Abstract
Context
HER2 is a membrane tyrosine kinase and oncogene that is overexpressed and gene amplified in about 20% of breast cancers. When activated it provides the cell with potent proliferative and anti-apoptosis signals and it is the major driver of tumor development and progression for this subset of breast cancer. When shown to be overexpressed or amplified by appropriate methods, HER2 is a valuable treatment target.
Objective
To review the basic biology of the HER2 signaling network, discuss various approved methods for its detection in clinical specimens and to describe the impressive results of therapies targeting HER2. Data Sources. Review selected literature searchable on PubMed as well as older studies revealed by the literature review.
Conclusion
HER2 is an important member of a complex signaling network and when gene amplified results in an aggressive subtype of breast cancer. Patients with tumors found to overexpress HER2 protein or to be amplified for the gene are candidates for therapy that significantly improves mortality.
Introduction
Human epidermal growth factor receptor-2 (HER2/neu, c-erbB2), one of a family of four membrane tyrosine kinases, was found to be amplified in a human breast cancer cell line twenty five years ago (1), and this amplification was shown to be important in the pathogenesis and progression of human breast cancer two years later (2). Since that time, HER2 amplification and resultant HER2 protein overexpression have been linked to important tumor cell proliferation and survival pathways; several drugs have been developed to target the pathway; and, the detection of HER2 has become a routine prognostic and predictive factor in breast cancer.
The aim of this review is to highlight important aspects of the biology of HER2, the current standards for its detection, and the clinical importance of its assessment.
Biological Significance of HER2
The estrogen receptor (ER) and the HER2 (c-erbB2, HER2/neu) signaling pathways are the dominant drivers of cell proliferation and survival in the majority (85%) of breast cancers. Targeting these pathways provides the most effective therapy in appropriately selected patients. Endocrine therapy to target ER and trastuzumab to target HER2 provide striking disease-free and overall survival benefits in the adjuvant setting when micrometastatic disease is present (50% reduction in risk of recurrence) (3–6). Remissions sometimes lasting years, although temporary, are seen in patients with metastatic disease treated with ER- and HER2-targeted therapy, the later usually combined with chemotherapy (7).
The HER2 pathway has been described in systems biology terms as a complex biological network comprised of three layers, an input layer of membrane receptors and their ligands to trigger the signal coming from outside the cell, a core system processing layer of protein kinases transmitting the signal to the nucleus, and an output layer of transcription factors regulating genes that affect various cellular functions (8) (Fig. 1). In turn, the genes and gene products regulating the activity of the pathway have been and are being defined. The input layer is comprised of 4 membrane receptors/tyrosine kinases (TKs) (HER1–4) and their many ligands (at least 11) (Table 1) (8). In breast cancer, HER2 is the dominant TK receptor, being amplified in 20% of cases (2). Upon ligand binding to their extracellular domains, HER proteins undergo dimerization and transphosphorylation of their intracellular domains. HER2 does not have a ligand and relies on heterodimerization with another family member or homodimerization with itself when expressed at very high levels to be activated. These phosphorylated tyrosine residues dock with numerous intracellular signaling molecules leading to activation of downstream second messenger pathways and crosstalk with other membrane signaling pathways (8–12). Transcription factors activated by the pathway regulate many genes involved in cell proliferation, survival, differentiation, angiogenesis, and invasion and metastasis. HER2 has the strongest catalytic kinase activity and HER2 containing heterodimers have the strongest signaling activity (8, 12–14). HER2 exists in an open conformation exposing its dimerization domains making it the dimerization partner of choice among the family members. HER3 is activated by ligand (heregulin) binding but it lacks TK activity, and, like HER2, must partner with another family member to be activated. Nevertheless, it has multiple docking sites for PI3K and when heterodimerized with HER2 is the most potent stimulator of the PI3K/AKT anti-apoptosis pathway (8, 12, 15, 16). HER2 can also be activated by complexing with other membrane receptors such as insulin-like growth factor receptor I (17). Even estrogen, working via the non-genomic activity of ER outside the nucleus has been shown to activate HER2 signaling (18). An aberrant form of HER2 missing the extracellular domain, so-called p95, is found in some breast cancers (19, 20). p95 is constitutively active since the external domain of these receptors acts as an inhibitor until they are bound by ligand. p95 can cause resistance to trastuzumab, an antibody that works by binding to a domain in the external domain of HER2. This domain is missing in p95. p95 is not detected by antibodies that target the external domain of HER2 for the same reason.
Figure 1. The HER signaling network and HER2-targeted therapy in breast cancer.
The HER network, recently described in systems biology terms, is a robust redundant network comprised of an input layer of 4 membrane (M) tyrosine kinase (TK) receptors (HER1/EGFR-HER4) and multiple ligands [e.g., EGF, TGFα, amphiregulin (AR), and heregulins (HRG)]; a signal core processing layer involving a series of phosphorylation (e.g., activation of the PI3K/AKT, RAS/MEK/MAPK, and STATs kinase cascades) that transmit signals from the receptor layer to the output layer; and an output layer of transcription and coregulator factors (TF, CoReg) that function in the nucleus (N) to alter expression of genes regulating tumor cell proliferation, survival, and other characteristics of the malignant phenotype (8, 12). Upon ligand binding, the receptors undergo conformational changes that allow their homo- and heterodimerization and transphosphorylation, following by the activation of a plethora of downstream signaling cascades. HER2, which is gene-amplified and/or overexpressed in 20% of breast cancers, does not have a ligand, but exists in an open conformation exposing its dimerization domain; it can be activated by hetrodimerization with other ligand-bound HER members or by homodimerization when it is overexpressed. HER3 lacks the TK activity (X). Several drugs have been developed to block the HER2 pathway, most aimed at the receptor level. The HER2 monoclonal antibody trastuzumab is FDA-approved in both the metastatic and the adjuvant settings and the dual HER1–HER2 small molecule TK inhibitor lapatinib is FDA-approved in metastatic HER2+ breast cancers.
Table 1.
HER Family of Membrane Receptors
| Receptor | Tyrosine Kinase Activity | Major Ligands |
|---|---|---|
| HER1 (EGFR) | Yes | EGF, amphiregulin, TGFα |
| HER2 | Yes | None |
| HER3 | No | Heregulin |
| HER4 | Yes | Heregulin |
HER2 Overexpression in Human Cancer
Normal tissues have a low complement of HER2 membrane protein. Overexpression of HER2 is seen in 20% of breast and in some ovarian and gastric cancers, and it confers worse biological behavior and clinical aggressiveness in breast cancer (2, 21). Breast cancers can have up to 25–50 copies of the HER2 gene, and up to 40–100 fold increase in HER2 protein resulting in 2 million receptors expressed at the tumor cell surface (22). The differential in HER2 expression between normal tissues and tumors helps to define HER2 as an ideal treatment target. Trastuzumab, the first treatment targeting HER2, is well-tolerated in patients with little toxicity, since its effects are relatively specific for cancer cells overexpressing HER2.
HER2 amplification is a relatively early event in human breast tumorigenesis, occurring in almost 50% of in situ carcinomas (23, 24). HER2 status is maintained during progression to invasive disease, and to nodal and distant metastasis (24–26). The fact that only 20% of invasive breast cancers are HER2-amplified suggests that many HER2-amplified in situ cancers never progress to the invasive stage. HER2 amplification defines a subtype of breast cancer with a unique signature of genes and this is maintained during progression (27, 28). Some tumors lose HER2 expression upon treatment with trastuzumab, presumably by selection of a HER2-negative clone not killed by treatment (29). Conversely, HER2 may become positive in some initially negative tumors over time, especially after endocrine therapy targeting ER (30). ER has been shown to downregulate HER2, and conversely, HER2 to downregulate ER expression and, therefore, it is not surprising that blocking ER might upregulate HER2 and that blocking HER2 might upregulate ER (31–35).
HER2 amplified breast cancers have unique biological and clinical characteristics (Table 2). They have increased sensitivity to certain cytotoxic agents such as doxorubicin, relative resistance to hormonal agents, and propensity to metastasize to the brain and viscera (36, 37). HER2-amplified tumors have an increased sensitivity to doxorubicin possibly due to co-amplification of the topoisomerase-2 gene which is near the HER2 locus on chromosome 17 and is the target of the drug (38). Half of HER2-positive breast cancers are ER-positive but they generally have lower ER levels, and many have p53 alterations (39, 40). These tumors have higher proliferation rates, more aneuploidy, and are associated with poorer patient prognosis (2, 23). The poor outcome is dramatically improved with appropriate chemotherapy combined with the HER2-targeting drug trastuzumab.
Table 2.
Characteristics of HER2-Amplified Breast Cancer
| Increased proliferation rates |
| High histologic and nuclear grade |
| Low ER and PR levels |
| More aneuploidy |
| Propensity to metastasize to CNS and viscera |
| Relative resistance to endocrine therapy |
| Increased sensitivity to doxorubicin; Co-amplification of topoisomerase 2 |
| Relative resistance to endocrine therapy |
| Response to HER2-targeted therapy |
ER indicates estrogen receptor; PR, progesterone receptor
HER2 Detection in Breast Cancer
HER2 testing has been problematic historically with error rates compared to a central, expert laboratory as high as 20% for immunohistochemistry and fluorescence in situ hybridization (FISH) (41, 42). Errors are unacceptable considering that the test is used to select patients for potentially curative and expensive therapy.
In 2007 the American Society of Clinical Oncology and the College of American Pathologists developed recommendations for HER2 testing performance to reduce assay errors (43). Currently HER2 testing is carried out by several methods: immunohistochemistry (IHC), fluorescence in situ hybridization (FISH), chromogenic in situ hybridization (CISH) and silver enhanced in situ hybridization (SISH). These tests are performed on tumor samples that are fixed in buffered formalin and embedded in paraffin. A new test to measure total HER2 as well as HER2 homodimers and heterodimers is also available as a predictor for efficacy of anti-HER2 therapies (44).
HER2 Immunohistochemistry
Immunohistochemistry (IHC) detects HER2 protein overexpression using monoclonal or polyclonal antibodies that bind to the protein. Currently in the United States, there are two Food and Drug Administration (FDA) approved methods for HER2 assessment: HerceptTest™ (DAKO, Glostrup Denmark) and HER2/neu (4B5) rabbit monoclonal primary antibody (Ventana, Tucson, Arizona). Current evidence suggests that home-brew assays can be less accurate than these approved assays (45). Accurate HER evaluation is determined not only by choice of antibody but also by other determinants such as tissue fixation, fixation time, and determination of thresholds for reporting positive results. According to the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines the optimal tissue handling requirements include: the time from tissue acquisition to fixation should be as short as possible; the formalin fixation in buffered formalin should range from 6–48 hours; and, the time to fixation and duration of fixation should be recorded for each sample (43). Additional IHC internal validation and quality assurance procedures, as well as external proficiency assessment are recommended (43). HER2 testing results by IHC fall into three categories, positive, equivocal and negative (Table 3, and Fig. 2). Each of these results triggers different patient management (43).
Table 3.
HER2 Testing by Validated IHC Assay
| Status | Score | Significance | Reflex HER2 FISH |
|---|---|---|---|
| Positive | 3+ | Uniform intense membrane staining of > 30% of invasive tumor cells | No |
| Equivocal | 2+ | Complete membrane staining, nonuniform or weak in intensity in at least 10% of the cells or intense complete membrane staining in 30% or less of tumor cells | Yes |
| Negative | 1+ | Weak or incomplete membrane staining in any proportion of tumor cells | No |
| Negative | 0 | No staining | No |
IHC indicates immunohistochemistry; FISH, fluorescence in situ hybridization;
Figure 2.

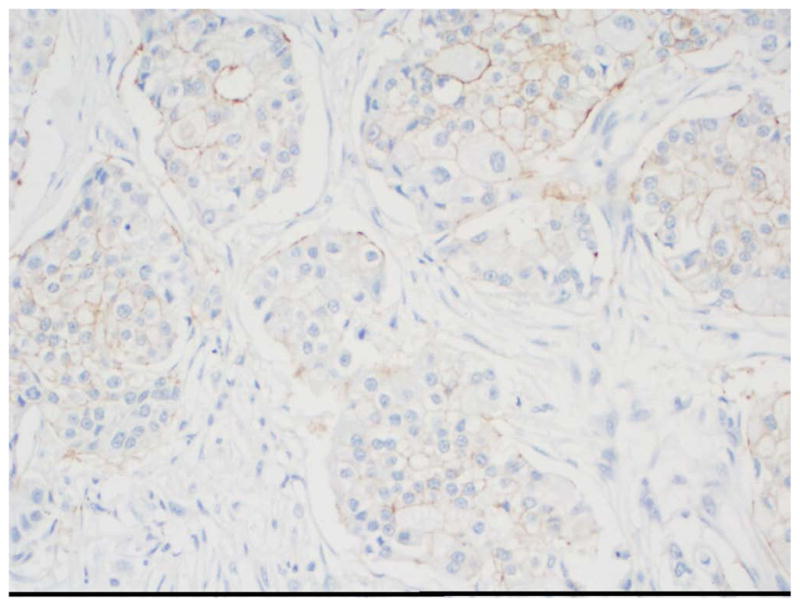

HER2 immunohistochemistry Scoring performed by the ASCO/CAP guidelines. 20x magnification. A. 1+; B. 2+; C. 3+ (Using the 4B5 rabbit monoclonal antibody, Ventana Medical Systems, Inc., Tucson, AZ)
Several additional interpretation criteria for HER2 IHC are also recommended. These include the quality of the controls; the staining of the sample; the scoring of only the invasive carcinoma and the status of adjacent normal tissue which should not stain. The report of the results must be standardized and include all of the above items discussed including fixation time, IHC method used, result and interpretation (43). The recommendations do not specify the primary antibody, the antigen retrieval method or the detection system, all of which can affect the result. It seems logical to use a method that has been validated on clinical samples and published in the literature.
HER2 Molecular Analysis
Fluorescent (FISH) or chromogenic (CISH) in situ hybridization for HER2 gene amplification has become an integral part of the diagnostic work up for patients with breast cancer. The principles of in situ hybridization are simple: DNA probes complementary to genomic sequences of interest are generated, labeled and then hybridized to the target tissue. FISH/CISH methods can be applied to a wide range of samples: cell lines, frozen tissue, paraffin embedded tissue and micro-tissue arrays. FISH utilizes fluorescence microscopy while CISH employs light microscopy.
HER2 FISH positive is defined as an average of >6 HER2 gene copies/nucleus for test systems without an internal control probe or HER2/CEP 17 ratio of more than 2.2, where CEP17 is a centromeric probe for chromosome 17 on which the HER2 gene resides (Fig. 3). The equivocal range for HER2 FISH assays is defined as HER/CEP ratios from 1.8 to 2.2 or average gene copy number between 4.0 and 6.0 for those systems without an internal control (43). Negative HER2 FISH amplification is defined as HER2/CEP17 ratio of less than 1.8 or an average of fewer than four copies of HER2 gene per nucleus for systems without an internal control probe. Tumor samples with a limited amount of invasive cancer, tissue not fixed in buffered formalin or fixed for more than 48 hours, and background auto fluorescence render a specimen uninterpretable. The report of HER2 FISH results must follow the standardized form recommended by ASCO/CAP guidelines (43).
Figure 3.

A. HER2 gene amplification using FISH by PathVysion (Vysis, Abbott Laboratories, Abbott Park, IL, USA), photo provided by Clarient,Inc.,Aliso Viejo, CA. B, C, D, HER2 gene amplification by Ultraview SISH Detection Kit (Ventana Medical Systems, Inc., Tucson, AZ); B. Chromosome 17 probe; C. HER2 probe not amplified; D. HER2 probe amplified.
Chromogenic in situ hybridization (CISH) uses chromogens for signal identification by means of indirect labeling (46). CISH has some advantages in the detection of gene amplification over FISH: 1) permanent staining, samples can be archived; 2) use of bright field microscopy; 3) easy identification of the target cells; 4) tumor heterogeneity is easily assessed (46). Fully automated systems such as bright field silver ISH (SISH) (Ventana) should help in method standardization. While CISH estimates of gene amplification correlate very well with FISH, CISH does not permit a determination of the actual gene copy number.
Both FISH and CISH protocols usually require 2 days to be completed, and the assays, like IHC, are difficult and can give erroneous results. FISH analysis requires specific training, a fluorescence microscope with multiband filters, a high resolution digital camera and manual counting which is time consuming. Automated FISH scanning is being developed. CISH on the other hand, is less time consuming, is more easily read by pathologists due to similarities with IHC, and requires only bright field microscopy. CISH methods such as the SPOT-Light HER2 CISH kit (Invitrogen) are FDA approved.
Because of the advantages over IHC, some have considered in situ hybridization the method of choice for HER2 gene testing (47). Two kits have been FDA approved: PathVysion (Vysis, Abbott Laboratories, Abbott Park, IL,), and HER2 FISH Pharm Dx (Dako) and Inform (Ventana) that has been submitted for FDA approval. The first two are dual color FISH tests and the latter uses only a probe specific for HER2. The first two use HER2/CEP17 ratios, and the third relies on the signal count of the HER2 probe. Other experts continue to recommend IHC with reflex to FISH in cases scored as 2+ by IHC (43). Both strategies have pluses and minuses and require knowledge in their interpretation.
Several studies have examined the impact of the ASCO/CAP guideline recommendations on HER2 interpretation. In the Mayo Clinic study, 144 cases of invasive carcinoma with IHC of 3+ scored according to the older HercepTest guidelines (strong membrane staining of more than 10% cells), were identified (48). The slides were re-reviewed and results were recorded according to the new ASCO/CAP guidelines of 3+, 2+, 1+ and 0. Of the 144 cases, 3 cases were reclassified as 2+ by the HercepTest and removed from the study. Analysis was done on 141 cases, of which 12 were classified as 2+ according to the new ASCO/CAP guidelines. Of the 12 cases, 6 were HER2 FISH amplified (HER2/CEP17 ratio >2.2), 4 cases were negative (ratio <1.8) and 2 cases were equivocal (ratio: 1.8–2.2). 119 tumors in this study showed 3+ staining by ASCO/CAP in a large proportion (70%) of the tumor. In 18 cases with 11–50% of the cells staining 3+ by IHC, 2 had non-concordant HER2 FISH results and were not amplified. All but 2 of the 119 cases showing 3+ staining in >70% cells were gene amplified. This study shows that the 2007 ASCO/CAP guidelines improve the concordance between IHC and FISH. Other studies have shown that standardized fixation and adherence to the ASCO/CAP guidelines resulted in a greater correlation between HER2 IHC and HER2 FISH and showed fewer of inconclusive FISH cases (49).
Several studies have shown that more automated quantitative analysis for HER2 IHC can provide standardized scoring (50–52). By using AQUA® technology and immunohistochemical/immuno-fluorescence HER2 staining, one study showed that the average percent coefficients of variation across instruments, operators and staining runs were 1.8%, 2.0% and 5.1% respectively (50). Positive/negative concordance rates ranged from 94.5% to 99.3% for all cases. Another study assessed the performance characteristics of the Automated Cellular Imaging System (ACIS III) for HER2 IHC analysis (51). The automated score was compared to the manual score and to HER2 by FISH. The automated score yielded fewer 2+ cases than the manual score and had a higher positive predictive value for HER2 amplification.
Silver enhanced in situ hybridization (SISH) is a highly sensitive technique that requires fewer reagents than FISH or CISH (Fig. 3). Like CISH, SISH staining is permanent and does not decay, allowing specimens to be archived. Unlike CISH, SISH allows quantitative scoring of gene copy number. In a recent study comparing HER2 FISH and HER2 SISH techniques in excisional biopsies, the results were identical in 50 out of 53 (94%) cases (52). Two cases were SISH−, FISH+ and one case SISH+, FISH−. SISH results on the core biopsy were identical to the corresponding excisional biopsy in 89% of the cases. Four cases (7%) were SISH negative in the core but positive in the excision specimen and two cases were the other way around. These are comparable with FISH and validate the use of SISH for measurement of HER2.
Influence of Polysomy 17
By using HER2 FISH that evaluates HER2 gene copy number and the centromeric region of chromosome 17, the results can be categorized as: 1) normal gene copy and ploidy status (2 signals of HER2 and 2 signals for chromosome 17); 2) HER 2 amplification; 3) chromosome 17 polysomy and; 4) chromosome 17 polysomy with HER2 amplification (53). Tumors with increased HER2 and chromosome 17 signals and with a ratio of <1.8 according to the ACSO/CAP guidelines are considered polysomy. These tumors have been associated with HER2+ IHC but rarely with 3+ (54). Patients with chromosome 17 polysomic breast cancer in the absence of HER2 amplification or 3+ IHC are not candidates for targeted therapy, and they have not been considered eligible for adjuvant trastuzumab trials or trials comparing different HER2 therapies (53). Recent studies have shown that true/complete chromosome 17 polysomy is rare and that a CEP17 copy number of >3 in FISH analysis is due to concomitant gain or amplification of the centromeric region in addition to HER2 (55, 56). Thus, patients may appear to have tumors with chromosome 17 polysomy, but in reality have HER2 amplification with the amplicon extending to the centromere. These patients might be denied HER-targeted therapy due to misinterpretation of the FISH results. Further investigation is needed to confirm these data and to clarify the importance, if any, of polysomy 17 in breast cancer.
Protein Proximity HER2 Assay
The HERmark® test is a new assay that uses the VeraTag system to identify total levels of a cellular protein or two similar or dissimilar proteins in close proximity (44). The technology has been used to measure total HER2 protein and the amount of HER2 homodimers in breast cancer tissue (57). For a total HER2 the technology uses two monoclonal antibodies specific for unique epitopes in the intracellular domain of the HER2 receptor protein. This results in the antibodies binding in close proximity to the same HER2 receptor proteins. One of the monoclonal antibodies is conjugated with a fluorescent reporter and the other to a photosensitizer molecule. The assay is done on paraffin embedded tissues and photo activation of the sample results in cleavage of the reporter which is then quantified using electrophoresis. The amount of cleaved reporter is proportional to the concentration of HER2 in the tumor. In a study in metastatic breast cancer in HER2+ patients treated with trastuzumab, the assay was superior to FISH and to immunohistochemistry in predicting benefit from trastuzumab (58). In a preliminary report from a recently completed adjuvant trial, the HERmark assay declassified 25% of cases originally classified as positive, equivocal, or negative when done by central IHC (59). Correlation with outcome has not yet been performed. The assay is now being developed to detect HER3, various homo- and hetero-dimer pairs, and other signaling molecules. Additional data are needed to know where this assay fits in the diagnostic evaluation of the breast cancer patient.
HER2 Targeted Therapy in the Clinic
The need for accurate determination of HER2 status is illustrated by the excellent results of therapies targeting HER2 in the clinic. These strategies have been shown to benefit only tumors overexpressing HER2 protein and usually HER2 gene amplified. Tumors with negative staining, 1+ or 2+, which is not gene amplified, do not benefit from HER2-targeted therapies.
Two drugs are currently FDA approved for treatment of HER2 positive cancers. Trastuzumab is a humanized monoclonal antibody that recognizes the external domain of HER2. Its mechanism of action is still not totally clear but it seems to have its greatest effects in tumors with increased HER2 homodimers (57). Although it does not block autophosphorylation of HER2, it does inhibit HER2 downstream signaling (60). In addition, it may disrupt the HER2/Src interaction, may cause internalization and downregulation of the receptor, and may enhance antibody mediated cytotoxicity. In patients with metastatic breast cancer undergoing first-line treatment, about 25% of those patients with HER2 overexpressing tumors have an objective response to trastuzumab when used alone (61).
Preclinically, trastuzumab has synergistic activity when combined with a variety of chemotherapy drugs, perhaps because it inhibits an important survival pathway making the cytotoxic affects of chemotherapy more potent. Doxorubicin may be more effective because of co-amplification with HER2 of its target topoisomerase 2 on chromosome 17 (62). In clinical trials, trastuzumab increased the objective response rate and time to progression when combined with doxorubicin, cyclophosphamide, or paclitaxel (63). The high rate of cardiac toxicity when trastuzumab was combined with doxorubicin containing regimens demanded that trastuzumab only be used sequentially with regimens containing anthracyclines. A variety of phase II and phase III studies confirmed the effectiveness of trastuzumab combined with a variety of chemotherapeutic agents, some showing an overall survival advantage (63). Trastuzumab is also beneficial when combined with endocrine therapy in patients with ER-positive and HER2-positive tumors (64).
More impressive results were observed when trastuzumab was combined with chemotherapy in the adjuvant treatment of patients with primary breast cancer following surgery. Several randomized trials using different chemotherapy regimens combined with trastuzumab all showed significant improvements in disease free survival and overall survival with reductions in the risk of recurrence ranging from 40–50% (65–68). Since prior studies of chemotherapy alone had shown a 40–50% reduction in the risk of recurrence, the addition of trastuzumab to chemotherapy in patients with HER2 overexpressing tumors has resulted in a marked improvement in the outcome of these patients with a tumor subtype noted for its aggressive behavior and poor prognosis in the absence of therapy.
A subset analysis of one of the large adjuvant trials performed by the NSABP showed an advantage for trastuzumab even in patients found at central laboratory testing to be HER2 negative (69). These investigators have done an exhaustive analysis of these patients to confirm that they are indeed not HER2 amplified. Whether this retrospective analysis on a small number of patients originally deemed HER2 positive in the local laboratory and found on central laboratory testing to be HER2 negative is merely a statistical fluke or whether it reflects real biology of the tumor remains to be clarified. Until then HER2 targeted therapy is reserved for patients whose tumor overexpresses the protein or is amplified for the gene.
Lapatinib is a new drug that works by a different mechanism to inhibit HER2 signaling (Fig. 1) (70). Lapatinib is a small molecule tyrosine kinase inhibitor that blocks the kinase activity of HER1 and HER2. This drug has been shown to cause remissions in patients whose tumors are resistant to trastuzumab and it may be more effective when given together with trastuzumab (71, 72). Lapatinib should still be effective in patients whose tumors express high levels of HER2 p95 and are trastuzumab resistant. This shortened form of HER2 has lost the extracellular domain to which trastuzumab binds; but, lapatinib would be expected to still block the tyrosine kinase activity. Lapatinib, like trastuzumab when combined with certain chemotherapy drugs in patients with metastatic disease, increases the patient benefit compared to the chemotherapy drug alone (73). Several trials of metastatic disease as well as in the adjuvant setting are now underway evaluating chemotherapy and lapatinib as a single agent or combined with trastuzumab. The combinations are of particular interest given the dramatic affects of combined HER-targeted therapies in preclinical xenograft models (74). Several other drugs are under development for HER2 overexpressing breast cancer. Among them are an irreversible dual tyrosine kinase inhibitor similar to lapatinib; a monoclonal antibody that blocks the dimerization domain on HER2 to prevent heterodimerization with other members of the HER receptor family; and, an antibody-drug conjugate that links trastuzumab with a cytotoxic agent (75, 76).
Summary and Conclusion
The HER2 oncogene is an important member of the HER family of membrane tyrosine kinases. The gene is amplified in a subset of breast cancers in which the HER network is the major driver of tumor cell proliferation and survival. Targeting this pathway therapeutically has remarkably improved the outlook of patients with these aggressive breast cancers, but treatment is only effective in patients whose tumors express high levels of the protein or are amplified for the gene. The pathologist’s role in accurately assessing the HER2 status of a tumor is critical for successful treatment. Diagnostic assessment of HER2 by IHC or gene amplification should follow established guidelines and procedures to avoid misclassification of patients who might not otherwise receive life-saving treatment.
Footnotes
The authors have no relevant financial interest in the products or companies described in this article.
Contributor Information
Carolina Gutierrez, Department of Pathology, Baylor ollege of Medicine in Houston, Texas
Rachel Schiff, Lester & Sue Smith Breast Center, Baylor ollege of Medicine in Houston, Texas
References
- 1.King CR, Kraus MH, Aaronson SA. Amplification of a novel v-erbB-related gene in a human mammary carcinoma. Science. 1985 Sep 6;229(4717):974–976. doi: 10.1126/science.2992089. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987 Jan 9;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 3.Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998 May 16;351(9114):1451–1467. [PubMed] [Google Scholar]
- 4.Joensuu H, Bono P, Kataja V, et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer Trial. J Clin Oncol. 2009 Dec 1;27(34):5685–5692. doi: 10.1200/JCO.2008.21.4577. [DOI] [PubMed] [Google Scholar]
- 5.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005 Oct 20;353(16):1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 6.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005 Oct 20;353(16):1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 7.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001 Mar 15;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 8.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006 Jul;7(7):505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 9.Barnes CJ, Kumar R. Biology of the epidermal growth factor receptor family. Cancer Treat Res. 2004;119:1–13. doi: 10.1007/1-4020-7847-1_1. [DOI] [PubMed] [Google Scholar]
- 10.Bazley LA, Gullick WJ. The epidermal growth factor receptor family. Endocr Relat Cancer. 2005 Jul;12(Suppl 1):S17–S27. doi: 10.1677/erc.1.01032. [DOI] [PubMed] [Google Scholar]
- 11.Yarden Y. Biology of HER2 and its importance in breast cancer. Oncology. 2001;61(Suppl 2):1–13. doi: 10.1159/000055396. [DOI] [PubMed] [Google Scholar]
- 12.Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007 Oct 4;26(45):6469–6487. doi: 10.1038/sj.onc.1210477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tzahar E, Waterman H, Chen X, et al. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol. 1996 Oct;16(10):5276–5287. doi: 10.1128/mcb.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997 Apr 1;16(7):1647–1655. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulze WX, Deng L, Mann M. Phosphotyrosine interactome of the ErbB-receptor kinase family. Mol Syst Biol. 2005;1:2005 0008. doi: 10.1038/msb4100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris JK, Lin W, Hauser C, Marchuk Y, Getman D, Lee KF. Rescue of the cardiac defect in ErbB2 mutant mice reveals essential roles of ErbB2 in peripheral nervous system development. Neuron. 1999 Jun;23(2):273–283. doi: 10.1016/s0896-6273(00)80779-5. [DOI] [PubMed] [Google Scholar]
- 17.Nahta R, Yuan LX, Zhang B, Kobayashi R, Esteva FJ. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005 Dec 1;65(23):11118–11128. doi: 10.1158/0008-5472.CAN-04-3841. [DOI] [PubMed] [Google Scholar]
- 18.Shou J, Massarweh S, Osborne CK, et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96(12):926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 19.Molina MA, Saez R, Ramsey EE, et al. NH(2)-terminal truncated HER-2 protein but not full-length receptor is associated with nodal metastasis in human breast cancer. Clin Cancer Res. 2002 Feb;8(2):347–353. [PubMed] [Google Scholar]
- 20.Scaltriti M, Rojo F, Ocana A, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007 Apr 18;99(8):628–638. doi: 10.1093/jnci/djk134. [DOI] [PubMed] [Google Scholar]
- 21.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989 May 12;244(4905):707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 22.Kallioniemi OP, Kallioniemi A, Kurisu W, et al. ERBB2 amplification in breast cancer analyzed by fluorescence in situ hybridization. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5321–5325. doi: 10.1073/pnas.89.12.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allred DC, Clark GM, Molina R, et al. Overexpression of HER-2/neu and its relationship with other prognostic factors change during the progression of in situ to invasive breast cancer. Hum Pathol. 1992 Sep;23(9):974–979. doi: 10.1016/0046-8177(92)90257-4. [DOI] [PubMed] [Google Scholar]
- 24.Park K, Han S, Kim HJ, Kim J, Shin E. HER2 status in pure ductal carcinoma in situ and in the intraductal and invasive components of invasive ductal carcinoma determined by fluorescence in situ hybridization and immunohistochemistry. Histopathology. 2006 May;48(6):702–707. doi: 10.1111/j.1365-2559.2006.02403.x. [DOI] [PubMed] [Google Scholar]
- 25.Latta EK, Tjan S, Parkes RK, O’Malley FP. The role of HER2/neu overexpression/amplification in the progression of ductal carcinoma in situ to invasive carcinoma of the breast. Mod Pathol. 2002 Dec;15(12):1318–1325. doi: 10.1097/01.MP.0000038462.62634.B1. [DOI] [PubMed] [Google Scholar]
- 26.Carlsson J, Nordgren H, Sjostrom J, et al. HER2 expression in breast cancer primary tumours and corresponding metastases. Original data and literature review. Br J Cancer. 2004 Jun 14;90(12):2344–2348. doi: 10.1038/sj.bjc.6601881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000 Aug 17;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 28.Weigelt B, Hu Z, He X, et al. Molecular portraits and 70-gene prognosis signature are preserved throughout the metastatic process of breast cancer. Cancer Res. 2005 Oct 15;65(20):9155–9158. doi: 10.1158/0008-5472.CAN-05-2553. [DOI] [PubMed] [Google Scholar]
- 29.Mittendorf EA, Wu Y, Scaltriti M, et al. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin Cancer Res. 2009 Dec 1;15(23):7381–7388. doi: 10.1158/1078-0432.CCR-09-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gutierrez MC, Detre S, Johnston S, et al. Molecular changes in tamoxifen-resistant breast cancer: relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. J Clin Oncol. 2005 Apr 10;23(11):2469–2476. doi: 10.1200/JCO.2005.01.172. [DOI] [PubMed] [Google Scholar]
- 31.Newman SP, Bates NP, Vernimmen D, Parker MG, Hurst HC. Cofactor competition between the ligand-bound oestrogen receptor and an intron 1 enhancer leads to oestrogen repression of ERBB2 expression in breast cancer. Oncogene. 2000 Jan 27;19(4):490–497. doi: 10.1038/sj.onc.1203416. [DOI] [PubMed] [Google Scholar]
- 32.Munzone E, Curigliano G, Rocca A, et al. Reverting estrogen-receptor-negative phenotype in HER-2-overexpressing advanced breast cancer patients exposed to trastuzumab plus chemotherapy. Breast Cancer Res. 2006;8(1):R4. doi: 10.1186/bcr1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez-Tarruella S, Schiff R. The dynamics of estrogen receptor status in breast cancer: re- shaping the paradigm. Clin Cancer Res. 2007 Dec 1;13(23):6921–6925. doi: 10.1158/1078-0432.CCR-07-1399. [DOI] [PubMed] [Google Scholar]
- 34.Xia W, Bacus S, Hegde P, et al. A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc Natl Acad Sci U S A. 2006 May 16;103(20):7795–7800. doi: 10.1073/pnas.0602468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo S, Sonenshein GE. Forkhead box transcription factor FOXO3a regulates estrogen receptor alpha expression and is repressed by the Her-2/neu/phosphatidylinositol 3-kinase/Akt signaling pathway. Mol Cell Biol. 2004 Oct;24(19):8681–8690. doi: 10.1128/MCB.24.19.8681-8690.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ross JS, Fletcher JA, Linette GP, et al. The Her-2/neu gene and protein in breast cancer 2003: biomarker and target of therapy. Oncologist. 2003;8(4):307–325. doi: 10.1634/theoncologist.8-4-307. [DOI] [PubMed] [Google Scholar]
- 37.Gabos Z, Sinha R, Hanson J, et al. Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J Clin Oncol. 2006 Dec 20;24(36):5658–5663. doi: 10.1200/JCO.2006.07.0250. [DOI] [PubMed] [Google Scholar]
- 38.Villman K, Sjostrom J, Heikkila R, et al. TOP2A and HER2 gene amplification as predictors of response to anthracycline treatment in breast cancer. Acta Oncol. 2006;45(5):590–596. doi: 10.1080/02841860500543182. [DOI] [PubMed] [Google Scholar]
- 39.Tsutsui S, Ohno S, Murakami S, Kataoka A, Kinoshita J, Hachitanda Y. Prognostic significance of the coexpression of p53 protein and c-erbB2 in breast cancer. Am J Surg. 2003 Feb;185(2):165–167. doi: 10.1016/s0002-9610(02)01203-5. [DOI] [PubMed] [Google Scholar]
- 40.Konecny G, Pauletti G, Pegram M, et al. Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J Natl Cancer Inst. 2003 Jan 15;95(2):142–153. doi: 10.1093/jnci/95.2.142. [DOI] [PubMed] [Google Scholar]
- 41.Paik S, Bryant J, Tan-Chiu E, Romond E, et al. Real-world performance of HER2 testing--National Surgical Adjuvant Breast and Bowel Project experience. J Natl Cancer Inst. 2002 Jun 5;94(11):852–854. doi: 10.1093/jnci/94.11.852. [DOI] [PubMed] [Google Scholar]
- 42.Perez EA, Suman VJ, Davidson NE, et al. HER2 testing by local, central, and reference laboratories in specimens from the North Central Cancer Treatment Group N9831 intergroup adjuvant trial. J Clin Oncol. 2006 Jul 1;24(19):3032–3038. doi: 10.1200/JCO.2005.03.4744. [DOI] [PubMed] [Google Scholar]
- 43.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131(1):18–43. doi: 10.5858/2007-131-18-ASOCCO. [DOI] [PubMed] [Google Scholar]
- 44.Shi Y, Huang W, Tan Y, et al. A novel proximity assay for the detection of proteins and protein complexes: quantitation of HER1 and HER2 total protein expression and homodimerization in formalin-fixed, paraffin-embedded cell lines and breast cancer tissue. Diagn Mol Pathol. 2009 Mar;18(1):11–21. doi: 10.1097/PDM.0b013e31818cbdb2. [DOI] [PubMed] [Google Scholar]
- 45.Kroese M, Zimmern RL, Pinder SE. HER2 status in breast cancer--an example of pharmacogenetic testing. J R Soc Med. 2007 Jul;100(7):326–329. doi: 10.1258/jrsm.100.7.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lambros MB, Natrajan R, Reis-Filho JS. Chromogenic and fluorescent in situ hybridization in breast cancer. Hum Pathol. 2007 Aug;38(8):1105–1122. doi: 10.1016/j.humpath.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 47.Sauter G, Lee J, Bartlett JM, Slamon DJ, Press MF. Guidelines for human epidermal growth factor receptor 2 testing: biologic and methodologic considerations. J Clin Oncol. 2009 Mar 10;27(8):1323–1333. doi: 10.1200/JCO.2007.14.8197. [DOI] [PubMed] [Google Scholar]
- 48.Shah SS, Ketterling RP, Goetz MP, et al. Impact of American Society of Clinical Oncology/College of American Pathologists guideline recommendations on HER2 interpretation in breast cancer. Hum Pathol. Jan;41(1):103–106. doi: 10.1016/j.humpath.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 49.Middleton LP, Price KM, Puig P, et al. Implementation of American Society of Clinical Oncology/College of American Pathologists HER2 Guideline Recommendations in a tertiary care facility increases HER2 immunohistochemistry and fluorescence in situ hybridization concordance and decreases the number of inconclusive cases. Arch Pathol Lab Med. 2009 May;133(5):775–780. doi: 10.5858/133.5.775. [DOI] [PubMed] [Google Scholar]
- 50.Gustavson MD, Bourke-Martin B, Reilly D, et al. Standardization of HER2 immunohistochemistry in breast cancer by automated quantitative analysis. Arch Pathol Lab Med. 2009 Sep;133(9):1413–1419. doi: 10.5858/133.9.1413. [DOI] [PubMed] [Google Scholar]
- 51.Minot DM, Kipp BR, Root RM, et al. Automated cellular imaging system III for assessing HER2 status in breast cancer specimens: development of a standardized scoring method that correlates with FISH. Am J Clin Pathol. 2009 Jul;132(1):133–138. doi: 10.1309/AJCPJV0SKAF2PCMY. [DOI] [PubMed] [Google Scholar]
- 52.Shousha S, Peston D, Amo-Takyi B, Morgan M, Jasani B. Evaluation of automated silver-enhanced in situ hybridization (SISH) for detection of HER2 gene amplification in breast carcinoma excision and core biopsy specimens. Histopathology. 2009 Jan;54(2):248–253. doi: 10.1111/j.1365-2559.2008.03185.x. [DOI] [PubMed] [Google Scholar]
- 53.Viale G. Be precise! The need to consider the mechanisms for CEP17 copy number changes in breast cancer. J Pathol. 2009 Sep;219(1):1–2. doi: 10.1002/path.2593. [DOI] [PubMed] [Google Scholar]
- 54.Downs-Kelly E, Yoder BJ, Stoler M, et al. The influence of polysomy 17 on HER2 gene and protein expression in adenocarcinoma of the breast: a fluorescent in situ hybridization, immunohistochemical, and isotopic mRNA in situ hybridization study. Am J Surg Pathol. 2005 Sep;29(9):1221–1227. doi: 10.1097/01.pas.0000165528.78945.95. [DOI] [PubMed] [Google Scholar]
- 55.Marchio C, Lambros MB, Gugliotta P, et al. Does chromosome 17 centromere copy number predict polysomy in breast cancer? A fluorescence in situ hybridization and microarray-based CGH analysis. J Pathol. 2009 Sep;219(1):16–24. doi: 10.1002/path.2574. [DOI] [PubMed] [Google Scholar]
- 56.Yeh IT, Martin MA, Robetorye RS, et al. Clinical validation of an array CGH test for HER2 status in breast cancer reveals that polysomy 17 is a rare event. Mod Pathol. 2009 Sep;22(9):1169–1175. doi: 10.1038/modpathol.2009.78. [DOI] [PubMed] [Google Scholar]
- 57.Desmedt C, Sperinde J, Piette F, et al. Quantitation of HER2 expression or HER2:HER2 dimers and differential survival in a cohort of metastatic breast cancer patients carefully selected for trastuzumab treatment primarily by FISH. Diagn Mol Pathol. 2009 Mar;18(1):22–29. doi: 10.1097/PDM.0b013e31818ebc69. [DOI] [PubMed] [Google Scholar]
- 58.Lipton A, Kostler WJ, Leitzel K. Quantitative HER2 protein levels predict outcome in FISH-positive patients with metastatic breast cancer treated with trastuzumab. Cancer. 2010 doi: 10.1002/cncr.25430. In Press. [DOI] [PubMed] [Google Scholar]
- 59.Huang W, Weidler J, Lie Y, et al. Correlation of quantitative total HER2 expression and HER2 homodimers with histopathologic characteristics of breast cancers in the FinHer study. J Clin Oncol. 2009;27(15S):Abstract 11061. [Google Scholar]
- 60.Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006 May;3(5):269–280. doi: 10.1038/ncponc0509. [DOI] [PubMed] [Google Scholar]
- 61.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002 Feb 1;20(3):719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 62.Slamon DJ, Press MF. Alterations in the TOP2A and HER2 genes: association with adjuvant anthracycline sensitivity in human breast cancers. J Natl Cancer Inst. 2009 May 6;101(9):615–618. doi: 10.1093/jnci/djp092. [DOI] [PubMed] [Google Scholar]
- 63.Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007 Jul 5;357(1):39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 64.Kaufman B, Mackey JR, Clemens MR, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol. 2009 Nov 20;27(33):5529–5537. doi: 10.1200/JCO.2008.20.6847. [DOI] [PubMed] [Google Scholar]
- 65.Perez EA, Romond EH, Suman VJ, et al. Updated results of the combined analysis of NCCTG N9831 and NSABP B-31 adjuvant chemotherapy with/without trastuzumab in patients with HER2-positive breast cancer. J Clin Oncol. 2007;25(18S):Abstract 512. [Google Scholar]
- 66.Smith I, Procter M, Gelber RD, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007 Jan 6;369(9555):29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 67.Slamon DJ, Eiermann W, Robert N, Pienkowski T, Martin M, editors. Breast Cancer Res Treat. Supp 1 Vol. 100. San Antonio, TX: 2006. BCIRG 006: 2nd interim analysis phase III randomized trial phase III comparing doxorubicin and cyclophosphamide followed by docetaxel (AC-T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC-TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2 positive early breast cancer patients San Antonio Breast Cancer Symposium. [Google Scholar]
- 68.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006 Feb 23;354(8):809–820. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 69.Paik S, Kim C, Wolmark N. HER2 status and benefit from adjuvant trastuzumab in breast cancer. N Engl J Med. 2008 Mar 27;358(13):1409–1411. doi: 10.1056/NEJMc0801440. [DOI] [PubMed] [Google Scholar]
- 70.Moy B, Goss PE. Lapatinib-associated toxicity and practical management recommendations. Oncologist. 2007 Jul;12(7):756–765. doi: 10.1634/theoncologist.12-7-756. [DOI] [PubMed] [Google Scholar]
- 71.Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28(7):1124–1130. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 72.Spector NL, Xia W, Burris H, 3rd, et al. Study of the biologic effects of lapatinib, a reversible inhibitor of ErbB1 and ErbB2 tyrosine kinases, on tumor growth and survival pathways in patients with advanced malignancies. J Clin Oncol. 2005 Apr 10;23(11):2502–2512. doi: 10.1200/JCO.2005.12.157. [DOI] [PubMed] [Google Scholar]
- 73.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006 Dec 28;355(26):2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 74.Arpino G, Gutierrez C, Weiss H, et al. Treatment of human epidermal growth factor receptor 2-overexpressing breast cancer xenografts with multiagent HER-targeted therapy. J Natl Cancer Inst. 2007 May 2;99(9):694–705. doi: 10.1093/jnci/djk151. [DOI] [PubMed] [Google Scholar]
- 75.Pal SK, Pegram M. Targeting HER2 Epitopes. Semin Oncol. 2006 Aug;33(4):386–391. doi: 10.1053/j.seminoncol.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 76.Jones KL, Buzdar AU. Evolving novel anti-HER2 strategies. Lancet Oncol. 2009 Dec;10(12):1179–1187. doi: 10.1016/S1470-2045(09)70315-8. [DOI] [PubMed] [Google Scholar]






