Abstract
More than 10 years after its discovery, the function of cyclooxygenase-2 (COX-2) in the cardiovascular system remains largely an enigma. Many scholars have assumed that the allegedly detrimental effects of COX-2 in other systems (e.g. proinflammatory actions and tumorigenesis) signify a detrimental role of this protein in cardiovascular homeostasis as well. This view, however, is ill-founded. Recent studies have demonstrated that ischemic preconditioning (PC) upregulates the expression and activity of COX-2 in the heart, and that this increase in COX-2 activity mediates the protective effects of the late phase of PC against both myocardial stunning and myocardial infarction. An obligatory role of COX-2 has been observed in the setting of late PC induced not only by ischemia but also by δ-opioid agonists and physical exercise, supporting the view that the recruitment of this protein is a central mechanism whereby the heart protects itself from ischemia. The beneficial actions of COX-2 appear to be mediated by the synthesis of PGE2 and/ or PGI2. Since inhibition of iNOS in preconditioned myocardium blocks COX-2 activity whereas inhibition of COX-2 does not affect iNOS activity, COX-2 appears to be downstream of iNOS in the protective pathway of late PC. The results of these studies challenge the widely accepted paradigm that views COX-2 activity as detrimental. The discovery that COX-2 plays an indispensable role in the anti-stunning and anti-infarct effects of late PC demonstrates that the recruitment of this protein is a fundamental mechanism whereby the heart adapts to stress, thereby revealing a novel, hitherto unappreciated cardioprotective function of COX-2. From a practical standpoint, the recognition that COX-2 is an obligatory co-mediator (together with iNOS) of the protection afforded by late PC has implications for the clinical use of COX-2 selective inhibitors as well as nonselective COX inhibitors. For example, the possibility that inhibition of COX-2 activity may augment myocardial cell death by obliterating the innate defensive response of the heart against ischemia/reperfusion injury needs to be considered and is the object of much current debate. Furthermore, the concept that the COX-2 byproducts, PGE2 and/ or PGI2, play a necessary role in late PC provides a basis for novel therapeutic strategies designed to enhance the biosynthesis of these cytoprotective prostanoids in the ischemic myocardium. From a conceptual standpoint, the COX-2 hypothesis of late PC expands our understanding of the function of this enzyme in the cardiovascular system and impels a critical reassessment of current thinking regarding the biologic significance of COX-2.
Keywords: Ischemia, Nitric oxide, Preconditioning, Reperfusion
1. Introduction
The phenomenon of ischemic preconditioning (PC), whereby brief episodes of sublethal ischemia render the myocardium resistant to subsequent ischemic stress, occurs in two phases: an early phase that starts within a few minutes after the initial ischemic stimulus and lasts for 2–3 h, and a late phase, which begins 12–24 h later and lasts for 3–4 days [1,2]. The late phase of ischemic PC is caused by the simultaneous activation of multiple stress-responsive signaling pathways, resulting in the shift of the heart to a phenotype that confers sustained protection against both reversible (stunning) and irreversible (infarction) myocardial ischemia / reperfusion injury [2]. During the past decade, late PC has become the focus of intense investigative efforts aimed at identifying the molecular mechanisms that underlie this powerful defensive adaptation of the heart. The clinical implications of these studies are potentially vast, since elucidation of the molecules that confer the preconditioned phenotype could conceivably enable therapeutic exploitation of this endogenous protective mechanism in patients with coronary artery disease who are at risk of acute myocardial infarction or other acute coronary events.
2. Mechanism of late PC
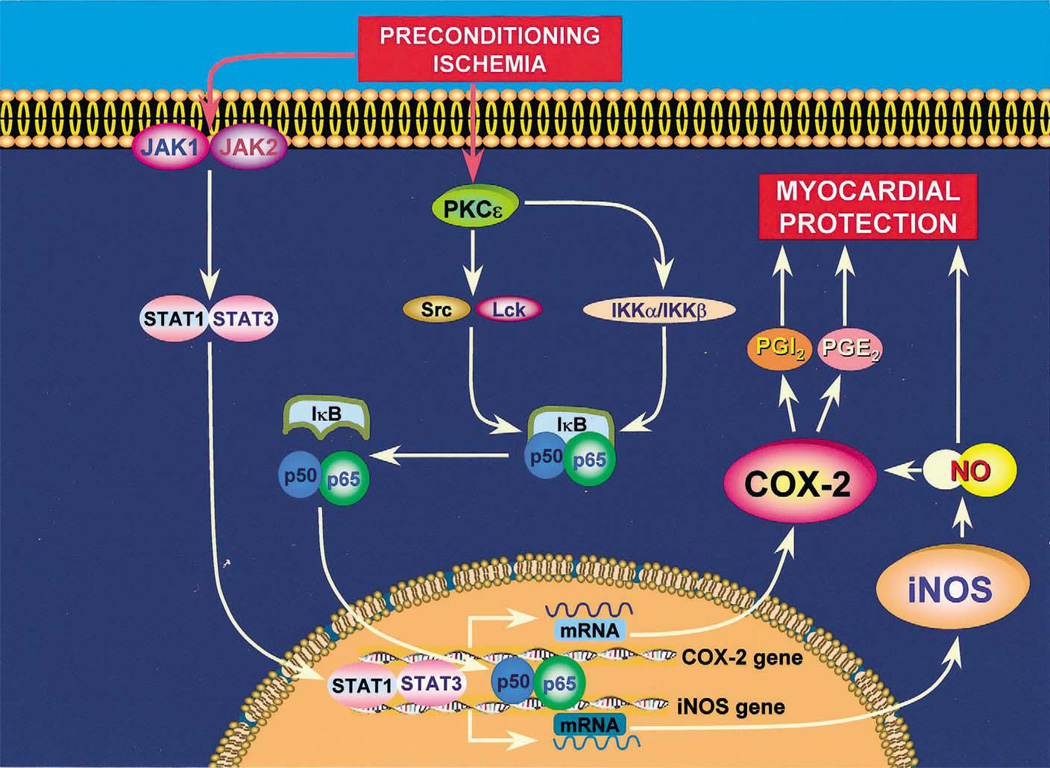
The shift of the heart to a late preconditioned (defensive) phenotype is the result of a complex cascade of molecular events that begins with an effective PC stimulus (such as reversible ischemia or pharmacologic and physical stimuli) and culminates in the synthesis of new proteins that render the heart tolerant to subsequent ischemic injury [2] (Fig. 1). The elements that constitute this molecular cascade can be conceptually subdivided into three major components: (i) ‘triggers’ or initiators of late PC, (ii) ‘mediators’ or effectors of late PC, and (iii) signaling pathways that connect these two groups of molecules [2] (Fig. 1). Several chemical species, including nitric oxide (NO), reactive oxygen species, adenosine, and opioids, have been shown to serve as triggers of the late PC response [2]. These triggers activate a series of kinases and transcription factors that include the ε isoform of PKC, the Src and/ or Lck isoform of the Src family of protein tyrosine kinases (PTKs), Janus kinases (JAK) 1 and 2, nuclear factor-κB (NF-κB), signal transducers and activators of transcription (STAT) 1 and 3, and probably other as-yet-unknown kinases and transcription factors [2] (Fig. 1). Recruitment of these pathways eventually culminates in transcriptional activation of cardioprotective genes and increased expression of proteins that confer resistance to ischemic injury (mediators of late PC). The first mediator of late PC to be identified was the inducible isoform of nitric oxide synthase (iNOS) [3–6]. However, given the complexity of the signaling pathways activated during late PC, it seemed implausible to us that iNOS would be the only cardio-protective protein involved. We postulated that late PC is a polygenic response that requires the coordinated upregulation of multiple proteins.
Fig. 1.
Schematic representation of our current understanding of the cellular mechanisms whereby COX-2 is upregulated by ischemic PC and participates in cardioprotection. A sublethal ischemic stress (ischemic PC) activates a complex signal transduction cascade that includes PKC (specifically, the ε isoform), PTKs (specifically, Src and/ or Lck kinases), and probably other as-yet-unknown kinases, leading to phosphorylation of IκBα and mobilization of the transcription factor NF-κB. In addition, ischemic PC activates the non-receptor tyrosine kinases JAK1 and JAK2 with subsequent tyrosine phosphorylation and activation of the transcription factors STAT1 and STAT3. Other, as yet unknown, transcription factors are most likely involved as well. The promoter of both the iNOS and the COX-2 genes contains cognate sequences for NF-κB and STAT1/STAT3. Binding of NF-κB and STAT1/STAT3 to these promoters results in a coordinated transcriptional activation of the iNOS and COX-2 genes with synthesis of new iNOS and COX-2 proteins. The activity of newly-synthetized COX-2 protein requires iNOS-dependent NO generation whereas the activity of iNOS does not require COX-2-dependent prostanoid generation. Thus, COX-2 is downstream of iNOS in the pathophysiological cascade of late PC. iNOS-derived NO can protect the myocardium from recurrent ischemia both via direct actions and via activation of COX-2-dependent synthesis of cardioprotective prostanoids. Among the products of COX-2, PGE2 and/ or PGI2 appear to be the most likely effectors of cytoprotection. A similar upregulation of COX-2 can be elicited pharmacologically by δ-opioid receptor agonists but not by adenosine A1 or A3 receptor agonists.
3. Rationale for the COX-2 hypothesis of late PC
Cyclooxygenase (COX)-2 is the rate-limiting enzyme in prostaglandin (PG) synthesis, catalyzing the conversion of arachidonic acid to PGH2 [7,8]. Two distinct COX isoforms have been characterized so far: COX-1, which is present in most cells and is responsible for constitutive prostanoid formation, and COX-2, which is induced in response to stress but is also constitutively expressed in some tissues (e.g. kidney, brain, endothelial cells) [7,8]. Our hypothesis that COX-2 is a co-mediator of the protection afforded by late PC (together with iNOS) was predicated on several considerations. First, COX-2 is known to be co-induced together with iNOS in various cell types, including cardiac myocytes, in response to stresses such as cytokines, hypoxia, and ischemia [9–21]. Second, the signaling elements that control the expression of COX-2 during stress appear to be similar to those that control the induction of iNOS, because they include reactive oxygen species [9,22], protein kinase C (PKC) [23,24], protein entyrosine kinases (PTKs) [25,26], and nuclear factor-kappa B (NF-κB) [10,22,27]. Third, an impressive body of evidence indicates that prostanoids (and their mimetics) exert salutary actions during myocardial ischemia / reperfusion, including attenuation of stunning and reduction in infarct size [28–45]. Despite these facts, however, virtually nothing was known regarding the role of COX-2 either in ischemic PC or in myocardial ischemia / reperfusion in general.
Accordingly, 4 years ago we formulated the COX-2 hypothesis of late PC and began a series of investigations to test it. The results of these studies have demonstrated that COX-2 activity is essential for late PC to occur, supporting a cytoprotective role of this protein and challenging the common paradigm that COX-2 activity is detrimental. The purpose of the present essay is to review the evidence supporting the recent recognition that COX-2 is a cardioprotective enzyme. We will first review the role of COX-2 in the late phase of ischemic and pharmacologic PC and then its role in the early phase of ischemic PC and in nonpreconditioned myocardium.
4. Role of COX-2 in ischemia-induced late PC
4.1. Ischemic PC upregulates COX-2
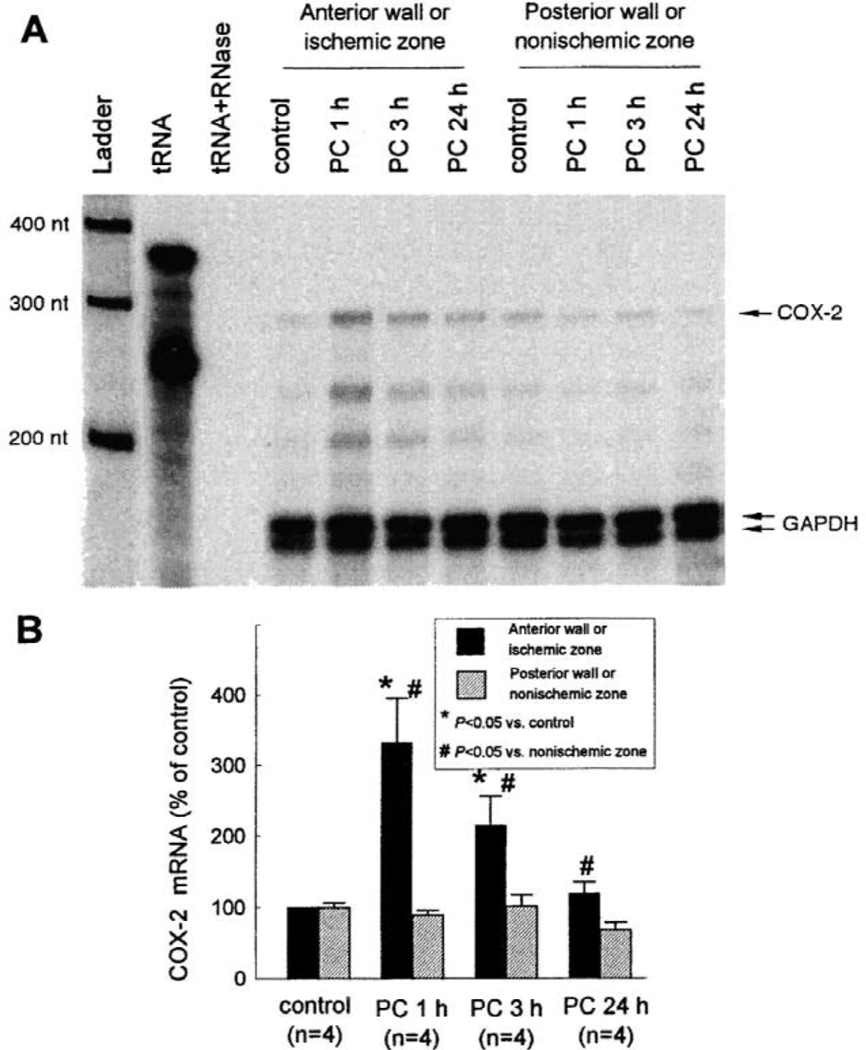
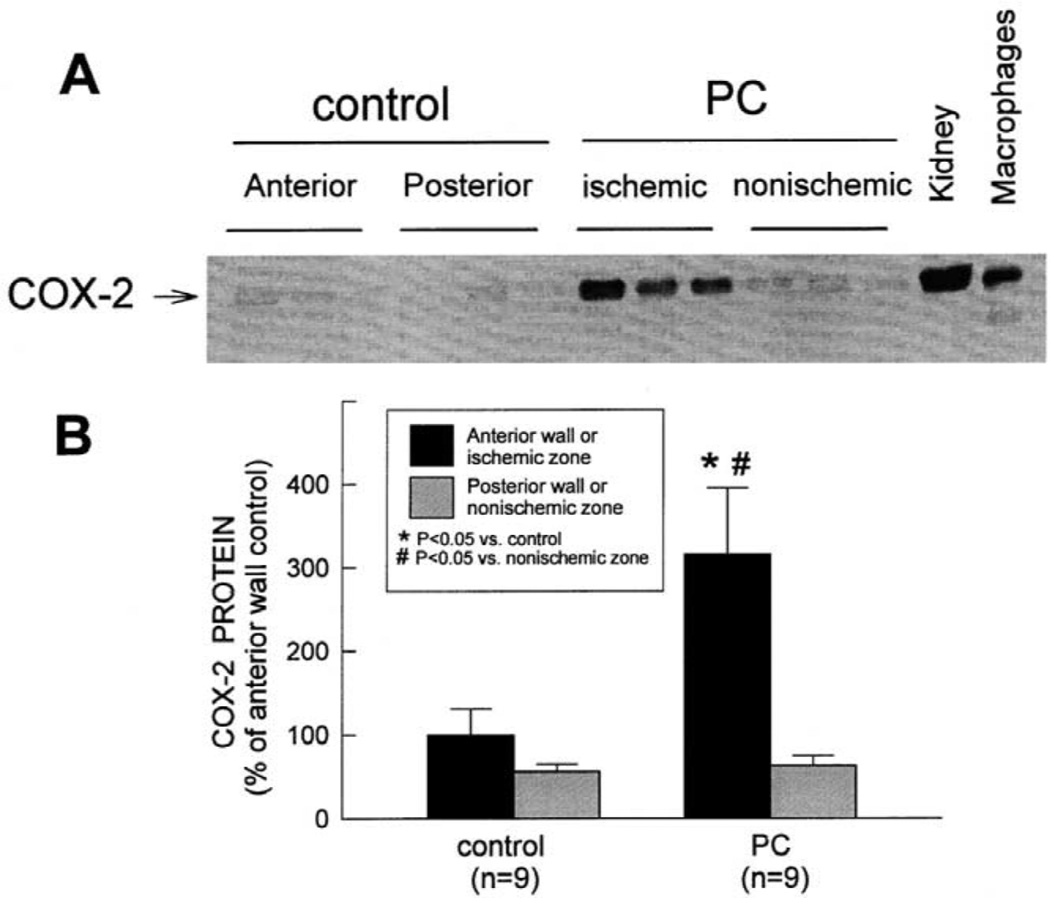
For this and other studies, we utilized a well-established conscious rabbit model of late PC [3,4,46–48]. The first question we addressed was: Is COX-2 induced by the brief episodes of ischemia that elicit late PC? Rabbits were preconditioned with a sequence of six 4-min coronary occlusion /4-min reperfusion cycles (a protocol that has been shown to induce late PC against both myocardial stunning and myocardial infarction [3,4,46–48]) and were euthanized at selected times thereafter. RNase protection assays were used to detect and quantitate COX-2 mRNA in myocardial tissue samples [46]. Low but detectable COX-2 mRNA levels were present in control rabbits. COX-2 mRNA levels were significantly increased in the ischemic-reperfused region at 1 h after ischemic PC (+231 ± 64%), remained elevated at 3 h, and returned to near control values by 24 h (Fig. 2). We then analyzed COX-2 protein expression in samples harvested 24 h after ischemic PC using standard Western immunoblotting. In control rabbits, over 99% of total COX-2 protein was found in the membranous fraction. Fig. 3 illustrates a representative Western immunoblotting analysis of COX-2. A weak COX-2 signal was detected in control hearts. When rabbits were preconditioned 24 h earlier, the expression of COX-2 in the ischemic-reperfused region increased significantly (+216 ± 79%) [10]. No COX-2 immunoreactivity was detectable in the cytosolic fractions of preconditioned hearts [46].
Fig. 2.
Effect of ischemic PC on COX-2 mRNA expression in rabbit myocardium. Tissue samples were obtained from the anterior and posterior LV wall of control rabbits and from the ischemic-reperfused and nonischemic regions of rabbits that underwent ischemic PC with six 4-min coronary occlusion /4-min reperfusion cycles and were euthanized 1 h, 3 h, or 24 h later. (A) Representative RPA gel; (B) densitometric analysis of COX-2 mRNA signals. Each COX-2 signal was normalized to the GAPDH signal from the same sample to control for RNA loading. The normalized values are expressed as percentage of the signal in the anterior LV wall of control hearts. Data are means±S.E.M. (Reproduced with permission of the National Academy of Sciences from Shinmura et al. [46]).
Fig. 3.
Effect of ischemic PC on the expression of COX-2 protein in rabbit myocardium. Tissue samples were obtained as described in the legend to in Fig. 2 from control rabbits and from rabbits that underwent ischemic PC 24 h earlier. (A) COX-2 immunoreactivity in the membranous fraction increased markedly in the ischemic-reperfused region 24 h after ischemic PC. Robust expression of COX-2 was observed in rabbit kidney and in murine macrophages stimulated with interferon-γ and lipopolysaccharide (positive controls). (B) Densitometric analysis of COX-2 signals in the membranous fraction. In all samples, the densitometric measurements of COX-2 immunoreactivity were expressed as a percentage of the average value measured in the anterior LV wall of control rabbits. Data are means±S.E.M. (Reproduced with permission of the National Academy of Sciences from Shinmura et al. [46]).
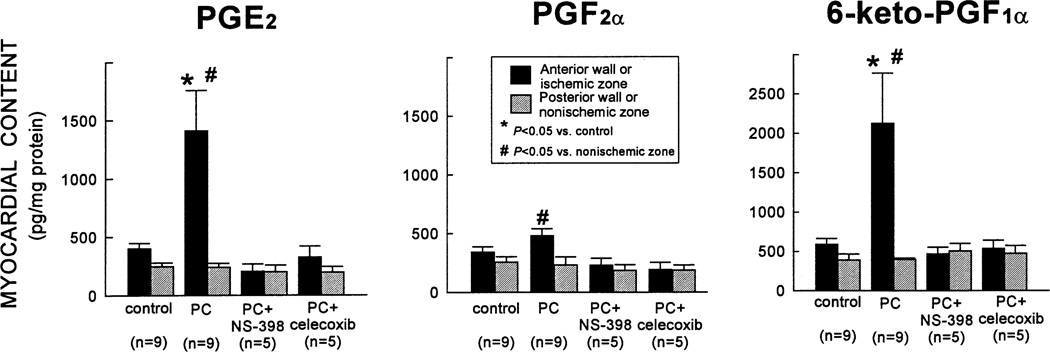
To determine whether the increased COX-2 protein expression was associated with increased COX-2 enzymatic activity, the myocardial content of the major arachidonic acid metabolites (PGD2, PGE2, PGF2α, 6-keto-PGF1α [stable metabolite of PGI2], and TXB2 [stable metabolite of TXA2]) was measured by enzyme immunoassay. Ischemic PC resulted in a robust increase in PGE2 and 6-keto-PGF1α (+250±85 and +259±107%, respectively, vs. controls) in the ischemic–reperfused region 24 h later (Fig. 4) [46]. PGF2α levels were also elevated but to a much lesser extent (Fig. 4). The increase in PGE2, PGF2α, and 6-keto-PGF1α was completely abrogated when rabbits preconditioned 24 h earlier were given the selective COX-2 inhibitors NS-398 or celecoxib 40 min before euthanasia (Fig. 4) [46]. Thus, the doses of NS-398 and celecoxib used in this study were effective in blocking the increase in COX activity associated with ischemic PC. Importantly, neither NS-398 nor celecoxib lowered prostanoid levels in the nonischemic region below control values (Fig. 4), indicating that constitutive COX activity (COX-1) was not suppressed by these drugs. There was no significant difference in the myocardial content of PGD2 or TXB2 between control and preconditioned hearts [46]. Taken together, these results (Figs. 2–4) demonstrate that ischemic PC upregulates the myocardial expression and activity of COX-2.
Fig. 4.
Effect of ischemic PC on the myocardial content of PGE2, PGF2α, and 6-keto-PGF1α (measured by EIA). In rabbits that underwent ischemic PC 24 h earlier, the levels of PGE2 and 6-keto-PGF1α in the ischemic-reperfused region increased markedly vs. control rabbits; the levels of PGF2α were higher than in the nonischemic region of the same group but did not differ significantly from controls. The increase in PGE2, PGF2α, and 6-keto-PGF1α was completely abrogated when rabbits were given NS-398 or celecoxib 24 h after ischemic PC (40 min before euthanasia). The myocardial content of PGE2 and 6-keto-PGF1α in the nonischemic region was similar in all groups, indicating that the COX-2 inhibitors did not affect constitutive production of these eicosanoids by COX-1. Data are means±S.E.M. (Reproduced with permission of the National Academy of Sciences from Shinmura et al. [46]).
4.2. COX-2 activity is necessary for the protective effects of ischemia-induced late PC against myocardial stunning
The next logical question was: Does the upregulation of COX-2 following ischemic PC play a necessary role in the manifestation of the preconditioned phenotype (i.e. of cardiac tolerance to ischemia) or is it merely an epi-phenomenon? This question is critical, as the number of proteins that are upregulated by ischemia is very large but only few of them are likely to be causally involved in late PC. As indicated above, the doses of NS-398 and celecoxib used in our study [46] effectively ablated the increased COX-2 activity associated with late PC (Fig. 4), thereby providing a pharmacologic tool to interrogate the functional significance of COX-2 in this phenomenon. Accordingly, we carried out studies to determine whether these same doses of NS-398 and celecoxib interfere with the cardioprotective effects of late PC. Because myocardial stunning and myocardial infarction are two different phenomena [49], we studied late PC against stunning and late PC against infarction separately.
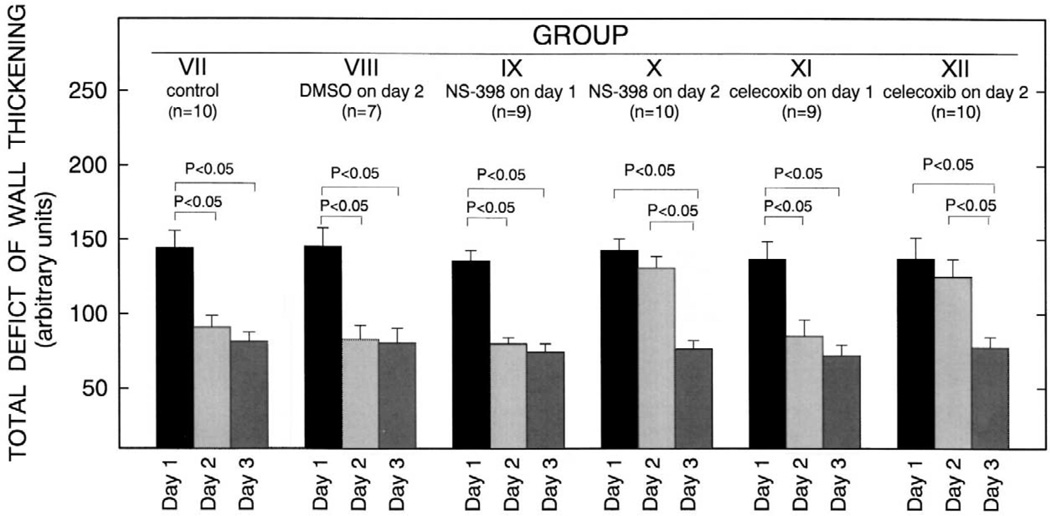
To examine late PC against stunning, conscious rabbits were subjected to a sequence of six 4-min coronary occlusions /4-min reperfusion cycles for three consecutive days (days 1, 2, and 3) [46]. The recovery of regional myocardial function was assessed as left ventricular (LV) systolic thickening fraction by using the pulsed Doppler probe [47]. The total deficit of systolic wall thickening (WTh) after reperfusion (an integrative assessment of the overall severity of myocardial stunning) [47] was calculated by measuring the area comprised between the systolic WTh-vs.-time line and the baseline (100% line) during the 5-h recovery phase after the sixth reperfusion. In all groups, thickening fraction on day 1 remained significantly depressed for 4 h after the sixth reperfusion and returned to values not significantly different from preocclusion values by 5 h. Thus, as previously observed in this model [3,46– 48], the sequence of six 4-min coronary occlusion /4-min reperfusion cycles resulted in severe stunning that lasted, on average, for 4 h. As expected [3,46–48], in control rabbits the recovery of WTh was improved on days 2 and 3 compared with day 1, resulting in a significant decrease in the total deficit of WTh on both days, compared with day 1 (Fig. 5). This indicates the development of late PC against stunning [3,46–48]. Similar results were obtained in rabbits given DMSO (the vehicle used for both NS-398 and celecoxib) on day 2 (Fig. 5).When rabbits were given NS-398 or celecoxib prior to the six occlusion / reperfusion cycles on day 2, however, the recovery of WTh during the 5-h reperfusion period was not improved on day 2 compared with day 1, so that the total deficit of WTh did not differ significantly between day 1 and day 2 (Fig. 5). Thus, the protective effects of late PC against stunning on day 2 were completely abrogated by the administration of either NS-398 or celecoxib. Administration of NS-398 or celecoxib on day 1 had no effect on the deficit of WTh on the same day (Fig. 5), indicating that these drugs, in themselves, do not affect the severity of myocardial stunning in nonpreconditioned myocardium.
Fig. 5.
Total deficit of WTh after the sixth reperfusion (a measure of the severity of myocardial stunning) on days 1, 2, and 3 in groups VII (control, n = 10), VIII (DMSO on day 2, n = 7), IX (NS-398 on day 1, n = 9), X (NS-398 on day 2, n = 10), XI (celecoxib on day 1, n = 9) and XII (celecoxib on day 2, n = 10). The total deficit of WTh was measured in arbitrary units, as described in the text. Data are means±S.E.M. (Reproduced with permission of the National Academy of Sciences from Shinmura et al. [46]).
4.3. COX-2 activity is necessary for the protective effects of ischemia-induced late PC against myocardial infarction
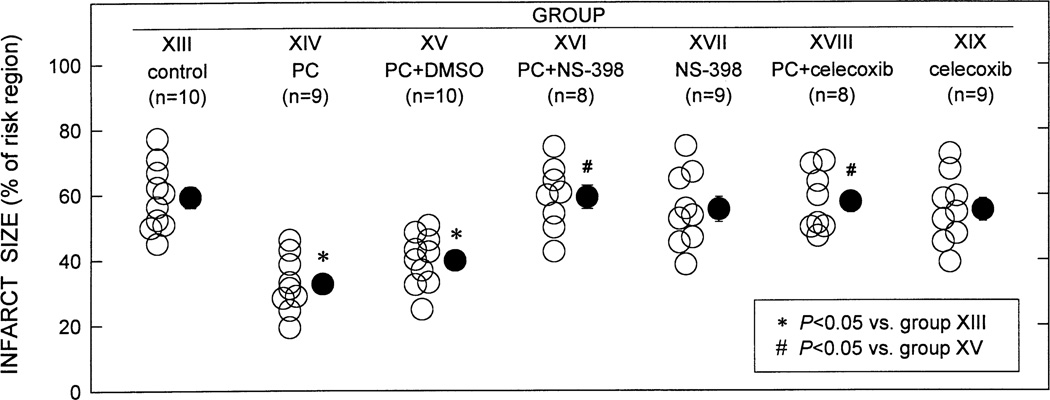
To examine late PC against infarction, conscious rabbits were preconditioned with a sequence of six 4-min coronary occlusion /4-min reperfusion cycles and then subjected, 24 underlyh later, to a 30-min coronary occlusion followed by 3 days of reperfusion. As expected [4,46,48,50], infarct size was significantly smaller in rabbits subjected to six occlusion / reperfusion cycles 24 h earlier (PC group) compared with controls, indicating a late PC effect against myocardial infarction (Fig. 6) [46]. In contrast, in rabbits treated with either NS-398 or celecoxib prior to the 30-min occlusion, infarct size was similar to that measured in controls (Fig. 6), indicating that both drugs abrogated late PC. When NS-398 or celecoxib were given in the absence of ischemic PC, infarct size did not differ from that observed in controls (Fig. 6), indicating that these drugs did not affect the extent of cell death in nonpreconditioned myocardium [46]. This finding suggests that COX-2 activity does not play an important role in modulating infarct size in naïve (nonpreconditioned) myocardium.
Fig. 6.
Myocardial infarct size in groups XIII (control, n = 10), XIV (PC, n = 9), XV(PC+DMSO, n = 10), XVI (PC+NS-398, n = 8), XVII (NS-398, n = 9), XVIII (PC+celecoxib, n = 8), and XIX (celecoxib, n = 9). Infarct size is expressed as a percentage of the region at risk of infarction. Solid circles represent means±S.E.M. (Reproduced with permission of the National Academy of Sciences from Shinmura et al. [46]).
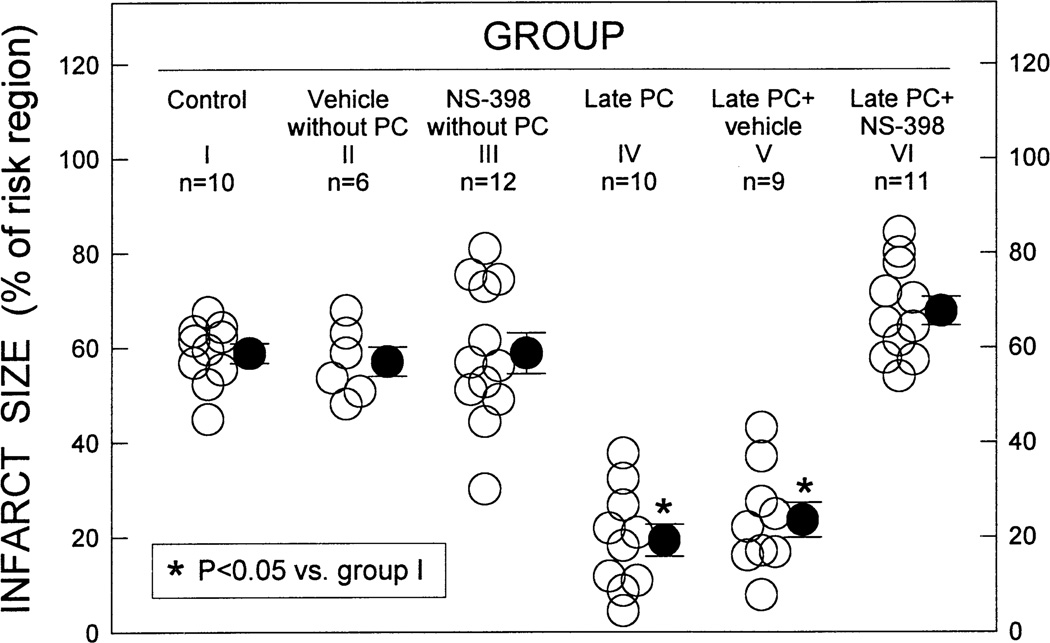
To determine whether the obligatory role of COX-2 in late PC is species-specific, we then examined the role of COX-2 in the mouse utilizing a well-established model of myocardial infarction [51] produced by a 30-min coronary occlusion followed by 24 h of reperfusion. As expected [51], ischemic PC (six 4-min coronary occlusion /4-min reperfusion cycles) resulted, 24 h later, in a significant reduction in infarct size, indicating a late PC effect analogous to that observed in the rabbit (Fig. 7) [52]. As we had found in rabbits, this cardioprotective effect was ablated when mice were given NS-398 prior to the 30-min coronary occlusion, indicating that COX-2 activity is essential for the infarct-sparing effects of late PC in mice (Fig. 7) [52]. Again, administration of NS-398 had no effect on infarct size in the absence of ischemic PC (Fig. 7).
Fig. 7.
Infarct size, expressed as a percent of the risk region, in the six groups of mice. Open circles indicate individual measurements, solid circles represent means±S.E.M. (Reproduced with permission of Steinkopff Verlag from Guo et al. [52]).
In summary, these two studies [46,52] demonstrate that COX-2 plays an obligatory role in the cardioprotective effects of ischemia-induced late PC in two different species (rabbits and mice), supporting the notion that the recruitment of this protein is a general mechanism underlying cardiac adaptation to stress. Furthermore, COX-2 is essential both for late PC against stunning and for late PC against infarction.
5. Role of COX-2 in pharmacologic PC
5.1. COX-2 activity is necessary for the protective effects of δ-opioid receptor- and NO donor-induced late PC
Having identified an obligatory role of COX-2 in ischemia-induced late PC, we sought to determine whether pharmacologic and other stimuli known to induce a late PC response also act via upregulation of COX-2 activity.
Activation of δ-opioid receptors has been shown to elicit a delayed cardioprotective effect against myocardial infarction that mimics that induced by ischemia [53,54]. In recent studies in conscious rabbits, we have found that administration of the δ-opioid receptor agonist BW373U86 alleviates the myocardial stunning induced 24 h later by a sequence of six 4-min occlusion / reperfusion cycles (δ-opioid-induced late PC against stunning). We used this model to test the role of COX-2. When rabbits were given the COX-2 inhibitors NS-398 or celecoxib prior to the consequence of six occlusion / reperfusion cycles 24 h after BW373U86, the recovery of WTh was indistinguishable from control rabbits, indicating that the late PC effect against stunning was ablated. In addition, BW373U86 upregulated the myocardial expression of COX-2 protein and the content of PGE2 and 6-keto-PGF1α 24 h later. Taken together, these data demonstrate that activation of δ-opioid receptors upregulates COX-2 activity in the myocardium and that this phenomenon plays an obligatory role in δ-opioid receptor-induced late PC against myocardial stunning.
Furthermore, in recent studies in mice subjected to a 30-min coronary occlusion followed by 24 h of reperfusion, we have found that administration of COX-2 inhibitors shortly before the 30-min occlusion blocks the late PC effect induced by pretreatment with NO donors (DETA/NO) as well as that induced by prior physical exercise, indicating that COX-2 is also a mediator of NO donor-induced and exercise-induced late PC.
5.2. COX-2 activity is not necessary for adenosine A1 or A3 receptor-induced late PC against myocardial infarction
The adenosine A1 receptor agonist 2-chloro-N6-cyclopentyladenosine (CCPA) and the adenosine A3 receptor agonist N6-3-iodobenzyladenosine-5′-N-methylcar-boxamide (IB-MECA) have been shown to elicit a delayed phase of protection against infarction similar to the late phase of ischemia-induced PC [55–57]. Using our conscious rabbit model, we found that neither CCPA nor IB-MECA upregulated COX-2 protein expression 24 h after their administration [50], despite the fact that both of these agonists were given in doses that elicit delayed cardioprotection [57]. Nevertheless, since COX-2 is constitutively expressed in the rabbit heart [46,50], it remains possible that this enzyme might be activated 24 h after CCPA or IB-MECA treatment and thus contribute to cardioprotection even though its protein expression is unchanged. To test this possibility, infarct size was measured in rabbits subjected to a 30-min coronary occlusion followed by 72 h of reperfusion [50]. Both CCPA and IB-MECA, given 24 h prior to the 30-min occlusion, resulted in a significant reduction in infarct size indicative of a late PC effect, consistent with prior observations [57]. Administration of NS-398 at the same dose that was previously shown to effectively abolish the enhanced COX-2 activity elicited by ischemia and the concomitant cardioprotection in the same conscious rabbit model [46], failed to block the infarct-sparing effects of either CCPA or IB-MECA, indicating that COX-2 activity is not necessary for these effects to occur [50]. Thus, unlike δ-opioid receptor-induced late PC against stunning, the mechanism of adenosine A1 and A3 receptor-induced late PC against infarction is independent of COX-2 activity.
6. Mechanism of upregulation of COX-2 by ischemia
Although COX-2 has been found to be upregulated by phorbol ester and oxidative stress in isolated neonatal myocytes [9], no information is available regarding the modulation of COX-2 in adult myocardium. Specifically, nothing is known regarding the signaling pathways where- by a sublethal ischemic stress leads to increased expression of COX-2 in the heart. Since the development of late PC is triggered by the formation of NO and reactive oxygen species during the initial PC stimulus and by the subsequent early activation of a cascade that involves the sequential recruitment of PKC, Src /Lck PTKs, and NF-κB [2], it seemed logical to hypothesize that these elements participate in the induction of COX-2 in response to sublethal myocardial ischemia.
Using our conscious rabbit model, we found [58] that the increase in COX-2 protein expression observed 24 h after ischemic PC was completely prevented when the animals were pretreated (before the PC protocol) with the PKC inhibitor chelerythrine, the tyrosine kinase inhibitor lavendustin-A, or the NF-κB inhibitor diethyldithiocarbamate (DDTC), given at doses which completely inhibit activation of PKCε [59], Src and Lck PTKs [60], and NF-κB [61], respectively, in this rabbit model. Thus, induction of COX-2 protein expression in preconditioned myocardium requires PKC-, Src /Lck PTK-, and NF-κB-dependent signaling (Fig. 1). In contrast, administration of the antioxidant N-2-mercaptopropionyl glycine (MPG) or the NOS inhibitor Nω-nitro-l-arginine (l-NA) prior to ischemic PC did not block the upregulation of COX-2 24 h later, indicating that the generation of NO and reactive oxygen species during the PC ischemia is not necessary for the increase in COX-2 protein expression to occur [58]. Since both l-NA and MPG were given in doses that completely block the development of late PC in this conscious rabbit model [3,47,48,62], the failure of these agents to block COX-2 induction [58] implies that NO and reactive oxygen species participate in the late phase of ischemic PC by upregulating proteins other than COX-2 (e.g. iNOS) [3–6], thereby supporting the notion [2] that the switch of the heart to a preconditioned phenotype is a polygenic response (i.e. COX-2 induction is necessary but not sufficient). As the activation of COX-2 gene transcription usually requires the combinatorial actions of various transcription factors [7,63], it seems very likely that the mechanism responsible for upregulating this enzyme in the heart is much more complex. Further studies will be needed to decipher the intricate network of kinases and transcription factors that underlie the recruitment of this cardioprotective protein.
7. COX-2 activity in preconditioned myocardium is modulated by iNOS
As indicated above, iNOS plays a necessary role in mediating the cardioprotective effects of late PC [3–6]. Since both iNOS and COX-2 are obligatory co-mediators of late PC, the question naturally arises to whether these two proteins act in series or are independent (i.e. parallel) effectors of cardioprotection. A possible ‘cross-talk’ between NOS and COX has been extensively examined in noncardiac tissues, with conflicting results (reviewed in Ref. [64]). While some studies suggest that NO enhances COX-2 activity [16,65–68], others have concluded that NO inhibits it [69–73] or has no effect [74–76]. The relationship between iNOS and COX-2 in the heart has not been evaluated.
In a recent study [58], we have examined this issue in the same conscious rabbit model in which the role of COX-2 was previously demonstrated [46]. We found that the increase in myocardial prostanoids (PGE2 and 6-keto-PGF1α) observed 24 h after ischemic PC (on day 2) was ablated when two selective iNOS inhibitors (SMT and 1400W) were given on day 2, 30–40 min prior to harvesting of tissue samples [58]. On the other hand, administration of NS-398 or celecoxib on day 2 did not have any appreciable effect on iNOS activity. These data indicate that the enhanced prostanoid biosynthesis associated with late PC is dependent upon iNOS-derived NO whereas the enhanced iNOS activity is independent on COX-2-derived prostanoids [58]. Thus, COX-2 is located downstream of iNOS in the protective pathway of late PC, implying that iNOS protects, at least in part, by recruiting COX-2. We propose that stimulation of COX-2 activity and production of cytoprotective prostanoids, such as PGE2 and PGI2, may be a previously unrecognized mechanism by which NO exerts its salubrious effects on the ischemic myocardium (Fig. 1).
Interestingly, the increase in prostanoid levels 24 h after ischemic PC was not affected by the administration on day 2 of the soluble guanylyl cyclase inhibitor ODQ (given at doses that block the increase in cGMP levels associated with late PC [77]), indicating that iNOS-derived NO activates cardiac COX-2 via cGMP-independent mechanisms [58]. This supports the hypothesis of a direct interaction between NO and the COX-2 molecule [65].
8. Does aspirin abrogate COX-2-dependent late PC?
Nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit COX activity and are widely used clinically. Acetylsalicylic acid (aspirin) is the most commonly used NSAID for relieving pain, inflammatory symptoms, and fever [78]. In addition, aspirin has established efficacy for preventing cardiovascular events [78–80]. Aspirin inhibits COX-1 activity by acetylating serine 530, which is located close to the active site (tyrosine 385) of COX-1; acetylation of this serine residue hinders the access of arachidonic acid to the catalytic site [81,82]. Aspirin also inhibits COX-2 by a similar mechanism but with less potency [83]. Although the ability of low doses of aspirin to inhibit COX-1 activity is well established [78,84], it is unknown whether these doses can also interfere with the cardio-protective effects of late PC by inhibiting COX-2 as well. Mounting evidence indicates that both ischemic and pharmacologic PC occur in patients with coronary artery disease [85,86]. Since aspirin at low doses is currently recommended for the prophylaxis of cardiac and cerebral ischemic events and is given to almost all patients with coronary artery disease [78], it is important to determine whether the use of aspirin affects the development of late PC.
We have recently examined this issue in conscious rabbits in which late PC against stunning was induced with a sequence of six 4-min coronary occlusion /4-min reperfusion cycles on three consecutive days [87]. We found that administration on day 2 of 5 mg/kg of aspirin, which was sufficient to inhibit platelet aggregation, did not inhibit either the increase in myocardial levels of PGE2 and 6-keto-PGF1α or the late PC protection against myocardial stunning; in contrast, administration on day 2 of 25 mg /kg of aspirin completely abrogated both the increase in myocardial prostanoids and the cardioprotective effects of late PC [87]. Thus, low doses of aspirin, which are widely used to prevent cardiovascular events in patients, do not interfere with the cardioprotective effects of late PC against myocardial stunning. In contrast, higher doses of aspirin, which are used for analgesic / antipyretic purposes, completely abrogate late PC, suggesting that they should be used with caution in patients with atherosclerotic cardiovascular disease, since they may deprive the heart of its innate defensive response. That low-dose aspirin inhibits platelet aggregation (a COX-1-dependent phenomenon) but not late PC (a COX-2-dependent phenomenon) probably results from the fact that aspirin exhibits less potency for COX-2 than for COX-1 inhibition [83] because the substrate channel of COX-2 is larger and more flexible than that of COX-1 [88]. For example, in intact cells aspirin is 166 times more active against COX-1 (IC50=0.3 µg/ml) than against COX-2 (IC50=50 µg/ml) [83]. The results summarized above [87] were obtained in the context of late PC against stunning; whether similar conclusions apply to late PC against infarction is not known.
9. Role of COX-2 in early PC
Studies of the effects of nonselective COX inhibitors on early PC have yielded conflicting results [89–91]. Perhaps the most conclusive study is that of Camitta et al. [92], who found that deletion of either COX-1 or COX-2 by gene targeting had no effect on early PC in isolated mouse hearts.
10. Effect of COX-2 on ischemia / reperfusion injury in nonpreconditioned myocardium
Our finding that COX-2 is an obligatory co-mediator of protection during late PC is consistent with an increasing number of reports in other systems suggesting a beneficial role of this enzyme in ischemia and other settings. Studies in isolated neonatal cardiomyocytes have shown that oxidative stress upregulates COX-2 and the addition of a COX-2-specific inhibitor enhances oxidative stress-induced injury and apoptosis, indicating that COX-2 is protective in this system [9]. A pro-apoptotic effect of inhibiting COX-2 has also been reported in other cell types [93–95]. In a recent study in isolated mouse hearts [92], Camitta et al. [92] found that the postischemic recovery of LV function was impaired in COX-2 null mice compared with controls, implying that constitutively-expressed COX-2 is cardio-protective. Interestingly, COX-2 protein was not detectable by Western immunoblotting in wild-type hearts; however, PGE2 and 6-keto-PGF1α were still present in COX-1 null hearts (albeit at lower levels than in controls), suggesting that sufficient COX-2 was present under baseline conditions to generate these prostanoids [92]. This concept is corroborated by the finding that loss of COX-2 aggravated ischemia–reperfusion injury [92], which implies that constitutively-expressed COX-2 is sufficient to produce cardioprotective prostanoids even though its protein expression may not be detectable. The fact that ablation of COX-2 exacerbated ischemia–reperfusion injury also implies that COX-1 cannot substitute for the loss of COX-2.
Taken together, the observation of Camitta et al. [92] support a cardioprotective role of constitutively-expressed COX-2 in the heart, a novel concept that warrants further investigation. Interestingly, acute inhibition of COX-2 with indomethacin failed to exacerbate ischemia–reperfusion injury while supplementation with prostaglandins failed to reverse the detrimental effects of COX-2 deletion, suggesting that in that study COX-2 protected the heart by indirect actions [e.g. altered expression of other gene(s)] rather than by the direct effects of its metabolites [92].
11. Mechanism of COX-2-mediated cardioprotection
COX-2 catalyzes the conversion of arachidonic acid to PGH2, which can be metabolized to various eicosanoids, including PGD2, PGE2, PGF2α, PGI2, and TXA2 [7,8]. The precise range of prostanoids generated by COX-2 depends on the cell types and their inherent prostanoid synthetic pathways [7,8]. Our published data in rabbits [46] as well as unpublished data in mice indicate that the two main products of COX-2 activity in preconditioned myocardium are PGE2 and PGI2 (Fig. 3), suggesting that the cardioprotective effects of COX-2 are related to the biological actions of these two prostanoids. A large number of studies have shown that PGE2 and PGI2 (and their analogs) exert salutary actions during myocardial ischemia / reperfusion, resulting in attenuation of stunning [96,97] and reduction in infarct size [29–31,35– 41,44,45,98]. The cytoprotective actions of PGE2 and PGI2 have been attributed to antagonism of adenylyl cyclase [28,34–36,42,99,100], activation of ATP-sensitive potassium channels [36,40,41,99], inhibition of Ca2+ influx [99], and attenuation of neutrophil infiltration [38,39,44,45] (Table 1). Interestingly, these actions are reminiscent of those of NO [101], suggesting that prostanoids and NO might possibly have additive or synergistic mechanisms of cytoprotection. Since activation of ATP-sensitive potassium channels is necessary for late PC against infarction [53,102,103] but not for late PC against stunning [103], this mechanism could account for the antiinfarct but not for the antistunning actions of COX-2; the latter must involve other mechanisms.
Table 1.
Mechanism for the cardioprotective effects of prostanoids
| Effect | Mechanism |
|---|---|
| More likely | |
| Inhibition of Ca2+ influx | |
| Antagonism of adenylyl cyclase | |
| Opening of KATP channels | |
| Less likely | |
| Attenuation of neutrophil infiltration |
12. COX-2 activity: friend or Foe?
The conclusion that COX-2 mediates the beneficial effects of late PC may seem surprising or even paradoxical, because the activity of COX-2 is generally thought to be detrimental [8,21]. Specifically, induction of COX-2 is believed to play a role in inflammation, toxic shock, cancer, and apoptosis [8,21,71,104–111]. A recent study has reported the expression of COX-2 in ischemic human myocardium and in dilated cardiomyopathy, but not in normal cardiomyocytes [112], a finding which has been interpreted (without proof) to indicate a role of COX-2 in cardiac disease. Evidence has been reported suggesting that COX-2 contributes to ischemia / reperfusion injury in the brain [113,114].
However, there is also evidence suggesting physiologically important or salutary actions of COX-2 in other situations [9,93–95,104,107–111,115–120]. For example, COX-2 protects cardiomyocytes against oxidative stress [9], exerts anti-apoptotic actions in various cell types [9,93–95,104,107–111], and has recently been identified as a major source of systemic PGI2 biosynthesis in humans [115]. Indeed, sheer stress induces COX-2 expression in endothelial cells and a substantial amount of eicosanoid production by endothelial cells results from the action of COX-2 [121]. COX-2-dependent production of PGI2 in endothelial cells may exert antithrombotic effects by counteracting COX-1-dependent production of TXA2 in platelets [119,122,123]. The finding that genetic disruption of COX-2 results in cardiac fibrosis [116] also suggests that COX-2 expression may be protective. Furthermore, it is now recognized that COX-2 is constitutively expressed in the kidney [120,124] and in the brain [125–127] and plays an important role in maintaining renal function [119,120] and in modulating neural responses [119]. Whether COX-2 exerts proinflammatory actions in reperfused myocardium remains unknown; even if it does, these actions would not necessarily be deleterious because inflammation is likely to be a consequence rather than a cause of myocardial ischemia / reperfusion injury.
We propose that the pathophysiological role of COX-2 is much more complex than hitherto appreciated, and that this enzyme may exert either beneficial or deleterious effects depending on the intensity of its induction, the pathophysiological setting, and the ability of specific cells to metabolize PGH2 produced by COX-2 into cytoprotective prostanoids. The experimental studies reviewed in this essay document an essential role of COX-2 as a mediator of cardioprotection during the late phase of ischemic or pharmacologic PC. Interestingly, the clinical experience accumulated with COX-2 inhibitors in the past three years suggests that COX-2 exert protective effects in patients with cardiovascular disease [123]. In the VIGOR trial [128], the relative risk of developing cardiovascular events with rofecoxib vs. naproxen was 2.38 (95% confidence interval, 1.39–4.00; P = 0.002). A recent meta-analysis of all randomized clinical trials of COX-2 antagonists (primarily VIGOR [128] and CLASS [129]) has concluded that these agents significantly increase the risk of myocardial infarction [123]. One explanation put forth for these differences was that COX-2 inhibitors may have a prothrombotic action, since they suppress endothelial production of PGI2 (which is mostly derived from COX-2 [115,121,122]) while leaving platelet production of TBA2 (which is exclusively due to COX-1 [8,119,122]) unaltered, thereby causing an imbalance between antithrombotic and prothrombotic prostanoids [122,123]. Another possibility, however, is that COX-2 antagonists may abrogate late PC, thereby increasing the severity of myocardial ischemia / reperfusion injury. Regardless of the mechanism involved, a prospective randomized trial of the effect of COX-2 inhibitors on cardiovascular events seems warranted [123].
13. Conclusions
More than 10 years after its discovery [130,131], the function of COX-2 in the cardiovascular system remains largely an enigma. Many scholars have assumed that the allegedly detrimental effects of COX-2 in other systems (e.g. proinflammatory actions, pain, tumorigenesis, among others) predict a detrimental role of this protein in cardiovascular homeostasis as well. This view, however, is ill-founded. Asides from the fact that a causative role of COX-2 activity in many of the aforementioned processes has not been proven but rather has been suspected on the basis of correlative data, tumorigenic and proinflammatory actions in other organs cannot be extrapolated to signify detrimental cardiovascular actions without data.
The evidence reviewed herein expands our understanding of this protein. Specifically, the discovery that COX-2 activity plays an indispensable role in the antiischemic effects of late PC has revealed a novel, heretofore-un-appreciated cardioprotective function of COX-2, thereby impelling a critical reassessment of current assumptions regarding the significance of this enzyme in the cardiovascular system. From a conceptual standpoint, the COX-2 hypothesis of late PC challenges the widely accepted view that this protein is detrimental to the heart. From a practical standpoint, the recognition that COX-2 is an obligatory co-mediator (together with iNOS) of the protection afforded by late PC has implications for the clinical use of COX-2 selective inhibitors as well as nonselective COX inhibitors. For example, the possibility that inhibition of COX-2 activity may augment myocardial cell death and dysfunction by obliterating the innate defensive response of the heart against ischemia / reperfusion injury (late PC) needs to be considered, particularly in light of recent clinical data suggesting an increase in cardiovascular events among patients treated with COX-2 inhibitors. Finally, the concept that the arachidonic acid metabolites, PGE2 and/or PGI2, play a necessary role in late PC provides a basis for novel therapeutic strategies aimed at enhancing the biosynthesis of these cytoprotective eicosanoids in the ischemic myocardium.
Acknowledgements
This study was supported in part by National Institutes of Health grants R01 HL-43151, HL-55757, HL-68088 (RB), and HL-65660 (YTX), the Medical Research Grant Program of the Jewish Hospital Foundation, Louisville, KY, and the Commonwealth of Kentucky Research Challenge Trust Fund. The expert secretarial assistance of Carla Hilse and Marcia Joines is gratefully appreciated.
References
- 1.Cohen MV, Baines CP, Downey JM. Ischemic preconditioning: From adenosine receptor to KATP channel. Annu Rev Physiol. 2000;62:79–109. doi: 10.1146/annurev.physiol.62.1.79. [DOI] [PubMed] [Google Scholar]
- 2.Bolli R. The late phase of preconditioning. Circ Res. 2000;87:972–983. doi: 10.1161/01.res.87.11.972. [DOI] [PubMed] [Google Scholar]
- 3.Bolli R, Manchikalapudi S, Tang XL, et al. The protective effect of late preconditioning against myocardial stunning in conscious rabbits is mediated by nitric oxide synthase. Evidence that nitric oxide acts both as a trigger and as a mediator of the late phase of ischemic preconditioning. Circ Res. 1997;81:1094–1107. doi: 10.1161/01.res.81.6.1094. [DOI] [PubMed] [Google Scholar]
- 4.Takano H, Manchikalapudi S, Tang XL, et al. Nitric oxide synthase is the mediator of late preconditioning against myocardial infarction in conscious rabbits. Circulation. 1998;98:441–449. doi: 10.1161/01.cir.98.5.441. [DOI] [PubMed] [Google Scholar]
- 5.Bolli R, Dawn B, Tang XL, et al. The nitric oxide hypothesis of late preconditioning. Basic Res Cardiol. 1998;93:325–338. doi: 10.1007/s003950050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo Y, Jones WK, Xuan Y-T, et al. The late phase of ischemic preconditioning is abrogated by targeted disruption of the iNOS gene. Proc Natl Acad Sci USA. 1999;96:11507–11512. doi: 10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and-2. J Biol Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- 8.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 9.Adderley SR, Fitzgerald DJ. Oxidative damage of cardiomyocytes is limited by extracellular regulated kinases 1/2-mediated induction of cyclooxygenase-2. J Biol Chem. 1999;274:5038–5046. doi: 10.1074/jbc.274.8.5038. [DOI] [PubMed] [Google Scholar]
- 10.Schmedtje JF, Jr, Ji YS, Liu WL, DuBois RN, Runge MS. Hypoxia induces cyclooxygenase-2 via the NF-κB p65 transcription factor in human vascular endothelial cells. J Biol Chem. 1997;272:601–608. doi: 10.1074/jbc.272.1.601. [DOI] [PubMed] [Google Scholar]
- 11.LaPointe MC, Sitkins JR. Phospholipase A2 metabolites regulate inducible nitric oxide synthase in myocytes. Hypertension. 1998;31:218–224. doi: 10.1161/01.hyp.31.1.218. [DOI] [PubMed] [Google Scholar]
- 12.LaPointe MC, Isenovic E. Interleukin-1β regulation of inducible nitric oxide synthase and cyclooxygenase-2 involves the p42/44 and p38 MAPK signaling pathways in cardiac myocytes. Hypertension. 1999;33:276–282. doi: 10.1161/01.hyp.33.1.276. [DOI] [PubMed] [Google Scholar]
- 13.Metais C, Li J, Simons M, Sellke FW. Serotonin-induced coronary contraction increases after blood cardioplegia–reperfusion: Role of COX-2 expression. Circulation. 1999;100:II328–II334. doi: 10.1161/01.cir.100.suppl_2.ii-328. [DOI] [PubMed] [Google Scholar]
- 14.Schuette R, LaPointe MC. Phorbol ester stimulates cyclooxygenase-2 expression and prostanoid production in cardiac myocytes. Am J Physiol. 2000;279:H719–H725. doi: 10.1152/ajpheart.2000.279.2.H719. [DOI] [PubMed] [Google Scholar]
- 15.Corbett JA, Kwon G, Turk J, McDaniel ML. IL-1 beta induces the coexpression of both nitric oxide synthase and cyclooxygenase by islets of Langerhans: Activation of cyclooxygenase by nitric oxide. Biochemistry. 1993;32:13767–13770. doi: 10.1021/bi00213a002. [DOI] [PubMed] [Google Scholar]
- 16.Nogawa S, Forster C, Zhang F, et al. Interaction between inducible nitric oxide synthase and cyclooxygenase-2 after cerebral ischemia. Proc Natl Acad Sci USA. 1998;95:10966–10971. doi: 10.1073/pnas.95.18.10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serou MJ, DeCoster MA, Bazan NG. Interleukin-1 beta activates expression of cyclooxygenase-2 and inducible nitric oxide synthase in primary hippocampal neuronal culture: Platelet-activating factor as a preferential mediator of cyclooxygenase-2 expression. J Neurosci Res. 1999;58:593–598. [PubMed] [Google Scholar]
- 18.Swierkosz TA, Mitchell JA, Warner TD, Botting RM, Vane JR. Co-induction of nitric oxide synthase and cyclo-oxygenase: Interactions between nitric oxide and prostanoids. Br J Pharmacol. 1995;114:1335–1342. doi: 10.1111/j.1476-5381.1995.tb13353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vane JR, Mitchell JA, Appleton I, et al. Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proc Natl Acad Sci USA. 1994;91:2046–2050. doi: 10.1073/pnas.91.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang X, Ma N, Szabolcs MJ, et al. Upregulation of COX-2 during cardiac allograft rejection. Circulation. 2000;101:430–438. doi: 10.1161/01.cir.101.4.430. [DOI] [PubMed] [Google Scholar]
- 21.Wu KK. Cyclooxygenase-2 induction in congestive heart failure: friend or foe? Circulation. 1998;98:95–96. doi: 10.1161/01.cir.98.2.95. [DOI] [PubMed] [Google Scholar]
- 22.von Knethen A, Callsen D, Brune B. Superoxide attenuates macrophage apoptosis by NF-kappa B and AP1 activation that promotes cyclooxygenase-2 expression. J Immunol. 1999;163:2858–2866. [PubMed] [Google Scholar]
- 23.Barry OP, Kazanietz MG, Pratico D, FitzGerald GA. Arachidonic acid in platelet microparticles up-regulates cyclooxygenase-2-dependent prostaglandin formation via a protein kinase C/mitogen-activated protein kinase-dependent pathway. J Biol Chem. 1999;274:7545–7556. doi: 10.1074/jbc.274.11.7545. [DOI] [PubMed] [Google Scholar]
- 24.Inoue H, Yokoyama C, Hara S, Tone Y, Tanabe T. Transcriptional regulation of human prostaglandin-endoperoxide synthase-2 gene by lipopolysaccharide and phorbol ester in vascular endothelial cells. Involvement of both nuclear factor for interleukin-6 expression site and cAMP response element. J Biol Chem. 1995;270:24965–24971. doi: 10.1074/jbc.270.42.24965. [DOI] [PubMed] [Google Scholar]
- 25.Blanco A, Habib A, Levy-Toledano S, Maclouf J. Involvement of tyrosine kinases in the induction of cyclo-oxygenase-2 in human endothelial cells. Biochem J. 1995;312:419–423. doi: 10.1042/bj3120419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chanmugam P, Feng L, Liou S, et al. Radicicol, a protein tyrosine kinase inhibitor, suppresses the expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide and in experimental glomerulonephritis. J Biol Chem. 1995;270:5418–5426. doi: 10.1074/jbc.270.10.5418. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto K, Arakawa T, Ueda N, Yamamoto S. Transcriptional roles of nuclear factor kappa B and nuclear factor-interleukin-6 in the tumor necrosis factor alpha-dependent induction of cyclooxygenase-2 in MC3T3-E1 cells. J Biol Chem. 1995;270:31315–31320. doi: 10.1074/jbc.270.52.31315. [DOI] [PubMed] [Google Scholar]
- 28.Hohlfeld T, Zucker TP, Meyer J, Schror K. Expression, function, and regulation of E-type prostaglandin receptors EP3 in the nonischemic and ischemic pig heart. Circ Res. 1997;81:765–773. doi: 10.1161/01.res.81.5.765. [DOI] [PubMed] [Google Scholar]
- 29.van der Giessen WJ, Schoutsen B, Tijssen JGP, Verdouw PD. Iloprost (ZK 36374) enhances recovery of regional myocardial function during reperfusion after coronary artery occlusion and reperfusion in the pig. Br J Pharmacol. 1986;87:23–27. doi: 10.1111/j.1476-5381.1986.tb10152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sturzebecher S, McDonald FM, Grundmann G, Hartmann S, Lammert C. Myocardial ischaemia and reperfusion in the anaesthetized pig: reduction of infarct size and myocardial enzyme release by the stable prostacyclin analogue iloprost. In: Schror K, Sinzinger H, editors. Prostaglandins in clinical research. 301st ed. New York: Alan R. Liss; 1988. pp. 155–160. [PubMed] [Google Scholar]
- 31.Hohlfeld T, Strobach H, Schror K. Stimulation of endogenous prostacyclin protects the reperfused ischemic pig myocardium from ischemic injury. J Pharmacol Exp Ther. 1993;264:397–405. [PubMed] [Google Scholar]
- 32.Zijlstra WG, Brusting JR, Ten Hoor F, Vergrosen AJ. Prostaglandin E1 and cardiac arrhythmia. Eur J Pharmacol. 1972;18:392–395. doi: 10.1016/0014-2999(72)90041-6. [DOI] [PubMed] [Google Scholar]
- 33.Mest HJ, Schror K, Forster W. Antiarrhythmic properties of PGE2: preliminary results. In: Bergstrom S, Bernhard S, editors. Advances in the biosciences, International Conference on Prostaglandins, Vienna, 1972. Oxford: Pergamon Press; 1973. pp. 385–393. [Google Scholar]
- 34.Schror K, Funke K. Prostaglandins and myocardial noradrenaline overflow after sympathetic nerve stimulation during ischemia and reperfusion. J Cardiovasc Pharmacol. 1985;7 Suppl 5:S50–S54. doi: 10.1097/00005344-198500075-00011. [DOI] [PubMed] [Google Scholar]
- 35.Hohlfeld T, Meyer-Kirchrath J, Vogel YC, Schror K. Reduction of infarct size by selective stimulation of prostaglandin EP3 receptors in the reperfused ischemic pig heart. J Mol Cell Cardiol. 2000;32:285–296. doi: 10.1006/jmcc.1999.1072. [DOI] [PubMed] [Google Scholar]
- 36.Zacharowski K, Olbrich A, Piper J, et al. Selective activation of the prostanoid EP3 receptor reduces myocardial infarct size in rodents. Arterioscler Thromb Vasc Biol. 1999;19:2141–2147. doi: 10.1161/01.atv.19.9.2141. [DOI] [PubMed] [Google Scholar]
- 37.Jugdutt BI, Hutchins GM, Bulkley BH, Becker LC. Dissimilar proteceffects of prostacyclin, prostaglandin E1, and prostaglandin E2 on myocardial infarct size after coronary occlusion in conscious dogs. Circ Res. 1981;49:685–700. doi: 10.1161/01.res.49.3.685. [DOI] [PubMed] [Google Scholar]
- 38.Schror K, Thiemermann C, Ney P. Protection of the ischemic myocardium from reperfusion injury by prostaglandin E1 inhibition of ischemia-induced neutrophil activation. Naunyn Schmiedeberg’s Arch Pharmacol. 1988;338:268–274. doi: 10.1007/BF00173399. [DOI] [PubMed] [Google Scholar]
- 39.Simpson PJ, Mickelson J, Fantone JC, Gallagher KP, Lucchesi BR. Reduction of experimental canine myocardial infarct size with prostaglandin E1: inhibition of neutrophil migration and activation. J Pharmacol Exp Ther. 1988;244:619–624. [PubMed] [Google Scholar]
- 40.Hide EJ, Ney P, Piper J, Thiemermann C, Vane JR. Reduction by prostaglandin E1 or prostaglandin E0 of myocardial infarct size in the rabbit by activation of ATP-sensitive potassium channels. Br J Pharmacol. 1995;116:2435–2440. doi: 10.1111/j.1476-5381.1995.tb15092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hide EJ, Thiemermann C. Sulprostone-induced reduction of myocardial infarct size in the rabbit by activation of ATP-sensitive potassium channels. Br J Pharmacol. 1996;118:1409–1414. doi: 10.1111/j.1476-5381.1996.tb15553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamamoto T, Habuchi Y, Tanaka H, et al. EP receptor-mediated inhibition by prostaglandin E1 of cardiac L-type Ca2+ current of rabbits. Am J Physiol. 1999;277:H1369–H1374. doi: 10.1152/ajpheart.1999.277.4.H1369. [DOI] [PubMed] [Google Scholar]
- 43.Coker SJ, Parratt JR. Prostacyclin: antiarrhythmic or arrhythmogenic? Comparison of the effects of intravenous and intracoronary prostacyclin and ZK 36374 during coronary artery occlusion and reperfusion in anaesthetised greyhounds. J Cardiovasc Pharmacol. 1983;5:557–567. doi: 10.1097/00005344-198307000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Simpson PJ, Mickelson J, Fantone JC, Gallagher KP, Lucchesi BR. Iloprost inhibits neutrophil function in vitro and in vivo and limits experimental infarct size in canine heart. Circ Res. 1987;60:666–673. doi: 10.1161/01.res.60.5.666. [DOI] [PubMed] [Google Scholar]
- 45.Smalling RW, Feld S, Ramanna N, et al. Infarct salvage with liposomal prostaglandin E1 administered by intravenous bolus immediately before reperfusion in a canine infarction–reperfusion model. Circulation. 1995;92:935–943. doi: 10.1161/01.cir.92.4.935. [DOI] [PubMed] [Google Scholar]
- 46.Shinmura K, Tang XL, Wang Y, et al. Cyclooxygenase-2 mediates the cardioprotective effects of the late phase of ischemic preconditioning in conscious rabbits. Proc Natl Acad Sci USA. 2000;97:10197–10202. doi: 10.1073/pnas.97.18.10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bolli R, Bhatti ZA, Tang XL, et al. Evidence that late precondition-functioning against myocardial stunning in conscious rabbits is triggered by the generation of nitric oxide. Circ Res. 1997;81:42–52. doi: 10.1161/01.res.81.1.42. [DOI] [PubMed] [Google Scholar]
- 48.Takano H, Tang XL, Qiu Y, et al. Nitric oxide donors induce late preconditioning against myocardial stunning and infarction in conscious rabbits via an antioxidant-sensitive mechanism. Circ Res. 1998;83:73–84. doi: 10.1161/01.res.83.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bolli R. The early and late phases of preconditioning against myocardial stunning and the essential role of oxyradicals in the late phase: an overview. Basic Res Cardiol. 1996;91:57–63. doi: 10.1007/BF00788866. [DOI] [PubMed] [Google Scholar]
- 50.Kodani E, Shinmura K, Xuan YT, et al. Cyclooxygenase-2 does not mediate late preconditioning induced by activation of adenosine A1 or A3 receptors. Am J Physiol Heart Circ Physiol. 2001;281:H959–H968. doi: 10.1152/ajpheart.2001.281.2.H959. [DOI] [PubMed] [Google Scholar]
- 51.Guo Y, Wu WJ, Qiu Y, et al. Demonstration of an early and a late phase of ischemic preconditioning in mice. Am J Physiol. 1998;275:H1375–H1387. doi: 10.1152/ajpheart.1998.275.4.H1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo Y, Bao W, Wu WJ, et al. Evidence for an essential role of cyclooxygenase-2 as a mediator of the late phase of ischemic preconditioning in mice. Basic Res Cardiol. 2000;95:479–484. doi: 10.1007/s003950070024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fryer RM, Hsu AK, Eells JT, Nagase H, Gross GJ. Opioid-induced second window of cardioprotection: Potential role of mitochondrial KATP channels. Circ Res. 1999;84:846–851. doi: 10.1161/01.res.84.7.846. [DOI] [PubMed] [Google Scholar]
- 54.Guo Y, Bao W, Tang XL, et al. Pharmacological preconditioning (PC) with adenosine A1 and opioid δ1 receptor agonists is iNOS-dependent [abstract] Circulation. 2000;102 II-121. [Google Scholar]
- 55.Baxter GF, Marber MS, Patel VC, Yellon DM. Adenosine receptor involvement in a delayed phase of myocardial protection 24 h after ischemic preconditioning. Circulation. 1994;90:2993–3000. doi: 10.1161/01.cir.90.6.2993. [DOI] [PubMed] [Google Scholar]
- 56.Baxter GF, Yellon DM. Time course of delayed myocardial protection after transient adenosine A1 receptor activation in the rabbit. J Cardiovasc Pharmacol. 1997;29:631–638. doi: 10.1097/00005344-199705000-00011. [DOI] [PubMed] [Google Scholar]
- 57.Takano H, Bolli R, Black RG, Jr, et al. A1 or A3 adenosine receptors induce late preconditioning against infarction in conscious rabbits by different mechanisms. Circ Res. 2001;88:520–528. doi: 10.1161/01.res.88.5.520. [DOI] [PubMed] [Google Scholar]
- 58.Shinmura K, Xuan Y-T, Tang XL. Inducible nitric oxide synthase modulates cyclooxygenase-2 activity in the heart of conscious rabbits during the late phase of ischemic preconditioning. Circ Res. 2002;90:602–608. doi: 10.1161/01.res.0000012202.52809.40. [DOI] [PubMed] [Google Scholar]
- 59.Qiu Y, Ping P, Tang XL, et al. Direct evidence that protein kinase C plays an essential role in the development of late preconditioning against myocardial stunning in conscious rabbits and that epsilon is the isoform involved. J Clin Invest. 1998;101:2182–2198. doi: 10.1172/JCI1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ping P, Zhang J, Zheng YT, et al. Demonstration of selective protein kinase C-dependent activation of Src and Lck tyrosine kinases during ischemic preconditioning in conscious rabbits. Circ Res. 1999;85:542–550. doi: 10.1161/01.res.85.6.542. [DOI] [PubMed] [Google Scholar]
- 61.Xuan YT, Tang XL, Banerjee S, et al. Nuclear factor-kappaB plays an essential role in the late phase of ischemic preconditioning in conscious rabbits. Circ Res. 1999;84:1095–1109. doi: 10.1161/01.res.84.9.1095. [DOI] [PubMed] [Google Scholar]
- 62.Tang XL, Takano H, Rizvi A. Oxidant species trigger late preconditioning against myocardial stunning in conscious rabbits. Am J Physiol. 2002;282:H281–H291. doi: 10.1152/ajpheart.2002.282.1.H281. [DOI] [PubMed] [Google Scholar]
- 63.Appleby SB, Ristimaki A, Neilson K, Narko K, Hla T. Structure of the human cyclo-oxygenase-2 gene. Biochem J. 1994;302:723–727. doi: 10.1042/bj3020723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goodwin DC, Landino LM, Marnett LJ. Effects of nitric oxide and nitric oxide-derived species on prostaglandin endoperoxide synthase and prostaglandin biosynthesis. FASEB J. 1999;13:1121–1136. doi: 10.1096/fasebj.13.10.1121. [DOI] [PubMed] [Google Scholar]
- 65.Salvemini D, Misko TP, Masferrer JL, et al. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci USA. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salvemini D, Seibert K, Masferrer JL, et al. Endogenous nitric oxide enhances prostaglandin production in a model of renal inflammation. J Clin Invest. 1994;93:1940–1947. doi: 10.1172/JCI117185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davidge ST, Baker PN, Laughlin MK, Roberts JM. Nitric oxide produced by endothelial cells increases production of eicosanoids through activation of prostaglandin H synthase. Circ Res. 1995;77:274–283. doi: 10.1161/01.res.77.2.274. [DOI] [PubMed] [Google Scholar]
- 68.Marnett LJ, Wright TL, Crews BC, Tannenbaum SR, Morrow JD. Regulation of prostaglandin biosynthesis by nitric oxide is revealed by targeted deletion of inducible nitric-oxide synthase. J Biol Chem. 2000;275:13427–13430. doi: 10.1074/jbc.275.18.13427. [DOI] [PubMed] [Google Scholar]
- 69.Stadler J, Harbrecht BG, Di Silvio M, et al. Endogenous nitric oxide inhibits the synthesis of cyclooxygenase products and interleukin-6 by rat Kupffer cells. J Leukoc Biol. 1993;53:165–172. doi: 10.1002/jlb.53.2.165. [DOI] [PubMed] [Google Scholar]
- 70.Amin AR, Attur M, Patel RN, et al. Superinduction of cyclooxygenase-2 activity in human osteoarthritis-affected cartilage. Influence of nitric oxide. J Clin Invest. 1997;99:1231–1237. doi: 10.1172/JCI119280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Habib A, Bernard C, Lebret M, et al. Regulation of the expression of cyclooxygenase-2 by nitric oxide in rat peritoneal macrophages. J Immunol. 1997;158:3845–3851. [PubMed] [Google Scholar]
- 72.Patel R, Attur MG, Dave M, Abramson SB, Amin AR. Regulation of cytosolic COX-2 and prostaglandin E2 production by nitric oxide in activated murine macrophages. J Immunol. 1999;162:4191–4197. [PubMed] [Google Scholar]
- 73.Clancy R, Varenika B, Huang W, et al. Nitric oxide synthase /COX cross-talk: nitric oxide activates COX-1 but inhibits COX-2-derived prostaglandin production. J Immunol. 2000;165:1582–1587. doi: 10.4049/jimmunol.165.3.1582. [DOI] [PubMed] [Google Scholar]
- 74.Tsai AL, Wei C, Kulmacz RJ. Interaction between nitric oxide and prostaglandin H synthase. Arch Biochem Biophys. 1994;313:367–372. doi: 10.1006/abbi.1994.1400. [DOI] [PubMed] [Google Scholar]
- 75.Curtis JF, Reddy NG, Mason RP, Kalyanaraman B, Eling TE. Nitric oxide: a prostaglandin H synthase 1 and 2 reducing cosubstrate that does not stimulate cyclooxygenase activity or prostaglandin H synthase expression in murine macrophages. Arch Biochem Biophys. 1996;335:369–376. doi: 10.1006/abbi.1996.0518. [DOI] [PubMed] [Google Scholar]
- 76.Hamilton LC, Warner TD. Interactions between inducible isoforms of nitric oxide synthase and cyclo-oxygenase in vivo: investigations using the selective inhibitors, 1400W and celecoxib. Br J Pharmacol. 1998;125:335–340. doi: 10.1038/sj.bjp.0702077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kodani E, Tang XL, Xuan YT, et al. Role of cyclic guanosine monophosphate in nitric oxide-dependent late preconditioning in conscious rabbits [abstract] Circulation. 2000;102 Suppl II doi: 10.1161/01.cir.0000019408.67709.b5. II-270. [DOI] [PubMed] [Google Scholar]
- 78.Calverley D, Roth G. Aspirin, prostaglandins and platelet function: pharmacology and thrombosis prevention. In: Rao G, editor. Hand-book of platelet physiology and pharmacology. Norwell, MA: Kluwer; 1999. pp. 478–494. [Google Scholar]
- 79.Cryer B, Feldman M. Cyclooxygenase-1 and cyclooxygenase-2 selectivity of widely used nonsteroidal anti-inflammatory drugs. Am J Med. 1998;104:413–421. doi: 10.1016/s0002-9343(98)00091-6. [DOI] [PubMed] [Google Scholar]
- 80.Xu XM, Sansores-Garcia L, Chen XM, et al. Suppression of inducible cyclooxygenase 2 gene transcription by aspirin and sodium salicylate. Proc Natl Acad Sci USA. 1999;96:5292–5297. doi: 10.1073/pnas.96.9.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roth GJ, Majerus PW. The mechanism of the effect of aspirin on human platelets. I. Acetylation of a particulate fraction protein. J Clin Invest. 1975;56:624–632. doi: 10.1172/JCI108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lecomte M, Laneuville O, Ji C, DeWitt DL, Smith WL. Acetylation of human prostaglandin endoperoxide synthase-2 (cyclooxygenase-2) by aspirin. J Biol Chem. 1994;269:13207–13215. [PubMed] [Google Scholar]
- 83.Mitchell JA, Akarasereenont P, Thiemermann C, Flower RJ, Vane J. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci USA. 1993;90:11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu KK. Aspirin and salicylate: An old remedy with a new twist. Circulation. 2000;102:2022–2023. doi: 10.1161/01.cir.102.17.2022. [DOI] [PubMed] [Google Scholar]
- 85.Yellon DM, Dana A. The preconditioning phenomenon: A tool for the scientist or a clinical reality? Circ Res. 2000;87:543–550. doi: 10.1161/01.res.87.7.543. [DOI] [PubMed] [Google Scholar]
- 86.Leesar MA, Stoddard MF, Dawn B, et al. Delayed preconditioning-mimetic action of nitroglycerin in patients undergoing coronary angioplasty. Circulation. 2001;103:2935–2941. doi: 10.1161/01.cir.103.24.2935. [DOI] [PubMed] [Google Scholar]
- 87.Shinmura K, Kodani E, Dawn B, Tang XL, Bolli R. The effect of aspirin on the late phase of ischemia preconditioning against myocardial stunning in conscious rabbits [abstract] Circulation. 2001;104 Suppl. II doi: 10.1016/s0735-1097(03)00086-x. II-227. [DOI] [PubMed] [Google Scholar]
- 88.Kurumbail RG, Stevens AM, Gierse JK, et al. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature. 1996;384:644–648. doi: 10.1038/384644a0. [DOI] [PubMed] [Google Scholar]
- 89.Vegh A, Szekeres L, Parratt JR. Protective effects of preconditioning of the ischaemic myocardium involve cyclo-oxygenase products. Cardiovasc Res. 1990;24:1020–1023. doi: 10.1093/cvr/24.12.1020. [DOI] [PubMed] [Google Scholar]
- 90.Li Y, Kloner RA. Cardioprotective effects of ischaemic preconditioning are not mediated by prostanoids. Cardiovasc Res. 1992;26:226–231. doi: 10.1093/cvr/26.3.226. [DOI] [PubMed] [Google Scholar]
- 91.Murphy E, Glasgow W, Fralix T, Steenbergen C. Role of lipoxygenase metabolites in ischemic preconditioning. Circ Res. 1995;76:457–467. doi: 10.1161/01.res.76.3.457. [DOI] [PubMed] [Google Scholar]
- 92.Camitta MG, Gabel SA, Chulada P, et al. Cyclooxygenase-1 and -2 knockout mice demonstrate increased cardiac ischemia / reperfusion injury but are protected by acute preconditioning. Circulation. 2001;104:2453–2458. doi: 10.1161/hc4401.098429. [DOI] [PubMed] [Google Scholar]
- 93.Hsu AL, Ching TT, Wang DS, et al. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J Biol Chem. 2000;275:11397–11403. doi: 10.1074/jbc.275.15.11397. [DOI] [PubMed] [Google Scholar]
- 94.Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 95.von Knethen A, Brune B. Cyclooxygenase-2: an essential regulator of NO-mediated apoptosis. FASEB J. 1997;11:887–895. [PubMed] [Google Scholar]
- 96.Farber NE, Pieper GM, Thomas JP, Gross GJ. Beneficial effects of iloprost in the stunned canine myocardium. Circ Res. 1988;62:204–215. doi: 10.1161/01.res.62.2.204. [DOI] [PubMed] [Google Scholar]
- 97.Farber NE, Gross GJ. Prostaglandin E1 attenuates postischemic contractile dysfunction after brief coronary occlusion and reperfusion. Am Heart J. 1989;118:17–24. doi: 10.1016/0002-8703(89)90066-5. [DOI] [PubMed] [Google Scholar]
- 98.Schror K, Hohlfeld T. Eicosanoids and the ischemic myocardium. In: Piper HM, editor. Pathophysiology of severe ischemic myocardial injury. Dordrecht: Kluwer; 1990. pp. 195–217. [Google Scholar]
- 99.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: Structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 100.Lopaschuk GD, Michalak M, Wandler EL, et al. Prostaglandin E receptors in cardiac sarcolemmal: Identification and coupling to adenylate cyclase. Circ Res. 1989;65:538–545. doi: 10.1161/01.res.65.3.538. [DOI] [PubMed] [Google Scholar]
- 101.Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: An overview of a decade of research. J Mol Cell Cardiol. 2001;33:1897–1918. doi: 10.1006/jmcc.2001.1462. [DOI] [PubMed] [Google Scholar]
- 102.Bernardo NL, D’Angelo M, Okubo S, Joy A, Kukreja RC. Delayed ischemic preconditioning is mediated by opening of ATP-sensitive potassium channels in the rabbit heart. Am J Physiol. 1999;276:H1323–H1330. doi: 10.1152/ajpheart.1999.276.4.H1323. [DOI] [PubMed] [Google Scholar]
- 103.Takano H, Tang XL, Bolli R. Differential role of KATP channels in late preconditioning against myocardial stunning and infarction in rabbits. Am J Physiol. 2000;279:H2350–H2359. doi: 10.1152/ajpheart.2000.279.5.H2350. [DOI] [PubMed] [Google Scholar]
- 104.Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. New Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 105.Lukiw WJ, Bazan NG. Strong nuclear factor-κB-DNA binding parallels cyclooxygenase-2 gene transcription in aging and in sporadic Alzheimer’s disease superior temporal lobe neocortex. J Neurosci Res. 1998;53:583–592. doi: 10.1002/(SICI)1097-4547(19980901)53:5<583::AID-JNR8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 106.Liu SF, Newton R, Evans TW, Barnes PJ. Differential regulation of cyclo-oxygenase-1 and cyclo-oxygenase-2 gene expression by lipopolysaccharide treatment in vivo in the rat. Clin Sci. 1996;90:301–306. doi: 10.1042/cs0900301. [DOI] [PubMed] [Google Scholar]
- 107.Morecki S, Yacovlev L, Slavin S. Effect of indomethacin on tumorigenicity and immunity induction in a murine model of mammary carcinoma. Int J Cancer. 1998;75:894–899. doi: 10.1002/(sici)1097-0215(19980316)75:6<894::aid-ijc12>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 108.Kinoshita T, Takahashi Y, Sakashita T, et al. Growth stimulation and induction of epidermal growth factor receptor by overexpression of cyclooxygenases 1 and 2 in human colon carcinoma cells. Biochim Biophys Acta. 1999;1438:120–130. doi: 10.1016/s1388-1981(99)00034-7. [DOI] [PubMed] [Google Scholar]
- 109.Kawamura T, Horie S, Maruyama T, et al. Prostaglandin E1 transported into cells blocks the apoptotic signals induced by nerve growth factor deprivation. J Neurochem. 1999;72:1907–1914. doi: 10.1046/j.1471-4159.1999.0721907.x. [DOI] [PubMed] [Google Scholar]
- 110.Liu XH, Yao S, Kirschenbaum A, Levine AC. NS398, a selective cyclooxygenase-2 inhibitor, induces apoptosis and down-regulates bcl-2 expression in LNCaP cells. Cancer Res. 1998;58:4245–4249. [PubMed] [Google Scholar]
- 111.Oshima M, Dinchuk JE, Kargman SL, et al. Suppression of intestinal polyposis in Apc δ716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 112.Wong SC, Fukuchi M, Melnyk P, Rodger I, Giaid A. Induction of cyclooxygenase-2 and activation of nuclear factor-kappaB in myocardium of patients with congestive heart failure. Circulation. 1998;98:100–103. doi: 10.1161/01.cir.98.2.100. [DOI] [PubMed] [Google Scholar]
- 113.Nogawa S, Zhang F, Ross ME, Iadecola C. Cyclo-oxygenase-2 gene expression in neurons contributes to ischemic brain damage. J Neurosci. 1997;17:2746–2755. doi: 10.1523/JNEUROSCI.17-08-02746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nakayama M, Uchimura K, Zhu RL, et al. Cyclooxygenase-2 inhibition prevents delayed death of CA1 hippocampal neurons following global ischemia. Proc Natl Acad Sci USA. 1998;95:10954–10959. doi: 10.1073/pnas.95.18.10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McAdam BF, Catella-Lawson F, Mardini IA, et al. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: The human pharmacology of a selective inhibitor of COX-2. Proc Natl Acad Sci USA. 1999;96:272–277. doi: 10.1073/pnas.96.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Morham SG, Langenbach R, Loftin CD, et al. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell. 1995;83:473–482. doi: 10.1016/0092-8674(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 117.Hao CM, Komhoff M, Guan Y, Redha R, Breyer MD. Selective targeting of cyclooxygenase-2 reveals its role in renal medullary interstitial cell survival. Am J Physiol. 1999;277:F352–F359. doi: 10.1152/ajprenal.1999.277.3.F352. [DOI] [PubMed] [Google Scholar]
- 118.Gilroy DW, Colville-Nash PR, Willis D, et al. Inducible cyclo-oxygenase may have anti-inflammatory properties. Nature Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 119.Lipsky PE, Brooks P, Crofford LJ, et al. Unresolved issues in the role of cyclooxygenase-2 in normal physiologic processes and disease [review] Arch Intern Med. 2000;160:913–920. doi: 10.1001/archinte.160.7.913. [DOI] [PubMed] [Google Scholar]
- 120.Harris RC, McKanna JA, Akai Y, et al. Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J Clin Invest. 1994;94:2504–2510. doi: 10.1172/JCI117620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Topper JN, Cai J, Falb D, Gimbrone MA., Jr Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: Cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc Natl Acad Sci USA. 1996;93:10417–10422. doi: 10.1073/pnas.93.19.10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Belton O, Byrne D, Kearney D, Leahy A, Fitzgerald DJ. Cyclooxygenase-1 and -2-dependent prostacyclin formation in patients with atherosclerosis. Circulation. 2000;102:840–845. doi: 10.1161/01.cir.102.8.840. [DOI] [PubMed] [Google Scholar]
- 123.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. J Am Med Assoc. 2001;286:954–959. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- 124.Guan Y, Chang M, Cho W, et al. Cloning, expression, and regulation of rabbit cyclooxygenase-2 in renal medullary interstitial cells. Am J Physiol. 1997;273:F18–F26. doi: 10.1152/ajprenal.1997.273.1.F18. [DOI] [PubMed] [Google Scholar]
- 125.Yamagata K, Andreasson KI, Kaufmann WE, Barnes CA, Worley PF. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron. 1993;11:371–386. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- 126.Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P. COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc Natl Acad Sci USA. 1996;93:2317–2321. doi: 10.1073/pnas.93.6.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tocco G, Freire-Moar J, Schreiber SS, et al. Maturational regulation and regional induction of cyclooxygenase-2 in rat brain: implications for Alzheimer’s disease. Exp Neurol. 1997;144:339–349. doi: 10.1006/exnr.1997.6429. [DOI] [PubMed] [Google Scholar]
- 128.Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. New Engl J Med. 2000;343:1520–1528. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]
- 129.Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs. nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. J Am Med Assoc. 2000;284:1247–1255. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- 130.Fu JY, Masferrer JL, Seibert K, Raz A, Needleman P. The induction and suppression of prostaglandin H2 synthase (cyclo-oxygenase) in human monocytes. J Biol Chem. 1990;265:16737–16740. [PubMed] [Google Scholar]
- 131.Kujubu DA, Fletcher BS, Varnum BC, Lim RW, Herschman HR. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase / cyclo-oxygenase homologue. J Biol Chem. 1991;266:12866–12872. [PubMed] [Google Scholar]