of Late PC
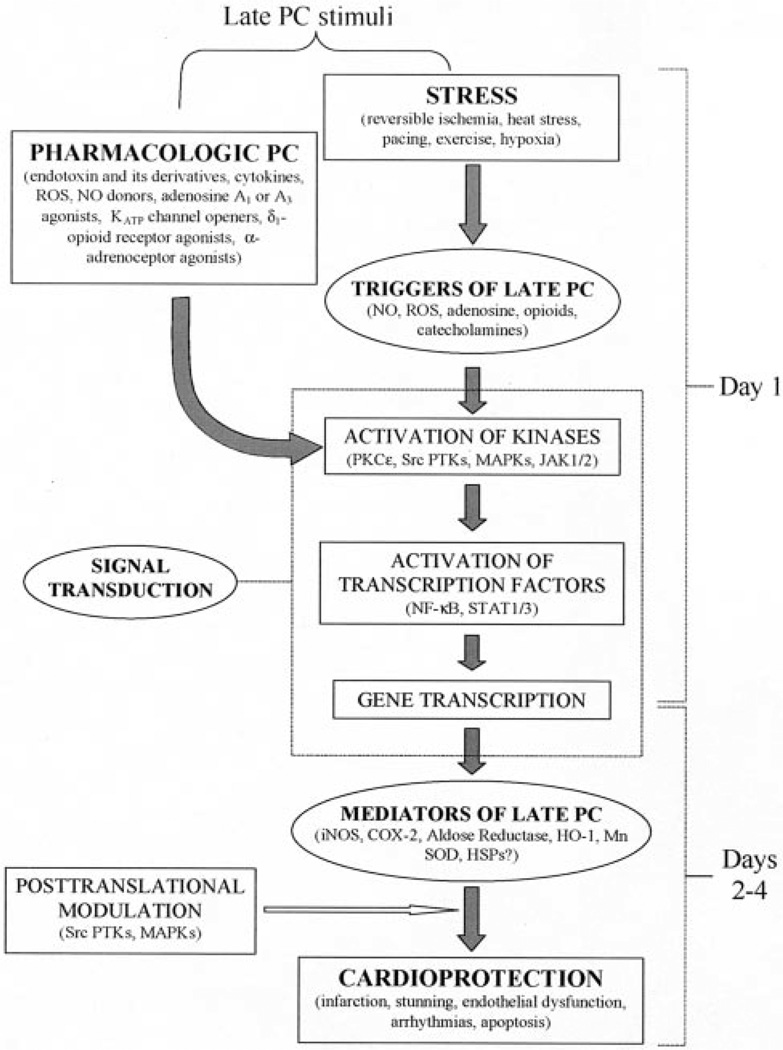
Late PC induced by ischemia is the result of a complex cascade of signaling events that ultimately results in cardioprotection. These signaling mechanisms have been broadly classified into 3 components: (1) the molecular species that are generated during the ischemic challenge and are responsible for initiating the adaptation (“triggers”) of late PC; (2) the molecular species that are expressed 24 to 72 hours later and are responsible for conferring the protection during the second ischemic insult (“mediators” of late PC); and (3) the signaling pathways that are activated by the triggers and culminate in the mediators (Figure).6
Figure.
Schematic representation of the cellular mechanisms underlying late PC. Physical stress (eg, reversible ischemia, heat stress, ventricular pacing, exercise, or hypoxia) causes release of chemical signals (NO, ROS, adenosine, and possibly catecholamine or opioid-like substance) that serve as triggers for the development of late PC. These substances activate a complex signal transduction cascade that includes PKC (specifically, the ε isoform), PTKs (specifically, Src and/or Lck), Janus kinases (JAKs; specifically, JAK1 and JAK2) and probably other as-yet-unknown kinases. The recruitment of PKC, JAKs, and distal kinases leads to activation of NF-κB, STATs, and almost certainly other transcription factors, resulting in increased transcription of multiple cardioprotective genes and synthesis of multiple cardioprotective proteins that serve as comediators of protection 2 to 4 days after the PC stimulus.
Triggers of Late PC
Brief episodes of myocardial ischemia/reperfusion, exercise, hypoxia, and heat stress are associated with major metabolic perturbations that result in the generation of a wide variety of metabolites and ligands. Among these, there is evidence that adenosine, NO, ROS, catecholamines, and endogenous opioids serve as chemical signals that trigger the development of the late phase of ischemic PC (Figure).6 These same agents can trigger pharmacological PC in the absence of ischemia (Figure).
Adenosine
Adenosine antagonists abolish the delayed protection of ischemic PC against myocardial infarction, whereas pretreatment with A1AR agonists mimics it.9 These data9 suggest that adenosine released during the PC stimulus triggers the development of late PC.
Nitric Oxide
The first indication that NO triggers late PC was provided by a study in which administration of Nω-nitro-l-arginine (l-NA), a nonselective nitric oxide synthase (NOS) inhibitor, before the ischemic PC stimulus was found to block the development of late PC against myocardial stunning,16 demonstrating that NO generation during the initial PC ischemia is necessary to trigger this cardioprotective phenomenon. A subsequent study demonstrated that NO is also necessary to trigger late PC against myocardial infarction.17 Importantly, exposure to exogenous NO is sufficient to reproduce late PC, because pretreatment with NO donors in the absence of ischemia induces a delayed protective effect against both myocardial stunning and infarction that is indistinguishable from that observed during the late phase of ischemic PC.8,18–20 The ability of NO-releasing agents such as nitrates to mimic the late phase of ischemic PC has been subsequently documented in patients,21 supporting the possibility of novel clinical applications of these drugs.
Subsequent studies22 have provided direct evidence of enhanced biosynthesis of NO in myocardium subjected to brief episodes of ischemia/reperfusion. The source of increased NO formation during the initial ischemia/reperfusion is likely to be the endothelial isoform of NOS (eNOS), because the development of late PC is blocked by pretreatment with the nonselective NOS inhibitor L-NA, but not with the relatively selective inducible NOS (iNOS) inhibitors aminoguanidine or S-methylisothiourea (SMT).23
Reactive Oxygen Species
Sun et al24 found that in conscious pigs the administration of a combination of antioxidants (superoxide dismutase [SOD] plus catalase plus n-2-mercaptopropionyl glycine [MPG]) during the PC ischemia prevented the development of late PC against stunning. MPG has also been found to prevent ischemia-induced late PC against infarction, arrhythmias, and coronary endothelial injury as well as heat stress–induced, NO donor–induced,8 exercise-induced, and opioid receptor agonist–induced late PC against infarction, thus implicating ROS as a common trigger for these forms of delayed protection as well.
The Signaling Pathway of Late PC
Chemical signals trigger the development of late PC by activating a series of complex signaling events that ultimately result in activation of cardioprotective genes. Among the many signaling pathways implicated, there is now convincing evidence that protein kinase C (PKC), Src protein tyrosine kinases (Src PTKs), mitogen-activated protein kinases (MAPKs), JAKs, nuclear factor-κB (NF-κB), and signal transducers and activators of transcription (STATs) play an essential role in the genesis of late PC.6
Nuclear Factor-κB
The involvement of NF-κB in late PC was first shown by Xuan et al.25
JAK Signal Transducers and Activators of Transcription Pathway
An essential role of the JAK-STAT pathway in late PC has been described by Xuan et al,26 who demonstrated that this pathway is necessary for the upregulation of iNOS during late PC and for the development of cardioprotection.26 More recently, Xuan et al27 have demonstrated that JAK-STAT signaling is also necessary for the upregulation of cyclooxygenase-2 (COX-2).
Mediators (or End-Effectors) of Late PC
Ischemic PC causes an increase in the rate of myocardial protein synthesis; if this increase is blocked with cycloheximide, the development of delayed protection is also blocked.28 Thus, unlike early PC, late PC requires increased synthesis of new proteins, not simply activation of preexisting proteins.28 The time course of the enhanced tolerance to ischemia, which requires 12 to 24 hours to develop and lasts for 3 to 4 days,2,3 is also consistent with the synthesis and degradation of cardioprotective proteins. Several proteins have been proposed as possible mediators (or end-effectors) of the protection afforded by late PC, including iNOS, COX-2, aldose reductase, Mn SOD, heme oxygenase-1 (HO-1), and heat-shock proteins (HSPs).6 In addition, considerable evidence implicates KATP channels as mediators of this defensive phenotype.
Inducible Nitric Oxide Synthase
The first demonstration that the cardioprotective effects of late PC are mediated by iNOS was provided by 2 studies in conscious rabbits.23,29 Guo et al30 then demonstrated in mice that the late phase of ischemic PC is associated with upregulation of myocardial iNOS and that targeted disruption of the iNOS gene completely abrogates the delayed infarct-sparing effect, providing unequivocal molecular genetic evidence for an obligatory role of iNOS in the cardioprotection afforded by late PC. Further studies using immunohistochemical and in situ hybridization have identified cardiac myocytes as the specific cell type that expresses iNOS during late PC.31
Thus, NO appears to play a dual role in the pathophysiology of the late phase of ischemic PC, acting initially as the trigger8,17–20 and subsequently as the mediator23,29–32 of this adaptive response.33 A recent study22 showing a biphasic regulation of NOS by ischemic PC provides direct evidence to support a dual role of NO in this phenomenon.
The precise mechanism by which iNOS-derived NO protects against ischemia remains to be elucidated, but it appears to involve the activation of soluble guanylate cyclase (sGC), given that both the alleviation of stunning and the reduction in infarct size are abrogated by the selective sGC inhibitor ODQ.34 We35 have also proposed that NO protects by upregulating and activating COX-2 (vide infra).
Cyclooxygenase-2
An obligatory role of COX-2 in late PC was first shown by Shinmura et al36 Their observations identified COX-2 as a cardioprotective protein and strongly pointed to prostaglandins PGE2 or PGI2, or both, as the likely effectors of COX-2–dependent protection.27 Interestingly, the synthesis of cardioprotective prostanoids by COX-2 is driven by iNOS-derived NO,35 indicating that iNOS and COX-2 form a functionally-coupled module.27
Conclusions
The heart reacts to a sublethal ischemic stress by genetically reprogramming itself in a manner that results in a shift from a naïve (nonpreconditioned) to a defensive (preconditioned) phenotype. Although the exact cellular and molecular mechanisms underlying the phenomenon of late PC remain to be deciphered, remarkable progress has been made in the last few years in our understanding of this powerful cardioprotective adaptation. A schematic representation of the mechanism of late PC is presented in the Figure. It is now clear that this is a polygenic process that requires the synthesis of multiple proteins. Specifically, chemical signals released by the ischemic stress (such as NO, ROS, and adenosine) are transduced by a cascade of signaling events that includes the translocation of PKC, Src PTKs, JAKs, NF-κB, and STATs to the nucleus where they direct the transcription of iNOS, COX-2, aldose reductase, HO-1, and probably other cardioprotective genes (Figure). An analogous sequence of events can be triggered by a wide variety of stimuli, including heat stress, exercise, and cytokines. Thus, late PC appears to be a universal response of the heart to stress in general. A sustained cardioprotection similar to that afforded by the late phase of ischemic PC can also be induced pharmacologically with clinically relevant agents, such as NO donors, A1 or A3AR agonists, endotoxin derivatives, α-adenoceptor agonists, and δ1-opioid receptor agonists (“PC mimetics”), suggesting that this endogenous adaptive response might be exploited for therapeutic purposes.
Deciphering the mechanism of late PC is important not only for our understanding of how the heart adapts to stress but also for its potential clinical implications. The identification of the cellular and molecular basis of this phenomenon should provide a conceptual framework for developing novel therapeutic strategies aimed at mimicking the cardioprotective effects of late PC with pharmacological agents (eg, PC-mimetic drugs) or genetic approaches (eg, transfer of cardioprotective genes) that can maintain the heart in a sustained or chronic defensive (preconditioned) state.
Acknowledgments
The work described in this article was supported in part by National Institutes of Health R01 grants HL-65660, HL-74351, HL-55757, HL-68088, HL-70897, HL-76794, and HL-72410, and by American Heart Association Grants 0325372B, 0150074N, 0355391B, 0265087B, and 0130146N.
References
- 1.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 2.Baxter GF, Goma FM, Yellon DM. Characterisation of the infarct-limiting effect of delayed preconditioning: timecourse and dose-dependency studies in rabbit myocardium. Basic Res Cardiol. 1997;92:159–167. doi: 10.1007/BF00788633. [DOI] [PubMed] [Google Scholar]
- 3.Tang XL, Qiu Y, Park SW, Sun JZ, Kalya A, Bolli R. Time course of late preconditioning against myocardial stunning in conscious pigs. Circ Res. 1996;79:424–434. doi: 10.1161/01.res.79.3.424. [DOI] [PubMed] [Google Scholar]
- 4.Kaszala K, Vegh A, Papp JG, Parratt JR. Time course of the protection against ischaemia and reperfusion-induced ventricular arrhythmias resulting from brief periods of cardiac pacing. J Mol Cell Cardiol. 1996;28:2085–2095. doi: 10.1006/jmcc.1996.0201. [DOI] [PubMed] [Google Scholar]
- 5.Bolli R. The early and late phases of preconditioning against myocardial stunning and the essential role of oxyradicals in the late phase: an overview. Basic Res Cardiol. 1996;91:57–63. doi: 10.1007/BF00788866. [DOI] [PubMed] [Google Scholar]
- 6.Bolli R. The late phase of preconditioning. Circ Res. 2000;87:972–983. doi: 10.1161/01.res.87.11.972. [DOI] [PubMed] [Google Scholar]
- 7.Tang XL, Takano H, Rizvi A, Turrens JF, Qiu Y, Wu WJ, Zhang Q, Bolli R. Oxidant species trigger late preconditioning against myocardial stunning in conscious rabbits. Am J Physiol. 2002;282:H281–H291. doi: 10.1152/ajpheart.2002.282.1.H281. [DOI] [PubMed] [Google Scholar]
- 8.Takano H, Tang XL, Qiu Y, Guo Y, French BA, Bolli R. Nitric oxide donors induce late preconditioning against myocardial stunning and infarction in conscious rabbits via an antioxidant-sensitive mechanism. Circ Res. 1998;83:73–84. doi: 10.1161/01.res.83.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takano H, Bolli R, Black RG, Jr, Kodani E, Tang XL, Yang Z, Bhattacharya S, Auchampach JA. A(1) or a(3) adenosine receptors induce late preconditioning against infarction in conscious rabbits by different mechanisms. Circ Res. 2001;88:520–528. doi: 10.1161/01.res.88.5.520. [DOI] [PubMed] [Google Scholar]
- 10.Kodani E, Xuan YT, Shinmura K, Takano H, Tang XL, Bolli R. δ-opioid receptor-induced late preconditioning is mediated by cyclooxygenase-2 in conscious rabbits. Am J Physiol. 2002;283:H1943–H1957. doi: 10.1152/ajpheart.00150.2002. [DOI] [PubMed] [Google Scholar]
- 11.Fryer RM, Hsu AK, Eells JT, Nagase H, Gross GJ. Opioid-induced second window of cardioprotection: potential role of mitochondrial KATP channels. Circ Res. 1999;84:846–851. doi: 10.1161/01.res.84.7.846. [DOI] [PubMed] [Google Scholar]
- 12.Wu S, Li HY, Wong TM. Cardioprotection of preconditioning by metabolic inhibition in the rat ventricular myocyte. Involvement of kappa-opioid receptor. Circ Res. 1999;84:1388–1395. doi: 10.1161/01.res.84.12.1388. [DOI] [PubMed] [Google Scholar]
- 13.Shinmura K, Nagai M, Tamaki K, Tani M, Bolli R. Cox-2-derived prostacyclin mediates opioid-induced late phase of preconditioning in isolated rat hearts. Am J Physiol. 2002;283:H2534–H2543. doi: 10.1152/ajpheart.00209.2002. [DOI] [PubMed] [Google Scholar]
- 14.Patel HH, Fryer RM, Gross ER, Bundey RA, Hsu AK, Isbell M, Eusebi LO, Jensen RV, Gullans SR, Insel PA, Nithipatikom K, Gross GJ. 12-lipoxygenase in opioid-induced delayed cardioprotection: gene array, mass spectrometric, and pharmacological analyses. Circ Res. 2003;92:676–682. doi: 10.1161/01.RES.0000065167.52922.F6. [DOI] [PubMed] [Google Scholar]
- 15.Patel HH, Hsu AK, Peart JN, Gross GJ. Sarcolemmal KATP channel triggers opioid-induced delayed cardioprotection in the rat. Circ Res. 2002;91:186–188. doi: 10.1161/01.res.0000029085.69891.f2. [DOI] [PubMed] [Google Scholar]
- 16.Bolli R, Bhatti ZA, Tang XL, Qiu Y, Zhang Q, Guo Y, Jadoon AK. Evidence that late preconditioning against myocardial stunning in conscious rabbits is triggered by the generation of nitric oxide. Circ Res. 1997;81:42–52. doi: 10.1161/01.res.81.1.42. [DOI] [PubMed] [Google Scholar]
- 17.Qiu Y, Rizvi A, Tang XL, Manchikalapudi S, Takano H, Jadoon AK, Wu WJ, Bolli R. Nitric oxide triggers late preconditioning against myocardial infarction in conscious rabbits. Am J Physiol. 1997;273:H2931–H2936. doi: 10.1152/ajpheart.1997.273.6.H2931. [DOI] [PubMed] [Google Scholar]
- 18.Banerjee S, Tang XL, Qiu Y, Takano H, Manchikalapudi S, Dawn B, Shirk G, Bolli R. Nitroglycerin induces late preconditioning against myocardial stunning via a PKC-dependent pathway. Am J Physiol. 1999;277:H2488–H2494. doi: 10.1152/ajpheart.1999.277.6.H2488. [DOI] [PubMed] [Google Scholar]
- 19.Hill M, Takano H, Tang XL, Kodani E, Shirk G, Bolli R. Nitroglycerin induces late preconditioning against myocardial infarction in conscious rabbits despite development of nitrate tolerance. Circulation. 2001;104:694–699. doi: 10.1161/hc3201.092218. [DOI] [PubMed] [Google Scholar]
- 20.Tang XL, Kodani E, Takano H, Hill M, Shinmura K, Vondriska TM, Ping P, Bolli R. Protein tyrosine kinase signaling is necessary for no donor-induced late preconditioning against myocardial stunning. Am J Physiol. 2003;284:H1441–H1448. doi: 10.1152/ajpheart.00789.2002. [DOI] [PubMed] [Google Scholar]
- 21.Leesar MA, Stoddard MF, Dawn B, Jasti VG, Masden R, Bolli R. Delayed preconditioning-mimetic action of nitroglycerin in patients undergoing coronary angioplasty. Circulation. 2001;103:2935–2941. doi: 10.1161/01.cir.103.24.2935. [DOI] [PubMed] [Google Scholar]
- 22.Xuan YT, Tang XL, Qiu Y, Banerjee S, Takano H, Han H, Bolli R. Biphasic response of cardiac no synthase isoforms to ischemic preconditioning in conscious rabbits. Am J Physiol. 2000;279:H2360–H2371. doi: 10.1152/ajpheart.2000.279.5.H2360. [DOI] [PubMed] [Google Scholar]
- 23.Bolli R, Manchikalapudi S, Tang XL, Takano H, Qiu Y, Guo Y, Zhang Q, Jadoon AK. The protective effect of late preconditioning against myocardial stunning in conscious rabbits is mediated by nitric oxide synthase. Evidence that nitric oxide acts both as a trigger and as a mediator of the late phase of ischemic preconditioning. Circ Res. 1997;81:1094–1107. doi: 10.1161/01.res.81.6.1094. [DOI] [PubMed] [Google Scholar]
- 24.Sun JZ, Tang XL, Park SW, Qiu Y, Turrens JF, Bolli R. Evidence for an essential role of reactive oxygen species in the genesis of late preconditioning against myocardial stunning in conscious pigs. J Clin Invest. 1996;97:562–576. doi: 10.1172/JCI118449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xuan YT, Tang XL, Banerjee S, Takano H, Li RC, Han H, Qiu Y, Li JJ, Bolli R. Nuclear factor-κB plays an essential role in the late phase of ischemic preconditioning in conscious rabbits. Circ Res. 1999;84:1095–1109. doi: 10.1161/01.res.84.9.1095. [DOI] [PubMed] [Google Scholar]
- 26.Xuan YT, Guo Y, Han H, Zhu Y, Bolli R. An essential role of the jak-stat pathway in ischemic preconditioning. Proc Natl Acad Sci U S A. 2001;98:9050–9055. doi: 10.1073/pnas.161283798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xuan YT, Guo Y, Zhu Y, Han H, Langenbach R, Dawn B, Bolli R. Mechanism of cyclooxygenase-2 upregulation in late preconditioning. J Mol Cell Cardiol. 2003;35:525–537. doi: 10.1016/s0022-2828(03)00076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rizvi A, Tang XL, Qiu Y, Xuan YT, Takano H, Jadoon AK, Bolli R. Increased protein synthesis is necessary for the development of late preconditioning against myocardial stunning. Am J Physiol. 1999;277:H874–H884. doi: 10.1152/ajpheart.1999.277.3.H874. [DOI] [PubMed] [Google Scholar]
- 29.Takano H, Manchikalapudi S, Tang XL, Qiu Y, Rizvi A, Jadoon AK, Zhang Q, Bolli R. Nitric oxide synthase is the mediator of late preconditioning against myocardial infarction in conscious rabbits. Circulation. 1998;98:441–449. doi: 10.1161/01.cir.98.5.441. [DOI] [PubMed] [Google Scholar]
- 30.Guo Y, Jones WK, Xuan YT, Tang XL, Bao W, Wu WJ, Han H, Laubach VE, Ping P, Yang Z, Qiu Y, Bolli R. The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible no synthase gene. Proc Natl Acad Sci U S A. 1999;96:11507–11512. doi: 10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Guo Y, Zhang SX, Wu WJ, Wang J, Bao W, Bolli R. Ischemic preconditioning upregulates inducible nitric oxide synthase in cardiac myocyte. J Mol Cell Cardiol. 2002;34:5–15. doi: 10.1006/jmcc.2001.1482. [DOI] [PubMed] [Google Scholar]
- 32.Imagawa J, Yellon DM, Baxter GF. Pharmacological evidence that inducible nitric oxide synthase is a mediator of delayed preconditioning. Br J Pharmacol. 1999;126:701–708. doi: 10.1038/sj.bjp.0702368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bolli R, Dawn B, Tang XL, Qiu Y, Ping P, Xuan YT, Jones WK, Takano H, Guo Y, Zhang J. The nitric oxide hypothesis of late preconditioning [comment] Basic Res Cardiol. 1998;93:325–338. doi: 10.1007/s003950050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kodani E, Xuan YT, Takano H, Shinmura K, Tang XL, Bolli R. Role of cyclic guanosine monophosphate in late preconditioning in conscious rabbits. Circulation. 2002;105:3046–3052. doi: 10.1161/01.cir.0000019408.67709.b5. [DOI] [PubMed] [Google Scholar]
- 35.Shinmura K, Xuan YT, Tang XL, Kodani E, Han H, Zhu Y, Bolli R. Inducible nitric oxide synthase modulates cyclooxygenase-2 activity in the heart of conscious rabbits during the late phase of ischemic preconditioning. Circ Res. 2002;90:602–608. doi: 10.1161/01.res.0000012202.52809.40. [DOI] [PubMed] [Google Scholar]
- 36.Shinmura K, Tang XL, Wang Y, Xuan YT, Liu SQ, Takano H, Bhatnagar A, Bolli R. Cyclooxygenase-2 mediates the cardioprotective effects of the late phase of ischemic preconditioning in conscious rabbits. Proc Natl Acad Sci U S A. 2000;97:10197–10202. doi: 10.1073/pnas.97.18.10197. [DOI] [PMC free article] [PubMed] [Google Scholar]