Abstract
Sphingolipids are essential structural components of cellular membranes, playing prominent roles in signal transduction that governs cell proliferation, differentiation and apoptosis. Ceramides, a family of distinct molecular species characterized by various acyl chains, are synthesized de novo at the cytosolic side of the endoplasmic reticulum serving as precursors for the biosynthesis of sphingolipids in the Golgi. Recently, mitochondria emerged as an important intracellular compartment of sphingolipid metabolism. Thus, several sphingolipid-metabolizing enzymes were found to be associated with mitochondria, including neutral ceramidase, novel neutral sphingomyelinase, and (dihydro) ceramide synthase, an important ceramide-generating enzyme in de novo ceramide synthesis and recycling pathway. Mitochondrial dysfunction appears to be essential in tissue damage after brain ischemia/reperfusion (IR). Mitochondria are known to be involved in both the necrosis and apoptosis detected in animal models of ischemic stroke, and treatments that ameliorate tissue infarction were associated with better recovery of mitochondrial function. Although mitochondrial injury in stroke has been extensively studied and key mitochondrial functions affected by IR are mainly characterized, the nature of the molecule that causes loss of mitochondrial integrity and function remains obscure. Emerging data indicate a deregulation of ceramide metabolism in mitochondria damaged by IR suggesting that ceramides could play critical roles in cerebral IR-induced mitochondrial damage. This review will examine the experimental evidence supporting the key role of ceramides in mitochondrial dysfunction in cerebral IR and highlight potential targets for development of novel therapeutic approaches for stroke treatment.
Keywords: Sphingolipid, ceramide, ceramide synthase, neutral ceramidase, mitochondria, stroke
Introduction
After more than a decade of extensive investigations, it has become clear that ceramides are essential sphingolipid messengers regulating a diverse range of cell-stress responses, including apoptosis, cell senescence, and autophagy [1]. Each ceramide is tightly regulated in cells, and its participation in cell death signaling pathways is controlled by rapid conversion of ceramide into less deleterious sphingolipids (Figure 1). Thus, ceramide can be metabolized into complex sphingolipids by glucosylceramide synthase or into SM by SM synthase, or into ceramide-1-phosphate by ceramide kinase [2, 3], or into sphingosine-1-phosphate by ceramidase and sphingosine kinase [4]. However, pathological conditions, including cerebral ischemia/reperfusion, could disturb ceramide metabolism resulting in ceramide accumulation that ultimately leads to cell death.
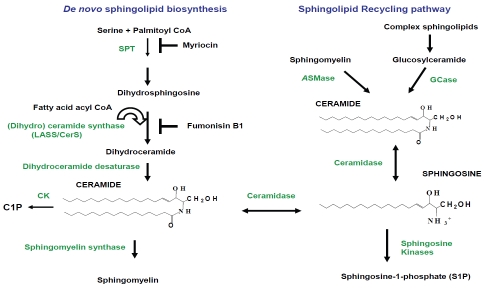
Figure 1.
Biosynthesis of ceramide and its conversion into other bioactive sphingolipids. De novo ceramide synthesis begins with the conversion of serine and fatty acyl CoA into 3-ketosphinganine by serine palmitoyl transferase (SPT), then 3-ketosphinganine is converted into dihydrosphingosine. Myriocin is a potent inhibitor of SPT activity. (Dihydro) ceramide synthase (LASS/CerS) acylates dihydrosphingosine to form dihydroceramide, which is then reduced to ceramide by dihydroceramide desaturase. Ceramide is also produced by SMases through SM degradation in SMase pathway. Ceramidase converts ceramide into sphingosine, which is phosphorylated by sphingosine kinase (SK) to generate sphingosine-1-phosphate. Ceramide is phosphorylated by ceramide kinase (CK) yielding ceramide-1-phosphate (C1P). In the salvage or recycling pathway, complex sphingolipids are broken down to ceramide by glucosylceramidase (GCase) and then by ceramidase to sphingosine, which is re-acylated to ceramide by LASS/CerS. Fumonisin B1 inhibits LASS/CerS activity.
Pathways of ceramide generation in mammalian cells
Ceramide is a family comprised of about 50 distinct molecular species characterized by various acyl chains, their desaturation, and hydroxylation. Ceramide is an N-acylsphingosine consisting of a fatty acid bound to the amino group of the sphingoid base, sphingosine. Ceramides can contain monounsaturated or saturated fatty acids of various lengths from 2 to 28 carbon atoms, and the fatty acid chain length profoundly alters ceramide's biophysical properties. Short-chain ceramides with fatty acyl chains of fewer than 12 carbons can be easily dispersed in water and serve as detergents [5]. In contrast, most ceramides found in mammalian cellular membranes contain long fatty acyl chains of 16-28 carbon atoms rendering them hydro-phobic lipids lacking detergent properties.
As a result, ceramide metabolism is restricted to cellular membranes and is highly compartmentalized. Hydrophobic ceramides are generated by membrane-associated enzymes, and exert their effects either in close proximity to the generation site or require specific transport mechanisms to reach their targets in another intracellular compartment [6]. Long-chain ceramides appear to be able to flip-flop across the membrane [7]; however, spontaneous inter-bilayer transfer is extremely slow [8]. Therefore, the transfer of ceramide between intracellular compartments is facilitated by vesicular transport pathways [9]. Alternatively, ceramide is transported by a non-vesicular pathway involving a transfer protein, CERT, from its generation site in the endoplasmic reticulum (ER) to the Golgi where it is required for SM synthesis [10]. In addition to de novo biosynthesis, ceramide is generated by sphingomyelinases (SMases) from SM in two major pathways: the neutral SMase (nSMase)-dependent pathway and aSMase (aSMase)-dependent pathway or salvage (recycling) pathway (Figure 1).
De novo ceramide biosynthesis
De novo ceramide biosynthesis occurs in the endoplasmic reticulum (ER) where all the participating enzymes have been found [11-13]. Ceramide is synthesized at the cytosolic side of the ER [13, 14], serving as a precursor for the biosynthesis of glycosphingolipids and SM in the Golgi [15, 16].
(Dihydro) ceramide synthase (EC 2.3.1.24) is a key enzyme in de novo ceramide synthesis, and it utilizes fatty acid acyl CoA for N-acylation of sphinganine (dihydrosphingosine) yielding dihydroceramide that is converted to ceramide by desaturase (Figure 1). Each of the 6 known mammalian ceramide synthases (CerS/LASS) appears to regulate synthesis of a specific subset of ceramides, and displays a unique substrate specificity profile for chain-length and/or saturation in fatty acid acyl CoA. Over-expression of any CerS protein in mammalian cells resulted in increased levels of a specific subset of ceramide species. It has been demonstrated that CerS1 exhibits high specificity for C18:0-CoA generating C18:0-ceramide [17]. CerS2, CerS4, and CerS3 appear to have broader specificity [18, 19]. CerS2 or CerS4 mainly synthesizes C20:0-, C22:0-, C24:1-, C24:0-, C26:1 and C26:0 ceramide, but is unable to synthesize C16:0- or C18:0-ceramide [17, 19]. CerS3 generates C18:0-, C20:0-, C22:0- and C24:0-ceramide [18]. It has been shown that CerS5 generates C14:0-, C16:0-, C18:0-,and C18:1-ceramide [17, 20]; and CerS6 produces C14:0-, C16:0-, and C18:0-ceramide [17].
The availability of certain fatty acyl-CoA species and the characteristic distribution pattern of CerS family members in tissues seem to regulate the tissue-specificity of the ceramide species. Northern blot and real-time RT-PCR analysis revealed broad expression of CerS5, CerS4, and CerS6 genes in mammalian tissues, but CerS1 expression was limited to the brain and skeletal muscle [18, 19]. Interestingly, CerS2 mRNA was more abundant than other CerS family members and had the broadest tissue distribution [19]. Except for a weak display in skin, CerS3 mRNA expression is limited almost solely to testis, implying that CerS3 plays an important role in this gland [18].
CerS are integral membrane proteins, but the exact number of transmembrane domains and their topology has not been resolved experimentally. All of the CerS genes have a highly conserved stretch of 52 amino acids known as the Lag1p motif which is essential for enzyme activity [21]. Some of the CerS proteins are post-translationally modified, and, for instance, CerS6 is expressed as a native and an Nglycosylated form. The N-glycosylation site is conserved in CerS6, CerS2, and CerS5, but this post-translational modification is not required for ceramide synthase activity [17]. Intriguingly, CerS1 phosphorylation appears to regulate the protein turnover [22]. All CerS except CerS1 contain a homeobox domain, suggesting involvement in developmental regulation [23]. De novo synthesis of ceramide is required for cell survival in vivo, and is widespread among cell types and tissues. Regulation of ceramide synthesis is only beginning to be understood. Regulation at the transcriptional level has been observed with a number of agents, including endotoxin and cytokines, UVB irradiation, and retinoic acid [14].
Sphingomyelin hydrolysis
SM hydrolysis by one of several SMases is another source of cellular ceramide. Three groups of SMases, acid, neutral, and alkaline, are distinguished by their primary structure, catalytic pH optimum, and localization.
A well-characterized enzyme, acid SMase (aSMase) contributes to the catabolism of SM and ceramide formation in lysosomes [24, 25]. aSMase could relocate from intracellular compartments to the plasma membrane where it plays an important role in SM hydrolysis and ceramide generation within lipid rafts [26]. aSMase is a soluble enzyme with no transmembrane domains, and the mechanism of aSMase association with the membrane, at which its substrate, SM, resides, remains unclear. aSMase is also secreted through the Golgi secre-tory pathway, and it is constitutively present in plasma [27] where it is involved in hydrolysis of lipoprotein-bound SM, the second most abundant lipid in human plasma.
Three mammalian closely related isoforms of neutral SMase (nSMase) have been recently cloned, including nSMase1, nSMase2 and mitochondria-associated nSMase (MA-nSMase) [28]. nSMase1 is localized to the ER and nucleus [29]. nSMase2 had a dynamic intracellular localization [30], having been found in the Golgi of sub-confluent cells, at the plasma membrane at the regions of cell-cell contact [31] and in recycling compartments [32]. MA-nSMase is found within the mitochondria and associated membranes [33]. The physiological role of neutral SMase isoforms may be dictated by their immediate environment in the specific intracellular compartment. Alkaline SMase lacks homology to neutral or aSMase and its mRNA is abundant in the intestine where the enzyme plays a major role in digestion of dietary SM [34].
Recycling or salvage pathway
Ceramide is also produced during the recycling of sphingosine in the process termed the “salvage pathway” [35]. In this process, complex sphingolipids are broken down to ceramide and then to sphingosine, which is then used by ceramide synthase to yield ceramide. SM is converted to ceramide by aSMase. Ceramide accumulation via the salvage pathway requires ceramide synthase which is important in de novo synthesis of ceramide. Complex sphingolipids undergo constitutive degradation in the late endosomes and the lysosomes yielding ceramide which does not leave the lysosomes [36] unless converted into sphingosine by acid ceramidase. Free sphingosine could be released from the lysosomes and re-acylated by ceramide synthase to form ceramide.
Ceramide generation in mitochondria
Mitochondria arise as important intracellular compartment for ceramide metabolism, and they have been shown to contain a variety of sphingolipids, including SM and ceramide [37,38]. Mounting evidence suggests a local action of ceramide on mitochondria in intact cells. Thus, selective hydrolysis of a mitochondrial pool of SM by overexpressed sphingomyelinase (bSMase) targeted to mitochondria resulted in apoptosis. In contrast, generation of ceramide in the plasma membrane, ER, or Golgi apparatus by bSMase targeted to these compartments had no effect on cell viability [39]. Recently, mitochondrial ceramide engagement in apoptosis has been shown using loss-of-function mutants of ceramide synthase in the germ cell line of C. elegans [40]. In this study, an ionizing radiation-induced apoptosis of germ cells was obliterated upon inactivation of ceramide synthase, and restored upon microinjection of long-chain ceramide. Radiation-induced increase in ceramide localized to mitochondria was required for activation of CED-3 caspase and apoptosis. Many studies underscore the physiological significance of the mitochondrial ceramide and SM pools [37, 38, 41-44].
Although several enzyme activities involved in ceramide metabolism have been demonstrated in mitochondria, the nature of enzymes generating ceramide in this organelle is still a matter of debate [19]. Mitochondria evolve as a specialized compartment of sphingolipid metabolism with their own subset of ceramide synthesizing and degrading enzymes. Three possibilities may account for ceramide generation in mitochondria.
First, experimental evidence suggests the presence of ceramide synthase activity in mitochondria. Thus, ceramide synthase activity was first detected [45, 46] and partially purified from a bovine brain mitochondria-enriched fraction [47]. Mitochondrial enzymes had higher specific ceramide synthase activity with C16:0- or C18:0-acyl CoA than the ceramide synthase from the ER [47]. Purification of ceramide synthase from bovine liver mitochondria yielded two major protein bands, 62 and 72 kDa on a gel [48]. Detailed analysis of ceramide synthase activity in highly purified mitochondria by Bionda et al. essentially confirmed previous findings [49]. Ceramide synthase activity was demonstrated in rat liver mitochondria and in the sub-compartment of the ER closely associated with mitochondria. Further sub-mitochondrial investigation of ceramide synthase activity revealed that both outer and inner mitochondrial membranes can synthesize ceramide [49].
Recent reports describing several ceramide synthase isoforms, including CerS1, CerS2, CerS4 and CerS6, in purified mouse brain mitochondria [50, 51] support the notion that several ceramide synthesizing enzymes could be localized in mitochondria [52]. No such association was found in HeLa cells [53], suggesting that this might be a cell type/tissue specific event. The intra-mitochondrial localization of CerS was examined in purified brain mitochondria by immunoprecipitation [51]. These studies reveal a selective CerS6 association with adenine nucleotide translocase, the inner membrane component of the mitochondrial permeability transition pore (MPTP). In contrast, CerS2 associated with the outer membrane resident protein Tom20, a receptor of the protein import complex. The data suggest CerS6/ceramide could regulate MPTP activity and mitochondrial Ca2+ homeostasis whereas CerS2/ceramide could modulate the mitochondrial protein import machinery.
Secondly, recent studies identified two novel SMases, which hydrolyze SM to ceramide, and phosphocholine in mitochondria from zebrafish [42] and mouse tissues [33]. Notably, in yeast, the mammalian nSMase ortholog Isc1p associates with mitochondria in the post-diauxic phase of yeast growth and regulates mitochondrial sphingolipid metabolism [54, 55].
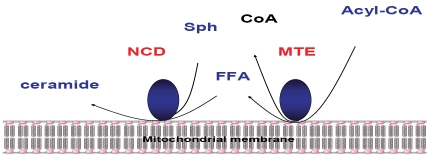
Thirdly, the additional source of ceramide in mitochondria is a reverse reaction of a neutral ceramidase (nCDase), e.g., formation of ceramide as a result of condensation of palmitate and sphingosine [56]. On the basis of molecular cloning and confocal microscopy data, this activity was ascribed to mitochondria [57], and it was demonstrated in purified mitochondria [49]. Recent studies describe the molecular mechanism of ceramide generation from palmi-tate and sphingosine in purified liver mitochondria that requires concerted action of two enzymes nCDase and thioesterase (which hydro-lyzes palmitoyl-CoA to CoA and fatty acid) [58]. Thus, mitochondria from nCDase-deficient mice have significantly decreased formation of ceramide from sphingosine and palmitoyl-CoA (or palmitate) compared to mitochondria from wild type mice, indicating that nCDase participates in ceramide formation in liver mitochondria, and that ceramide formation may occur from sphingosine and palmitoyl-CoA from coupled activities of a mitochondrial thioesterase and nCDase catalyzing the reverse reaction (Figure 2). Another possibility is that ceramide could be also transported from the ER to mitochondria through the contact sites between them [59].
Figure 2.
Ceramide formation from Acyl-CoA and sphingosine (Sph) mediated by coupled activities of mitochondrial thioesterase (MTE) and nCDase (NCD).
Ceramide accumulation in cerebral ischemia/reperfusion (IR)
Ceramide accumulation has been demonstrated in various in vivo models of IR and it has been implicated as an important mediator of apoptosis in the injured tissue, but mechanisms of ceramide generation are not well-defined and the downstream targets of ceramide remain unresolved. The IR-induced accumulation of ceramide appears to be a general phenomenon for heart, kidney, liver and brain.
Ischemic stroke occurs when cerebral or pre-cerebral arteries are occluded or significantly stenosed by emboli or by local atherosclerotic disease. Within minutes of interrupted blood flow, mitochondrial energy production is shut down due to lack of oxygen resulting in membrane depolarization and excessive release of neurotransmitters, specifically, glutamate. Extracellular glutamate accumulation over-stimulates glutamate receptors, promoting cytosolic Ca2+ overload, the generation of ROS, and mitochondrial dysfunction leading to cell death.
A few studies reported ceramide accumulation during cerebral ischemia and IR [60, 61], and it appears that the mechanism of ceramide accumulation depends on the severity of the insult to the brain. Thus, severe and lethal cerebral IR resulted in ceramide accumulation via activation of aSMase and SM hydrolysis [60-62] or inhibition of ceramide utilization by glucosylceramide synthase [63]. Consistent with these data, the extent of brain tissue damage was decreased in mice lacking aSMase [64]. In a recent study, severe cerebral IR induced SM hydrolysis and increased ceramide and sphingosine in the ischemic brain [65]. Similarly, chronic cerebral ischemia caused ceramide accumulation due to activation of SM degradation accompanied by reduced ceramide utilization via glucosylceramide synthase [66]. The data highlight IR-induced deregulation of complex sphingolipids metabolism.
In mild IR, ceramide accumulation resulted from de novo ceramide biosynthesis rather from hydrolysis of SM [50]. There is apparent tissue specificity in the expression of individual ceramide species that might reflect the tissue specificity of the ceramide synthases. In brain, C18:0-, C18:1- and C24:1-ceramide are the major species expressed (39.5%, 34%, and 12.5% of total ceramide, respectively) whereas C16:0- ceramide contributes only 4% of total ceramide. All ceramide species were elevated in the ischemic brain about 1.5-2-fold. The enhanced accumulation of sphingolipids seems to occur during the reperfusion phase; there were no changes in sphingolipid content after ischemia without reperfusion. This finding is in line with data which show that both ischemia and the restoration of blood flow to ischemic tissue (reperfusion) causes cellular damage by different molecular mechanisms [67, 68].
Investigation of intracellular sites of ceramide accumulation after mild IR revealed the elevation of ceramide species both in purified mitochondria and in the ER [50]. In mitochondria, only C18:0-, C18:1- and C16:0-ceramides were increased, but all ceramide species increased in the ER suggesting activation of different ceramide synthases in these intracellular compartments. Indeed, several ceramide synthases were identified in mitochondria and the ER, including CerS1, CerS2, and CerS6, but CerS5 was localized only in the ER in the brain. Activity measurements indicated activation of CerS6 in ischemic mitochondria apparently via post-translational mechanisms; IR did not affect the CerS6 protein expression [50].
It appears that CerS6 is developmentally regulated and primarily generates C16:0-ceramide in brain mitochondria [51]. An investigation into the role of CerS6 in mitochondria revealed that ceramide synthase down-regulation during brain development is associated with dramatically decreased mitochondrial Ca2+-loading capacity (CLC) which could be rescued by addition of ceramide. Ceramide-mediated blockade of MPTP opening seems to be the underlying mechanism of the increased CLC in brain mitochondria isolated from young animals. In fact, mitochondria maintain low cytosolic Ca2+ levels by sequestering Ca2+ inside the mitochondrial matrix complexed with phosphate. Energized mitochondria take up Ca2+ via the mitochondrial calcium uniporter which has been recently described as a highly selective, inwardly rectifying channel [69]. Excessive accumulation of Ca2+ in the mitochondrial matrix could trigger opening of MPTP at a high conductance state, which would be accompanied by dissipation of the transmembrane potential and mitochondrial swelling. In brain mitochondria, Ca2+ may also activate a limited permeability state of MPTP opening [70] that only depolarizes mitochondria without causing swelling [71]. This depolarization dramatically reduces the driving force for Ca2+ influx via mitochondrial Ca2+ uniporter, thus limiting the mitochondrial ability to sequester Ca2+ [72]. The lower CerS6 expression and C16:0-ceramide content were associated with reduced mitochondrial CLC in adult brain mitochondria, whereas exogenous C16:0-ceramide restored CLC to that of young brain mitochondria. This is in line with the finding that long-chain ceramides, including C16:0-ceramide, are potent inhibitors of MPTP activity [73]. This suggests that CerS6-generated ceramide could prevent MPTP opening, leading to increased Ca2+ accumulation in the mitochondrial matrix.
The role of CerS6 in cell survival was examined in primary oligodendrocyte (OL) precursor cells, which undergo apoptotic cell death during early postnatal brain development or following cerebral IR. Exposure of OLs to glutamate resulted in apoptosis that was prevented by inhibitors of de novo ceramide biosynthesis, myriocin and fumonisin B1. Knockdown of CerS6 with siRNA reduced glutamate-triggered OL apoptosis, whereas knockdown of CerS5 had no effect. Importantly, blocking mitochondrial Ca2+ uptake or decreasing Ca2+-dependent protease calpain activity with specific inhibitors prevented OL apoptosis. Finally, knocking down CerS6 decreased calpain activation. The data suggest a novel role for CerS6 in the regulation of both mitochondrial Ca2+ homeostasis and calpain, which could be important in cell death after cerebral IR (Figure 3). These studies illuminate a novel determinant in cerebral IR, mitochondrial ceramide synthase CerS6 which could be an important future target for neuroprotection. In vitro, de novo synthesized ceramide increased after brief exposure of cultured brain cells to hypoxia, oxygen/glucose deprivation, or TNF [74, 75]. In neuronal precursor cells, hypoxia/reoxygenation triggered a robust elevation in C14:0- and C16:0-ceramides, and a small increase in C18:0-, C18:1- and C20:0-ceramides, and no increase in C24:0- and C24:1-ceramides [76]. The elevations in ceramides were primarily due to the actions of aSMase and ceramide synthase CerS5, demonstrating the involvement of the salvage pathway. Interestingly, C2-ceramide infusion protected the brain against IR injury [77, 78]. However, this effect could be also attributed to the intracellular/extracellular conversion of ceramide into sphingosine-1-phosphate, which is known to protect cells from apoptosis [24, 79, 80].
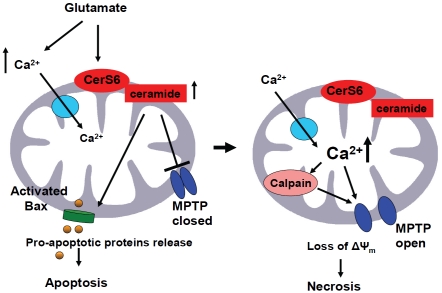
Figure 3.
Hypothetical role of CerS6/ceramide in mitochondria after cerebral IR. IR triggers glutamate-induced cytosolic Ca2+ influx into the mitochondria and an activation of mitochondrial CerS6 that elevates ceramide. Ceramide blocks the MPTP opening at a low conductance state, leading to increased Ca2+ in the mitochondrial matrix. This MPTP inactivation would allow mitochondria to support adequate ATP production for formation of the apoptosome, and might be responsible for the initial raise in ATP production (and hence Δψ) during apoptosis [135], an observation that corresponds well with the reported transient mitochondrial hyper-polarization in the apoptosis induced by IL-3 withdrawal [136]. Rising mitochondrial Ca2+ activates calpain 10, which could cleave protein components of the MPTP [137] resulting in the MPTP opening at a high conductance state, swelling, and rupture of the outer mitochondrial membrane leading to necrotic cell death.
Ceramide and mitochondrial injury in stroke
Although IR-induced mitochondrial injury has been extensively studied and mitochondrial functions affected by IR are characterized [81], crucial information is needed regarding the cause of mitochondrial dysfunction. Our studies suggest that exogenously added ceramide could provoke mitochondrial dysfunctions similar to that occurring in cerebral IR [82]. Of note, some data on mitochondrial effects of ceramide have been obtained using synthetic short-chain analogs, which may not fully mimic the properties of naturally occurring long-chain ceramides.
Respiratory chain
The restriction on mitochondrial respiratory chain function has been shown in various rodent models of stroke [81]. An impairment of Complex III has been implicated (Figure 4), but the mechanisms remain unresolved, and a Complex I defect has not been ruled out. We and others have reported that short-chain ceramide could directly suppress respiratory chain Complex III activity [83, 84]. Also, ceramide seems to participate in displacement of cyto-chrome c from its binding site on Complex III [85] corresponds to an apparent mitochondrial Complex III defect in IR.
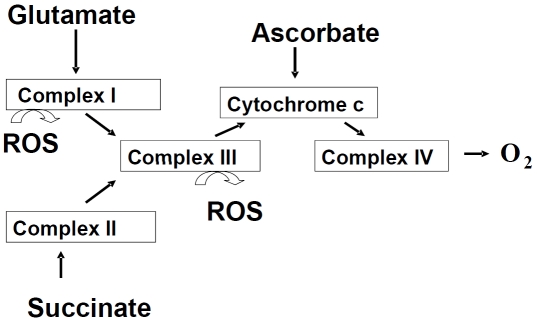
Figure 4.
Mitochondrial respiratory chain complexes. Mitochondrial respiratory chain consists of four multi-protein complexes Complex I-IV. The respiratory chain function is determined using substrates such as glutamate, succinate or ascorbate, which are oxidized via different complexes of respiratory chain. An inhibition of the electron transport through the Complex I or III could result in generation of ROS.
Reactive oxygen species (ROS)
Free radical formation occurs during cerebral IR. In fact, mitochondria are the major site of production of ROS, and are the likely source for the generation of peroxynitrite, formed from nitric oxide and superoxide during IR [86]. Studies in isolated mitochondria indicated that Complex I and III are potential sites of superoxide formation [87]. Complex III deficiency observed in cerebral IR strongly implicates Complex III as the major and relevant site of ROS generation; however, Complex I remains to be ruled out. Short-chain ceramide could increase the generation of ROS in isolated mitochondria [88].
Mitochondrial permeability transition pore (MPTP)
Excessive accumulation of Ca2+ in mitochondrial matrix could trigger opening of the MPTP at a high conductance state that is accompanied with dissipation of transmembrane potential and swelling of mitochondria. In brain mitochondria, Ca2+ may also activate a limited permeability state of MPTP opening that only depolarizes mitochondria without swelling [70]. This depolarization dramatically reduces the driving force for Ca2+ influx via the uniporter channel in the inner membrane, thus limiting the mitochondrial ability to sequester Ca2+.
MPTP opening at a high conductance state appears to be a crucial event leading to cell death by necrosis [89-91]. Severe insult causes widespread opening of the MPTP in mitochondria. Cell death proceeds through necrosis when the MPTP remains open, causing the inhibition of ATP production. If the initial insult is not too severe and MPTP does not open, cellular ATP can be maintained to support the energy demand of apoptosis. This provides an explanation for the coexistence of apoptotic and necrotic cell death in IR-injured tissue. It has been emphasized that MPTP regulates necrotic, but not apoptotic cell death in cardiac and cerebral IR [92, 93]. Mice deficient in cyclophilin D (CyD), the main regulatory component of MPTP, developed up to 37% smaller brain infarcts after IR. CyD-deficient cells died normally in response to various apoptotic stimuli, but were resistant to necrotic cell death induced by ROS and Ca2+ overload. MPTP opening at a high conductance state has been proposed to occur in cerebral IR, but the evidence is largely indirect [81, 94]. The opening of the MPTP at a high conductance state has been implicated as another mechanism of cyto-chrome c release from mitochondria due to short-chain ceramide [95-98]. However, it has been demonstrated that long-chain ceramide is a potent inhibitor of MPTP [73].
Release of pro-apoptotic proteins from mitochondria
The release of mitochondrial cytochrome c and/ or Smac, which antagonizes apoptotic protein inhibitor, into the cytosol initiates the activation of caspase-9 leading to the proteolytic activation of executioner caspase-3 and -7. Cyto-chrome c release from mitochondria is well documented in different models of ischemia [91]. Recent studies have showed caspase-independent apoptosis involving the release of mitochondrial proteins, apoptosis-inducing factor (AIF), and endonuclease G (EndoG) and their translocation to the nucleus in brain IR [91, 99]. Emerging evidence indicates an important role of Bax in cytochrome c and Smac (but not AIF and EndoG) release from brain mitochondria [100]. Importantly, mitochondrial calpain has been implicated in AIF release from mitochondria [101]. The release of mitochondrial proteins implies that the outer and /or the inner mitochondrial membrane is compromised in IR, but the precise mechanisms of the protein release remain unclear. Short-chain ceramide accelerated the release of cytochrome c and AIF from heart mitochondria [102], and natural C16-ceramide has been shown to form large channels in the outer mitochondrial membrane permeable to cytochrome c [103].
Moreover, current research is focused on the ability of ceramides to release cytochrome c or other pro-apoptotic proteins from the mitochondrial inter-membrane space. Within the model of Colombini and co-workers, pro-apoptotic protein release is due to formation of large pores in the outer mitochondrial membrane by ceramide itself, whereas the inner membrane is viewed as being ceramide-insensitive. This model is supported by extensive experimental material using isolated mitochondria [102, 104-106] and artificial membranes (liposomes and black lipid membranes) [104, 107]. Importantly, it was recently shown that anti-apoptotic Bcl-2 can disassemble ceramide channels in the outer mitochondrial membrane and black lipid membranes [108], thus providing the mechanistic explanation for the original observation of Ghafourifar et al. [106] that Bcl-2 suppresses ceramide-induced cytochrome c release from isolated mitochondria. However, the formation of ceramide channels seems to be highly dependent on the conditions employed, and has been questioned in a number of publications [95, 98, 109, 110]. Besides, a few reports suggest that the permeabilization of the inner mitochondrial membrane via the opening of the MPTP could be a primary event in initiation of cytochrome c release in the presence of ceramides [95, 111]. The switch between selective permeabilization of the outer membrane vs. permeabilization of the inner membrane in the presence of ceramide appears to depend on the composition of incubation medium and the nature of ceramide employed [102].
Emerging evidence suggests involvement of ceramides in reorganization of the mitochondrial network. Both exogenous and endogenously generated ceramides induce mitochondrial fission [112, 113], which may contribute to apoptotic cell death [114]. What particular effectors of mitochondrial fission (DRP1, Fis1, or Bax) or fusion (OPA-1, Mitofusins) machineries are the targets of ceramide in this process remains to be determined. However, in cardiomyocytes exposed to exogenous C2-ceramide, an increased expression of mitochondrial resident Fis1 and enhanced recruitment of cytosolic DRP1 to mitochondrial fission foci may contribute to disintegration of the mitochondrial network [113].
Ceramide and mitochondria in cell death
Irrespective of the type of IR, IR-related physiological events have a common final consequence: alteration of mitochondrial function and release of mitochondrial proteins, leading to cell death. Cells with hallmarks of necrosis or apoptosis have been detected in animal models of IR [115]. The mitochondrial changes appear to be one essential step in tissue damage in IR, and treatments that ameliorate tissue infarction were associated with better recovery of mitochondrial function [116]. Multiple studies show intimate connections between ceramide signaling and functioning of mitochondria [117, 118], which play central role in integration of cellular signals to determine the outcome among apoptosis, necrosis, or proliferation [119-121].
Several lines of evidence have implicated changes in mitochondrial function as an intermediate step in transduction of ceramide signals that culminate in apoptotic or necrotic cell death [80, 118]. First, ceramide-induced apoptosis is accompanied by release of pro-apoptotic proteins from mitochondria [39, 122, 123], increased generation of mitochondrial ROS [124], and discharge of mitochondrial transmembrane potential, Δψ [122, 125-127]. Second, interventions that specifically prevent mitochondrial dysfunction suppress ceramide-induced apoptosis: inhibitors of the MPTP bongkrekic acid [125, 128] and cyclosporin A [128-130]; and over-expression of Bcl-2 [125, 127, 131-133]. Third, TNF-α-, ischemia/reperfusion-, etoposide-, or UV-induced apoptosis is associated with simultaneous increase in mitochondrial ceramide [41, 50, 88, 134].
Conclusion
Mitochondria are being appreciated as vital intracellular compartments for sphingolipid metabolism. Emerging data suggest that the sub-cellular location of ceramide generation plays a fundamental role in dictating its downstream targets and cell responses to stress stimuli. A cardinal feature of brain tissue injury in stroke is mitochondrial dysfunction and the release of mitochondrial proteins leading to cell death. It has become increasingly clear that ceramide, a membrane sphingolipid and a key mediator of cell-stress responses, could play a critical role in cerebral IR - induced mitochondrial injury. Continued research efforts are required to better understand the pathophysiological mechanisms of cerebral IR injury, to identify and test new protective agents. Further studies of the molecular basis of the role of ceramide in the ischemic brain are warranted. Because many assumptions regarding ceramide functions in IR -induced tissue injury were based on in vitro studies employing artificial ceramides, we must critically evaluate the mitochondrial dysfunctions in IR-injured brain and define a possible role of long-chain ceramides as causes of the mitochondrial impairment. This will allow the discovery of novel and groundbreaking therapeutic approaches to mitigate diseases that may result from elevations in ceramide and its metabolites.
Acknowledgements
We thank Dr. Jennifer G Schnellmann for help with preparation of the manuscript. This work is supported by the NIH/NCCR P20 RR 17677, VA Merit Awards from RRD and BMLRD (TIG).
References
- [1].Hannun YA, Obeid LM. Many Ceramides. J Biol Chem. 2011;286:27855–62. doi: 10.1074/jbc.R111.254359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hannun YA, Obeid LM. The Ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem. 2002;277:25847–50. doi: 10.1074/jbc.R200008200. [DOI] [PubMed] [Google Scholar]
- [3].Ogretmen B, Hannun YA, Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–16. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- [4].Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–50. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- [5].Sot J, Goni FM, Alonso A. Molecular associations and surface-active properties of short-and long-N-acyl chain ceramides. Biochim Biophys Acta. 2005;1711:12–9. doi: 10.1016/j.bbamem.2005.02.014. [DOI] [PubMed] [Google Scholar]
- [6].Futerman AH, Riezman H. The ins and outs of sphingolipid synthesis. Trends Cell Biol. 2005;15:312–8. doi: 10.1016/j.tcb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- [7].López-Montero I, Rodriguez N, Cribier S, Pohl A, Vélez M, Devaux PF. Rapid transbilayer movement of ceramides in phospholipid vesicles and in human erythrocytes. J Biol Chem. 2005;280:25811–9. doi: 10.1074/jbc.M412052200. [DOI] [PubMed] [Google Scholar]
- [8].Contreras FX, Sánchez-Magraner L, Alonso A, Goñi FM. Transbilayer (flip-flop) lipid motion and lipid scrambling in membranes. FEBS Lett. 2010;584:1779–86. doi: 10.1016/j.febslet.2009.12.049. [DOI] [PubMed] [Google Scholar]
- [9].Perry RJ, Ridgway ND. Molecular mechanisms and regulation of ceramide transport. Biochim Biophys Acta. 2005;1734:220–34. doi: 10.1016/j.bbalip.2005.04.001. [DOI] [PubMed] [Google Scholar]
- [10].Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, Fukasawa M, Nishijima M. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 2003;426:803–9. doi: 10.1038/nature02188. [DOI] [PubMed] [Google Scholar]
- [11].Michel C, van Echten-Deckert G. Conversion of dihydroceramide to ceramide occurs at the cytosolic face of the endoplasmic reticulum. FEBS Lett. 1997;416:153–5. doi: 10.1016/s0014-5793(97)01187-3. [DOI] [PubMed] [Google Scholar]
- [12].Hirschberg K, Rodger J, Futerman AH. The long-chain sphingoid base of sphingolipids is acylated at the cytosolic surface of the endoplasmic reticulum in rat liver. Biochem J. 1993;290:751–7. doi: 10.1042/bj2900751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mandon EC, Ehses I, Rother J, van Echten G, Sandhoff K. Subcellular localization and membrane topology of serine palmitoyltransferase, 3-dehydrosphinganine reductase. and sphinganine N-acyltransferase in mouse liver. J Biol Chem. 1992;267:11144–8. [PubMed] [Google Scholar]
- [14].Merrill AH., Jr De novo sphingolipid biosynthesis: a necessary, but dangerous. pathway. J Biol Chem. 2002;277:25843–6. doi: 10.1074/jbc.R200009200. [DOI] [PubMed] [Google Scholar]
- [15].Kolter T, Proia RL, Sandhoff K. Combinatorial ganglioside biosynthesis. J Biol Chem. 2002;277:25859–62. doi: 10.1074/jbc.R200001200. [DOI] [PubMed] [Google Scholar]
- [16].Futerman AH, Stieger B, Hubbard AL, Pagano RE. Sphingomyelin synthesis in rat liver occurs predominantly at the cis and medial cisternae of the Golgi apparatus. J Biol Che, 1990;265:8650–7. [PubMed] [Google Scholar]
- [17].Mizutani Y, Kihara A, Igarashi Y. Mammalian Lass6 and its related family members regulate synthesis of specific ceramides. Biochem J. 2005;390:263–71. doi: 10.1042/BJ20050291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mizutani Y, Kihara A, Igarashi Y. LASS3 (longevity assurance homologue 3) is a mainly testis-specific (dihydro)ceramide synthase with relatively broad substrate specificity. Biochem J. 2006;398:531–8. doi: 10.1042/BJ20060379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Laviad EL, Albee L, Pankova-Kholmyansky I, Epstein S, Park H, Merrill AH, Jr, Futerman AH. Characterization of ceramide synthase 2: tissue distribution, substrate specificity. and inhibition by sphingosine 1-phosphate. J Biol Chem. 2008;283:5677–84. doi: 10.1074/jbc.M707386200. [DOI] [PubMed] [Google Scholar]
- [20].Lahiri S, Futerman AH. LASS5 is a bona fide dihydroceramide synthase that selectively utilizes palmitoyl-CoA as acyl donor. J Biol Chem. 2005;280:33735–8. doi: 10.1074/jbc.M506485200. [DOI] [PubMed] [Google Scholar]
- [21].Spassieva S, Seo JG, Jiang JC, Bielawski J, Alvarez-Vasquez F, Jazwinski SM, Hannun YA, Obeid LM. Necessary role for the Lag1p motif in (dihydro)ceramide synthase activity. J Biol Chem. 2006;281:33931–8. doi: 10.1074/jbc.M608092200. [DOI] [PubMed] [Google Scholar]
- [22].Sridevi P, Alexander H, Laviad EL, Pewzner-Jung Y, Hannink M, Futerman AH, Alexander S. Ceramide synthase 1 is regulated by proteasomal mediated turnover. Biochim Biophys Acta. 2009;1793:1218–27. doi: 10.1016/j.bbamcr.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Venkataraman K, Futerman AH. Do longevity assurance genes containing Hox domains regulate cell development via ceramide synthesis? FEBS Lett. 2002;528:3–4. doi: 10.1016/s0014-5793(02)03248-9. [DOI] [PubMed] [Google Scholar]
- [24].Tani M, Ito M, Igarashi Y. Ceramide/sphingosine/sphingosine 1-phosphate metabolism on the cell surface and in the extracellular space. Cell Signal. 2007;19:229–37. doi: 10.1016/j.cellsig.2006.07.001. [DOI] [PubMed] [Google Scholar]
- [25].Tani M, Igarashi Y, Ito M. Involvement of neutral ceramidase in ceramide metabolism at the plasma membrane and in extracellular milieu. J Biol Chem. 2005;280:36592–600. doi: 10.1074/jbc.M506827200. [DOI] [PubMed] [Google Scholar]
- [26].Bollinger CR, Teichgraber V, Gulbins E. Ceramide-enriched membrane domains. Biochim Biophys Acta. 2005;1746:284–94. doi: 10.1016/j.bbamcr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- [27].Spence MW, Byers DM, Palmer FB, Cook HW. A new Zn2+-stimulated sphingomyelinase in fetal bovine serum. J Biol Chem. 1989;264:5358–63. [PubMed] [Google Scholar]
- [28].Clarke CJ, Wu BX, Hannun YA. The neutral sphingomyelinase family: identifying biochemical connections. Adv Enzyme Regul. 2011;51:51–8. doi: 10.1016/j.advenzreg.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tomiuk S, Zumbansen M, Stoffel W. Characterization and subcellular localization of murine and human magnesium-dependent neutral sphingomyelinase. J Biol Chem. 2000;275:5710–7. doi: 10.1074/jbc.275.8.5710. [DOI] [PubMed] [Google Scholar]
- [30].Clarke CJ, Snook CF, Tani M, Matmati N, Marchesini N, Hannun YA. The extended family of neutral sphingomyelinases. Biochemistry. 2006;45:11247–56. doi: 10.1021/bi061307z. [DOI] [PubMed] [Google Scholar]
- [31].Marchesini N, Osta W, Bielawski J, Luberto C, Obeid LM, Hannun YA. Role for mammalian neutral sphingomyelinase 2 in confluence-induced growth arrest of MCF7 cells. J Biol Chem. 2004;279:25101–11. doi: 10.1074/jbc.M313662200. [DOI] [PubMed] [Google Scholar]
- [32].Milhas D, Clarke CJ, Hannun YA. Sphingomyelin metabolism at the plasma membrane: implications for bioactive sphingolipids. FEBS Lett. 2010;584:1887–94. doi: 10.1016/j.febslet.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wu BX, Rajagopalan V, Roddy PL, Clarke CJ, Hannun YA. Identification and characterization of murine mitochondria-associated neutral sphingomyelinase (MA-nSMase) the mammalian sphingomyelin phosphodiesterase 5. J Biol Chem. 2010;285:17993–8002. doi: 10.1074/jbc.M110.102988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nilsson A, Duan RD. Absorption and lipoprotein transport of sphingomyelin. J Lipid Res. 2006;47:154–71. doi: 10.1194/jlr.M500357-JLR200. [DOI] [PubMed] [Google Scholar]
- [35].Kitatani K, Idkowiak-Baldys J, Hannun YA. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal. 2008;20:1010–8. doi: 10.1016/j.cellsig.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chatelut M, Leruth M, Harzer K, Dagan A, Marchesini S, Gatt S, Salvayre R, Courtoy P, Levade T. Natural ceramide is unable to escape the lysosome. in contrast to a fluorescent analogue. FEBS Lett. 1998;426:102–6. doi: 10.1016/s0014-5793(98)00325-1. [DOI] [PubMed] [Google Scholar]
- [37].Ardail D, Popa I, Alcantara K, Pons A, Zanetta JP, Louisot P, Thomas L, Portoukalian J. Occur-rence of ceramides and neutral glycolipids with unusual long-chain base composition in purified rat liver mitochondria. FEBS Lett. 2001;488:160–4. doi: 10.1016/s0014-5793(00)02332-2. [DOI] [PubMed] [Google Scholar]
- [38].Tserng KY, Griffin R. Quantitation and molecular species determination of diacylglycerols, phosphatidylcholines, ceramides. and sphingomyelins with gas chromatography. Anal Biochem. 2003;323:84–93. doi: 10.1016/j.ab.2003.08.026. [DOI] [PubMed] [Google Scholar]
- [39].Birbes H, El Bawab S, Hannun YA, Obeid LM. Selective hydrolysis of a mitochondrial pool of sphingomyelin induces apoptosis. Faseb J. 2001;15:2669–79. doi: 10.1096/fj.01-0539com. [DOI] [PubMed] [Google Scholar]
- [40].Deng X, Yin X, Allan R, Lu DD, Maurer CW, Haimovitz-Friedman A, Fuks Z, Shaham S, Kolesnick R. Ceramide biogenesis is required for radiation-induced apoptosis in the germ line of C. elegans. Science. 2008;322:110–5. doi: 10.1126/science.1158111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dai Q, Liu J, Chen J, Durrant D, McIntyre TM, Lee RM. Mitochondrial ceramide increases in UV-irradiated HeLa cells and is mainly derived from hydrolysis of sphingomyelin. Oncogene. 2004;23:3650–8. doi: 10.1038/sj.onc.1207430. [DOI] [PubMed] [Google Scholar]
- [42].Yabu T, Shimuzu A, Yamashita M. A novel mitochondrial sphingomyelinase in zebrafish cells. J Biol Chem. 2009;284:20349–63. doi: 10.1074/jbc.M109.004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Andreyev AY, Fahy E, Guan Z, Kelly S, Li X, McDonald JG, Milne S, Myers D, Park H, Ryan A, Thompson BM, Wang E, Zhao Y, Brown HA, Merrill AH, Raetz CR, Russell DW, Subramaniam S, Dennis EA. Subcellular organelle lipidomics in TLR-4-activated macrophages. J Lipid Res. 2010;51:2785–97. doi: 10.1194/jlr.M008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Monette JS, Gómez LA, Moreau RF, Bemer BA, Taylor AW, Hagen TM. Characteristics of the rat cardiac sphingolipid pool in two mitochondrial subpopulations. Biochem Biophys Res Commun. 2010;398:272–7. doi: 10.1016/j.bbrc.2010.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Morell P, Radin NS. Specificity in ceramide biosynthesis from long chain bases and various fatty acyl coenzyme A's by brain micro-somes. J Biol Chem. 1970;245:342–50. [PubMed] [Google Scholar]
- [46].Ullman MD, Radin NS. Enzymatic formation of hydroxy ceramides and comparison with enzymes forming nonhydroxy ceramides. Arch Biochem Biophys. 1972;152:767–77. doi: 10.1016/0003-9861(72)90272-x. [DOI] [PubMed] [Google Scholar]
- [47].Shimeno H, Soeda S, Yasukouchi M, Okamura N, Nagamatsu A. Fatty acyl-Co A: sphingosine acyltransferase in bovine brain mitochondria: its solubilization and reconstitution onto the membrane lipid liposomes. Biol Pharm Bull. 1995;18:1335–9. doi: 10.1248/bpb.18.1335. [DOI] [PubMed] [Google Scholar]
- [48].Shimeno H, Soeda S, Sakamoto M, Kouchi T, Kowakame T, Kihara T. Partial purification and characterization of sphingosine Nacyltransferase (ceramide synthase) from bovine liver mitochondrion-rich fraction. Lipids. 1998;33:601–5. doi: 10.1007/s11745-998-0246-2. [DOI] [PubMed] [Google Scholar]
- [49].Bionda C, Portoukalian J, Schmitt D, Rodriguez-Lafrasse C, Ardail D. Subcellular compartmentalization of ceramide metabolism: MAM (mitochondria-associated membrane) and/or mitochondria? Biochem J. 2004;382:527–33. doi: 10.1042/BJ20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yu J, Novgorodov SA, Chudakova D, Zhu H, Bielawska A, Bielawski J, Obeid LM, Kindy MS, Gudz TI. JNK3 signaling pathway activates ceramide synthase leading to mitochondrial dysfunction. J Biol Chem. 2007;282:25940–9. doi: 10.1074/jbc.M701812200. [DOI] [PubMed] [Google Scholar]
- [51].Novgorodov SA, Chudakova DA, Wheeler BW, Bielawski J, Kindy MS, Obeid LM, Gudz TI. Developmentally regulated ceramide synthase 6 increases mitochondrial Ca2+ loading capacity and promotes apoptosis. J Biol Chem. 2011;286:4644–58. doi: 10.1074/jbc.M110.164392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Futerman AH. Intracellular trafficking of sphingolipids: relationship to biosynthesis. Biochim Biophys Acta. 2006;1758:1885–92. doi: 10.1016/j.bbamem.2006.08.004. [DOI] [PubMed] [Google Scholar]
- [53].Mesicek J, Lee H, Feldman T, Jiang X, Skobeleva A, Berdyshev EV, Haimovitz-Friedman A, Fuks Z, Kolesnick R. Ceramide synthases 2, 5. and 6 confer distinct roles in radiation-induced apoptosis in HeLa cells. Cell Signal. 2010;22:1300–7. doi: 10.1016/j.cellsig.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Vaena de Avalos S, Okamoto Y, Hannun YA. Activation and localization of inositol phosphosphingolipid phospholipase C, Isc1p, to the mitochondria during growth of Saccharomyces cerevisiae. J Biol Chem. 2004;279:11537–45. doi: 10.1074/jbc.M309586200. [DOI] [PubMed] [Google Scholar]
- [55].Kitagaki H, Cowart LA, Matmati N, Vaena de Avalos S, Novgorodov SA, Zeidan YH, Bielawski J, Obeid LM, Hannun YA. Isc1 regulates sphingolipid metabolism in yeast mitochondria. Biochim Biophys Acta. 2007;1768:2849–61. doi: 10.1016/j.bbamem.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].El Bawab S, Birbes H, Roddy P, Szulc ZM, Bielawska A, Hannun YA. Biochemical characterization of the reverse activity of rat brain ceramidase. A CoA-independent and fumonisin B1-insensitive ceramide synthase. J Biol Chem. 2001;276:16758–66. doi: 10.1074/jbc.M009331200. [DOI] [PubMed] [Google Scholar]
- [57].El Bawab S, Roddy P, Qian T, Bielawska A, Lemasters JJ, Hannun YA. Molecular cloning and characterization of a human mitochondrial ceramidase. J Biol Chem. 2000;275:21508–13. doi: 10.1074/jbc.M002522200. [DOI] [PubMed] [Google Scholar]
- [58].Novgorodov SA, Wu BX, Gudz TI, Bielawski J, Ovchinnikova TV, Hannun YA, Obeid LM. Novel pathway of ceramide production in mitochondria: thioesterase and neutral ceramidase produce ceramide from sphingosine and acylCoA. J Biol Chem. 2011;286:25352–62. doi: 10.1074/jbc.M110.214866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Stiban J, Caputo L, Colombini M. Ceramide synthesis in the endoplasmic reticulum can permeabilize mitochondria to proapoptotic proteins. J Lipid Res. 2008;49:625–34. doi: 10.1194/jlr.M700480-JLR200. [DOI] [PubMed] [Google Scholar]
- [60].Nakane M, Kubota M, Nakagomi T, Tamura A, Hisaki H, Shimasaki H, Ueta N. Lethal fore-brain ischemia stimulates sphingomyelin hydrolysis and ceramide generation in the gerbil hippocampus. Neurosci Lett. 2000;296:89–92. doi: 10.1016/s0304-3940(00)01655-4. [DOI] [PubMed] [Google Scholar]
- [61].Kubota M, Kitahara S, Shimasaki H, Ueta N. Accumulation of ceramide in ischemic human brain of an acute case of cerebral occlusion. Jpn J Exp Med. 1989;59:59–64. [PubMed] [Google Scholar]
- [62].Kubota M, Narita K, Nakagomi T, Tamura A, Shimasaki H, Ueta N, Yoshida S. Sphingomyelin changes in rat cerebral cortex during focal ischemia. Neurol Res. 1996;18:337–41. doi: 10.1080/01616412.1996.11740432. [DOI] [PubMed] [Google Scholar]
- [63].Takahashi K, Ginis I, Nishioka R, Klimanis D, Barone FC, White RF, Chen Y, Hallenbeck JM. Glucosylceramide synthase activity and ceramide levels are modulated during cerebral ischemia after ischemic preconditioning. J Cereb Blood Flow Metab. 2004;24:623–7. doi: 10.1097/01.WCB.0000119990.06999.A9. [DOI] [PubMed] [Google Scholar]
- [64].Yu ZF, Nikolova-Karakashian M, Zhou D, Cheng G, Schuchman EH, Mattson MP. Pivotal role for acidic sphingomyelinase in cerebral ischemia-induced ceramide and cytokine production. and neuronal apoptosis. J Mol Neurosci. 2000;15:85–97. doi: 10.1385/JMN:15:2:85. [DOI] [PubMed] [Google Scholar]
- [65].Chudakova DA, Zeidan YH, Wheeler BW, Yu J, Novgorodov SA, Kindy MS, Hannun YA, Gudz TI. Integrin-associated Lyn kinase promotes cell survival by suppressing acid sphingomyelinase activity. J Biol Chem. 2008;283:28806–16. doi: 10.1074/jbc.M803301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ohtani R, Tomimoto H, Kondo T, Wakita H, Akiguchi I, Shibasaki H, Okazaki T. Upregulation of ceramide and its regulating mechanism in a rat model of chronic cerebral ischemia. Brain Res. 2004;1023:31–40. doi: 10.1016/j.brainres.2004.07.024. [DOI] [PubMed] [Google Scholar]
- [67].Chan PH. Future targets and cascades for neuroprotective strategies. Stroke. 2004;35:2748–50. doi: 10.1161/01.STR.0000143325.25610.ac. [DOI] [PubMed] [Google Scholar]
- [68].Gustafsson AB, Gottlieb RA. Eat your heart out: Role of autophagy in myocardial ischemia/reperfusion. Autophagy. 2008;4:416–21. doi: 10.4161/auto.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–4. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- [70].Novgorodov SA, Gudz TI. Permeability transition pore of the inner mitochondrial membrane can operate in two open states with different selectivities. J Bioenerg Biomembr. 1996;28:139–46. doi: 10.1007/BF02110644. [DOI] [PubMed] [Google Scholar]
- [71].Brustovetsky N, Dubinsky JM. Limitations of cyclosporin A inhibition of the permeability transition in CNS mitochondria. J Neurosci. 2000;20:8229–37. doi: 10.1523/JNEUROSCI.20-22-08229.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Brustovetsky N, Dubinsky JM. Dual responses of CNS mitochondria to elevated calcium. J Neurosci. 2000;20:103–13. doi: 10.1523/JNEUROSCI.20-01-00103.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Novgorodov SA, Gudz TI, Obeid LM. Long-chain ceramide is a potent inhibitor of the mitochondrial permeability transition pore. J Biol Chem. 2008;283:24707–17. doi: 10.1074/jbc.M801810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ginis I, Schweizer U, Brenner M, Liu J, Azzam N, Spatz M, Hallenbeck JM. TNF-alpha pre-treatment prevents subsequent activation of cultured brain cells with TNF-alpha and hypoxia via ceramide. Am J Physiol. 1999;276:C1171–83. doi: 10.1152/ajpcell.1999.276.5.C1171. [DOI] [PubMed] [Google Scholar]
- [75].Liu J, Ginis I, Spatz M, Hallenbeck JM. Hypoxic preconditioning protects cultured neurons against hypoxic stress via TNF-alpha and ceramide. Am J Physiol Cell Physiol. 2000;278:C144–53. doi: 10.1152/ajpcell.2000.278.1.C144. [DOI] [PubMed] [Google Scholar]
- [76].Jin J, Hou Q, Mullen TD, Zeidan YH, Bielawski J, Kraveka JM, Bielawska A, Obeid LM, Hannun YA, Hsu YT. Ceramide generated by sphingomyelin hydrolysis and the salvage pathway is involved in hypoxia/reoxygenation-induced Bax redistribution to mitochondria in NT-2 cells. J Biol Chem. 2008;283:26509–17. doi: 10.1074/jbc.M801597200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Furuya K, Ginis I, Takeda H, Chen Y, Hallen-beck JM. Cell permeable exogenous ceramide reduces infarct size in spontaneously hyper-tensive rats supporting in vitro studies that have implicated ceramide in induction of tolerance to ischemia. J Cereb Blood Flow Metab. 2001;21:226–32. doi: 10.1097/00004647-200103000-00006. [DOI] [PubMed] [Google Scholar]
- [78].Chen Y, Ginis I, Hallenbeck JM. The protective effect of ceramide in immature rat brain hypoxia-ischemia involves up-regulation of bcl-2 and reduction of TUNEL-positive cells. J Cereb Blood Flow Metab. 2001;21:34–40. doi: 10.1097/00004647-200101000-00005. [DOI] [PubMed] [Google Scholar]
- [79].Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S. Sphingosine kinases, sphingosine 1 -phosphate. apoptosis and diseases. Biochim Biophys Acta. 2006;1758:2016–26. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- [80].Taha TA, Mullen TD, Obeid LM. A house divided: Ceramide, sphingosine. and sphingosine-1-phosphate in programmed cell death. Biochim Biophys Acta. 2006;1758:2027–36. doi: 10.1016/j.bbamem.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Sims NR, Anderson MF. Mitochondrial contributions to tissue damage in stroke. Neurochem Int. 2002;40:511–26. doi: 10.1016/s0197-0186(01)00122-x. [DOI] [PubMed] [Google Scholar]
- [82].Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, de Cabo R, Sauve AA, Sinclair DA. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Gudz TI, Tserng KY, Hoppel CL. Direct inhibition of mitochondrial respiratory chain complex III by cell-permeable ceramide. J Biol Chem. 1997;272:24154–8. doi: 10.1074/jbc.272.39.24154. [DOI] [PubMed] [Google Scholar]
- [84].Di Paola M, Cocco T, Lorusso M. Ceramide interaction with the respiratory chain of heart mitochondria. Biochemistry. 2000;39:6660–8. doi: 10.1021/bi9924415. [DOI] [PubMed] [Google Scholar]
- [85].Yuan H, Williams SD, Adachi S, Oltersdorf T, Gottlieb RA. Cytochrome c dissociation and release from mitochondria by truncated Bid and ceramide. Mitochondrion. 2003;2:237–44. doi: 10.1016/S1567-7249(02)00106-X. [DOI] [PubMed] [Google Scholar]
- [86].Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- [87].Votyakova TV, Reynolds IJ. DeltaPsi(m)-Dependent and -independent production of reactive oxygen species by rat brain mitochondria. J Neurochem. 2001;79:266–77. doi: 10.1046/j.1471-4159.2001.00548.x. [DOI] [PubMed] [Google Scholar]
- [88].García-Ruiz C, Colell A, Marí M, Morales A, Fernández-Checa JC. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J Biol Chem. 1997;272:11369–77. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- [89].Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–42. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- [90].Fiskum G. Mitochondrial participation in ischemic and traumatic neural cell death. J Neurotrauma. 2000;17:843–55. doi: 10.1089/neu.2000.17.843. [DOI] [PubMed] [Google Scholar]
- [91].Galluzzi L, Blomgren K, Kroemer G. Mitochondrial membrane permeabilization in neuronal injury. Nat Rev Neurosci. 2009;10:481–94. doi: 10.1038/nrn2665. [DOI] [PubMed] [Google Scholar]
- [92].Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–8. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- [93].Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci USA. 2005;102:12005–10. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Stavrovskaya IG, Kristal BS. The powerhouse takes control of the cell: is the mitochondrial permeability transition a viable therapeutic target against neuronal dysfunction and death? Free Radic Biol Med. 2005;38:687–97. doi: 10.1016/j.freeradbiomed.2004.11.032. [DOI] [PubMed] [Google Scholar]
- [95].Szalai G, Krishnamurthy R, Hajnoczky G. Apoptosis driven by IP(3)-linked mitochondrial calcium signals. Embo J. 1999;18:6349–61. doi: 10.1093/emboj/18.22.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Pastorino JG, Tafani M, Rothman RJ, Marcinkeviciute A, Hoek JB, Farber JL. Functional consequences of the sustained or transient activation by Bax of the mitochondrial permeability transition pore. J Biol Chem. 1999;274:31734–9. doi: 10.1074/jbc.274.44.31734. [DOI] [PubMed] [Google Scholar]
- [97].Arora AS, Jones BJ, Patel TC, Bronk SF, Gores GJ. Ceramide induces hepatocyte cell death through disruption of mitochondrial function in the rat. Hepatology. 1997;25:958–63. doi: 10.1002/hep.510250428. [DOI] [PubMed] [Google Scholar]
- [98].Novgorodov SA, Szulc ZM, Luberto C, Jones JA, Bielawski J, Bielawska A, Hannun YA, Obeid LM. Positively charged ceramide is a potent inducer of mitochondrial permeabilization. J Biol Chem. 2005;280:16096–105. doi: 10.1074/jbc.M411707200. [DOI] [PubMed] [Google Scholar]
- [99].Joza N, Pospisilik JA, Hangen E, Hanada T, Modjtahedi N, Penninger JM, Kroemer G. AIF: not just an apoptosis-inducing factor. Ann N Y Acad Sci. 2009;1171:2–11. doi: 10.1111/j.1749-6632.2009.04681.x. [DOI] [PubMed] [Google Scholar]
- [100].Brustovetsky N, Dubinsky JM, Antonsson B,Jemmerson R. Two pathways for tBID-induced cytochrome c release from rat brain mitochondria: BAK- versus BAX-dependence. J Neurochem. 2003;84:196–207. doi: 10.1046/j.1471-4159.2003.01545.x. [DOI] [PubMed] [Google Scholar]
- [101].Kar P, Samanta K, Shaikh S, Chowdhury A, Chakraborti T, Chakraborti S. Mitochondrial calpain system: an overview. Arch Biochem Biophys. 2010;495:1–7. doi: 10.1016/j.abb.2009.12.020. [DOI] [PubMed] [Google Scholar]
- [102].Di Paola M, Zaccagnino P, Montedoro G, Cocco T, Lorusso M. Ceramide induces release of pro-apoptotic proteins from mitochondria by either a Ca2+ -dependent or a Ca2+ -independent mechanism. J Bioenerg Biomembr. 2004;36:165–70. doi: 10.1023/b:jobb.0000023619.97392.0c. [DOI] [PubMed] [Google Scholar]
- [103].Siskind LJ, Colombini M. The lipids C2- and C16-ceramide form large stable channels. Implications for apoptosis. J Biol Chem. 2000;275:38640–4. doi: 10.1074/jbc.C000587200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Siskind LJ, Kolesnick RN, Colombini M. Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. J Biol Chem. 2002;277:26796–803. doi: 10.1074/jbc.M200754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Siskind LJ, Kolesnick RN, Colombini M. Ceramide forms channels in mitochondrial outer membranes at physiologically relevant concentrations. Mitochondrion. 2006;6:118–25. doi: 10.1016/j.mito.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Ghafourifar P, Klein SD, Schucht O, Schenk U, Pruschy M, Rocha S, Richter C. Ceramide induces cytochrome c release from isolated mitochondria. Importance of mitochondrial redox state. J Biol Chem. 1999;274:6080–4. doi: 10.1074/jbc.274.10.6080. [DOI] [PubMed] [Google Scholar]
- [107].Montes LR, Ruiz-Argüello MB, Goñi FM, Alonso A. Membrane restructuring via ceramide results in enhanced solute efflux. J Biol Chem. 2002;277:11788–94. doi: 10.1074/jbc.M111568200. [DOI] [PubMed] [Google Scholar]
- [108].Siskind LJ, Feinstein L, Yu T, Davis JS, Jones D, Choi J, Zuckerman JE, Tan W, Hill RB, Hard-wick JM, Colombini M. Anti-apoptotic Bcl-2 Family Proteins Disassemble Ceramide Channels. J Biol Chem. 2008;283:6622–30. doi: 10.1074/jbc.M706115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Yuan H, Williams S., Adachi S, Oltersdorf T, Gottlieb RA. Cytochrome c dissociation and release from mitochondria by trancated Bid and ceramide. Mitochondrion. 2003;2:237–244. doi: 10.1016/S1567-7249(02)00106-X. [DOI] [PubMed] [Google Scholar]
- [110].Kristal BS, Brown AM. Apoptogenic ganglioside GD3 directly induces the mitochondrial permeability transition. J Biol Chem. 1999;274:23169–75. doi: 10.1074/jbc.274.33.23169. [DOI] [PubMed] [Google Scholar]
- [111].Pastorino JG, Tafani M, Rothman RJ, Marcinkeviciute A, Hoek JB, Farber JL. Functional consequences of the sustained or transient activation by Bax of the mitochondrial permeability transition pore. J Biol Chem. 1999;274:31734–9. doi: 10.1074/jbc.274.44.31734. [DOI] [PubMed] [Google Scholar]
- [112].Zeidan YH, Wu BX, Jenkins RW, Obeid LM, Hannun YA. A novel role for protein kinase Cdelta-mediated phosphorylation of acid sphingomyelinase in UV light-induced mitochondrial injury. Faseb J. 2008;22:183–93. doi: 10.1096/fj.07-8967com. [DOI] [PubMed] [Google Scholar]
- [113].Parra V, Eisner V, Chiong M, Criollo A, Moraga F, Garcia A, Härtel S, Jaimovich E, Zorzano A, Hidalgo C, Lavandero S. Changes in mitochondrial dynamics during ceramide-induced cardiomyocyte early apoptosis. Cardiovasc Res. 2008;77:387–97. doi: 10.1093/cvr/cvm029. [DOI] [PubMed] [Google Scholar]
- [114].Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22:1577–90. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Li Y, Chopp M, Jiang N, Zaloga C. In situ detection of DNA fragmentation after focal cerebral ischemia in mice. Brain Res Mol Brain Res. 1995;28:164–8. doi: 10.1016/0169-328x(94)00220-9. [DOI] [PubMed] [Google Scholar]
- [116].Nakai A, Kuroda S, Kristián T, Siesjö BK. The immunosuppressant drug FK506 ameliorates secondary mitochondrial dysfunction following transient focal cerebral ischemia in the rat. Neurobiol Dis. 1997;4:288–300. doi: 10.1006/nbdi.1997.0146. [DOI] [PubMed] [Google Scholar]
- [117].Mimeault M. New advances on structural and biological functions of ceramide in apoptotic/necrotic cell death and cancer. FEBS Lett. 2002;530:9–16. doi: 10.1016/s0014-5793(02)03432-4. [DOI] [PubMed] [Google Scholar]
- [118].Morales A, Lee H, Goñi FM, Kolesnick R, Fernandez-Checa JC. Sphingolipids and cell death. Apoptosis. 2007;12:923–39. doi: 10.1007/s10495-007-0721-0. [DOI] [PubMed] [Google Scholar]
- [119].Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nat Cell Biol. 2001;3:E255–63. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- [120].Brenner C, Kroemer G. Apoptosis. Mitochondria-the death signal integrators. Science. 2000;289:1150–1. doi: 10.1126/science.289.5482.1150. [DOI] [PubMed] [Google Scholar]
- [121].Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- [122].Hearps AC, Burrows J, Connor CE, Woods GM, Lowenthal RM, Ragg SJ. Mitochondrial cyto-chrome c release precedes transmembrane depolarisation and caspase-3 activation during ceramide-induced apoptosis of Jurkat T cells. Apoptosis. 2002;7:387–94. doi: 10.1023/a:1020034906200. [DOI] [PubMed] [Google Scholar]
- [123].Zhang X, Li B, Zhang Y, Liu J. Ceramide induces release of mitochondrial proapoptotic proteins in caspase-dependent and -independent manner in HT-29 cells. Sci China C Life Sci. 2008;51:66–71. doi: 10.1007/s11427-008-0015-y. [DOI] [PubMed] [Google Scholar]
- [124].Won JS, Singh I. Sphingolipid signaling and redox regulation. Free Radic Biol Med. 2006;40:1875–88. doi: 10.1016/j.freeradbiomed.2006.01.035. [DOI] [PubMed] [Google Scholar]
- [125].Gendron MC, Schrantz N, Métivier D, Kroemer G, Maciorowska Z, Sureau F, Koester S, Petit PX. Oxidation of pyridine nucleotides during Fas- and ceramide-induced apoptosis in Jurkat cells: correlation with changes in mitochondria, glutathione depletion. intracellular acidification and caspase 3 activation. Biochem J. 2001;353:357–67. [PMC free article] [PubMed] [Google Scholar]
- [126].Lin CF, Chen CL, Chang WT, Jan MS, Hsu LJ, Wu RH, Tang MJ, Chang WC, Lin YS. Sequential caspase-2 and caspase-8 activation upstream of mitochondria during ceramideand etoposide-induced apoptosis. J Biol Chem. 2004;279:40755–61. doi: 10.1074/jbc.M404726200. [DOI] [PubMed] [Google Scholar]
- [127].Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsch T, Susin SA, Petit PX, Mignotte B, Kroemer G. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med. 1995;182:367–77. doi: 10.1084/jem.182.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Stoica BA, Movsesyan VA, Lea PM, Faden AI. Ceramide-induced neuronal apoptosis is associated with dephosphorylation of Akt, BAD, FKHR, GSK-3beta, and induction of the mitochondrial-dependent intrinsic caspase pathway. Mol Cell Neurosci. 2003;22:365–82. doi: 10.1016/s1044-7431(02)00028-3. [DOI] [PubMed] [Google Scholar]
- [129].Pastorino JG, Simbula G, Yamamoto K, Glascott PA, Jr, Rothman RJ, Farber JL. The cytotoxicity of tumor necrosis factor depends on induction of the mitochondrial permeability transition. J Biol Chem. 1996;271:29792–8. doi: 10.1074/jbc.271.47.29792. [DOI] [PubMed] [Google Scholar]
- [130].Pacher P, Hajnoczky G. Propagation of the apoptotic signal by mitochondrial waves. Embo J. 2001;20:4107–21. doi: 10.1093/emboj/20.15.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Geley S, Hartmann BL, Kofler R. Ceramides induce a form of apoptosis in human acute lymphoblastic leukemia cells that is inhibited by Bcl-2. but not by CrmA. FEBS Lett. 1997;400:15–8. doi: 10.1016/s0014-5793(96)01284-7. [DOI] [PubMed] [Google Scholar]
- [132].Zhang J, Alter N, Reed JC, Borner C, Obeid LM, Hannun YA. Bcl-2 interrupts the ceramide-mediated pathway of cell death. Proc Natl Acad Sci USA. 1996;93:5325–8. doi: 10.1073/pnas.93.11.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Scaffidi C, Schmitz I, Zha J, Korsmeyer SJ, Krammer PH, Peter ME. Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J Biol Chem. 1999;274:22532–8. doi: 10.1074/jbc.274.32.22532. [DOI] [PubMed] [Google Scholar]
- [134].Birbes H, Luberto C, Hsu YT, El Bawab S, Hannun YA, Obeid LM. A mitochondrial pool of sphingomyelin is involved in TNFalpha-induced Bax translocation to mitochondria. Biochem J. 2005;386:445–51. doi: 10.1042/BJ20041627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Atlante A, Giannattasio S, Bobba A, Gagliardi S, Petragallo V, Calissano P, Marra E, Passarella S. An increase in the ATP levels occurs in cerebellar granule cells en route to apoptosis in which ATP derives from both oxidative phosphorylation and anaerobic glycolysis. Biochim Biophys Acta. 2005;1708:50–62. doi: 10.1016/j.bbabio.2005.01.009. [DOI] [PubMed] [Google Scholar]
- [136].Vander Heiden MG, Chandel NS, Williamson EK, Schumacker PT, Thompson CB. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997;91:627–37. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- [137].Arrington DD, Van Vleet TR, Schnellmann RG. Calpain 10: a mitochondrial calpain and its role in calcium-induced mitochondrial dysfunction. Am J Physiol Cell Physiol. 2006;291:C1159–71. doi: 10.1152/ajpcell.00207.2006. [DOI] [PubMed] [Google Scholar]