Summary
Background
Microparticles (MPs), small vesicles shed from stimulated cells, permit cross-talk between cells within a particular environment. Their composition is thought to reflect their cell of origin, and differs according to whether they are produced by stimulation or by apoptosis. Whether MP properties vary according to stimulus is not yet known.
Methods
We studied the characteristics of MPs produced from monocytic THP-1 cells upon stimulation with lipopolysaccharide or a soluble P-selectin chimera, using proteomics, flow cytometry, western blotting, and electron microscopy.
Results
Utilizing a novel criterion of calcein-AM staining to define MPs, we found that MP populations were similar with respect to size, presence and organization of cytoskeleton, and expression of certain antigens. The MPs shared the same level of procoagulant activity. We found that MPs also have distinct characteristics, depending on stimuli. These include differences in phosphatidylserine expression and expression of proteins from specific subcellular locations such as the mitochondria, and of unique antigens such as leukocyte-associated immunoglobin-like-receptor (LAIR)-1, which was found only upon stimulation with the soluble P-selectin chimera.
Conclusion
We found that the properties of MPs depend on the stimulus that produced them. This supports the concept that monocytic MPs differentially modulate thrombosis, inflammation and immune regulation according to stimulus.
Keywords: LPS, microparticles, monocytes, phosphatidylserine, proteomics, P-selectin
Introduction
Microparticles (MPs), small vesicles (0.05–1 µm) shed from the plasma membrane of cells in response to stress, are believed to act as messengers delivering antigens and lipids from parent cells to elicit effects on downstream targets [1]. The numbers of MPs are increased in many diseases, including sickle-cell disease, pre-eclampsia, and diabetes mellitus [2]. Monocytes shed MPs that promote inflammation [3] and are highly procoagulant [4], primarily due to the presence of tissue factor (TF) and phosphatidylserine (PS) [1].
P-selectin – an adhesion molecule found in endothelial cells and platelets [5] – can be shed from the plasma membrane, resulting in increased levels of soluble P-selectin in the bloodstream [6]. Our laboratory has shown that soluble P-selectin induces the formation of procoagulant MPs in vivo from monocytes upon interaction with its cognate receptor, P-selectin glycoprotein ligand-1 (PSGL-1) [7,8]. PSGL-1, expressed on leukocyte surfaces, interacts with P-selectin to mediate leukocyte rolling [9] and recruit leukocytes to growing thrombi [10]. Monocyte-derived MPs can also be induced by lipopolysaccharide (LPS), a component of Gram-negative bacterial membranes. Both the stimulated cells and MPs express TF and are procoagulant [4,11].
Studies suggest that MPs produced via activating stimuli have different protein expression patterns from those produced via apoptosis [12,13]. The present study addresses the hypothesis that different activating stimuli produce MPs that vary in composition and biological function, even when they originate from the same parent cell. Using a proteomic approach, we compared the protein composition of four different populations of MPs generated from a human monocytic cell line (THP-1), stimulating the cells with a physiologic agonist [soluble P-selectin-Ig chimera (P-sel-Ig)], a pathologic agonist (LPS), a control IgG, and no stimulus (spontaneously produced MPs). We found that MPs differ when produced by different stimuli; however, they have common characteristics that are independent of stimulus.
Materials and methods
Proteins/antibodies
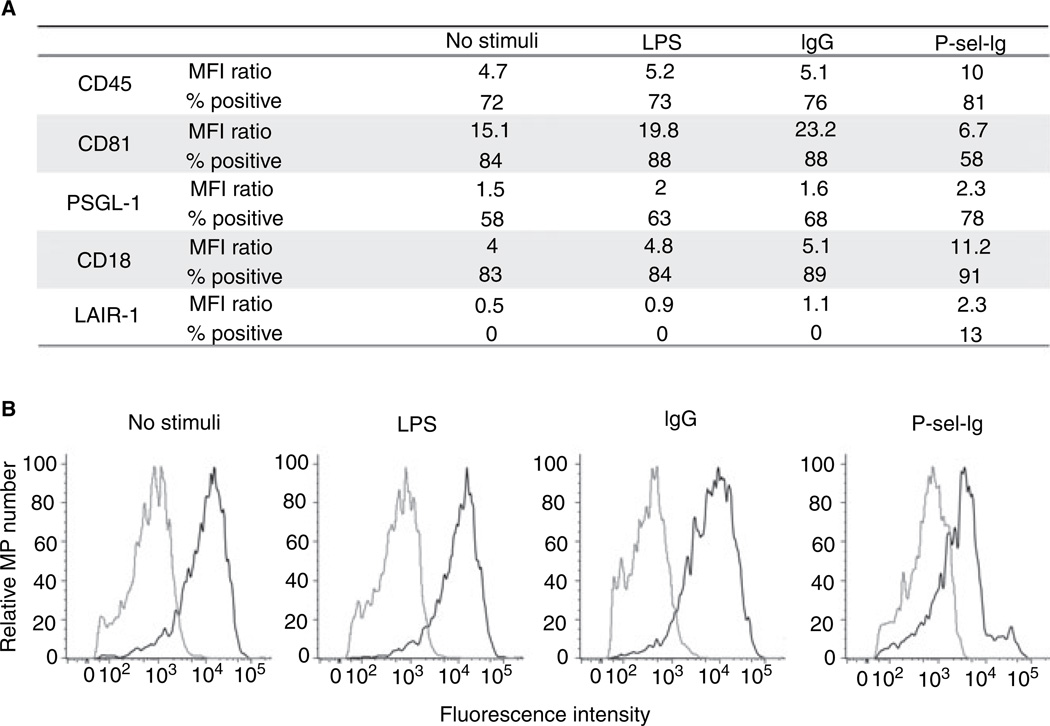
Two P-sel-Ig [14] chimeras with the same biological activity were provided by Wyeth Laboratories (Cambridge, MA, USA) and by Jian-Guo Geng (University of Minnesota Medical School, Minneapolis, MN, USA) [15] with the latter chimera used solely to generate MPs used in the fluorescence-activated cell sorting (FACS) analysis of Fig. 4. IgG1κ was from Calbiochem/EMD Biosciences (Gibbstown, NJ, USA). Endotoxin contamination, if any, was removed prior to use (Supplementary Materials and methods). Antibodies for flow cytometry were as follows: mouse IgG1 and IgG2 isotype controls, anti-CD45 (clone HI30), anti-CD18 (clone 6.7) and anti-leukocyte-associated immunoglobin-like-receptor-1 (LAIR-1) (clone 14) from BD Biosciences (San Diego, CA, USA); and anti-PSGL-1 (clone PL-2) and anti-CD81 (clone JS64) from Beckman Coulter (Miami, FL, USA). Antibodies for western blotting were as follows: rabbit anti-α-actinin-4 was a gift from M. R. Pollack (Renal Medicine Division, Brigham and Women’s Hospital, Boston, MA, USA); mouse anti-β-actin (clone AC-15) was from Sigma-Aldrich (St Louis, MO, USA).
Fig. 4.
Flow cytometry analysis. Microparticles (MPs) generated by all four stimuli were analyzed for the presence of various proteins, using flow cytometry. (A) Data are representative results of two or three analyses for each antigen. Mean fluorescence intensity (MFI) ratio indicates MFIspecific antibody/MFIcontrol; % positive refers to percentage of MPs positive for the specific antigen. For each antigen and MP population tested, except for leukocyte-associated immunoglobin-like-receptor-1 (LAIR-1) staining on spontaneous, IgG, and LPS MPs, the difference between antibody and isotype control histograms was statistically significant as determined by chi-square test. (B) Representative histograms showing CD81 expression (black line) compared to isotype control (gray line) for each MP population as indicated. LPS, lipopolysaccharide; P-sel-Ig, P-selectin-Ig chimera; PSGL-1, P-selectin glycoprotein ligand-1.
MP generation and isolation
THP-1 cells harvested in log growth phase were reseeded at 4 × 106 cells mL−1 in complete medium. See Supplementary Materials and methods for culture conditions. Sixteen hours later, cells were centrifuged and resuspended in complete medium. Cells were stimulated with LPS (10 µg mL−1; Escherichia coli 055:B5; Sigma-Aldrich, St Louis, MO, USA), P-sel-Ig (100 µg mL−1), IgG (100 µg mL−1), or phosphate-buffered saline (PBS), and rotated continuously at 37 °C for 6 h [8]. After stimulation, cell viability was measured using Trypan blue. Only samples with < 5% cell death were accepted. Cells were removed by centrifugation at 750 × g and 1500 × g for 15 min and 5 min, respectively; MPs were pelleted by centrifugation at 16 000 × g for 45 min [13].
Flow cytometry analysis
MP number; PS expression
To determine MP number and measure PS expression, pelleted MPs were resuspended in HEPES buffer [7] with 2.5 mm CaCl2 and stained with calcein-AM and Alexa Fluor 647 annexin V conjugate (5 µg mL−1 each) (Molecular Probes/Invitrogen, Carlsbad, CA, USA) for 20 min at ambient temperature. Stained MPs were diluted in HEPES buffer and analyzed using FACSCalibur (Becton Dickinson, Franklin Lakes, NJ, USA).
Antigen expression; size distribution
MPs were costained with Alexa Fluor 647-labeled antibodies and calcein-AM. For size assessment, size-calibrated, Dragon Green-coupled polystyrene microspheres (Bangs Laboratories; Fishers, IN, USA) were used. MPs and microspheres were analyzed using FACSAriaII (BD Biosciences, San Jose, CA, USA).
For all experiments, MP samples were accepted if stimulation produced at least three-fold more MPs, defined as calcein-positive events, than non-stimulation. Flow cytometry data were analyzed using flow jo software version 8.5.2 (Tree Star Inc., Ashland, OR, USA). See Supplementary Materials and methods for information on gating and analysis.
Proteomic analysis
MPs for each population were batch-produced, pelleted and pooled in order to generate 50 µg of total protein for proteomic analysis (see Supplementary Materials and methods for determination of protein concentration); 4%–20% 7-cm sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel lanes were cut into 24 bands, which were trypsin-digested and subjected to liquid chromatography coupled to tandem mass spectrometry as before [16,17]. Spectra were searched using the Sequest algorithm [18] against the International Protein Index database (http://www.ebi.a-c.uk/IPI/IPIhelp.html). The probability-based evaluation program Protein Prophet (PP) was used for filtering identifications [19]. Comparisons between the MP populations were based on a strict present/absent approach. A protein was defined as present in a given population if the PP score was ≥ 0.9, and absent if it was undetected or the PP score was ≤ 0.5. A protein was not considered to be differentially expressed if it had a score of ≥ 0.9 in a given population, but at the same time had a score between > 0.5 and < 0.9 in another. Babelomics (http://babelomics.bioinfo.cipf.es/EntryPoint?loadForm=fatigo) and swissprot (http://www.expasy.org/sprot/) searches were used to extract Gene Ontology annotations (see Supplementary Materials and methods for more details).
Electron microscopy of MPs
Samples were prepared for transmission electron microscopy by applying highly concentrated MPs in PBS for 15 min to a coverslip precoated with 1 mg mL−1 poly(l-lysine) (Sigma-Aldrich). Coverslips were rapidly frozen on a helium-cooled copper block without fixation, and transferred to a Cressington freeze–fracture machine (CFE-50; Watford, UK) at − 170 °C. Samples were fractured with a liquid nitrogen-cooled knife, and either immediately rotary-coated with 2 nm of platinum at a 20° angle and then coated with 3 nm of carbon without rotation (metal cast), or fractured and warmed to − 98 °C for 5 min and metal cast. Replicas were floated off the coverslip using 25% hydrofluoric acid, washed in water, and collected on 200-mesh copper grids coated with Formvar. Grids were photographed in a JEOL 1200-EX electron microscope (JEOL Ltd, Tokyo, Japan) at 100 kV.
Western blot analysis
MPs were solubilized in sodium dodecylsulfate lysis buffer and incubated at 95 °C for 5 min. Lysed MPs were separated using SDS-PAGE and transferred to poly(vinylidene difluoride) membranes. Western blots were performed under standard conditions, using the appropriate antibodies listed above, and developed using an enhanced chemiluminescence system (Pierce, Rockford, IL, USA).
MP procoagulant activity and TF expression
TF expression on lysed MPs was measured using the IMUBIND Tissue Factor enzyme-linked immunosorbent assay (ELISA) kit from American Diagnostica (Stamford, CT, USA), following the manufacturer’s instructions. The absorbance at 450 nm was compared with that of a standard curve fitted to a quadratic equation, in order to determine the amount of TF in each sample. Procoagulant activity was measured using a STart 4 coagulometer (Diagnostica Stago, Parsippany, NJ, USA). An equal volume of MPs in Tris-buffered saline (125 mm Tris-HCl, pH 7.4, 150 mm NaCl) and pooled normal plasma (George King Bio-Medical, Overland Park, KS, USA) were incubated for 2 min at 37 °C. Coagulation was initiated with 50 µL of 25 mm CaCl2 (at 37 °C), and time to clot formation was measured.
Results
We generated MPs from THP-1 cells, comparing four populations – those stimulated for 6 h with LPS or P-sel-Ig (LPS MPs and P-sel-Ig MPs, respectively) and those considered ‘non-stimulated’, incubated with IgG1κ control or PBS (IgG MPs and spontaneous MPs, respectively). We developed a new criterion to define an MP, using calcein-positive events identified by flow cytometry. Calcein-AM, a non-fluorescent calcein acetomethyl ester, is membrane-permeable, with negligible membrane binding [20]. Upon cleavage by cytosolic esterases, calcein binds to calcium, becomes fluorescent, and is retained within the cytoplasm-containing cell/vesicle. Because only intact MPs will fluoresce, we avoid concomitant staining of debris seen with lipid dyes. When we disrupted either THP-1 cells or MPs by rapid freeze–thaw cycles, we saw a dramatic reduction in calcein-AM staining, validating our method of staining intact MPs (Fig. S1 for THP-1 cells).
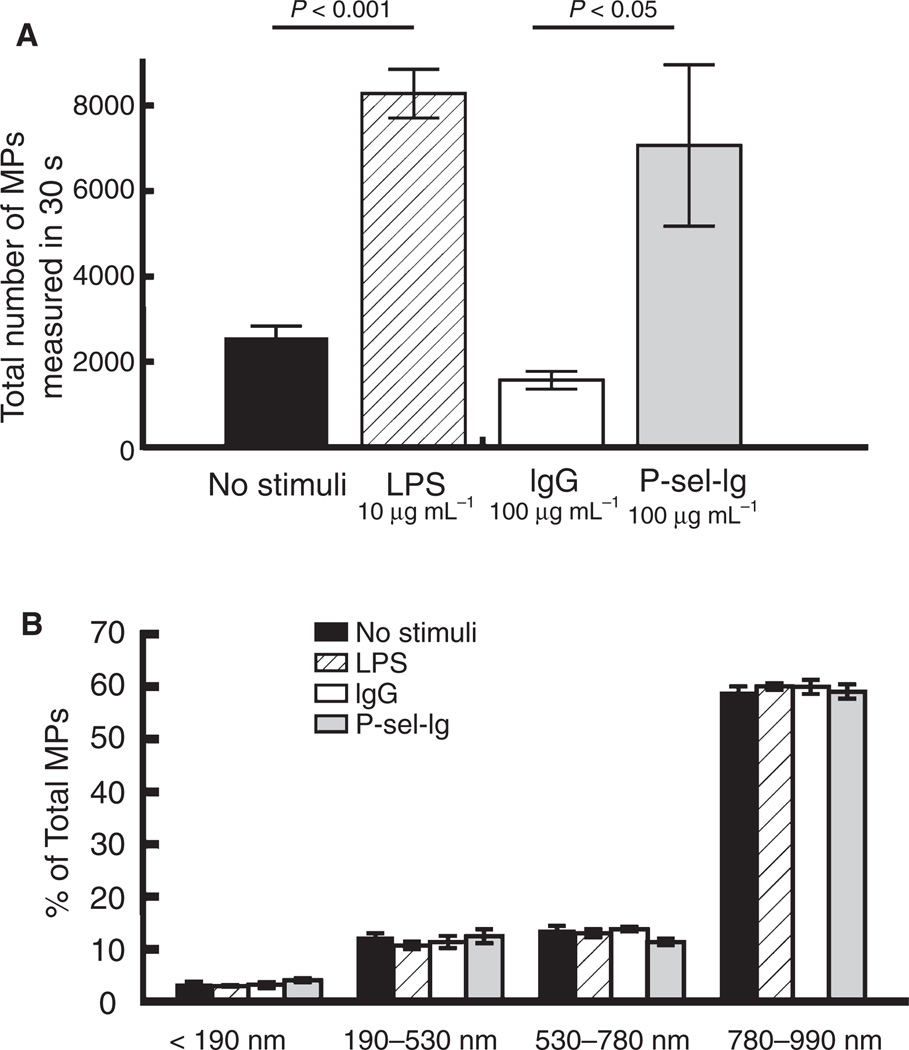
Both LPS and P-sel-Ig stimulated the production of three-fold to four-fold more MPs than controls (Fig. 1A). After stimulation, we evaluated cell viability, accepting only samples from cells with greater than 95% viability to ensure that our MPs were predominantly due to stimulation and not cell death. We determined whether different stimuli affect the size distribution of the resulting MPs by flow cytometry. Size-calibrated beads showed discrimination between diameters 190, 530, 780 and 990 nm (Fig. S2). The size distribution was similar in all four MP populations (Fig. 1B).
Fig. 1.
Microparticle (MP) number and size distribution. MP numbers and size distribution were measured by flow cytometry. Data represent mean ± standard error of the mean. (A) Lipopolysaccharide (LPS) and P-selectin-Ig chimera (P-sel-Ig) stimulations (6 h) produced three-fold to four-fold more MPs than controls (6 h) (n = 6–7). (B) The size distribution of calcein-positive MPs was similar in all four populations, using forward scatter (FSC)-A gates defined by size-calibrated polystyrene beads (n = 6).
PS expression on MP surface
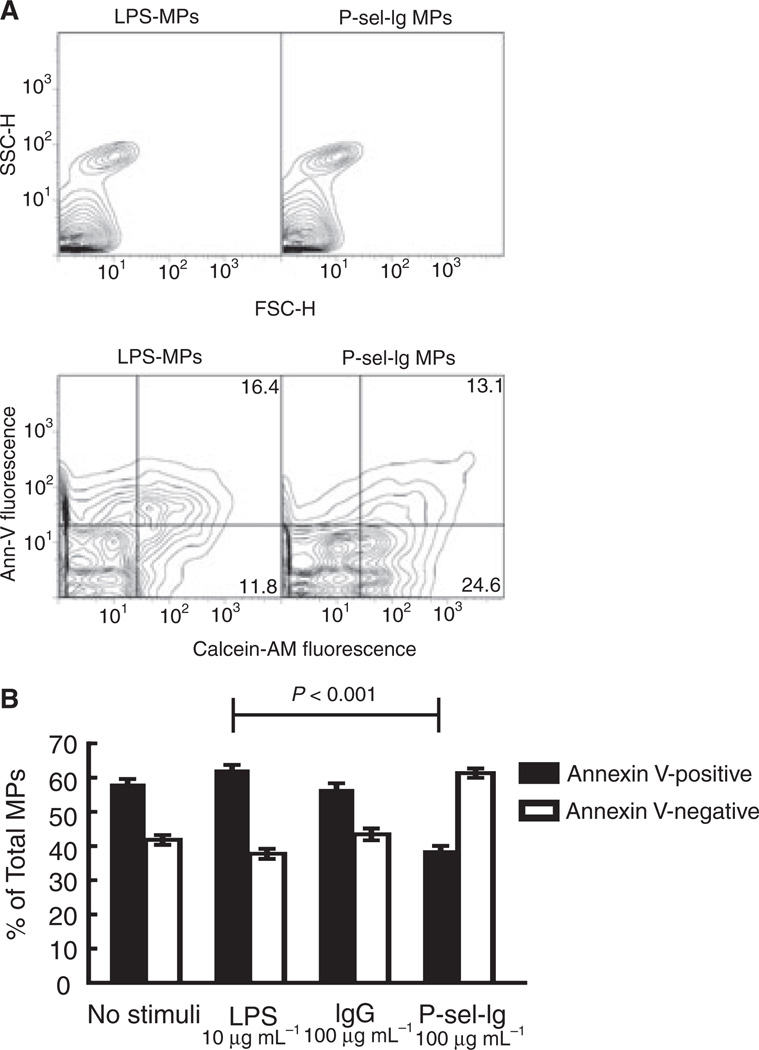
Many groups use PS expression to measure MP generation; therefore, we assayed our MPs for PS expression by labeling them with fluorescently labeled annexin V, a protein that specifically binds to PS. All four MP populations had a portion that bound annexin V (PS-positive) and a portion that failed to bind annexin V (PS-negative). A large percentage of P-sel-Ig MPs (~ 60%) were PS-negative, in contrast to the other MP populations, with only ~ 40% PS-negative MPs (Fig. 2). This was our first evidence that MPs generated from the same parental cells via different stimuli differ, and led us to investigate further those differences.
Fig. 2.
Representative flow cytometry plots and phosphatidylserine (PS) expression. (A) Representative flow cytometry plots of microparticles (MPs) produced by lipopolysaccharide (LPS) (left) or P-selectin-Ig chimera (P-sel-Ig) (right) obtained with FACSCalibur. Top panel: side scatter (SSC) vs. forward scatter (FSC) scattergrams; MP populations appear similar. Bottom panel: annexin V (Ann-V) vs. calcein-AM fluorescence for these same populations. LPS and P-sel-Ig MPs have similar proportions of MPs that stain positive for both annexin V and calcein-AM (16.4% and 13.1%, respectively); however, P-sel-Ig MPs have a greater proportion of MPs in the lower right quadrangle that are positive for calcein-AM but are annexin V-negative (24.6% vs. 11.8% for LPS MPs). (B) LPS and P-sel-Ig MP populations differ in PS expression. P-sel-Ig stimulation generated fewer PS-positive MPs than the other stimuli. Data represent mean ± standard error of the mean, n = 14–16.
Proteomic analysis of MPs
We utilized a proteomic approach to identify the protein composition of each MP population. SDS-PAGE-separated proteins were trypsin-digested and subjected to peptide tandem mass spectrometry. In total, 1076 proteins were identified across the four populations, with 199 proteins in the IgG subgroup, 331 in the P-sel-Ig subgroup, 457 in the spontaneous fraction, and 830 in the LPS population (Table 1; see Table S1 for a complete list of identified proteins in each population). The identified proteins represented many different protein subtypes, including cytoskeletal proteins, adhesion receptors, and signaling molecules. Table 2 highlights proteins of interest chosen to represent subcellular locations and functions, and their distribution among the MP populations.
Table 1.
Protein numbers from microparticle populations
| Total | Unique to one |
Common to stimulated or unstimulated |
Common to all |
||
|---|---|---|---|---|---|
| Stimulated | P-sel-Ig | 331 | 52 | 42 | 100 |
| LPS | 830 | 408 | |||
| Unstimulated | IgG No stimuli | 199 | 34 | 3 | |
| 457 | 87 |
LPS, lipopolysaccharide; P-sel-Ig, P-selectin-Ig chimera.
Table 2.
Select proteins identified via proteomics with International Protein Index (IPI) accession numbers, function, and mass spectrometry Protein Prophet (PP) scores
| PP score† |
||||||
|---|---|---|---|---|---|---|
| Accession number | Protein | Function* | IgG | P-sel-Ig | No stimulus | LPS |
| IPI00021439 | β-Actin | Cytoskeletal structure | 1 | 1 | 1 | 1 |
| IPI00013808 | α-Actinin-4 | Actin binding | 0.99 | 1 | 1 | 1 |
| IPI00298994 | Talin | Actin, integrin binding | 1 | 1 | 1 | 1 |
| IPI00005161 | p34arc | Arp 2/3 subunit; actin filament branching |
ND | 1 | ND | ND |
| IPI00000477 | ND | 0 | ND | 1 | ||
| IPI00554811 | p20arc | Arp 2/3 subunit; actin filament branching |
ND | ND | ND | 1 |
| IPI00642191 | 1 | 1 | 1 | 0 | ||
| IPI00657756 | LAIR-1 | Inhibitory immune regulation | ND | 0.99 | ND | ND |
| IPI00000190 | CD81 | Proliferation regulation | 1 | 1 | ND | 1 |
| IPI00155168 | CD45 | Tyrosine phosphatase | 1 | 1 | 1 | 1 |
| IPI00291792 | β2-Integrin | Cell adhesion/signaling | 1 | 1 | 1 | 1 |
| IPI00644788 | αL-Integrin | Cell adhesion/signaling | 1 | 1 | 1 | 1 |
| IPI00217563 | β1-Integrin | Fibronectin receptor | 0 | 0.95 | ND | 0 |
| IPI00645194 | 1 | ND | ND | 1 | ||
| IPI00306604 | α5-Integrin | Fibronectin receptor | 0.9 | 1 | 0.97 | 1 |
| IPI00412656 | β7-Integrin | Cell adhesion | ND | ND | 0 | 1 |
| IPI00299412 | CD97 | Inflammatory response regulator |
ND | 0.99 | ND | ND |
| IPI00253036 | CD99 | Cell adhesion | ND | 0.97 | ND | ND |
| IPI00021854 | ApoAII | Lipoprotein metabolism | ND | 1 | ND | ND |
| IPI00414264 | Sorcin | Ca2+ binding | ND | 0.96 | ND | 1 |
| IPI00002460 | Annexin a7 | Ca2+ gate regulation | ND | ND | 1 | 1 |
| IPI00218733 | Superoxide dismutase | Oxidative stress response | ND | 1 | ND | 1 |
| IPI00216231 | Snap23b | t-SNARE activity | ND | 1 | ND | 0.95 |
| IPI00465315 | Cytochrome c | Electron transport | ND | 0.99 | ND | 1 |
| IPI00514407 | HMGCL | Metabolic activity | ND | 0.99 | ND | 0.99 |
| IPI00294779 | VDAC3 | Mitochondrial voltage gate regulation |
ND | 0.86 | 1 | 1 |
| IPI00420014 | 200-kDa helicase | Nuclear splicing | ND | ND | ND | 0.99 |
ApoAII, apolipoprotein AII; Arp, actin-related protein; Snap23b, synaptosomal-associated protein 23; HMGCL, 3-hydroxymethyl-3-methyl-glutaryl-coenzyme A lyase; LPS, lipopolysaccharide; P-sel-Ig, P-selectin-Ig chimera; VDAC3, voltage-dependent anion-selective channel protein 3.
Functions based on Gene Ontology analysis.
PP scores range from ND (not detected) and 0 to 1 [19].
Proteins with PP scores ≥ 0.9 were considered to be present.
Using a present/absent approach, we found 100 proteins common to all four MP populations. An additional 42 proteins were common to only the LPS and P-sel-Ig MPs (Table 1), suggesting that these proteins may be particular to MPs obtained through stimulation. Notably, we found proteins unique to each MP subtype, in particular 52 proteins unique to the P-sel-Ig MPs and 408 unique to the LPS MPs (Table 1). Thus, it appears that the compositions of MPs obtained through P-sel-Ig and LPS stimulation may be distinct.
Similarities among MPs
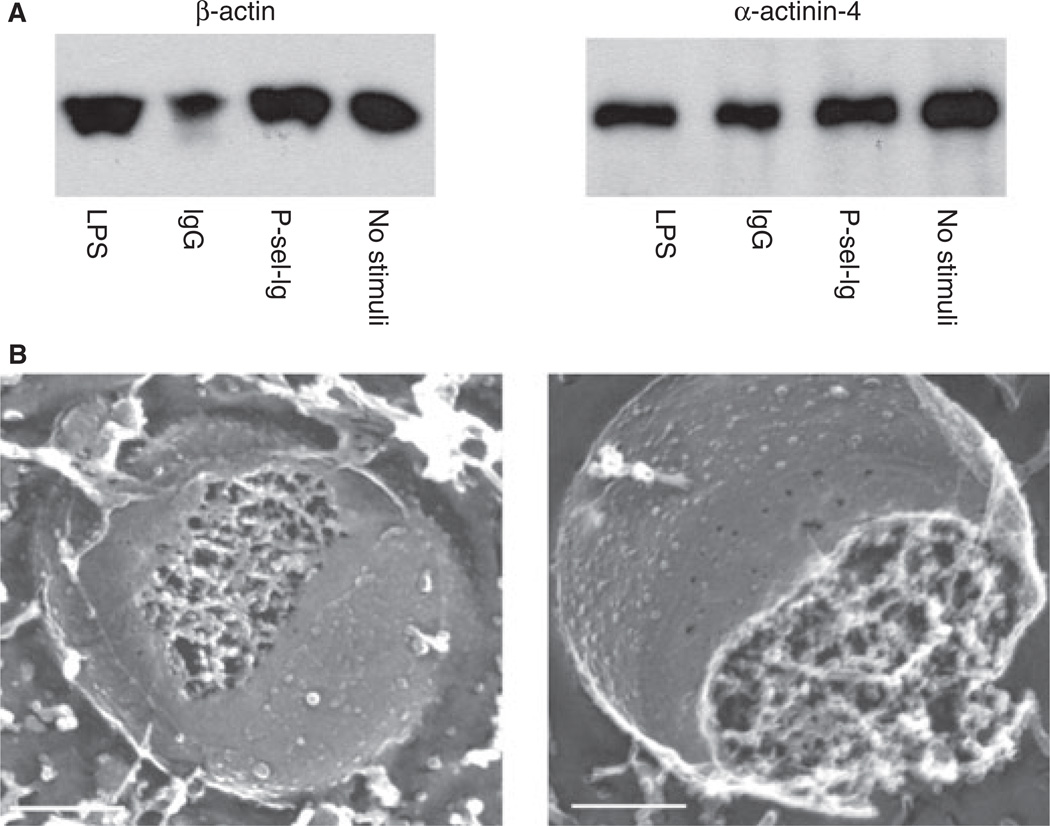
Of the 100 proteins shown by proteomics to be common to all MPs, many were cytoskeletal proteins, such as β-actin and α-actinin 4 (Table 2); we confirmed this using western blotting (Fig. 3A). In addition, subunits of the actin-related protein 2/3 complex involved in the branching of actin filaments were found in all four populations (Table 2). The presence of other proteins common to all four populations, such as CD18, CD81, and CD45, was confirmed using flow cytometry, as was the presence of proteins of particular interest not identified by proteomics, such as PSGL-1 (Fig. 4). CD11a, a subunit of the integrin complex αLβ2 formed with CD18, was also identified by proteomics in all four MP populations (Table 2). Well-known THP-1 antigens, such as PSGL-1, were not identified in our proteomics approach, possibly missing detection by mass spectrometry because of their extensive post-translational modifications. We tested PSGL-1 expression on our MPs using flow cytometry analysis, and demonstrated its expression on all of the MP classes (Fig. 4A).
Fig. 3.
Cytoskeleton. (A) Western blots for β-actin (left) and α-actinin-4 (right) in all four microparticle (MP) populations. Equal amounts of total protein for each MP sample were loaded on polyacrylamide gels. (B) Freeze--fracture electron microscopy of an MP obtained by P-selectin-Ig chimera (P-sel-Ig) (left) and lipopolysaccharide (LPS) (right) stimulation. Scale bars are 0.2 µm.
Owing to the large number of identified cytoskeletal proteins, we postulated that MPs could contain organized cytoskeleton, and investigated this in the electron microscope after fracturing and etching rapidly frozen MPs. Metal-cast images of the LPS and P-sel-Ig MPs showed that MPs contain an internal three-dimensional protein scaffolding similar in structure to the highly branched cytoskeleton of cells. The internal cytoskeletons of the MPs appeared identical, independently of the stimulus used to produce the MPs (Fig. 3B).
Differences among MPs
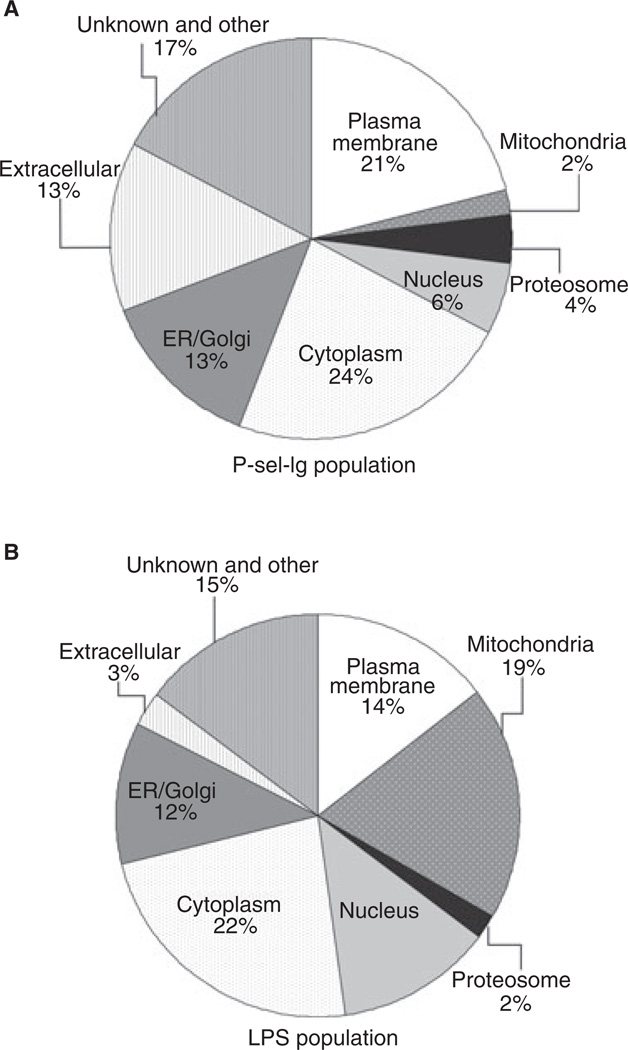
Despite the similarities, there were also significant differences among the four MP populations, in addition to the difference in PS expression (Fig. 2). When comparing proteomic identifications of those proteins that were only present in LPS MPs (408) or only present in P-sel-Ig MPs (52), we found that a large number of these LPS proteins were mitochondrial or nuclear and associated with metabolism and energy pathways, whereas a larger fraction of the P-sel-Ig MP proteins were part of the plasma membrane and involved in signal transduction and cell communication (Fig. 5; Fig. S3). In addition, LAIR-1 – a cell surface protein – was identified by proteomics in only the P-sel-Ig MPs (Table 2), and we confirmed this by flow cytometry (Fig. 4A).
Fig. 5.
Subcellular location of microparticle (MP) proteins. Proteins identified in only (A) P-selectin-Ig chimera (P-sel-Ig) or (B) lipopolysaccharide (LPS) populations were categorized on the basis of subcellular location as a result of Gene Ontology analysis. The largest differences are the higher percentage of plasma membrane proteins found in P-sel-Ig MPs, and mitochondrial and nuclear proteins in LPS MPs. ER, endoplasmic reticulum.
We also used flow cytometry to define the relative abundances of several proteins identified by proteomics across the four MP populations. CD45, a leukocyte marker, was more highly expressed on P-sel-Ig MPs than on the other MP populations, as shown by a mean fluorescence intensity ratio twice that of the other populations (Fig. 4A). Similarly, CD18, a β2-integrin protein, had higher expression levels on P-sel-Ig MPs (Fig. 4A).
MP and procoagulant activity
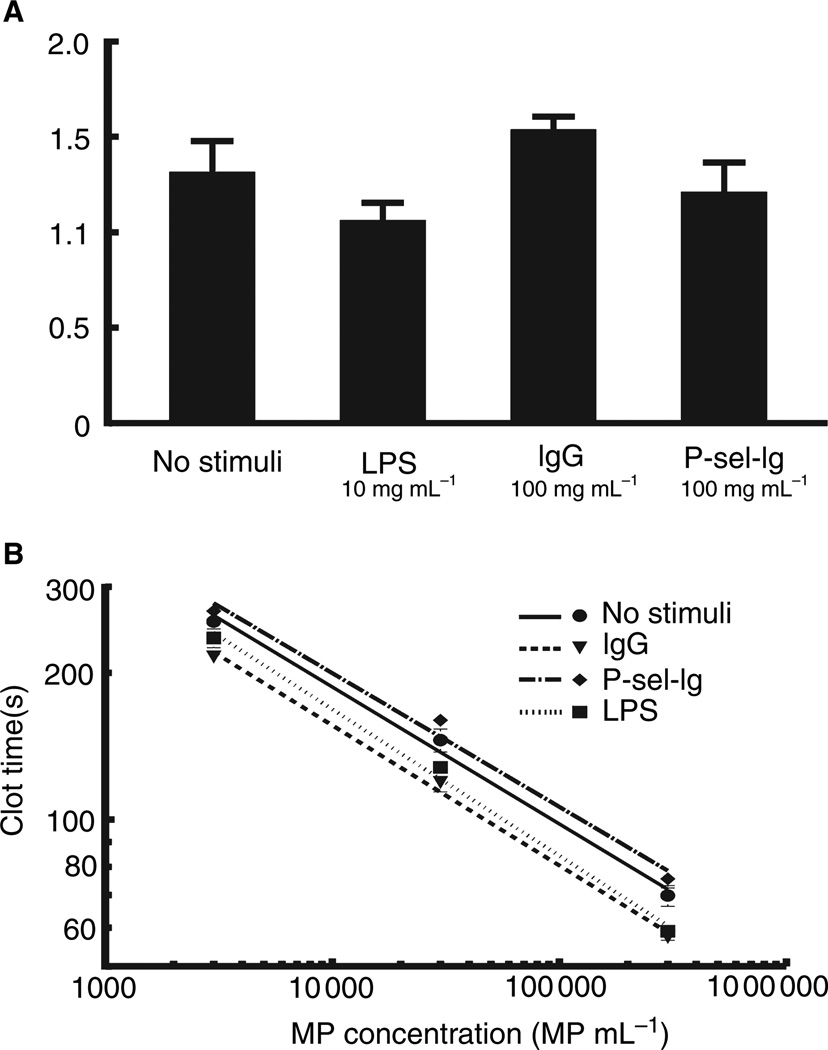
Functional comparisons of the biological activities of these MPs are difficult, because the LPS cannot be removed completely from the MPs, and the contamination is likely to generate cellular responses. We ascertained the procoagulant nature of the MPs in a cell-free environment using platelet-poor plasma. The coexpression of PS and TF on the MP surface renders them highly procoagulant [4,11]. The four MP populations were assayed for TF content by ELISA. TF was not detected in the proteomic analysis, but was present in all four MP populations at nearly identical levels (expressed as ng/MP; Fig. 6A). The procoagulant activity was measured, and for all MP populations, clotting was dose-dependent, with higher MP concentrations yielding shorter clot times, with no major differences in activity among the four populations (Fig. 6B). The addition of corn trypsin inhibitor – an inhibitor of the contact pathway – had no effect on clot times at the MP concentrations tested (not shown).
Fig. 6.
Tissue factor (TF) expression and procoagulant activity. (A) TF content was measured by enzyme-linked immunosorbent assay of lysed microparticles (MPs). There were no significant differences in TF expression among the four MP populations. Data represent mean ± standard error of the mean (SEM); n = 4. (B) Procoagulant activity was determined using a two-stage clotting assay. Clot times decreased with increasing MP concentration. Clot times were similar for all four MP populations. Data represent mean ± SEM; n = 4. LPS, lipopolysaccharide; P-sel-Ig, P-selectin-Ig chimera.
Discussion
Cellular changes upon stimulation have been extensively studied, providing insights into the pathophysiology of the cell. MPs recently gained interest because they are elevated in numerous diseases and possess biological functions [1]. This current study characterizes MPs produced from THP-1 cells upon different pathophysiologic stimuli. Our goal was to unveil proteins common to all MPs or unique to MPs generated through specific stimuli, in order to gain insights into the biology of MP formation and predict common and unique effects of these MPs. Using a proteomic approach, we identified 1076 proteins across four MP populations, and we complemented this analysis with flow cytometry, western blotting, and electron microscopy.
MPs’ small size places them at the limit of resolution for most investigational tools used for cell studies, and they are difficult to distinguish from exosomes and debris. We addressed these limits by centrifuging the MPs at 16 000 × g, not ultracentrifuging, in order to pellet only MPs and retain small MPs indistinguishable in size from exosomes in the supernatant. We employed a new criterion of calcein-AM to define an MP, allowing us to measure only intact vesicles and avoid cellular fragments. By diminishing calcein-AM staining through disruption of THP-1 cells and MPs, we verified that calcein-AM preferentially stains intact cells/vesicles, supporting previous work showing that disrupted erythrocytes lose calcein-AM staining [21]. We also found that platelet-derived MPs stain positive for calcein-AM.
Non-differentiated THP-1 cells lack CD14, the coreceptor for LPS; [22] therefore, higher LPS doses are necessary for stimulation. Use of this well-established monocyte-like cell line nevertheless allows high uniformity among cells and higher MP – and therefore protein – yields than are obtainable from monocyte preparations. A higher concentration of P-sel-Ig (100 µg mL−1) was also needed to stimulate THP-1 cells than to stimulate human blood [8].
Similarities among MPs
The four MP populations had a similar size distribution, reflecting uniformity in structure and a potentially similar mechanism of formation. This similar size profile allowed us to compare MPs using flow cytometry, with the resulting fluorescence intensities reflecting antigen density and not particle size. This similarity in size was also verified by the electron microscopy studies, where the mean sizes of MPs in the different populations were found to be comparable.
Cell morphology and vesicle formation depend on the presence of an intact cytoskeleton. Previous studies have shown that cytoskeletal remodeling drugs diminish MP production [23], suggesting that actin remodeling is necessary for MP formation. Proteomic analysis and western blotting identified several components of the cytoskeleton in all four populations. Using electron microscopy imaging, we demonstrated that not only do MPs contain cytoskeletal proteins, but these are organized into a complex cytoskeletal network. The presence of an intact cytoskeleton suggests that the cytoskeleton of the parent cell must break down to allow the MP to form, and then must re-form during MP generation. The cell biology of MP formation and the precise role that the cytoskeleton plays require further investigation.
Of particular interest to this study was the identification of lymphocyte function-associated antigen-1 (LFA-1; CD11a/CD18) on the MPs. LFA-1 binds to intercellular adhesion molecule-1 on antigen-presenting cells or endothelial cells [24]. Both proteomic and flow cytometry analyses showed high levels of CD18, with more being expressed on P-sel-Ig MPs. This agrees with observations of increased CD18 expression on monocyte surfaces upon interaction with platelets [25]. Proteomics also identified CD11a on all four MP populations. The presence of LFA-1 on the MP surface suggests that these MPs could be involved in the immune response. In addition, LFA-1 may bring MPs to the endothelium, causing endothelial activation/dysfunction [3,26].
TF ELISA demonstrated equal amounts of TF among the four MP populations, reflecting the constitutive expression of TF on THP-1 cells. Even upon LPS stimulation, TF expression on the THP-1 cells was not dramatically upregulated (not shown). This is in contrast to experiments utilizing freshly isolated monocytes, where TF is highly upregulated upon stimulation with LPS or P-selectin [27]. We can conclude that TF is not targeted differentially to the MPs according to the stimulus.
The procoagulant assay showed only minimal differences in clotting times among the four MP populations. As corn trypsin inhibitor had no effect, it is evident that MP-initiated clotting occurs through the extrinsic pathway, and is therefore dependent on the presence of PS and TF. As TF expression is equal among the MPs, the procoagulant activity of the P-sel-Ig MPs was expected to be lower, owing to the smaller proportion of PS-expressing MPs. This somewhat surprising result can be explained by the fact that there is still a population of P-sel-Ig MPs that do express PS on their surface, and even though the procoagulant activity of the MPs was similar in all four populations, the P-sel-Ig MPs had slightly longer clotting times at each concentration tested, especially as compared with the LPS and IgG MP populations.
Differences among MPs
Our study uncovered significant differences among the MP populations, emphasizing the importance of specific stimulation of the MPs. Although all four populations contained MPs expressing PS, P-sel-Ig MPs had fewer PS-positive MPs overall. Many investigators use PS expression to define an MP; however, our results – and those of others [28] – suggest that not all MPs express PS, and limiting selection to those that do excludes an entire MP population; in our model, this represents 40–60% of the MPs generated. The biological importance of PS-negative MPs is as yet unknown, but we can hypothesize that translocation of PS from the inner to the outer membrane bilayer is modulated differentially according to the stimulus.
LAIR-1, an inhibitory receptor that is constitutively expressed on almost all immune cells, binds to collagen directly, inhibiting immune cell activation in order to regulate the immune response [29,30]. The engagement of LAIR-1 with collagen suggests a functional role for LAIR-1 as an adhesion molecule, aiding in the adherence of cells to the extracellular matrix [30]. Our analysis showed LAIR-1 on the surface of only P-sel-Ig MPs, reflecting selective incorporation of LAIR-1 into MPs only upon P-sel-Ig stimulation, permitting accumulation of MPs at sites of vascular injury where collagen is exposed, and localizing procoagulant MPs to these sites, thus promoting hemostasis. Thus, P-selectin, a hemostatically important molecule [31], may induce formation of MPs that are most suitable for this purpose.
MPs are believed to originate from lipid rafts [32] and, indeed, we identified the raft-associated protein CD81 [33] in all MP populations. The presence of CD45, a transmembrane protein on nucleated hematopoietic cells that modulates lymphocyte maturation and activation [34,35], is regulated in lipid rafts [36]. We found an increase in CD45 expression specifically in the P-sel-Ig MPs, suggesting that P-sel-Ig stimulation may result in translocation of CD45 into the lipid rafts of THP-1 cells, probably affecting the biological activity of the cell and consequently of the resulting MPs [37].
Proteomic analysis identified mitochondrial and nuclear proteins predominantly in LPS MPs. Further analysis of the mitochondrial proteins showed the presence of intermembrane proteins such as cytochrome c, released by either increased permeability of the mitochondrial membrane or membrane damage. The simultaneous finding of matrix proteins favors mitochondrial damage as the explanation for elevated mitochondrial proteins, with more extensive damage upon LPS stimulation. Analysis of the nuclear proteins identified by proteomics revealed large proteins such as histones and helicases. Because nucleoplasmic proteins are separated from the cytoplasm by pores that are impermeable to proteins larger than 60–110 kDa [38], the presence of these larger nuclear proteins indicates dysfunction of nuclear retention in LPS-treated cells.
Our study demonstrates that the characteristics of MPs are dependent on the stimulus used for generation. Some characteristics are common to all MPs, independently of stimulus, such as size similarity and an organized cytoskeleton. However, the differences between these MPs, for example the presence of LAIR-1 in only P-sel-Ig MPs, could be of great consequence. Differential stimulation of cells resulting in distinct MPs means that these MPs could have different functions within the vascular processes of hemostasis, inflammation, and immune regulation.
Supplementary Material
Acknowledgements
We thank the Conway Mass Spectrometry Core Facility, particularly G. Elia and K. Wynne, for technical assistance, J. Polgar for contributions to preliminary MP studies, L. Burke for assistance with the FACSAria II, and L. Cowan for help with manuscript preparation. This work was supported by the National Heart, Lung and Blood Institute of the National Institutes of Health grants P01 HL056949 (D. D. Wagner and J.H. Hartwig) and T32HL066987 (E. K. Waters), European Hematology Association Fellowship 2006/04 (M. Bernimoulin), Swiss National Science Foundation Fellowship PBLAB – 109593 (M. Bernimoulin), and research grants from the Health Research Board of Ireland (P. B. Maguire) and funding from the Programme for Research in Third-Level Institutions (PRTLI), administered by the Higher Education Authority of Ireland (P. B. Maguire).
Footnotes
Addendum
M. Bernimoulin, E. K. Waters, P. B. Maguire and D. D. Wagner designed the research, analyzed data, and cowrote the manuscript; M. Bernimoulin, E. K. Waters, M. Foy, B. M. Steele, M. Sullivan, M. T. Walsh and P. B. Maguire performed the research; M. Sullivan designed software; H. Falet performed western blots; N. Barteneva assisted with performance of flow cytometry experiments and analysis of the results; J.-G. Geng provided important reagents; and J. H. Hartwig performed electron microscopy and helped with cytoskeletal analysis.
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Supplementary Materials and methods.
Fig. S1. Effect of freeze–thaw on calcein-AM labeling of THP-1 cells.
Fig. S2. Forward scatter (FSC-H) resolution with size-calibrated fluorescent beads.
Fig. S3. Cellular processes of MP proteins.
Table S1. A complete list of the 1076 proteins identified across the four MP populations.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Hugel B, Martinez MC, Kunzelmann C, Freyssinet JM. Membrane microparticles: two sides of the coin. Physiology (Bethesda) 2005;20:22–27. doi: 10.1152/physiol.00029.2004. [DOI] [PubMed] [Google Scholar]
- 2.Lynch SF, Ludlam CA. Plasma microparticles and vascular disorders. Br J Haematol. 2007;137:36–48. doi: 10.1111/j.1365-2141.2007.06514.x. [DOI] [PubMed] [Google Scholar]
- 3.Mesri M, Altieri DC. Leukocyte microparticles stimulate endothelial cell cytokine release and tissue factor induction in a JNK1 signaling pathway. J Biol Chem. 1999;274:23111–23118. doi: 10.1074/jbc.274.33.23111. [DOI] [PubMed] [Google Scholar]
- 4.Satta N, Toti F, Feugeas O, Bohbot A, Dachary-Prigent J, Eschwege V, Hedman H, Freyssinet JM. Monocyte vesiculation is a possible mechanism for dissemination of membrane-associated procoagulant activities and adhesion molecules after stimulation by lipopolysaccharide. J Immunol. 1994;153:3245–3255. [PubMed] [Google Scholar]
- 5.McEver RP. GMP-140: a receptor for neutrophils and monocytes on activated platelets and endothelium. J Cell Biochem. 1991;45:156–161. doi: 10.1002/jcb.240450206. [DOI] [PubMed] [Google Scholar]
- 6.Michelson AD, Barnard MR, Hechtman HB, MacGregor H, Connolly RJ, Loscalzo J, Valeri CR. In vivo tracking of platelets: circulating degranulated platelets rapidly lose surface P-selectin but continue to circulate and function. Proc Natl Acad Sci USA. 1996;93:11877–11882. doi: 10.1073/pnas.93.21.11877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andre P, Hartwell D, Hrachovinova I, Saffaripour S, Wagner DD. Pro-coagulant state resulting from high levels of soluble P-selectin in blood. Proc Natl Acad Sci USA. 2000;97:13835–13840. doi: 10.1073/pnas.250475997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hrachovinova I, Cambien B, Hafezi-Moghadam A, Kappelmayer J, Camphausen RT, Widom A, Xia L, Kazazian HH, Jr, Schaub RG, McEver RP, Wagner DD. Interaction of P-selectin and PSGL-1 generates microparticles that correct hemostasis in a mouse model of hemophilia A. Nat Med. 2003;9:1020–1025. doi: 10.1038/nm899. [DOI] [PubMed] [Google Scholar]
- 9.Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74:541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 10.Palabrica T, Lobb R, Furie BC, Aronovitz M, Benjamin C, Hsu YM, Sajer SA, Furie B. Leukocyte accumulation promoting flbrin deposition is mediated in vivo by P-selectin on adherent platelets. Nature. 1992;359:848–851. doi: 10.1038/359848a0. [DOI] [PubMed] [Google Scholar]
- 11.Robinson RA, Worfolk L, Tracy PB. Endotoxin enhances the expression of monocyte prothrombinase activity. Blood. 1992;79:406–416. [PubMed] [Google Scholar]
- 12.Jimenez JJ, Jy W, Mauro LM, Soderland C, Horstman LL, Ahn YS. Endothelial cells release phenotypically and quantitatively distinct microparticles in activation and apoptosis. Thromb Res. 2003;109:175–180. doi: 10.1016/s0049-3848(03)00064-1. [DOI] [PubMed] [Google Scholar]
- 13.Miguet L, Pacaud K, Felden C, Hugel B, Martinez MC, Freyssinet JM, Herbrecht R, Potier N, van Dorsselaer A, Mauvieux L. Proteomic analysis of malignant lymphocyte membrane microparticles using double ionization coverage optimization. Proteomics. 2006;6:153–171. doi: 10.1002/pmic.200500133. [DOI] [PubMed] [Google Scholar]
- 14.Sako D, Chang XJ, Barone KM, Vachino G, White HM, Shaw G, Veldman GM, Bean KM, Ahern TJ, Furie B, Cumming DA, Larsen GR. Expression cloning of a functional glycoprotein ligand for P-selectin. Cell. 1993;75:1179–1186. doi: 10.1016/0092-8674(93)90327-m. [DOI] [PubMed] [Google Scholar]
- 15.Wang HB, Wang JT, Zhang L, Geng ZH, Xu WL, Xu T, Huo Y, Zhu X, Plow EF, Chen M, Geng JG. P-selectin primes leukocyte integrin activation during inflammation. Nat Immunol. 2007;8:882–892. doi: 10.1038/ni1491. [DOI] [PubMed] [Google Scholar]
- 16.Coppinger JA, Cagney G, Toomey S, Kislinger T, Belton O, McRedmond JP, Cahill DJ, Emili A, Fitzgerald DJ, Maguire PB. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood. 2004;103:2096–2104. doi: 10.1182/blood-2003-08-2804. [DOI] [PubMed] [Google Scholar]
- 17.Coppinger JA, O’Connor R, Wynne K, Flanagan M, Sullivan M, Maguire PB, Fitzgerald DJ, Cagney G. Moderation of the platelet releasate response by aspirin. Blood. 2007;109:4786–4792. doi: 10.1182/blood-2006-07-038539. [DOI] [PubMed] [Google Scholar]
- 18.Yates JR, 3rd, Eng JK, McCormack AL, Schieltz D. Method to correlate tandem mass spectra of modified peptides to amino acid sequences in the protein database. Anal Chem. 1995;67:1426–1436. doi: 10.1021/ac00104a020. [DOI] [PubMed] [Google Scholar]
- 19.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 20.Kendall DA, MacDonald RC. A fluorescence assay to monitor vesicle fusion and lysis. J Biol Chem. 1982;257:13892–13895. [PubMed] [Google Scholar]
- 21.Bratosin D, Mitrofan L, Palii C, Estaquier J, Montreuil J. Novel fluorescence assay using calcein-AM for the determination of human erythrocyte viability and aging. Cytometry A. 2005;66:78–84. doi: 10.1002/cyto.a.20152. [DOI] [PubMed] [Google Scholar]
- 22.Pugin J, Kravchenko VV, Lee JD, Kline L, Ulevitch RJ, Tobias PS. Cell activation mediated by glycosylphosphatidylinositol-anchored or transmembrane forms of CD14. Infect Immun. 1998;66:1174–1180. doi: 10.1128/iai.66.3.1174-1180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yano Y, Kambayashi J, Shiba E, Sakon M, Oiki E, Fukuda K, Kawasaki T, Mori T. The role of protein phosphorylation and cytoskeletal reorganization in microparticle formation from the platelet plasma membrane. Biochem J. 1994;1:303–308. doi: 10.1042/bj2990303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marlin SD, Springer TA. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1) Cell. 1987;51:813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- 25.da Costa Martins PA, van Gils JM, Mol A, Hordijk PL, Zwaginga JJ. Platelet binding to monocytes increases the adhesive properties of monocytes by up-regulating the expression and functionality of beta1 and beta2 integrins. J Leukoc Biol. 2006;79:499–507. doi: 10.1189/jlb.0605318. [DOI] [PubMed] [Google Scholar]
- 26.Mesri M, Altieri DC. Endothelial cell activation by leukocyte microparticles. J Immunol. 1998;161:4382–4387. [PubMed] [Google Scholar]
- 27.Edwards RL, Rickles FR, Bobrove AM. Mononuclear cell tissue factor: cell of origin and requirements for activation. Blood. 1979;54:359–370. [PubMed] [Google Scholar]
- 28.Shet AS, Aras O, Gupta K, Hass MJ, Rausch DJ, Saba N, Koopmeiners L, Key NS, Hebbel RP. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102:2678–2683. doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- 29.Lebbink RJ, de Ruiter T, Adelmeijer J, Brenkman AB, van Helvoort JM, Koch M, Farndale RW, Lisman T, Sonnenberg A, Lenting PJ, Meyaard L. Collagens are functional, high affinity ligands for the inhibitory immune receptor LAIR-1. J Exp Med. 2006;203:1419–1425. doi: 10.1084/jem.20052554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyaard L. The inhibitory collagen receptor LAIR-1 (CD305) J Leukoc Biol. 2008;83:799–803. doi: 10.1189/jlb.0907609. [DOI] [PubMed] [Google Scholar]
- 31.Cambien B, Wagner DD. A new role in hemostasis for the adhesion receptor P-selectin. Trends Mol Med. 2004;10:179–186. doi: 10.1016/j.molmed.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Del Conde I, Shrimpton CN, Thiagarajan P, Lopez JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106:1604–1611. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- 33.Wolf Z, Orso E, Werner T, Boettcher A, Schmitz G. A flow cytometric screening test for detergent-resistant surface antigens in monocytes. Cytometry A. 2006;69:192–195. doi: 10.1002/cyto.a.20238. [DOI] [PubMed] [Google Scholar]
- 34.Koretzky GA, Picus J, Schultz T, Weiss A. Tyrosine phosphatase CD45 is required for T-cell antigen receptor and CD2-mediated activation of a protein tyrosine kinase and interleukin 2 production. Proc Natl Acad Sci USA. 1991;88:2037–2041. doi: 10.1073/pnas.88.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kishihara K, Penninger J, Wallace VA, Kundig TM, Kawai K, Wakeham A, Timms E, Pfeffer K, Ohashi PS, Thomas ML, Furlonger C, Paige CJ, Mak TW. Normal B lymphocyte development but impaired T cell maturation in CD45-exon6 protein tyrosine phosphatase-deficient mice. Cell. 1993;74:143–156. doi: 10.1016/0092-8674(93)90302-7. [DOI] [PubMed] [Google Scholar]
- 36.Giurisato E, McIntosh DP, Tassi M, Gamberucci A, Benedetti A. T cell receptor can be recruited to a subset of plasma membrane rafts, independently of cell signaling and attendantly to raft clustering. J Biol Chem. 2003;278:6771–6778. doi: 10.1074/jbc.M210758200. [DOI] [PubMed] [Google Scholar]
- 37.Edmonds SD, Ostergaard HL. Dynamic association of CD45 with detergent-insoluble microdomains in T lymphocytes. J Immunol. 2002;169:5036–5042. doi: 10.4049/jimmunol.169.9.5036. [DOI] [PubMed] [Google Scholar]
- 38.Wang R, Brattain MG. The maximal size of protein to diffuse through the nuclear pore is larger than 60kDa. FEBS Lett. 2007;581:3164–3170. doi: 10.1016/j.febslet.2007.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.