Abstract
Erlin1 and erlin2 are highly homologous, ~ 40kDa, endoplasmic reticulum membrane proteins that assemble into a ring-shaped complex with a mass of ~2MDa. How this complex is formed is not understood, but appears to involve multiple interactions, including a coiled-coil region that mediates lower-order erlin assembly, and a short hydrophobic region, termed the “assembly domain”, that mediates higher-order assembly into ~2MDa complexes. Here we have used molecular modeling, mutagenesis and cross-linking to examine the role of the assembly domain in higher-order assembly. We find (i) that the assembly domains of erlin1 and erlin2 are amphipathic helices, (ii) that erlin1 alone and erlin2 alone can assemble into ~2MDa complexes, (iii) that higher-order assembly is strongly inhibited by point mutations to the assembly domain, (iv) that three interacting hydrophobic residues in the assembly domain and aromaticity are essential for higher-order assembly, and (iv) that while erlins1 and 2 are equally capable of forming lower-order homo- and hetero-oligomers, hetero-oligomers are the most prevalent form when erlin1 and erlin2 are co-expressed. Overall, we conclude that the ~2MDa erlin1/2 complex is composed of an assemblage of lower-order hetero-oligomers, probably heterotrimers, linked together by assembly domain hydrophobic residues.
Keywords: erlin1, erlin2, amphipathic helix, assembly, crosslinking
INTRODUCTION
Erlin1 (E1) and erlin2 (E2), which are also known as SPFH1 and SPFH2, are integral endoplasmic reticulum (ER) membrane proteins that belong to a family of ~ 100 mammalian proteins that contain an “SPFH” domain [1]. This domain is an ~ 200 amino acid long motif named because of minor sequence similarities in the proteins Stomatin, Prohibitin, Flotillin, and HflC/K [1–3]. SPFH domain-containing proteins share several structural and biochemical properties, including a size of 30–50kDa, localization to membranes, and a propensity to assemble into very large oligomeric complexes [1,3], but have diverse functions, ranging from regulation of plasma membrane ion channels (stomatins) [4] to control of inner mitochondrial membrane functional integrity (prohibitins) [5].
E1 (348 amino acids) and E2 (340 amino acids) assemble into a complex that has been estimated to be ~2MDa in size and is composed of ~ 40 subunits in an ~ 1:1 ratio [6]. The complex is found in the ER membrane and is ring-shaped [6]. It plays a role in ER-associated degradation [6,7], the pathway by which aberrant or misfolded proteins, or unassembled subunits of multimeric ER proteins, are degraded [8]. Specifically, the E1/2 complex has a clearly defined role in mediating the ER-associated degradation of activated inositol 1,4,5-trisphosphate receptors [6,7,9,10], contributes to the degradation of some model ER-associated degradation substrates [9], and may play a role in 3-hydroxy-3-methyl-glutaryl-CoA reductase degradation [11]. Assembly of the E1/2 complex appears to be mediated at two levels; lower-order oligomers are formed primarily via interactions between coiled-coil regions (residues 179–276 in E1 and 177–274 in E2), and “assembly domains” (residues 301–311 in E1 and 299–309 in E2), that are enriched in hydrophobic residues (Figure 1A) [6,12], play a key role in higher-order assembly. Indeed, mutation of an individual hydrophobic residue (F305) within the assembly domain of E2, blocks higher-order assembly, without affecting lower-order oligomerization [12]. Interestingly, a similar situation appears to exist for stomatin, for which the assembly of ~0.3MDa complexes [13] is mediated by a short hydrophobic domain located near the C-terminus [14]. Prohibitins 1 and 2 and flotillins 1 and 2 also form large oligomeric complexes, but in these cases no clear role for a hydrophobic domain in assembly has been defined [15–17].
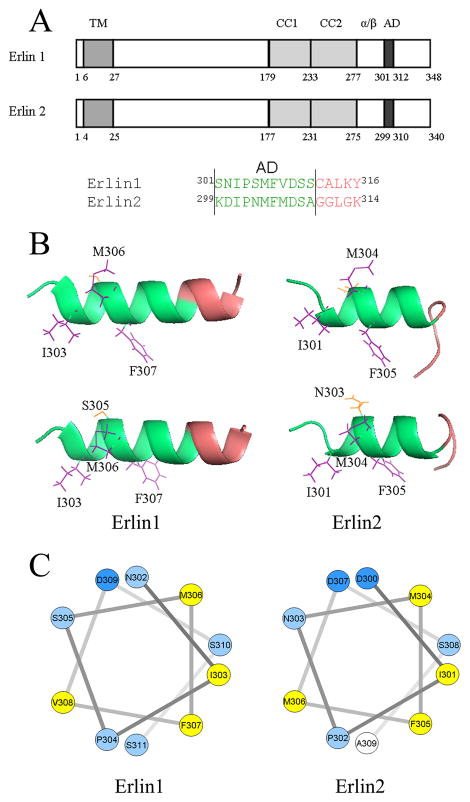
Figure 1. Modeling of assembly domain structure.
A. Domain organization of E1 and E2, with numbering according to mouse proteins, and indicating the first amino acid of each domain. TM, transmembrane domain; CC1 and CC2, coiled-coil regions 1 and 2; α/β, α/β domain; AD, assembly domain. B. Predicted structures with assembly domain amino acids shaded green and the side chains of selected residues highlighted. For E1 and E2, two images are shown, rotated differentially along the helical axis to best illustrate side chain orientation. C. Helical wheel projections of helical parts of the assembly domains with amino acids color-coded; yellow = more hydrophobic than alanine, light blue = less hydrophobic than alanine, dark blue = less hydrophobic than alanine and charged [19].
A critical unresolved question for SPFH domain-containing proteins concerns how lower-order oligomers are assembled into higher-order complexes. Here we have used molecular modeling, mutagenesis and cross-linking to examine this question for E1 and E2.
MATERIALS AND METHODS
Molecular modeling
The assembly domains of E1 and E2 were modeled with the server I-Tasser (zhanglab.ccmb.med.umich.edu/I-TASSER/). For the assembly domain amino acid sequences submitted, the program retrieves template proteins with homologous folds from the Protein Data Bank library. The fragments obtained are then re-assembled into full-length models by replica-exchange Monte Carlo simulations. The decoys generated during the simulation are clustered and the top five cluster centroids are used to generate full length models. The models are ranked based on the cluster density, with higher cluster density signifying that the structure occurs more often in the simulation trajectory and therefore is the most likely structure. Helical wheels were obtained using rzlab.ucr.edu/scripts/wheel/wheel.cgi.
Constructs, transfection, sample preparation and PAGE
cDNAs encoding mouse E1HA, E2HA and deletion mutants, E1flag and E2flag have been described previously [6]. Point mutations in E1HA and E2HA were created by mutagenic PCR. Briefly, a 50 μl mix containing 50ng of template DNA, PfuUltraII buffer, dNTPs, primers (10 μM), and PfuUltraII enzyme, was cycled (initial denaturation step of 95°C for 2 minutes, followed by 23 cycles of 95°C for 30 seconds, 66°C for 1 minute, 68°C for 6.5 minutes, and a final extension step of 72°C for 10 minutes). The reaction products were then digested with DpnI to remove template DNA and the presence of mutations was confirmed by DNA sequencing. HeLa cells (~6 × 105/well of a 6-well plate) were transiently transfected using Lipofectamine 2000 (10 μl plus 4.8 μg total cDNA), and ~24h later cells were detached with 155 mM NaCl, 10mM HEPES, 1mM EDTA, pH 7.4, were centrifuged (1000 × g for 2 min), were disrupted for 30 min at 4°C with lysis buffer (150 mM NaCl, 50 mM Tris-HCl, 1 mM EDTA, 1% CHAPS, 10 μM pepstatin, 0.2 mM PMSF, 0.2 μM soybean trypsin inhibitor, 1mM dithiothreitol, pH 8.0), were centrifuged (16,000 × g for 10 min at 4 C), and supernatant samples were subjected to native or SDS-PAGE and immunoblotting as described [18]. Antibodies used for immunodetection were mouse monoclonal anti-hemagglutinin (HA) epitope clone HA11 (Covance), anti-flag epitope clone M2 (Sigma), anti-E1 clone 7D3 [12], and rabbit polyclonal anti-E1 or anti-E2 [6].
Cross-linking
Transfected HeLa cells in 6-well plates were rinsed once with PBS and were then incubated for 1 min at 22°C with 1mM disuccinimidyl suberate (DSS, Thermo Scientific) in PBS. Cells were then detached and incubated for 30 min at 4°C with 1% CHAPS lysis buffer supplemented with 10mM lysine and 25 mM glycine, were centrifuged (16,000 × g for 10 min at 4°C), and supernatant samples were subjected to SDS-PAGE. Immunoprecipitated αT3-1 cell E1/2 complex, prepared using anti-E2 [6], was incubated for 2 min at 22°C with 1mM DSS in PBS, reactions were stopped by adding gel-loading buffer supplemented with 10mM lysine and 25 mM glycine, and samples were subjected to SDS-PAGE.
RESULTS
Molecular modeling of the assembly domain
The domain structures of E1 and E2 are shown in Figure 1A, together with the amino acid sequences of the assembly domain regions. Previous studies have indicated that E1/2 complex assembly is mediated by two distinct interactions; the primary interaction (lower-order oligomerization) being mediated by the two coiled-coil motifs and the α/β domain, and the secondary interaction (the linkage of lower-order oligomers into higher-order ~2MDa complexes) being mediated by the assembly domain [6,12]. Evidence for the existence of the assembly domain came from experiments showing that deletion of amino acids 299–309 of E2 blocked ~2MDa complex formation [6], but not lower-order assembly, and also that mutation of hydrophobic amino acids within the E2 assembly domain to alanine, or mutation of just F305 to alanine had the same effect [12]. To further explore assembly mechanisms we derived structures of the assembly domain regions of E1 and E2 (Fig 1B). Helical regions that encompass the assembly domains are predicted, with a preponderance of hydrophobic residues aligned towards one face of the helices (amino acids I303, M306 and F307 in E1, and I301, M304 and F305 in E2). Helical wheel projections (Figure 1C) confirm this distribution and indicate that the helices are amphipathic.
Mutation of the Assembly Domain
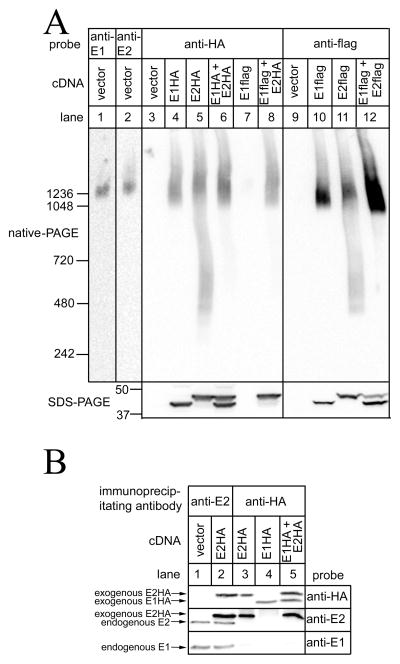
The extent of E1 or E2 assembly was assessed by native-PAGE, which allows for the separation of proteins under native conditions, and preserves protein complexes [20]. When expressed in HeLa cells, E1HA and E2HA predominantly migrated as a smear at ~2MDa, (Figure 2A, lanes 4 and 5), similarly to endogenous E1 and E2 (lanes 1 and 2), indicating that each exogenous erlin alone is capable of oligomerizing into higher-order complexes. There was a tendency, however, for some E2HA to migrate in the ~0.3–1MDa range (lane 5). Interestingly, when E1HA and E2HA were co-expressed, much less immuoreactivity was detected in the ~0.3–1MDa range, suggesting that higher-order complexes are most stable when composed of both erlins. These findings were recapitulated when E1flag and E2flag were expressed (lanes 10–12). Importantly, there was little or no comingling of endogenous and exogenous erlins, as immunoprecipitation of exogenous E1HA or E2HA with anti-HA did not co-immunoprecipitate endogenous E1 or E2 (Figure 2B, lanes 3–5), while both endogenous E1 and E2 were readily immunoprecipitatable from vector-transfected, or E2HA-expressing cells with anti-E2 (lanes 1 and 2). Thus, the signals seen after native-PAGE (e.g. Figure 2A) reflect the properties of exogenous proteins only.
Figure 2. Exogenous erlins form higher-order complexes.
Hela cells were transfected with vectors encoding epitope-tagged erlins, or empty vector, and cell lysates were prepared. In A, lysates were subjected to either native-PAGE or SDS-PAGE and were probed as indicated, with the migration positions of molecular mass markers indicated (masses in kDa). In B, lysates were incubated with anti-E2 or rabbit polyclonal anti-HA [10] and immunoprecipitates were subjected to SDS-PAGE and were probed as indicated. Note that mouse monoclonal anti-E1 is human specific [9,12] and recognizes endogenous E1, but not exogenous E1HA. E1, E2, E1HA and E2HA migrated, respectively, at 41,43, 42 and 44 kDa.
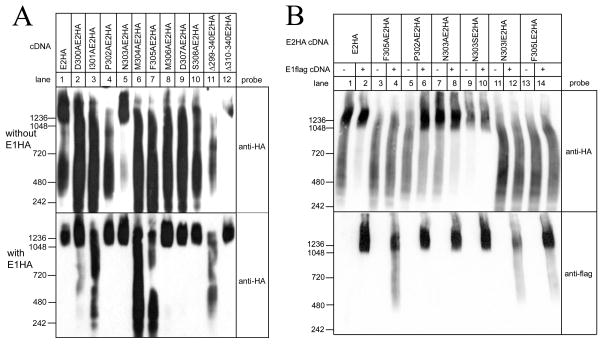
Systematic point mutation of each residue within the E2 assembly domain to alanine, which has a relatively small hydrophobic side chain and does not disrupt helical motifs [21], showed that all mutants, with the exception of N303AE2HA, failed to migrate predominantly at ~2MDa (Figure 3A, upper panel, lanes 2–10), indicating that assembly is extremely sensitive to perturbation of the assembly domain. Consistent with these results, Δ310–340E2HA migrated at ~2MDa (lane 12), while Δ299–340E2HA, which lacks the assembly domain, did not (lane 11). Interestingly, E1HA co-expression reversed the effects of several of the point mutations (lower panel, lanes 2–10), but the migration of three E2HA mutants, I301A (lane 3), M304A (lane 6) and F305A (lane 7) was not substantially altered. The ability of E1HA to facilitate the assembly of some of the E2HA mutants suggests that it combines with E2 in such a way to buffer the effects of mutations, whereas the resistance of I301AE2HA, M304AE2HA and F305AE2HA to E1HA indicates that I301, M304 and F305 are indispensable for assembly. Each of these residues are hydrophobic and are clustered on one face of the assembly domain helix (Figure 1B and C). The same appears to be true for E1, in which an identical cluster is found, and F307AE1HA fails to migrate at ~2MDa, in the absence or presence of E2HA (data not shown).
Figure 3. Effects of mutations to the E2 assembly domain on higher-order complex formation.
Hela cells were transfected with vectors encoding E2HA and its mutants, (A) either without E1HA or with E1HA, or (B) either without or with E1flag. Cell lysates were prepared and were subjected to native-PAGE and were probed with anti-HA or anti-flag as indicated. The migration positions of molecular mass markers are indicated (masses in kDa).
Additional information was obtained from co-expression of the different types of E2HA mutant with E1flag, since this allowed for effects of E2 mutants on E1 assembly to be observed directly (Figure 3B). Notably, the ability of E1flag to form ~2MDa complexes was disrupted by resistant mutants like F305AE2HA, but not by reversible mutants like P302AE2HA (lower panel, lanes 3–6). Thus, the influence of E1 and E2 on each other is bi-directional, indicative of an intimate interaction between the assembly domains of the two proteins.
Other mutations were made to further probe the assembly domain. To examine why N303AE2HA appears to migrate more predominantly in the ~2MDa region than wild-type E2HA (Figure 3A, upper panel, compare lanes 1 and 5; Figure 3B, upper panel, compare lanes 1 and 7), we replaced N303 (relatively large, polar, uncharged) with serine (relatively small, polar, uncharged), or with isoleucine (relatively large, hydrophobic). When expressed alone, N303SE2HA migrated very similarly to N303AE2HA, while N303IE2HA failed to form an ~2MDa complex (Figure 3B, upper panel, lanes 7, 9 and 11). This suggests that there are steric constraints at position 303 of E2, with small amino acid side chains (those of alanine and serine) enhancing assembly, and large side chains (that of isoleucine) inhibiting assembly. Interestingly, a serine (S305) is found in E1 in the position equivalent to N303 of E2 (Figure 1), which may explain why E1 alone migrates more predominantly at ~2MDa than E2 alone (Figure 2A).
To examine whether the aromaticity of phenylalanine at position 305 in E2 is critical to assembly, we replaced F305 (large hydrophobic, aromatic) with leucine (large hydrophobic, non-aromatic) and found that F305LE2HA does not assemble into ~2MDa complexes in the absence or presence of E1flag, suggesting that aromaticity, rather than just hydrophobicity at position 305, is required for assembly (Figure 3B, lanes 13 and 14). These data also show that the inability of I301AE2HA, M304AE2HA and F305AE2HA to assemble is not simply because the number of residues in the hydrophobic cluster is reduced from 3 to 2, since F305LE2HA possesses 3 clustered hydrophobic residues.
Overall, these data indicate that the formation of higher-order erlin complexes is extremely sensitive to perturbation of the assembly domain, that three hydrophobic residues oriented to one side of the assembly domain form a hydrophobic patch that drives assembly, that aromaticity of F305 contributes to assembly, and that even residues on the non-hydrophobic side of the assembly domain (e.g. at position 303 in E2) influence assembly through steric effects.
Cross-linking of the E1/2 complex
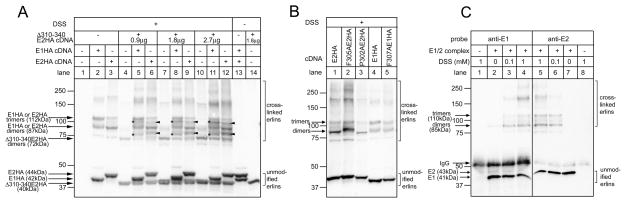
To examine whether there is a lower-order assemblage or “basic unit” from which the E1/2 complex is constructed, we employed chemical cross-linking. Previous immunoprecipitation studies have shown that E1 and E2 can form homo-oligomers when expressed individually, can form hetero-oligomers when co-expressed, and that the formation of these oligomers does not require ~2MDa complex assembly [6,12]. Brief exposure of cells expressing exogenous E1HA or E2HA to DSS, a homobifunctional primary amine-specific cross-linker that couples adjacent lysine residues, yielded species indicative of partial cross-linking (Figure 4A, lanes 1–12). For E1HA, clear bands at ~112kDa and ~87kDa were seen, corresponding in size to dimers and trimers, respectively, with the ~112kDa band predominating (lane 2). For E2HA, bands of similar size were seen, but with the ~87kDa band predominating (lane 3). Thus, erlins appear capable of forming homodimers and homotrimers when expressed alone. To examine whether there is a preference for the formation of homomers or heteromers when both erlins are expressed, E1HA and E2HA were co-expressed with Δ310–340E2HA, which contains the AD and forms ~2MDa complexes (Figure 3A, lane 12), but which is substantially smaller than E1HA and E2HA, and which when expressed alone is cross-linked into an ~72kDa band, corresponding in size to dimers (Figure 4A, lanes 4, 7 and 10). When Δ310–340E2HA was co-expressed with E1HA, new bands were formed at ~75 and 100kDa (lanes 5, 8 and 11, marked with asterisks), indicative of the formation of heterodimers and heterotrimers between E1HA and the E2HA mutant. Interestingly, similarly sized bands were formed with the same efficiency when Δ310–340E2HA was co-expressed with E2HA (lanes 6, 9 and 12, marked with arrowheads), indicating that Δ310–340E2HA interacts equally well with E1HA and E2HA. These data indicate that there is no preference for homomeric versus heteromeric interactions in the formation in lower-order erlin oligomers, and that when co-expressed, E1 and E2 associate in an unbiased manner. That these lower-order interactions were independent of higher-order assembly is demonstrated by the observations that E1HA and E2HA mutants incapable of forming ~2MDa complexes (F305AE2HA, P302AE2HA and F307AE1HA) were cross-linked into dimers and trimers similarly to wild-type E2HA and E1HA (Figure 4B).
Figure 4. Cross-linking of erlins.
A and B. Hela cells were transfected with the vectors indicated, were exposed to 1mM DSS for 1min, and cell lysates were subjected to SDS-PAGE and were probed with anti-HA. C. E1/E2 complex immunopurified from αT3 cells (lanes 2–7) and control material lacking E1/E2 complex (lanes 1 and 8) were exposed to DSS for 2 min as indicated and subjected to SDS-PAGE and probed with anti-E2 and rabbit polyclonal anti-E1 [6]. The migration positions of molecular mass markers are indicated (masses in kDa) as are the positions of unmodified and cross-linked erlins and IgG heavy chain.
Lower-order assembly was also examined using endogenous E1/2 complex immunopurified from αT3-1 cells (Figure 4C), that is composed of an ~1:1 mixture of E1 and E2 [6]. While E1 and E2 migrate at 41 and 43kDa, respectively, brief exposure to DSS caused the formation of anti-E1 and anti-E2 immunoreactive bands at ~85 and 110kDa, corresponding in size to dimers and trimers. The co-migration of anti-E1 and anti-E2 immunoreactivity suggests that these bands correspond to heterodimers and heterotrimers, and again support the notion that there is no preference for homomeric lower-order assemblages. Thus, it is reasonable to conclude that the basic unit from which the ~2MDa complex is assembled is a lower order hetero-oligomer, either a heterodimer or a heterotrimer.
DISCUSSION
Our studies have provided insight into both the nature of the assembly domain that is critical for erlin1/2 complex assembly and the basic unit from which the complex is assembled. A fundamental conclusion is that the assembly of E1 and E2 appears to be an unbiased process. This stems from the findings that E1 alone and E2 alone can form ~2MDa complexes, and that Δ310–340E2HA cross-links equally well with E1HA and E2HA. Further support for this conclusion comes from data showing that exogenous E1 and E2 can form homo-oligomers equally as well as hetero-oligomers [12], and that essentially any ratio of E1:E2 will assemble into ~2MDa complexes [6]. Thus, it appears that any E1 and E2 present in the ER membrane will randomly associate. This lack of bias is most probably a reflection of the great similarity between E1 and E2; the amino acid sequences of mouse E1 and E2 are 76% identical, and the region from the transmembrane domain to the end of the assembly domain (amino acids 27–311 in E1 and 25–309 in E2), that includes all of the determinants for assembly, are 85% identical [6]. Finally, these conclusions are consistent with the observation that E1 is not dependent upon E2 for stability, and vice versa [6,12]. Interestingly, this contrasts with the situation for prohibitins 1 and 2 and flotillins 1 and 2, that are destabilized in the absence of their counterparts [5,22]. This may be because, for these proteins, there are constraints to how oligomers form; for prohibitins, at least, it appears that hetero-oligomers are the only viable lower-order assemblage [5].
Our cross-linking data also provide evidence for the existence of lower-order E1/E2 assemblages that may represent the basic unit that is multimerized into higher-order complexes. For both wild-type erlins, and mutant erlins incapable of assembling into ~2MDa complexes, DSS caused the formation of species corresponding in size to dimers and trimers. Thus, these lower-order oligomers exist independently of higher-order assembly and are likely held together by the coiled-coil and α/β domains of E1 and E2 (Figure 1A), deletion of which inhibits interaction between E1 and E2 [6]. While there are currently no structural data for E1 or E2, the crystal structure of a prokaryotic stomatin has been solved, revealing a trimeric assembly [23]. Given the similarities between the erlins and stomatin [1], it is quite plausible that erlins too are trimeric. Thus, the basic unit may well be erlin trimers, and as E1 and E2 are co-expressed in all cell types so far examined [6,9,12], heterotrimers (E1+E1+E2 or E2+E2+E1) will be the most likely assemblage.
It appears that these basic units are linked into ~2MDa complexes by the assembly domains of E1 and E2. Molecular modeling and mutagenesis indicate that assembly domains are amphipathic helices and that a cluster of 3 interacting hydrophobic residues are essential to this process. Mutation of any one of these 3 residues to alanine may reduce the mutual attraction between the hydrophobic/aromatic faces of the assembly domains of adjacent erlins, such that linkage into higher–order complexes becomes impossible. The importance of the hydrophobic cluster is consistent with the notion of “hot spots” in protein interfaces – amino acids or small regions whose mutation leads to a significant drop in binding free energy [24]. The requirement for phenylalanine for ~2MDa complex assembly raises the possibility that phenylalanine aromaticity in adjacent assembly domains provides binding free energy, perhaps by stacking, as has been seen in other proteins [25]. Interestingly, computational analysis of protein structures shows that clusters of aromatic residues commonly participate in protein-protein interactions; for example, a cluster of 20 interacting aromatic residues interact in epoxide hydrolase oligomers [26]. The same ideas may apply to stomatin, in which mutation of any of the amino acids in a small hydrophobic stretch near the C-terminus (STIVFPLPI) inhibits higher-order oligomerization [14].
As the E1/E2 complex is ring-shaped and is composed of ~40 E1 and E2 subunits [6], a model that accommodates our new data would be that ~14 E1/E2 heterotrimers are linked into a ring by the three critical hydrophobic residues on the hydrophobic faces of assembly domains. Such a model would be consistent with the data showing that lower-order assembly is independent of higher-order assembly (Figure 4B, [6,12]), that higher-order assembly is mediated by the 3 hydrophobic residues on one face of the assembly domain helices (Figures 1 and 3), and that the non-hydrophobic side of the helices can also influence higher-order assembly (Figure 3). Finally, the importance of the assembly domain to erlin function appears to be exemplified by a recently described autosomal recessive disease that is caused by a frameshift mutation in E2 that truncates the protein at residue 272 [27]. This mutant should be able to assemble into lower-order oligomers [6], but lacks the assembly domain (Figure 1A), and thus will be incapable of forming ~2MDa complexes.
*Highlights.
Erlins 1 and 2 are ER membrane proteins that form a large complex via unknown means.
We find that there are multiple determinants of complex assembly.
We focus on mutation of a key amphipathic helical domain that mediates assembly.
This provides the first clear insight into how the erlin1/2 complex is assembled.
Acknowledgments
The authors thank Dr Stephen M. Robbins for mouse monoclonal anti-E1, Danielle Sliter, Justine Lu, Forrest Wright and Cherry Ignacio for helpful suggestions, and National Institutes of Health Grant DK049194 for financial support.
ABBREVIATIONS
- E1
erlin1
- E2
erlin2
- ER
endoplasmic reticulum
- HA
hemagglutinin
- DSS
disuccinimidyl suberate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Browman DT, Hoegg MB, Robbins SM. The SPFH domain-containing proteins: more than lipid raft markers. Trends Cell Biol. 2007;17:394–402. doi: 10.1016/j.tcb.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Tavernarakis N, Driscoll M, Kyrpides NC. The SPFH domain: implicated in regulating targeted protein turnover in stomatins and other membrane-associated proteins. Trends Biochem Sci. 1999;24:425–427. doi: 10.1016/s0968-0004(99)01467-x. [DOI] [PubMed] [Google Scholar]
- 3.Morrow IC, Parton RG. Flotillins and the PHB domain family proteins: rafts, worms and anaesthetics. Traffic. 2005;6:725–740. doi: 10.1111/j.1600-0854.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 4.Lapatsina L, Brand J, Poole K, et al. Stomatin-domain proteins. Eur J Cell Biol. 2011 doi: 10.1016/j.ejcb.2011.01.018. in press. [DOI] [PubMed] [Google Scholar]
- 5.Merkwirth C, Langer T. Prohibitin function within mitochondria: Essential roles for cell proliferation and cristae morphogenesis. Biochim Biophys Acta. 2009;1793:27–32. doi: 10.1016/j.bbamcr.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Pearce MMP, Wormer DB, Wilkens S, et al. An endoplasmic reticulum (ER) membrane complex composed of SPFH1 and SPFH2 mediates the ER-associated degradation of inositol 1,4,5-trisphosphate receptors. J Biol Chem. 2009;284:10433–10445. doi: 10.1074/jbc.M809801200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wojcikiewicz RJH, Pearce MMP, Sliter DA, et al. When worlds collide: IP3 receptors and the ERAD pathway. Cell Calcium. 2009;46:147–153. doi: 10.1016/j.ceca.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vembar SS, Brodsky JL. One step at a time: endoplasmic reticulum-associated degradation. Nat Rev Mol Cell Biol. 2008;9:944–957. doi: 10.1038/nrm2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Pearce MMP, Sliter DA, et al. SPFH1 and SPFH2 mediate the ubiquitination and degradation of inositol 1,4,5-trisphosphate receptors in muscarinic receptor-expressing HeLa cells. Biochim Biophys Acta. 2009;1793:1710–1718. doi: 10.1016/j.bbamcr.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu JP, Wang Y, Sliter DA, et al. RNF170, an endoplasmic reticulum membrane ubiquitin ligase, mediates inositol 1,4,5-trisphosphate receptor ubiquitination and degradation. J Biol Chem. 2011;286:24426–24433. doi: 10.1074/jbc.M111.251983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jo Y, Sguigna PV, DeBose-Boyd RA. Membrane-associated ubiquitin ligase complex containing gp78 mediates sterol-accelerated degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J Biol Chem. 2011;286:15022–15031. doi: 10.1074/jbc.M110.211326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoegg MB, Browman DT, Resek ME, et al. Distinct regions within the erlins are required for oligomerization and association with high molecular weight complexes. J Biol Chem. 2009;284:7766–7776. doi: 10.1074/jbc.M809127200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snyers L, Umlauf E, Prohaska R. Oligomeric nature of the integral membrane protein stomatin. J Biol Chem. 1998;273:17221–17226. doi: 10.1074/jbc.273.27.17221. [DOI] [PubMed] [Google Scholar]
- 14.Umlauf E, Mairhofer M, Prohaska R. Characterization of the stomatin domain involved in homo-oligomerization and lipid raft association. J Biol Chem. 2006;281:23349–23356. doi: 10.1074/jbc.M513720200. [DOI] [PubMed] [Google Scholar]
- 15.Back JW, Artal Sanz M, De Jong L, et al. A structure for the yeast prohibitin complex: structure prediction and evidence from chemical crosslinking and mass spectrometry. Protein Sci. 2002;11:2471–2478. doi: 10.1110/ps.0212602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tatsuta T, Model K, Langer T. Formation of membrane-bound ring complexes by prohibitins in mitochondria. Mol Biol Cell. 2005;16:248–259. doi: 10.1091/mbc.E04-09-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salzer U, Prohaska R. Stomatin, flotillin-1 and flotillin-2 are major integral proteins of erythrocyte lipid rafts. Blood. 2001;97:1141–1143. doi: 10.1182/blood.v97.4.1141. [DOI] [PubMed] [Google Scholar]
- 18.Sliter DA, Kubota K, Kirkpatrick DS, et al. Mass spectral analysis of type I inositol 1,4,5-trisphosphate receptor ubiquitination. J Biol Chem. 2008;283:35319–35328. doi: 10.1074/jbc.M807288200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 20.Krause F. Detection and analysis of protein-protein inetractions in organellar and prokaryotic proteomes by native gel electrophoresis: (membrane) protein complexes and supercomplexes. Electrophoresis. 2006;27:2759–2781. doi: 10.1002/elps.200600049. [DOI] [PubMed] [Google Scholar]
- 21.Pace CN, Scholtz JM. A helix propensity scale based on experimental studies of peptides and proteins. Biophys J. 1998;75:422–427. doi: 10.1016/s0006-3495(98)77529-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solis GP, Hoegg M, Munderloh C, et al. Reggie/flotillin proteins are organized into stable tetramers in membrane microdomains. Biochem J. 2007;403:313–322. doi: 10.1042/BJ20061686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yokoyama H, Fujii S, Matsui I. Crystal structure of a core domain of stomatin from Pyrococcus horikoshii illustrates a novel trimeric and coiled-coil fold. J Mol Biol. 2008;376:868–878. doi: 10.1016/j.jmb.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 24.Keskin O, Gursoy A, Ma B, et al. Principles of protein-protein interactions: what are the preferred ways for proteins to interact ? Chem Rev. 2008;108:1225–1244. doi: 10.1021/cr040409x. [DOI] [PubMed] [Google Scholar]
- 25.Chelli R, Gervasio L, Procacci P, et al. Stacking and T-shape competition in aromatic-aromatic amino acid interactions. J Am Chem Soc. 2002;124:6133–6143. doi: 10.1021/ja0121639. [DOI] [PubMed] [Google Scholar]
- 26.Lanzarotti E, Biekofsky RR, Estrin DA, et al. Aromatic-aromatic inetactions in proteins: beyond the dimer. J Chem Inf Model. 2011;51:1623–1633. doi: 10.1021/ci200062e. [DOI] [PubMed] [Google Scholar]
- 27.Yildirim Y, Orhan EK, Iseri SAU, et al. A frameshift mutation of ERLIN2 in recessive intellectual disability, motor dysfunction and multiple joint contactures. Hum Mol Genet. 2011;20:1886–1892. doi: 10.1093/hmg/ddr070. [DOI] [PubMed] [Google Scholar]