Abstract
COX-2 inhibition prevents adenoma formation in humans and mouse models of colon cancer. The selective COX-2 inhibitor, celecoxib, reduces COX-2 and PGE2 expression and adenomas in the intestine of Min/+ mice after treatment for several weeks, but prolonged treatment increases PGE2 production, resulting in drug-resistant tumor formation and TGFβ-dependent intestinal fibrosis. In this study, we examined pathways that regulate COX-2 expression and suppress chronic intestinal inflammation. We show that NF-κB signaling was inhibited in the ileum of Min/+ mice receiving long-term treatment with celecoxib. This effect was associated with inhibition of TAK-1 and IKKα/β activities and reduced expression of the Toll-like receptors TLR2 and TLR4 that enhance colonic barrier function. Additionally, we observed reduced activities of protein kinases JNK1 and PKA and transcription factor CREB, regulators of COX-2 expression which crosstalk with NF-κB. In ileum subjected to long-term celecoxib treatment, we noted relatively higher expression of COX-2, VEGF, and IL-1β in Paneth cells, while NF-κB and COX-2 were more strongly expressed by an expanded population of stromal myofibroblasts. Our findings argue that celecoxib resistance is an acquired adaptation to changes in the crypt microenvironment that is associated with chronic intestinal inflammation and impaired acute wound-healing responsiveness.
Keywords: COX-2, celecoxib, colon cancer, NF-κB
INTRODUCTION
Chronic inflammation promotes tumorigenesis by altering the intestinal microenvironment in ways that both activate the stroma and enhance epithelial cell growth and survival. Cyclooxygenase-2 (COX-2) and its downstream prostaglandin, PGE2, are driving factors in colorectal cancer (CRC) and inflammatory bowel disease (IBD) (1). COX-2 inhibition by non-steroidal anti-inflammatory drugs (NSAIDs) effectively prevents the formation of pre-cancerous adenomas in humans (2).
We showed that responses to celecoxib in the intestine change with the duration of treatment. Using the Apc-deficient Min/+ mouse, a CRC model, we reported that resistance occurred with long term treatment (3). Short term (3 weeks) celecoxib treatment of these mice inhibited intestinal adenoma formation, COX-2 expression, and PGE2 production, but long term (5 months) treatment accelerated tumor growth and progression. Importantly, high levels of PGE2 and COX-2 expression recurred in tumors and non-tumor mucosa of long term-treated mice. Recently, we showed that long term celecoxib treatment increased the number of COX-2-expressing myofibroblasts in the ileum and resulted in TGFβ-mediated intestinal fibrosis resembling patients with and rodent models of inflammatory bowel disease (IBD) (4).
The transcription factor, NF-κB, regulates inflammation and plays a role in CRC promotion (5). NF-κB signaling is activated by bacterial products, such as lipopolysaccharides (LPS), and inflammatory cytokines, and its transcriptional targets include the genes encoding COX-2, ICAM-1, VEGF, and inflammatory cytokines IL-1β and TNFα. NF-κB signaling is needed to orchestrate wound healing responses to acute intestinal injury, and its long term inhibition is associated with chronic intestinal inflammation (6, 7). NF-κB signaling positively regulates the expression of Toll-like receptors (TLRs), which when activated, promote signals required for innate immunity (8). Several studies demonstrated the relevance of TLRs to COX-2 expression. For instance, LPS-induced TLR4 activation caused NSAID-suppressible, NF-κB-dependent expression of COX-2 in enterocytes (8). Also, several lines of evidence link TLR signaling to intestinal tumorigenesis. TLR signaling in response to gut microbial flora modulated the number of tumors in Min/+ mice (9). Signal transduction from TLRs depends on the NF-κB upstream adaptor, MyD88, and TLR signaling-deficient MyD88−/−Min/+ mice developed fewer intestinal tumors with reduced COX-2 expression than MyD88+/+Min/+ littermates (10). Importantly, under conditions of tissue injury, MyD88 function was required to position COX-2-expressing mesenchymal cells around the base of crypts in the colon to stimulate enterocyte proliferation (11).
NF-κB is integrated with other pathways and activated by inflammatory cytokines during wound healing to allow proper temporal sequence and eventual termination of responses. For instance, receptor binding of the cytokines TNFα, IL-1β, and TGFβ activates TGFβ-associated kinase-1 (TAK-1) and subsequently NF-κB (12). Demonstrating pathway cross-activation, TGFβ enhanced IL-1β-mediated TAK-1 activity, and IL-1β activated canonical TGFβ signaling (13). Consistent with the requirement of TLR signaling and NF-κB activity for homeostasis, inducible TAK1 ablation in enterocytes caused colitis (14). While in certain contexts, TGFβ induces NF-κB-dependent COX-2 expression, it inhibits COX-2 activity in others (15). Moreover, PGE2 both stimulates inflammation and promotes wound healing resolution. PGE2 elicits this negative-feedback by autocrine signals initiated from its G-protein-coupled receptors (EP2/4) that modulate intracellular cAMP (16). In these circumstances, PGE2 induces cAMP- and PKA-dependent inhibition of NF-κB and TGFβ signaling that limits inflammation (17, 18).
Intestinal wound healing and inflammatory responses also involve cross talk between COX-2 and Wnt signaling, a crucial pathway for enterocyte proliferation and tumorigenesis (19). Increased Wnt signaling stimulates enterocyte proliferation allowing rapid coverage of surface lesions (20). PKA activates the transcription factor, CREB, which positively regulates COX-2 expression (21) and also activates certain Wnt target genes (22). PGE2 stimulates Wnt signaling downstream of the Epidermal Growth Factor Receptor (EGFR); activity of this receptor tyrosine kinase is constitutively increased in the Min/+ mucosa relative to Apc+/+ littermates (23). Finally, the mitogen-activated kinases (MAPKs), c-Jun NH2-terminal kinases (JNKs), which phosphorylate certain transcription factors, also promote COX-2 expression (24). After specific MKK-mediated activation, JNKs and other MAPKs cross talk extensively with the NF-κB pathway.
In the intestine of Min/+ mice, celecoxib resistance likely is not intrinsic, but rather is a reversible adaptation to targeted therapy (25). Therefore, acquired resistance should alter the microenvironment of the intestinal crypt comprised of epithelial and mesenchymal cells. COX-2 expression in this modified niche by any or all of its cell types then should exceed the bioavailable drug concentration. Here, we asked what effects long term COX-2 inhibition produced in pathways that regulate inducible COX-2 expression. We expected to find changes in NF-κB activity and other pathways known to promote COX-2 expression and chronic intestinal inflammation. A better understanding of the biology underlying this type of drug resistance will inform the design of safer and more effective CRC chemoprevention strategies.
MATERIALS AND METHODS
Materials
C57BL/6J-Min/+ mice were purchased from The Jackson Laboratory (Bar Harbor, MN). Ten mice per treatment group were fed AIN-76A diet (Research Diets (New Brunswick, NJ) with/without celecoxib (1,500 ppm) for timed intervals (3, 4). Antibodies are listed in Supplementary Table 1.
Immunohistochemical (IHC) analyses
IHC used standard techniques (3); for CD34, IL-1β, TLR2, TLR4, and VEGF, all solutions were prepared in Antibody Diluent (Invitrogen, Carlsbad, CA). IHC of control and treatment groups were performed in parallel. Experiments were repeated using tissues from 3 different mice of each treatment group. Quantitative analysis and cell counting of IHC used only well-oriented crypt-villus units from non-tumor ileum of 3 different mice per treatment group, and was performed by an investigator blinded to treatment status. Twelve high-powered fields (120 crypt villus units) for vWF + cell counting were analyzed per treatment group. For MPO+ cell counting, 50 crypt-villus units were analyzed/treatment group. For comparison of the mean number of NF-κB p65+ myofibroblasts, simultaneous IHC for Vimentin+ and p65+ cells was performed on serial sections, and doubly-positive cells were counted in 50 crypt-villus units. Statistical evaluations used an unpaired Student’s t test.
Immunoblotting (IB)
Ileum of Min/+ mice untreated or treated with celecoxib for 4 days, 3 weeks, or 5 months was processed, lysates prepared, and IBs performed as described (26). Total cell lysates from scraped small bowel mucosa mainly reflect expression patterns in enterocytes because they outnumber stromal cells by >100-fold (26). IBs were replicated 3 times. All experiments were repeated using tissues from 2 different mice of each treatment group. For NF-κB, ImageJ software from the NIH determined relative band intensities (27). Data were pooled and quantitatively analyzed from 3 independently performed experiments for total vs. active NF-κB p65. Band intensity was normalized to the internal β-actin control, then compared relative to the untreated control as a fold-difference, using the unpaired Student’s t test (P<0.05 was considered statistically significant).
RESULTS
Long term celecoxib treatment suppressed TLR-TAK-1-NF-κB signaling in the ileum of Min/+ mice
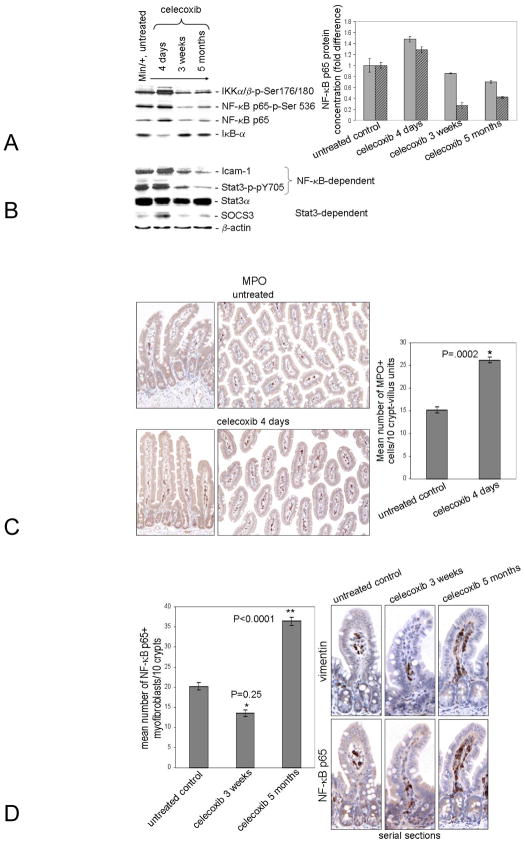
NF-κB signaling in enterocytes shortly after injury induces the expression of cytokines that recruit myeloid cells from the circulation to initiate healing (7). To learn the effect of celecoxib treatment on NF-κB expression and activity, IB analyses were performed using lysates prepared from mucosa of the non-tumor ileum of Min/+ mice that were untreated vs. celecoxib-treated (4 days, 3 weeks, and 5 months). Expression of total NF-κB p65 protein (filled bars) and its active form, NF-κB p65-p-Ser536 (hatched bars), increased after 4 days of treatment, but declined thereafter to amounts lower than were found in untreated controls (Fig. 1A, Left). Importantly, NF-κB activity was significantly lower in animals treated long term (P=0.006). The active forms of IKKα/β kinases phosphorylate the NF-κB inhibitor, IκB-α, causing its turnover. Expression of these kinases was elevated after 4 days, but decreased after 3 weeks. Sustained inhibition of NF-κB p65 was confirmed by the reduced active IKKα/β levels in the long term-treated lysate relative to the untreated one. After 4-day treatment, IκB-α expression was reduced, consistent with an initial NF-κB signaling induction. IκB-α expression was increased in the long term-treated sample to a level that resembled the untreated control. Confirming that IκB-α expression in the untreated lysate was similar in the long term-treated one, the amount of NF-κB p65 protein was not significantly different (P=0.06).
Figure 1. Long term celecoxib treatment suppressed NF-κB activity in enterocytes, a condition associated with chronic intestinal inflammation.
IBs used lysates prepared as detailed (37) from Min/+ mice that were untreated or treated with celecoxib for 4 days, 3 weeks, or 5 months (A). The antibodies used in this study are listed in Supplementary Methods Table 1. In this and subsequent IBs, the protein concentration of each sample was the same except where indicated, β-actin served as a control for the uniformity of sample loading. IBs of total vs. phosphorylated proteins were performed in parallel. The fold difference at each treatment time for NF-κB p65 and p65-p-Ser536 is shown (Right); filled bars denote total NF-κB; hatched bars denote active phospho-NF-κB. In this and subsequent figures, error bars represent standard error of the mean (SEM). P values for NF-κB p65: untreated vs. 4 day: P=0.0194, 4 day vs. 3 week: P=0.0002, 3 week vs. 5 month: P=.0030, and untreated vs. 5 months: P=0.06. P values for NF-κB p65-p-Ser356: untreated vs. 4 day: P=0.0207, 4 day vs. 3 week: P=0.0001, 3 week vs. 5 month: P=0.0363, and untreated vs. 5 months: P=0.0006. IBs of NF-κB and STAT3 downstream signaling targets are shown (B). Representative photomicrographs of IHC for MPO using sectioned ileum from Min/+ mice untreated or treated with celecoxib for 4 days is shown (C). The number of myeloid cells (MPO+ or F4/80+) were significantly reduced at subsequent treatment times (data not shown & 4). Representative photomicrographs of serial sections of ileum from Min/+ mice untreated or treated with celecoxib for indicated times were immunostained for Vimentin and p65 to show the expression and location of NF-κB+ in myofibroblasts (D).
Expression of ICAM-1 is NF-κB-inducible in enterocytes in vivo (7); the level of this pro-angiogenic protein was increased after 4-day treatment (Fig. 1B). NF-κB activity triggered by chemical wounding is known to cause STAT-3 activation (STAT-3-p-Y705), stimulating enterocyte proliferation and survival (7). In our model, active STAT-3 was increased in 4 day-treated Min/+ ileum relative to the untreated control, but was decreased in samples from 3 week-and 5 month-treated mice (Fig. 1B). Consistent with STAT3 activation, its transcriptional target Suppressor of Cytokine Signaling 3 (SOCS3) was induced after 4 day treatment (Fig. 1B). NF-κB signaling in enterocytes immediately after injury also induces the expression of cytokines that recruit myeloid cells (macrophages and neutrophils) from the circulation to initiate healing (7). We used an antibody for myeloperoxidase (MPO), a myeloid cell-specific marker to identify these cells by IHC. We found that MPO+ cells were significantly increased in the lamina propria of Min/+ mice treated with celecoxib for 4 days relative to the control (Fig. 1C).
Recently, we reported that the number of COX-2-expressing myofibroblasts was increased in ileum of Min/+ mice treated long term with celecoxib (4). IHC of serial sections for NF-κB p65 and Vimentin, a myofibroblast-specific marker, was performed on ileum of Min/+ mice treated with and without celecoxib. Doubly positive cells were counted. As observed previously, treatment of Min/+ mice for 3 weeks significantly reduced the number of NF-κB p65+ myofibroblasts in the lamina propria, whereas long term treatment increased their number (Fig. 1D). The nuclear expression of NF-κB p65 was not observed in crypt enterocytes by this method.
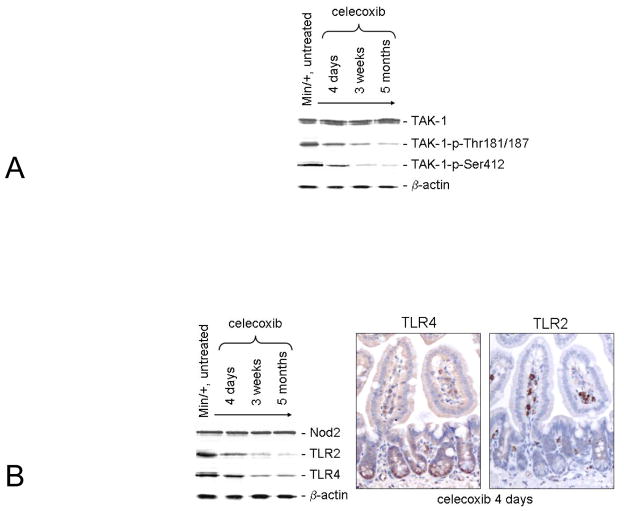
Active TAK-1 (TAK-1-p-Thr180/187) phosphorylates IKKβ, both stimulating NF-κB signaling and protecting enterocytes against chronic inflammation (12, 14). IBs showed that levels of total TAK-1 protein were invariant with respect to treatment duration. However, levels of active TAK-1 were inversely associated with the duration of celecoxib treatment. As expected, TAK-1-p-Thr180/187 levels correlated with those of IKKα/β-p-Ser176/180 (Fig. 2A). TAK-1-p-Ser412 is a different isoform associated with a cAMP-PKA-dependent pathway (28). Relative to the untreated control, TAK-1-p-Ser412 expression also was decreased with lengthened treatment time suggesting a corresponding decrease in PKA activity.
Figure 2. NF-κB inhibition upon long term celecoxib exposure was associated with reduced TLR2 and TLR4 expression, and TAK-1 activity.
IBs examined treatment time-dependent changes in expression of TAK-1 vs. its active isoforms (A). IB analysis is shown of treatment time-dependent changes in TLR2, and TLR4 but not in NOD-2 expression (B, Left). Representative photomicrographs of serial sections of ileum from Min/+ mice treated with celecoxib for 4 days are shown that were immunostained separately for TLR4 and TLR2 (B, Right).
TLR4, TLR2, and NOD2 expression is NF-κB-dependent and enhances enterocyte barrier function (8, 10). IB analysis of these receptors in the tissue lysates showed that 3 week and long term drug exposures reduced TLR4 and TLR2 expression (Fig. 2B), whereas NOD2 expression was unchanged. IHC of serially sectioned ileum showed differential staining of crypt base enterocytes for TLR4, but low overall staining of the entire crypt-villus unit for TLR2. Consistent with these results, twice as much protein was needed to produce band intensities for TLR2 that were similar to that of TLR4, indicating that Min/+ enterocytes contain a lowers level of TLR2 compared to TLR4. In contrast, however, more stromal cells were positively stained for TLR2 at all treatment times relative to TLR4. The complete treatment series are shown in Supplementary data Fig. 1 and 2. These results suggest that innate immunity was reduced in long term-treated Min/+ mice.
Long term celecoxib treatment increased microvessel density, the number of CD34+ vessels in the submucosa, and VEGF expression in Paneth cells
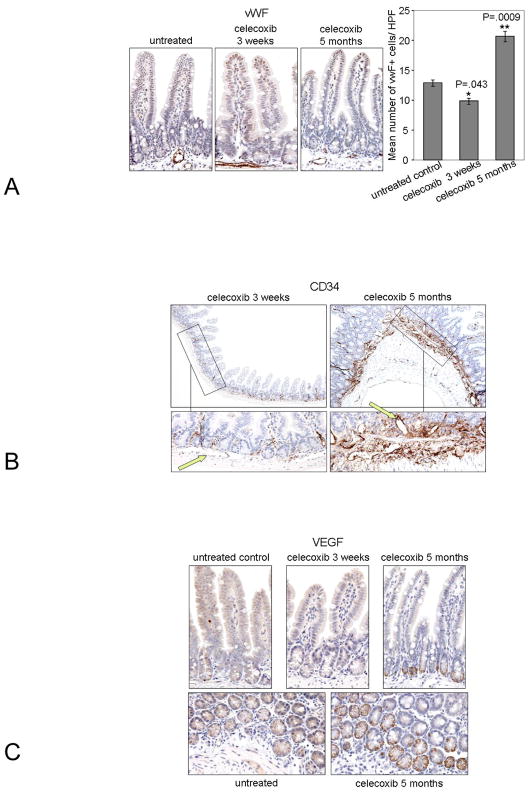
Angiogenesis is an essential feature of intestinal inflammation, and short-term and celecoxib treatment is known to produce anti-angiogenic effects (29, 30). As further evidence for chronic inflammation following long-term celecoxib treatment, we examined the effect of celecoxib treatment duration upon angiogenesis in the ileum of Min/+ mice. Using antibody for the endothelial cell marker, von Willibrand Factor (vWF), to perform IHC, we counted the microvessel density (MVD) in the ileum of study animals (Fig. 3A). MVD was significantly reduced after 3-week treatment relative to untreated mice, but was increased after 5-month treatment. This result correlated the inhibition of angiogenesis by short term celecoxib treatment with previously observed inhibition of COX-2 and PGE2 expression, and confirmed the opposite effects after long term treatment (3).
Figure 3. Long term celecoxib treatment increased microvessel density, the number of CD34+ vessels in the submucosa, and VEGF expression in Paneth cells.
Representative photomicrographs of sectioned ileum from Min/+ mice untreated and treated for indicated times are shown that were immunostained for vWF (Left). A graph of the mean number of vWF+ cells per high power magnification (40X) field in crypt-villus units is shown (Right) (A). P values: untreated vs. 3 week: 0.0432; untreated vs. 5 months: 0.0009; 3 weeks vs. 5 months: P<0.0001. Representative photomicrographs of sectioned ileum from Min/+ mice treated with celecoxib for indicated times are shown that were immunostained for CD34 at original magnifications of 10X (Top) and 40X (Bottom) (B). Green arrows point to blood vessels in the submucosa. Representative photomicrographs of sectioned ileum from Min/+ mice untreated and treated for indicated show crypt-villus units that were immunostained for VEGF (Top). Images of ileum cross-sectioned at the base of crypts at 40X show positive staining for VEGF in Paneth cells of Min/+ mice that were untreated or treated with celecoxib for 5 months (Bottom) (C).
To confirm and extend this result, we performed IHC for CD34, an endothelial progenitor cell marker of new blood vessels. Overall CD34 staining of the mucosa was low in short term but strong in long term-treated Min/+ mice (Fig. 3B). Low magnification images best demonstrated the staining intensity differences. Arrows in the high magnification images indicate CD34− vessels of short term-treated and CD34+ vessels of long term-treated Min/+ ileum. These results suggest that chronic exposure to celecoxib promoted dynamic vessel remodeling. The entire treatment series is shown in Supplementary data Fig. 3.
Vascular endothelial growth factor (VEGF) is a key regulator of angiogenesis. IHC for VEGF expression in untreated and celecoxib-treated ileum of Min/+ mice revealed positively stained enterocytes at the base of crypts. Tissue sections are shown in two orientations. As in the case of TLR4 (Fig. 3C), VEGF-positive cells had the appearance and localization of Paneth cells.
The number of Paneth cells and their expression of IL-1β and COX-2 were modulated by the duration of celecoxib treatment
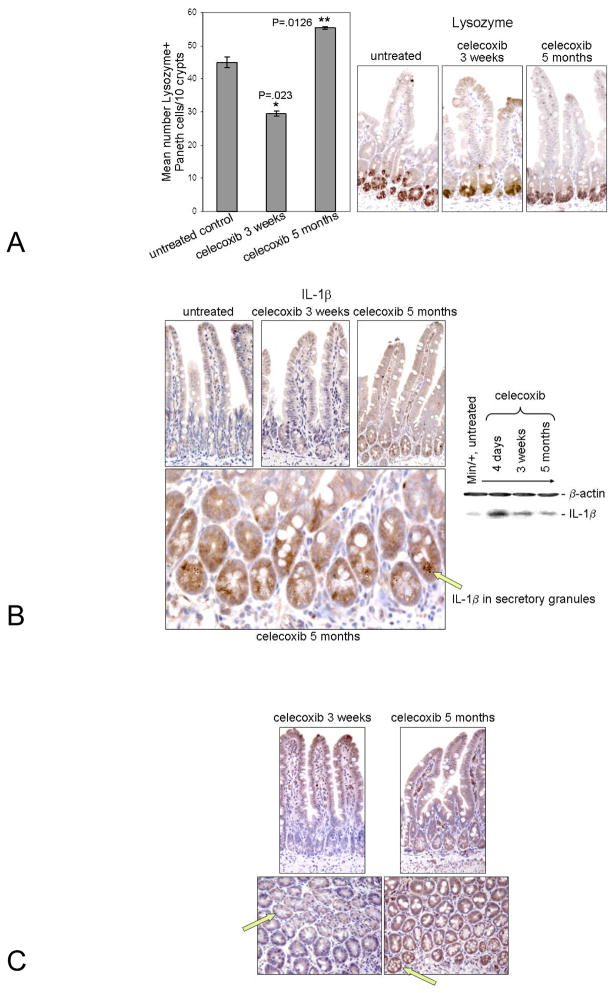
Residing subjacent to the submucosa, Paneth cells are appropriately placed to modulate angiogenesis and were shown to secrete other pro-angiogenic factors (31, 32). We therefore examined the effect of celecoxib treatment time on these secretory enterocytes. Using IHC to identify the Paneth cell marker, Lysozyme, we counted the relative number of positively stained cells (Fig, 4A) in treated and untreated tissues. Consistent with the known anti-angiogenic effect of celecoxib, the pro-angiogenic role of Paneth cells, and our MVD data (Fig. 3A), treatment for 3 weeks reduced the number of Paneth cells, whereas long term treatment increased their number relative to the control.
Figure 4. Paneth cell number and IL-1β and COX-2 expression were modulated by the duration of celecoxib treatment.
Representative photomicrographs of sectioned ileum from Min/+ mice untreated or treated for indicated times are shown that were immunostained for Lysozyme (Right). A graph of the mean number of Paneth cells/10 crypt length of ileum vs. the duration of treatment time is shown (Left) (A). Representative photomicrographs of ileum from Min/+ mice untreated or treated for indicated times are shown that were immunostained for IL- 1β (Top, Left). Below is a 40X magnification image of ileum from Min/+ treated with celecoxib for 5 months; an arrow points to IL-1β in the secretory granules of Paneth cells (Bottom, Right). IB analysis of IL-1β expression in lysates of ileum from Min/+ mice untreated or treated for indicated times is shown (Left) (B). Representative photomicrographs of ileum from Min/+ mice untreated or treated for indicated times are shown that were immunostained for COX-2 is shown; arrows indicate COX-2 expression, or the lack of its expression, in Paneth cells (C).
IL-1β, a potent inflammatory cytokine, is inducible by NF-κB and can perpetuate NF-κB activation via autocrine signaling. Production of active IL-1β requires processing in lysosomes for secretion. IHC showed that IL-1β was expressed in the secretory granules of Paneth cells, implying that this cytokine is released into the lumen and acts on enterocytes (Paneth cells, stem cells, and progenitors) at the base of crypts (Fig. 4B, Left). IB analysis also showed that expression of mature 17 kDa IL-1β was increased by all celecoxib treatments relative to the untreated control (Fig. 4B, Right). Consistent with the initial induction of NF-κB activity (Fig. 1A), overall IL-1β expression in ileum was highest in the 4-day treatment lysate. Finally, we found that COX-2 was also expressed in Paneth cells of long term-treated Min/+ mice. Fig. 4C shows 3-week and 5 month-treated ileum cut in 2 orientations. By IHC, Cox-2 was more highly expressed in Paneth cells (green arrows) and stromal cells from long term-treated mice when compared with those treated short term. These results indicate that Paneth cells of Min/+ mice contribute to celecoxib resistance and modulate inflammation and angiogenesis.
Long term celecoxib treatment suppressed alternate pathways that crosstalk with NF-κB to activate COX-2 expression
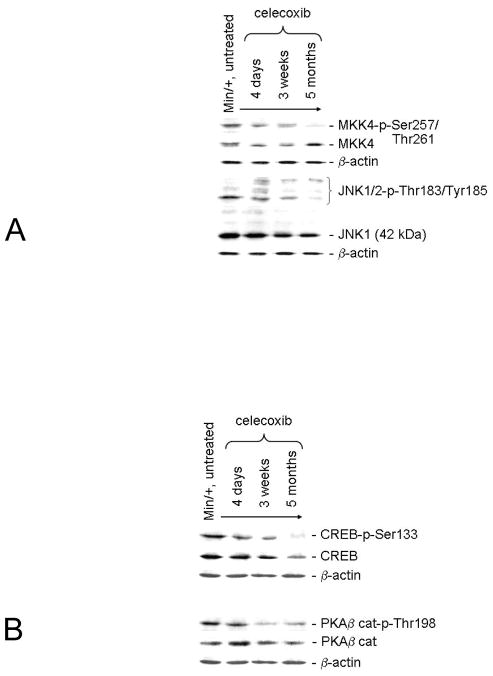
JNK1 and 2 phosphorylate transcription factors, are activated by a Ras-Rac1-MAPK kinase 4 (MKK4) pathway, and stimulate COX-2 expression (33). By IB analysis, we evaluated the relative expression of active MKK4 (MKK4-p-Ser80) and JNK1/2 (JNK-p-Thr183/Tyr185) when compared to total MKK4 and JNK1, respectively (Fig. 5A). The activity of MKK4 was strongly reduced in the mucosa of Min/+ mice after 5-months treatment, but not the steady-state expression of this protein. Overall expression of 42 kDa JNK1 declined slightly with treatment duration; activity of this kinase was reduced more severely. We did not pursue the possibility of COX-2 expression via MKK7-JNK2 activity because of the low intensity of the 54 kDa JNK2 band.
Figure 5. Long term celecoxib treatment was associated with decreased PKA-CREB and MKK4-JNK1 activities, pathways that crosstalk with NF-κB.
IB is shown of the relative expression of total MKK4 vs. its active phosphorylated isoform and of total JNK1 vs. its active 42 kDa phosphorylated isoform (Left) (A). IB is shown of the relative expression of total CREB vs. its active phosphorylated isoform and of total PKA β catalytic subunit vs. its active phosphorylated isoform (Right) (B).
CREB is a transcription factor that promotes COX-2 expression (21). CREB transactivation requires phosphorylation of Ser133 by PKA or other kinases. IB analysis of total CREB versus CREB-p-Ser133 levels in treatment samples showed that activity of this regulator declined in parallel with its overall expression (Fig. 5B). Since levels of PKA-targeted TAK-1-p-Ser412 were reduced in long term-treated vs. untreated Min/+ mice (Fig. 2A), and PKA is a positive regulator of CREB activity, we expected that celecoxib resistance would be associated with PKA inhibition. Of the three PKA catalytic subunits, PKAβ was the predominant isoform expressed in our mucosal lysates (data not shown). Active PKAβ cat-p-Thr198 was reduced in mice treated with celecoxib for 3 weeks and longer. This result associated short term celecoxib-mediated COX-2 and PGE2 inhibition with a reduction of PKA activity and downstream NF-κB signaling, suggesting crosstalk between these pathways. Since the level of PGE2 was increased but PKA activity remained low after the 5 month treatment, chronic exposure of enterocytes to celecoxib may have altered calcium signaling, as suggested previously (16). Taken together, celecoxib resistance was associated with inhibition of pathways that stimulate COX-2 expression in most enterocytes, but not in Paneth cells or in stromal myofibroblasts. Moreover, associated with chronic intestinal inflammation produced by long term celecoxib treatment was the suppression of signaling necessary for acute wound healing.
DISCUSSION
These data add to our previous findings, supporting the hypothesis that short term administration of celecoxib in Min/+ mice is anti-inflammatory and tumor suppressive, whereas long term administration results in chronic inflammation and tumor promotion (3, 4). We showed that resistance to the anti-tumor effects of celecoxib in Min/+ mice evolved by adaptations that altered enterocyte-stromal interactions and extracellular matrix (ECM) composition (3, 4). Our present work revealed that NF-κB activation in small bowel enterocytes of Min/+ mice occurred rapidly (4 days) after initiating celecoxib treatment and induced a cellular program of NF-κB-dependent wound healing (Fig. 1A–C). In contrast, long term celecoxib treatment inhibited NF-κB signaling in enterocytes, a condition associated with chronic intestinal inflammation.
As indicated by the expression of target gene products (VEGF, COX-2, and IL-1β), NF-κB signaling was up-regulated in Paneth cells and myofibroblasts of long term treated mice (Fig. 1A and D, Fig. 4B and C). It is reasonable to propose that Paneth cells and myofibroblasts produced the >2-fold increase in COX-2 expression in the mucosa of long term-treated Min/+ mice compared to controls to overcome the concentration of bioavailable drug in the stem/progenitor cell compartment (3). The close apposition of myofibroblasts expressing COX-2 may also produce a physical barrier limiting celecoxib bioavailability in the stem cell niche. Also, it is plausible that celecoxib resistance is limited to the crypt base since this is where COX-2 cooperates with Wnt signaling. For instance stem, progenitor, and Paneth cells maintain high levels of stabilized β-catenin to effect Wnt signaling, and this oncoprotein can promote COX-2 gene transcription and stabilize its mRNA (34, 35). Furthermore, PGE2 stimulates enterocyte proliferation by transactivating EGFR (19, 23), and COX-2 enhances Wnt signaling by increasing integrin-dependent adhesion to ECM (36, 37).
The increased number of Paneth cells in the celecoxib resistant Min/+ mucosa likely reflects enhanced survival of these long-lived cells (Fig. 4A). Several other observations support a role for Paneth cells in this chronic inflammation model. Paneth cells regulate the number and type of commensal microbes and limit pathogen contact with the mucosa (38). These enterocytes exclusively express matrix metalloproteinase MMP7, an enzyme that both promotes tumorigenesis and inhibits chronic intestinal inflammation (39, 40). Germline mutations predisposing to IBD modulate NF-κB signaling in Paneth cells, as well as their relative number, survival, and expression of inflammatory cytokines (41). To induce the expression of defensins, Paneth cells activate NF-κB via a TLR2-NOD2-RIP2 pathway (42). The importance of this pathway for the expression of defensins and survival signaling was confirmed by the hypersensitivity to chemically-induce colitis displayed in mice bearing a conditional deletion of NF-κB p65 in enterocytes (43). Finally, Paneth cells regulate angiogenesis in the intestine during development and inflammation by secreting β-defensins and angiogenins (31, 32).
NSAIDs including celecoxib produce COX-2-independent effects that are likely modulated by the duration of treatment time. For instance, genome expression and proteome analyses identified numerous changes in mRNA expression of non-tumor mucosa from Familial Adenomatous Polyposis patients treated with celecoxib for 1 year (44). COX-2-independent pharmacological effects were reported including inhibition of 5-lipoxygenase and phosphodiesterase activities; suppression of the latter caused increased intracellular levels of cyclic nucleotides and PKG activity (45, 46). Interestingly, IKKβ is a secondary pharmacological target of celecoxib (47). Here, we identified TAK-1 as the most upstream kinase in the NF-κB pathway inhibited by long term celecoxib exposure (Fig. 2A). We showed that IL-1β was more highly expressed in celecoxib long-term-treated relative to untreated Min/+ mice (Fig. 4B). IL-1β over expression was associated with chronic intestinal inflammation after prolonged treatment of mice with the selective IKKβ inhibitor, ML120B, (6). Also, we found that long term celecoxib treatment inhibited IKKβ activity (Fig. 1A), a result consistent with the essential role of enterocyte-intrinsic IKKβ expression in maintaining intestinal homeostasis (48).
We do not yet know the extent to which the adaptive processes here are present in FAP patients treated long term with NSAIDs for prevention of colorectal adenomas. In addition, the relationship of this response to germline Apc mutation is not yet known, and studies of long term NSAID administration in animals that are wild type at the Apc locus are underway. Based upon the results presented here, it may be possible to maximize chemoprevention effectiveness while limiting toxicity by using lower NSAID doses or by providing drug holidays. Chemoprevention might also be achieved by alternating NSAIDs such as celecoxib with other agents. For example, statins inhibit isoprenoid synthesis, in particular geranyl geranylpyrophosphate, thereby inhibiting RhoA and its downstream kinase, ROCK (49). Statins also are direct extracellular integrin antagonists (50). The statin, simvastatin, demonstrated antiinflammatory effects in animal models of chemically induced colitis and tumor formation, and inhibited RhoA and induced NF-κB activities in doxorubicin-treated colon cancer cells (51). Since we previously showed that enterocytes of Min/+ mice constitutively maintain increased RhoA activity, alternating cycles of celecoxib and simvastatin may inhibit acquired resistance to celecoxib in this model. A selective Focal Adhesion Kinase (FAK) inhibitor may produce a similar effect. For example, in non-transformed cells, FAK activity is strictly integrin adhesion-dependent. If celecoxib resistance is associated with TGFβ signaling downstream of integrin-ECM engagement, then a FAK inhibitor such as PF-562,271 may enhance chemoprevention efficacy in combination with celecoxib.
In conclusion, the tissue-specific effects of chemopreventive NSAIDs such as celecoxib change over time. In association with an altered intestinal microenvironment, acquired resistance may occur in non-tumor IBD mucosa or pre-malignant adenomas, just as in invasive cancers (25). Signaling pathway cross-activation may promote drug resistance (13). The more selective the drug and the longer the treatment time, the greater will be the pressure driving resistance. This view is consistent with the model of dynamic reciprocity which posits that the microenvironment, especially the extracellular matrix, exerts an influence on gene expression in epithelial cells. Importantly, this type of drug resistance is reversible. Our results emphasize that more comprehensive knowledge of stromal-epithelial interactions, signaling crosstalk, and negative feedback mechanisms operating during different stages of inflammation and tumorigenesis is needed to develop safe and effective CRC chemoprevention regimens.
Supplementary Material
References
- 1.Rhodes JM, Campbell BJ. Inflammation and colorectal cancer: IBD-associated and sporadic cancer compared. Trends Mol Med. 2002;8:10–16. doi: 10.1016/s1471-4914(01)02194-3. [DOI] [PubMed] [Google Scholar]
- 2.Bertagnolli MM. Chemoprevention of colorectal cancer with cyclooxygenase-2 inhibitors: two steps forward, one step back. Lancet Oncol. 2007;8:439–43. doi: 10.1016/S1470-2045(07)70139-0. [DOI] [PubMed] [Google Scholar]
- 3.Carothers AM, Moran AE, Cho NL, et al. Changes in the anti-tumor response in C57BL/6J-Min/+ mice during long-term administration of a selective cyclooxygenase-2 inhibitor. Cancer Res. 2006;66:6432–8. doi: 10.1158/0008-5472.CAN-06-0992. [DOI] [PubMed] [Google Scholar]
- 4.Davids JS, Carothers AM, Damas BC, Bertagnolli MM. Chronic cyclooxygenase-2 inhibition promotes myofibroblasts-associated intestinal fibrosis. Cancer Prev Res. 2010 doi: 10.1158/1940-6207.CAPR-09-0146. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006;441:431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 6.Greten FR, Arkan MC, Bollrath J, et al. NF-κB is a negative regulator of IL-1β secretion as revealed by genetic and pharmacological inhibition of IKKβ. Cell. 2007;130:918–31. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckmann L, Nebelsiek T, Fingerle AA, et al. Opposing functions of IKKβ during acute and chronic intestinal inflammation. Proc Natl Acad Sci USA. 2008;105:15058–63. doi: 10.1073/pnas.0808216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukata M, Abreu MT. Role of Toll-like receptors in gastrointestinal malignancies. Oncogene. 2008;27:234–43. doi: 10.1038/sj.onc.1210908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman JV, Kosaka T, Sheppard BJ, Fox JG, Schauer DB. Bacterial infection promotes colon tumorigenesis in ApcMin/+ mice. J Infect Dis. 2001;184:227–30. doi: 10.1086/321998. [DOI] [PubMed] [Google Scholar]
- 10.Rakoff-Nahous S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124–27. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- 11.Brown SL, Riehl TE, Walker MR, Geske MJ, Doherty JM, Stenson WF, Stappenbeck TS. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J Clin Invest. 2007;117:258–69. doi: 10.1172/JCI29159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shim J-H, Xiao C, Paschal AE, et al. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes & Development. 2005:2668–81. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu T, Tian L, Han Y, Vogelbaum M, Stark GR. Dose-dependent cross-talk between the transforming growth factor-β and interleukin-1 signaling pathways. Proc Natl Acad Sci USA. 2007;104:4365–70. doi: 10.1073/pnas.0700118104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kajino-Sakamoto R, Inagaki M, Lippert E, et al. Enterocyte-derived TAK1 signaling prevents epithelium apoptosis and the development of ileitis and colitis. J Immunol. 2008;181:1143–52. doi: 10.4049/jimmunol.181.2.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan M, Rerko RM, Platzer P, et al. 15-Hydroxyprostaglandin dehydrogenase, a Cox-2 oncogene antagonist is a TGFβ-induced suppressor of human gastrointestinal cancers. Proc Natl Acad Sci USA. 2004;101:17468–73. doi: 10.1073/pnas.0406142101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen-Petrik MB, McEntee MF, Jull B, Shi H, Zemel MB, Whelan J. Prostaglandin E2 protects intestinal tumors from nonsteroidal anti-inflammatory drug-induced regression in ApcMin/+ mice. Cancer Res. 2002;62:403–8. [PubMed] [Google Scholar]
- 17.Wall EA, Zavzavadjian JR, Chang MS, et al. Suppression of LPS-induced TNF-α production in macrophages by cAMP is mediated by PKA-AKAP95-p105. Sci Signal. 2009;2:ra28. doi: 10.1126/scisignal.2000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiller M, Dennler S, Anderegg U, et al. Increased cAMP levels modulate Transforming Growth Factor-β (TGF-β)/SMAD-induced expression of extracellular matrix components and other key fibroblast effector functions. J Biol Chem. 2010;285:409–21. doi: 10.1074/jbc.M109.038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchanan FG, DuBois RN. Connecting Cox-2 and Wnt in cancer. Cancer Cell. 2006;9:6–8. doi: 10.1016/j.ccr.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 20.Seno H, Miyoshi H, Brown SL, Geske MJ, Colonna M, Stappenbeck TS. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc Natl Acad Sci USA. 2009;106:256–61. doi: 10.1073/pnas.0803343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Q, Chen W, Gonzales MS, Finch J, Inoue H, Bowden GT. Role of cyclic AMP response element in the UVB induction of cyclooxygenase-2 transcription in human keratinocytes. Oncogene. 2001;20:5164–72. doi: 10.1038/sj.onc.1204667. [DOI] [PubMed] [Google Scholar]
- 22.Chen AE, Ginty DD, Fan CM. Protein kinase A signaling via CREB controls myogenesis induced by Wnt proteins. Nature. 2005;433:317–22. doi: 10.1038/nature03126. [DOI] [PubMed] [Google Scholar]
- 23.Moran AE, Hunt DH, Javid SH, Redston M, Carothers AM, Bertagnolli MM. Apc deficiency is associated with increased Egfr activity in the intestinal enterocytes and adenomas of C57BL/6J-Min/+ mice. J Biol Chem. 2004;279:43281–72. doi: 10.1074/jbc.M404276200. [DOI] [PubMed] [Google Scholar]
- 24.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 25.Berger G, Hannahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carothers AM, Melstrom KA, Mueller JD, Weyant MJ, Bertagnolli MM. Progressive changes in adherens junction structure during intestinal adenoma formation in Apc mutant mice. J Biol Chem. 2001;276:39094–102. doi: 10.1074/jbc.M103450200. [DOI] [PubMed] [Google Scholar]
- 27.Image Processing and Analysis in Java Program (Image J) retrieved from the National Institutes of Health official website: http://rsbweb.nih.gov/ij/
- 28.Kobayashi Y, Mizoguchi T, Take I, Kuihara S, Udagawa N, Takahashi N. Prostaglandin E2 enhances osteoclastic differentiation of precursor cells through protein kinase A-dependent phosphorylation of TAK-1. J Biol Chem. 2005;280:11395–403. doi: 10.1074/jbc.M411189200. [DOI] [PubMed] [Google Scholar]
- 29.Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gasteroenterol Hepatol. 2006;3:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 30.Masferrer JL, Leahy KM, Koki AT, et al. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–11. [PubMed] [Google Scholar]
- 31.Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci USA. 2002;99:15451–5. doi: 10.1073/pnas.202604299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crabtree B, Holloway DE, Baker MD, Acharya KR, Subramanian V. Biological and structural features of murine angiogenin-4, an angiogenic protein. Biochemistry. 2007;46:2431–43. doi: 10.1021/bi062158n. [DOI] [PubMed] [Google Scholar]
- 33.Guan Z, Buckman SY, Miller BW, Springer LD, Morrison AR. Interleukin-1β-induced cyclooxygenase-2 expression requires activation of both c-Jun NH2-terminal kinase and p38 MAPK signal pathways in rat renal mesangial cells. J Biol Chem. 1998;273:28670–6. doi: 10.1074/jbc.273.44.28670. [DOI] [PubMed] [Google Scholar]
- 34.Araki Y, Okamura S, Perwez Hussain S, et al. Regulation of cyclooxygenase-2 expression by the Wnt and Ras pathways. Cancer Res. 2005;65:728–34. [PubMed] [Google Scholar]
- 35.Lee HK, Jeong S. β-Catenin stabilizes cyclooxygenase-2 mRNA by interacting with AU-rich elements of 3’-UTR. Nucleic Acid Res. 2006;34:5705–14. doi: 10.1093/nar/gkl698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaric J, Rüegg CJ. Integrin-mediated adhesion and soluble ligand binding stabilize COX-2 protein levels in endothelial cells by inducing expression and preventing degradation. J Biol Chem. 2005;280:1077–85. doi: 10.1074/jbc.M410006200. [DOI] [PubMed] [Google Scholar]
- 37.Yazawa K, Tsuno NH, Kitayama J, et al. Selective inhibition of cycylooxygenase-2 inhibits colon cancer cell adhesion to extracellular matrix by expression of β1 integrin. Cancer Sci. 2005;96:93–99. doi: 10.1111/j.1349-7006.2005.00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci USA. 2008;105:20858–63. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson CL, Heppner KJ, Labosky PA, Hogan BL, Matrisian LM. Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc Natl Acad Sci USA. 1997;94:1402–7. doi: 10.1073/pnas.94.4.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson CL, Ouellett AJ, Satchell DP, et al. Regulation of intestinal α-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286:113–7. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- 41.Kaser A, Blumberg RS. Paneth cells and inflammation dance together in Crohn’s disease. Cell Res. 2008;18:1160–2. doi: 10.1038/cr.2008.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nuñez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–4. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 43.Steinbrecher KA, Harmel-Laws E, Sitcheran R, Baldwin AS. Loss of epithelial RelA results in deregulated intestinal proliferative/apoptotic homeostasis and susceptibility to inflammation. J Immunol. 2008;180:2588–99. doi: 10.4049/jimmunol.180.4.2588. [DOI] [PubMed] [Google Scholar]
- 44.Glebov OK, Rodriquez LM, Lynch P, et al. Celecoxib treatment alters the gene expression profile of normal colonic mucosa. Cancer Epidemiol Biomarkers Prev. 2006;15:1382–91. doi: 10.1158/1055-9965.EPI-04-0866. [DOI] [PubMed] [Google Scholar]
- 45.Maier TJ, Tausch L, Hoernig M, et al. Celecoxib inhibits 5-lipoxygenase. Biochem Pharmacol. 2008;76:862–72. doi: 10.1016/j.bcp.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 46.Soh JW, Kazi JU, Li H, Thompson WJ, Weinstein IB. Celecoxib-induced growth inhibition in SW480 colon cancer cells is associated with activation of protein kinase G. Mol Carcinog. 2008;47:519–25. doi: 10.1002/mc.20409. [DOI] [PubMed] [Google Scholar]
- 47.Sethi G, Tergaonkar V. Potential pharmacological control of the NF-κB pathway. Trends Pharmacol Sci. 2009;30:313–21. doi: 10.1016/j.tips.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 48.Zaph C, Troy AE, Taylor BC, et al. Epithelial-cell-intrinsic IKK-β expression regulates intestinal immune homeostasis. Nature. 2007;446:552–6. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 49.Zhou Q, Liao JK. Rho kinase: an important mediator of atherosclerosis and vascular disease. Curr Pharm Des. 2009;15:3108–15. doi: 10.2174/138161209789057986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weltz-Schmidt G, Welzenbach K, Brinkmann V, et al. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nature Med. 2001;7:687–92. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- 51.Riganti C, Doublier S, Costamagna C, Aldieri E, Pescarmona G, Ghigo D, Bosia A. Activation of nuclear factor-κB pathway by simvastatin and RhoA silencing increases doxorubicin cytotoxicity in human colon cancer HT29 cells. Mol Pharmacol. 2008;74:476–84. doi: 10.1124/mol.108.045286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.