Abstract
The dual functionality of a C60+-Q-Star hybrid instrument allows for the examination of complex lipid profiles with both MALDI and SIMS methodologies.[1] The difficulties associated with characterizing lipids in situ – isobaric interference and salt contamination – are discussed. The inherent advantages of both methodologies were used to deconvolute complex lipid spectra and establish characteristic fragmentation pathways. The high lateral resolution of SIMS allows for single cell images of aplysia californica neurons, while the established protocols were utilized to identify a major lipid component.
Keywords: lipid profile, C60+, MALDI, yandem MS, aplysia neuron, trimethylamine, mass spectrometry imaging (MSI)
Introduction
Glycerophospholipids are a diverse and versatile class of biomolecules responsible for a vast array of biological functions. Their complexity stems from an assortment of headgroups and fatty acid chains attached to a glycerol backbone. The headgroup, typically attached to the sn-3 site of the glycerol molecule, consists of a phosphate attached to a choline, serine, ethanolamine, glycerol or inositol functional group. The sn-1 and sn-2 glycerol sites are typically attached to the fatty acid functional groups. There are three type of fatty acid–glycerol linkage – 1-2-diacyl, 1-alkyl-2-acyl and 1-alk-1-enyl-2-acyl. These linkages are described as diacyl, ether and plasmalogen lipids, respectively. Variations within the fatty acid moieties also contribute to the diverse nature of lipids. The length of the fatty acid chain, degree of unsaturation and double bond location are all variable constituents of the fatty acid functional group. Altogether, variability in the headgroup, fatty acid composition and fatty acid–glycerol linkage produces a diverse compilation of lipids.
Recent papers have described complex lipid profiles obtained from various tissues, including liver,[2] adipose,[3] skeletal muscle[4] and the aorta[5] using ToF-SIMS. With all in situ mass spectrometry techniques, overlapping peaks in the lipid region, m/z 600 to 1200, lead to convoluted spectra making it difficult to directly identify and quantify individual lipid species. In general, the number of potential phospholipids at each mass unit in this spectral region can range from 2 to 200. To help mitigate these issues, we utilize the simplicity of MALDI spectra, common motifs in lipid profiles, and tandem MS to deconvolute lipid profiles obtained directly from tissue and even from a single cell.
Experimental
The experiments were performed using a hybrid SIMS-C60+ Q-Star instrument, details of which are described elsewhere.[1] The 20 keV C60+ direct current (d.c.) ion beam had an estimated current of 50 pA.The beam width was defined by the diameter of the opening of a 10 μm nose cone that resulted in a 15 μm beam size on the target. The nitrogen laser (337 nm) was operated with energy of 55 μJoules at a frequency of 30 Hz. The laser beam width was approximately 250 μm.
Porcine lipid extract (Cat. No. 131 101) was purchased from Avanti Polar Lipids, Inc. The brain extract was dissolved in glycerol to reduce damage accumulation (Shelton Scientific, Inc.). SIMS spectra were collected for 300 s with a stationary ion beam. For the matrix-assisted SIMS and MALDI analysis, porcine brain extract was dissolved in chloroform (EMD, Inc.) and dropped onto a clean silicon wafer (Ted Pella, Inc). After the film was dry, 5 μl of 10 μM 2,5-dihydroxybenzoic acid (MP Biomedicals, LLC) matrix solution in methanol (EMD, Inc.) was dropped on top of the lipid film.
The 10-μm-thick coronal slice of mouse brain tissue was sectioned directly onto an indium tin-oxide slide using a cryostat at −20 °C and stored in a −80 °C freezer. To avoid contamination associated with condensation, the tissue section was acclimated to ambient temperature under vacuum before analysis. A SIMS image was collected using 150 μm step size and SIMS spectra were accumulating for 1 s per pixel. After the SIMS analysis, the sample was removed and the MALDI matrix was applied using the sublimation technique described by Hankin and coworkers.[6] The same sample area was probed with the MALDI laser at 100 shots per pixel.
The aplysia californica sea slugs were humanely euthanized with magnesium chloride. The ganglia were sequentially removed and incubated in a protease solution. Individual cells were extracted and cultured on coated silicon shards. A positive-mode SIMS image was collected using 25 μm step size with accumulation time of 1 s per pixel.
Results and Discussion
Porcine lipid extract analysis
With both MALDI and SIMS techniques, the glycerophosphocholine in the extract exhibiteda lossof trimethlyamine (−N(CH3)3) associated with cation–lipid adduct [M+cation−N(CH3)3]+. For example, the sodiated–DPPC species (m/z 756.5) fragments into DPPC+Na−N(CH3)3 and is observed at mass 697.5 Da. Also observed, the potassiated–DPPC species (m/z 772.5) fragments into DPPC+K−N(CH3)3 and is observed at mass 713.5 Da. This characteristic fragment pathway was predominate in the MALDI and the matrix-assisted SIMS analyses of the lipid film coated with matrix. In the absence of biological and matrix-related salts, the protonated glycerophosophocholine species dominates the mass spectrum as shown in Fig. 1. The major peaks at m/z 734, 760 and 788, correspond to glycerophosphocholines with fatty acid constituents with a carbon-to-double-bond ratio of 32 : 0, 34 : 1 and 36 : 1, respectively. Although these peaks are predominately composed of the aforementioned glycerophosphocholine species, it is imperative to mention that other minor lipid species are most likely also present at these masses.
Figure 1.
Lipid profiles of porcine brain extract with SIMS and MALDI techniques are directly compared. Spectral data ranging from 731 to 736 Da, 758 to 764 Da and 782 to790 Da were reduced by a factor of 10 in the SIMS domain.
When directly compared to the MALDI spectrum, the SIMS spectrum has an extra layer of complexity that needs to be resolved. However, the lipid profile obtained with MALDI complement the profile obtained with SIMS, since nearly every peak in the MALDI spectrum can be matched to one in the SIMS spectrum. The exception involves cationized species which appear in the MALDI spectra, for example at m/z 782 (m/z 760−H+Na+), since these species are not seen in SIMS using a glycerol matrix.
Mouse brain MSI
A tissue section of mouse brain was imaged using SIMS and MALDI consecutively and the two lipid profiles were overlaid as shown in Fig. 2. An excess of cationized-lipid adducts were observed in both the MALDI and SIMS spectra due to the presence of biological salts in the tissue. The glycerophosphocholine species exhibit loss of–N(CH3)3 as noted above. For example, sodiated and potassiated lipid adducts for 32 : 0 and 34 : 1 glycerophophocholine produced fragmentation peaks at m/z 697, 713, 723 and 739, respectively. In the presence of biological salts, this fragmentation pathway is more predominate in the SIMS analysis compared to the MALDI study.
Figure 2.
Lipid profiles of mouse brain tissue with SIMS and MALDI. The MALDI spectrum is dominated by m/z 734 and 760 and their cation–lipid adducts; m/z 756 (m/z 734 – H+Na+), m/z 772 (m/z 734–H K+), m/z 782 (m/z 760–H+Na+), m/z 798 (m/z 760–H K+). While the SIMS spectra contains these peaks plus the fragments of these lipids associated with the loss of a trimethylamine; m/z 697 (m/z 734–H+Na+ –N(CH3)3), m/z 713 (m/z 734–H+K+ –N(CH3)3), m/z 723 (m/z 760–H+Na+ –N(CH3)3), m/z 739 (m/z 760–H+K+ –N(CH3)3). The SIMS image of the tissue is shown in the lower right.
Aplysia neuron MSI
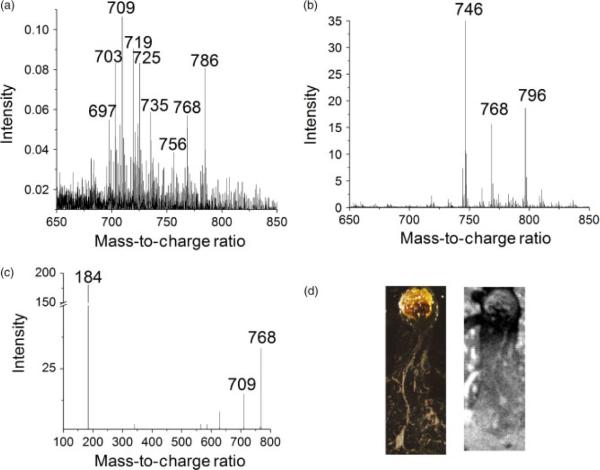
The higher resolution of SIMS imaging allows imaging of a single neuron from an aplysia californica sea slug as shown in Fig. 3. The major peaks in the SIMS spectra are found at m/z 709.5, 719.5, 725.5, 768.5 and 784.5, whereas the major peaks in the MALDI spectrum were m/z 746.5, 768.5 and 796.5. Sweedler and coworkers also identified m/z 746.5 as a major constituent of the lipid profile in their analysis of aplysia cells.[7]
Figure 3.
A. Lipid profile obtained from a single neuron with SIMS and from a compilation of neurons with MALDI, B. C. The tandem MS spectrum shows that m/z 709 and 184 are major fragments of m/z 768.5, the sodiated adduct of major lipid component m/z 746.5. Optical image (D, left) and black and white SIMS total ion image (D, right) of cultured aplysia neuron on silicon wafer (image size 2.00 × 4.75 mm).
Major peaks in SIMS and MALDI spectra are seen at m/z 709.5 and 746.5, respectively. These peaks are related via the glycerophosphocholine fragmentation pathway described in in a previous section. The lipid molecular ion at m/z 746.5 produces a peak at m/z 768.5 when it attaches to a sodium ion, and this species is observed in both spectra. Loss of–N(CH3)3 yields the expected fragment at m/z 709.5.
In order to confirm this idea, tandem MS spectra of the m/z 746.5 and 768.5 peaks were performed with MALDI. The tandem MS spectra produced 184.07 fragments, typically associated with glycerophosphocholine and sphinglomyelin. Although sphingomyelin lipids also produce a characteristic 184.07 fragment group and are abundant in neurons, the nitrogen group in the sphingosine moiety produces only odd molecular ion species.[8] Therefore, from the characteristic fragmentation peak and the odd–even parity, we are able to deduce that the m/z 746 is most likely a glycerophosphocholine lipid. Tandem MS of the m/z 768.5 peak produces an m/z 709.5 peak and confirms our fragmentation pathway theory. On the basis of information obtained thus far in the analysis, we are able to reduce the possible lipid contributing to peak m/z 746.5 to the following lipids; a diacyl glycerophospholipid with fatty acid constituents consisting of 33 carbons and one double bond (33a:1 GPCho, 746.5779 Da), an ether glycerophospholipid (34e:1 GPCho, 746.6143 Da) and a plasmalogen glycerophospholipid (34p:0 GPCho, 746.6143 Da).
Conclusion
SIMS and MALDI methodologies were used to investigate the variety of lipid species in biological materials. Characterizing lipids in situ is extremely difficult because lipid profiles obtained with mass spectrometry directly without extraction, purification and separation techniques are typically complex and convoluted. However, by utilizing the simplicity of MALDI spectra, common motifs in lipid profiles, and tandem MS, we were able to deconvolute lipid profiles and mitigate these issues.
Acknowledgments
The authors would like to thank Dr Robert Murphy and Dr Joseph Hankin at the University of Colorado for insight on lipid chemistry and preparation of the mouse brain section. We would also like to thank Stanislav S. Rubakhin from Dr Sweedler's group at the University of Illinois for the preparation of the aplysia cell. The authors acknowledge the Lipid MAPS Consortium (GM 069338-07) for financial support. Also additional financial support from the National Institutes of Health (2R01 EB002016-16) and the National Science Foundation (# CHE-0908226) is appreciated.
References
- [1].Carado A, Passarelli MK, Kozole J, Wingate JE, Winograd N, Loboda AV. Anal. Chem. 2008;80:7921. doi: 10.1021/ac801712s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Debois D, Bralet MP, Le Naour F, Brunelle A, Laprevote O. Anal. Chem. 2009;81:2823. doi: 10.1021/ac900045m. [DOI] [PubMed] [Google Scholar]
- [3].Malmberg P, Nygren H, Richter K, Chen Y, Dangardt F, Friberg P, Magnusson Y. Microsc. Res. Tech. 2007;70:828. doi: 10.1002/jemt.20481. [DOI] [PubMed] [Google Scholar]
- [4].Magnusson Y, Friberg P, Sjovall P, Dangardt F, Malmberg P, Chen Y. Clinical Physiology and Functional Imaging. 2008;28:202. doi: 10.1111/j.1475-097X.2008.00796.x. [DOI] [PubMed] [Google Scholar]
- [5].Malmberg P, Borner K, Chen Y, Friberg P, Hagenhoff B, Mansson JE, Nygren H. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids. 2007;1771:185. doi: 10.1016/j.bbalip.2006.12.003. [DOI] [PubMed] [Google Scholar]
- [6].Hankin JA, Barkley RM, Murphy RC. J. Am. Soc. Mass Spectrom. 2007;18:1646. doi: 10.1016/j.jasms.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Garden RW, Sweedler JV. Anal. Chem. 2000;72:30. doi: 10.1021/ac9908997. [DOI] [PubMed] [Google Scholar]
- [8].Murphy RC, Harrison KA. Mass Spectrom. Rev. 1994;13:57. [Google Scholar]