Abstract
Melanocortin-3 receptor (MC3R), expressed in the hypothalamus and limbic systems of the brain, as well as by peripheral sites, plays an important role in the regulation of energy homeostasis and other physiological functions. Past work shows that MC3R-deficiency resulted in fat mass increase, feeding efficiency increase, hyperleptinemia and mild hyperinsulinemia in mice and human. MC3R belongs to G-protein coupled receptor (GPCR) family and many studies indicate that some cysteine residues in GPCR play key roles in maintaining receptor tertiary structure and function. In this study, we examined the role of cysteine residues in MC3R on receptor function. Human MC3R (hMC3R) has eighteen cysteine residues where they are located in the extracellular loops (ELs), the transmembrane domains (TMs) and the intracellular loops (ILs). We replaced these cysteines with serine and expressed these receptors in HEK-293 cells which lack endogenous MC3R. Our results indicate that five cysteines in eighteen of the hMC3R are important for hMC3R function. Mutations, C305S, C311S, and C313S in EL3, resulted in significant decrease in receptor expression and receptor function while two other mutations C115S and C162S in TM3 significantly decreased NDP-MSH binding affinity and potency. These results suggest that extracellular cysteine residue 305, 311 and 313 are crucial for receptor expression and the transmembrane cysteine residue, C115 and 162 are important for ligand binding and signaling. These findings provide important insights into the importance of cysteine residues of hMC3R on receptor tertiary structure and function.
Keywords: MSH, MC3R, cysteine, MCR, GPCR
Introduction
Obesity has become one of the most significant public health problems facing the world today [12, 18, 19]. Type 2 diabetes, associated with obesity, is today the most common form of diabetes. In the United States the prevalence of obesity has risen by 32% in adults and 40% in children over last two decades [15]. Patients face an increased risk of mortality and morbidity due to obesity associated diseases such as Type 2 diabetes mellitus, hypertension, obstructive sleep apnea, coronary artery diseases and cancer [1, 19, 23]. The melanocortin system has been identified to play an important role in the regulation of food intake and body weight in animals and humans. Genetic studies demonstrate that both MC3R and MC4R deficient mice are obese but their obese phenotypes are different. MC4R knock-out (KO) mice are hyperphagia but obese MC3R KO mice are not [3, 5]. Further extensive studies indicate that MC4R is mainly involved in the regulation of food intake but MC3R is mainly associated with the regulation of energy homeostasis [2, 7, 8]. Great effort and progress has been made in determining the role of MC4R in regulating food intake, much still remains to be learned regarding the molecular basis of MC3R in energy homeostasis. Previous pharmacological studies indicate that alpha melanocyte-stimulating hormone (α-MSH) is equally potent at MC3R and MC4R but γ-MSH has higher affinity at MC3R than MC4R. Agouti related protein (AGRP) is a potent antagonist at MC3R and MC4R but agouti inhibitory activity is significantly decreased at MC3R [33, 35, 36]. These pharmacological differences indicate that these two receptors, though members of the same system, are likely to possess functional and structural differences. Structure-function studies of MC3R should therefore provide important insights into the mechanism of MC3R for regulating energy homeostasis.
Cysteine residues within GPCR have been identified important for receptor function, which maintain the three-dimensional receptor conformation by forming critical inter- and intramolecular disulfide bond [9]. The members of this family contain a cysteine in the putative first extracellular loop (EC-1) near the top of the third transmembrane domain (TM-3) and another cysteine in EC-2. This pair of Cys residues forms a disulfide bond in most GPCRs and this linkage has been proposed to be important for receptor expression function [9, 11, 13]. MC3R belongs to GPCR family. In this study, we have performed extensive studies to determine the structural role of cysteine in MC3R on receptor function. The hMC3R contains eighteen cysteine residues, including six cysteine residues within external loops (ELs) (C42, C43, C72, C305, C311 and C313) and nine cysteine residues within transmembrane domains (TMs) (C115, C162, C170, C208, C209, C212, C228, C291 and C327), one cysteine C274 within third intracellular loop (IL3) and two cysteine residues, C252 and C254 in C terminus. All of these residues are conserved through melanocortin receptors except C42, C43 in N terminus, C274 and C354 in C terminus (Figure 1). Our results suggest that three extracellular cysteine residues, C305, C311 and C313 are crucial for high-level receptor expression but two TM cysteine residues C115 and C162 are important for ligand binding and signaling.
Figure 1.
Alignment of MC3R with other member of human MCR family. Conserved cysteines of the hMC3R with other MCRs are marked by black.
Methods
Peptides
NDP-MSH was purchased from Peninsula Laboratories, Inc. (Belmont, CA). Murine anti-FLAG M1 monoclonal antibody was obtained from Sigma (St. Louis, MO). Quick-Change Site-Directed Mutagenesis kit was from Stratagene ( La Jolla, CA). PolyJet in Vitro DNA transfection reagent was from SignaGen Laboratories (Ijamsville, MD 21754). DMEM medium was from fisher scientific (Suwanee GA 30024). cAMP assay kit was from Amersham (Arlington Heights, IL).
Site-directed mutagenesis
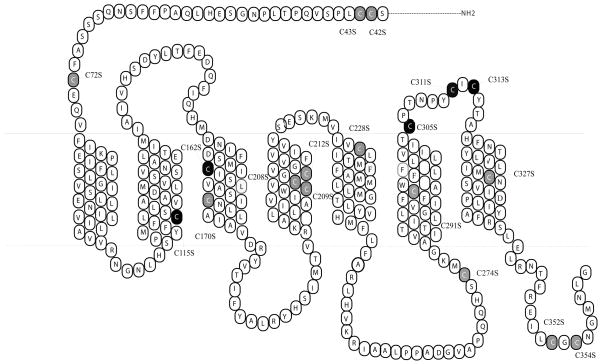
Single mutations were constructed using the Quick-Change Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA). The entire coding region of the mutated receptors was sequenced by the University of Alabama at Birmingham Sequence Core to confirm that the desired mutation sequences were present and that no sequence errors had been introduced. The mutated receptors are shown in Figure 2. The mutant receptors were subcloned into the eukaryotic expression vector pCDNA 3.1 (Invitrogen; Carlsbad, CA).
Figure 2.
Two-dimensional representation of the seven TM structure of the hMC3R. The residues mutated in these experiments are denoted by gray or black highlighting. The residues with mutation significantly affected ligand binding affinity and potency as determined are highlighted in black.
Cell culture and transfection
The HEK-293 cells, lacking endogenous MC3R, were cultured in DMEM medium containing 10% bovine fetal serum, 2% CBS and HEPES. Cells at 80% confluence were washed twice with DMEM, and the receptor constructs were transfected into cells (5 million) using PolyJet in vitro DNA transfection reagent. Experiments were performed 24 hour after transfection. Untransfected HEK3 cells exhibit no response to melanocortin stimulation, and therefore there is no significant background.
Binding Assays
After removal of media, cells were incubated with various non-radioligand in 0.5 mL MEM (Fisher Scientific, Pittsburgh, PA) containing 0.2% BSA and radioligand. Binding experiments were performed using conditions previously described (19). Briefly, 2 × 105 cpm of 125I-NDP-MSH was used in combination with non-radiolabeled MSH. Binding reactions were terminated by removing the media and washing the cells twice with MEM containing 0.2% BSA. The cells were lysed with 0.2 N NaOH, and the radioactivity in the lysate was quantified in an analytical gamma counter. Nonspecific binding was determined by measuring the amount of 125I-label bound in the presence of 10−6 M unlabeled ligand. Specific binding was calculated by subtracting nonspecifically bound radioactivity from total bound radioactivity. Data were analyzed using Graphpad Prism.
cAMP Assay
cAMP generation was measured using a competitive binding assay (TRK 432, Amersham, Arlington Heights, IL). Briefly, HEK cells stably expressing hMC3R were used in these assays (19). Cell culture media was removed, and cells were incubated with 0.5 mL Earle’s Balanced Salt Solution (EBSS), containing NDP-MSH (10−10-10−6 M), for one hour at 37°C in the presence of 10−3 M isobutylmethylxanthine. The reaction was stopped by adding ice-cold 100% ethanol (500μl/well). The cells in each well were scraped, transferred to a 1.5 mL tube, and centrifuged for 10 min at 1900 × g, and the supernatant was evaporated in a 55°C water bath with pre-purified nitrogen gas. cAMP content was measured according to instructions accompanying the assay kit. Each experiment was performed a minimum of three times with duplicate wells.
Receptor expression
For receptor protein expression studies, a FLAG tag was inserted into the NH2 terminus of hMC3R in order to characterize receptor protein cell surface expression by flow cytometry (FACS). The FLAG protein is an eight amino acid peptide (Asp-Tyr-Lys-Asp-Asp-Asp-Asp-Lys), useful for immunoaffinity purification of fusion proteins. hMC3R-FLAG or mutant receptor-FLAG transfected cells were harvested using 0.2% EDTA and washed twice with phosphate buffer saline (PBS). Aliquots of 3X106 cells were centrifuged and fixed with 3% paraformaldehyde in PBS (pH 7.4). The cells were incubated with 50 μl of 10 μg/mL murine anti-FLAG M1 monoclonal antibody (Sigma, St. Louis, MO) in incubation buffer for 45 minutes. Under these conditions, the primary antibody binds only to receptors located at the cell surface. The cells were collected by centrifugation and washed three times with incubation buffer. The cell pellets were suspended in 100 μl of incubation buffer containing CY™3-conjugated Affinity Pure Donkey Anti-Mouse Ig G (ImmunoResearch Lab, Inc., West Grove, PA) and incubated at room temperature for 30 minutes. Flow cytometry was performed on a fluorescence-activated cell sorter (Becton Dickinson FACStar plus six parameter cytometer/sorter with a dual Argon ion laser, San Jose, California). The results were analyzed using the software CellQuest (Beckton-Dickinson Immunocytometry Systems, San Jose, California).
Statistical analysis
Each experiment was performed at three separate times with duplicated wells. Data are expressed as mean ± SEM. The mean value of the dose-response data of binding and cAMP production was fit to a sigmoid curve with a variable slope factor using non-linear squares regression analysis (Graphpad Prism, Graphpad Software, San Diego, CA). Significant differences were assessed by one-way ANOVA, with p < 0.05 considered to be statistically significant.
Results
Substitutions hMC3R extracellular loops cysteine residues on receptor function
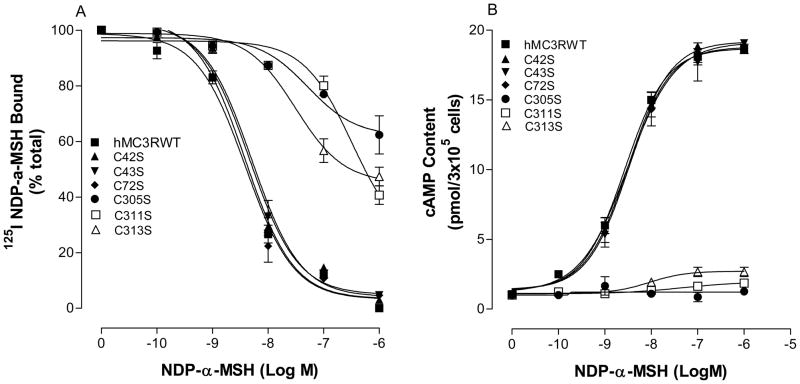
To examine the role of the extracellular loop cysteines on hMC3R function, we substituted the extracellular cysteine residues, C42, C43, C72, C305, C311 and C313, with serine. These mutant receptor expressions were examined by flow cytometry (FACs). Our results show that all these mutations were expressed at cell surface but their expressions are different. The expression levels of mutation C42S, C43S and C72S are similar to that of hMC3R wild type but the expression levels of the mutation C305S, C311S and C313S were significantly decreased (Table 1). MC3R mutant function was then determined by examining NDP-MSH binding affinity. Cells expressing these MC3R mutants were incubated with 125I-NDP-MSH and various concentrations of unlabeled NDP-MSH. As shown in Figure 3A, unlabeled NDP-MSH dose dependently displaces 125 I NDP-MSH binding at the mutated receptor C42S, C43S and C72S and NDP-MSH binding affinity at these mutated receptors were not significantly altered (Table 1). However, mutations of C305S, C311S and C313S significantly reduced NDP-MSH binding affinity. To determine whether these mutations alter receptor signaling, NDP-MSH induced cAMP production was determined. Cell expressing these mutations were incubated with various concentrations of NDP-MSH and cAMP production was measured. Our results indicate that NDP-MSH induced cAMP production at C42S, C43S and C72S was similar to that of hMC3R wild type but significantly reduced at mutation C305S, C311S, and C313S (figure 3B). The maximal response stimulated by NDP-MSH at C305S, C311S and C313S was only 12, 21 and 24% of that of the hMC3R-WT, respectively (Table 1). Their EC50 are shown in Table 1.
Table 1.
Effect of the substitutions of the cysteine residues of hMC3R on 125I -NDP-α-MSH binding and cAMP production
| R eceptor expression(%WT) | 125I - NDP binding K i (nM) | cAM P production EC50 (nM) | Emax (%WT) | |
|---|---|---|---|---|
| hMC3R-WT | 100 ± 0 | 6.3 ± 1.0 | 5.6 ± 0.1 | 100 ± 0 |
| C42S | 88 ± 8.4 | 7.8 ± 0.4 | 5.2 ± 0.4 | 89 ± 8.4 |
| C43S | 96 ± 10.5 | 5.6 ± 0.5 | 5.7 ± 0.5 | 92 ± 7.5 |
| C72S | 92 ± 6.4 | 8.4 ± 0.4 | 4.9 ± 0.3 | 95 ± 9.4 |
| C115S | 65 ± 0.5 | 85.5 ± 0.5* | 71.5 ± 0.5* | 65 ± 10.5* |
| C162S | 52 ± 7.3 | 66.2 ± 0.3* | 68.3 ± 0.3* | 52 ± 5.3* |
| C170S | 93 ± 0.3 | 7.3 ± 0.3 | 5.8 ± 0.3 | 93 ± 11.3 |
| C208S | 93 ± 9.5 | 8.6 ± 0.5 | 6.2 ± 0.5 | 95 ± 13.5 |
| C209S | 94 ± 11.6 | 7.5 ± 0.6 | 6.5 ± 0.6 | 92 ± 12.6 |
| C212S | 90 ± 8.6 | 8.5 ± 0.4 | 5.9 ± 0.6 | 89 ± 7.6 |
| C228S | 96 ± 11.6 | 6.7 ± 0.6 | 6.7 ± 0.6 | 87 ± 9.2 |
| C274S | 93 ± 7.7 | 7.6 ± 0.7 | 7.6 ± 0.7 | 93 ± 12.2 |
| C291S | 78 ± 0.7 | 7.8 ± 0.7 | 7.8 ± 0.7 | 95 ± 11.3 |
| C305S | 26 ± 5.8 | >103* | >103* | 12 ± 2.3* |
| C311S | 38 ± 6.9 | >103 * | >103* | 21 ± 1.4* |
| C313S | 45 ± 6.6 | 98 ± 0.5* | 121 ± 11.5* | 24 ± 0.2* |
| C327S | 87 ± 4.8 | 8.7 ± 1.6 | 7.3 ± 0.5 | 87 ± 7.6 |
| C352S | 91 ± 8.7 | 7.7 ± 1.4 | 7.7 ± 0.3 | 77 ± 16.5 |
| C354S | 89 ± 9.3 | 8.5 ± 1.3 | 8.5 ± 0.6 | 85 ± 9.2 |
P<0.05 compared with WT receptor.
Figure 3.
Effects of the hMC3R extracellular loop cysteine residue mutation on NDP-MSH binding and signaling. Panel A shows the cysteine mutation on NDP-MSH binding affinity. Panel B shows the ability of NDP-MSH stimulated cAMP production at these mutants. Data points represent the mean ± SE of three independent experiments with duplicate wells.
Substitutions of transmembrane domain cysteine residues in on receptor function
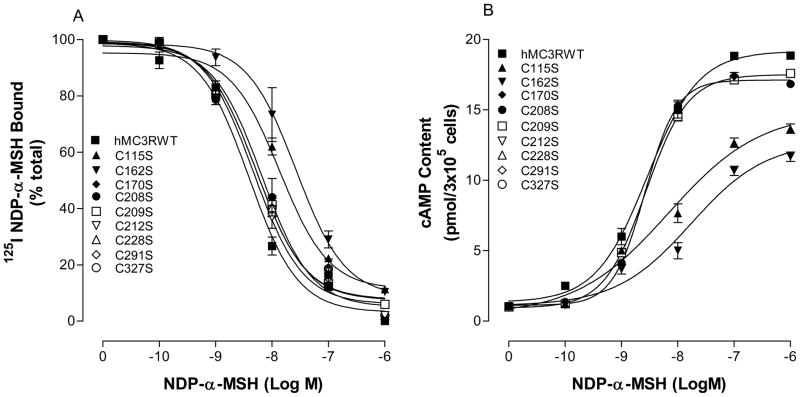
To determine whether transmembrane domain (TM) cysteines are important for hMC3R function, nine cysteine residues (C115 C162, C170, C208, C209, C212, C228, C291 and C327) in the TMs of the hMC3R were mutated and examined. Our results show that all of the mutations were expressed at cell surface but their expression levels are different. The expression levels of the mutations C170S, C208S, C209S, C212S, C228S, C291S and C327S are similar to that of hMC3R WT but the expression levels of the mutation C115S and C162S were significantly decreased. Their expression levels are shown in Table 1. To determine whether these mutations alter ligand NDP-MSH binding and potency, cell expressing these mutations were incubated with 125I-NDP-MSH and various concentrations of unlabeled NDP-MSH. As shown in Figure 3A, unlabeled NDP-MSH dose dependently displaces 125 I NDP-MSH binding at mutated receptors C115S C162S, C170S, C208S, C209S, C212S, C228S, C291S and C327S. Mutations of C170S, C208S, C209S, C212S, C228S, C291S and C327S did not significantly alter ligand binding affinity but mutation C115S and C162S significantly decreased NDP-MSH binding affinity (Figure 4A) (Table 1). Further study indicate that NDP-MSH induced cAMP production at C170S, C208S, C209S, C212S, C228S, C291S and C327S was similar to that of hMC3R WT but significantly reduced at mutations C115S and C162S (Figure 4B). The maximal response stimulated by NDP-MSH at C115S and C162S was only 65 and 52% of that of the hMC3R-WT, respectively (Table 1). Their EC50 are shown in Table 1.
Figure 4.
Effects of the hMC3R transmembrane domain cysteine residue mutations on NDP-MSH binding affinity and potency. Panel A shows the NDP-MSH binding affinity at these mutants. Panel B shows the ability of NDP-MSH stimulated cAMP production at these mutants. Data points represent the mean ± SE of three independent experiments with duplicate wells.
Substitutions of cysteine residues in the intracellular loop on receptor function
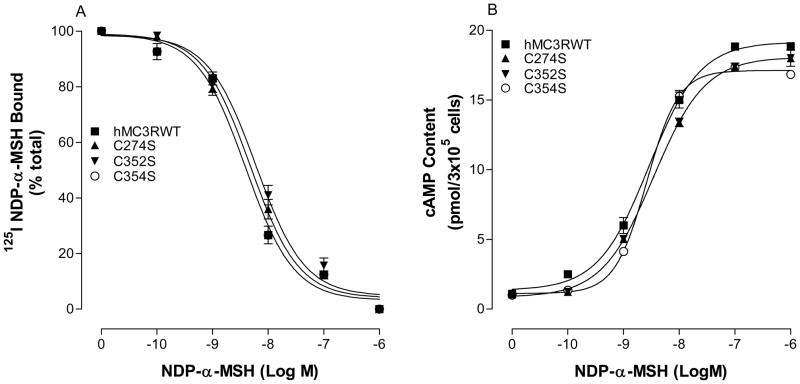
There is one cysteine C274 in IL3 and two cysteine residues (C352 and C354) in the C terminus of the hMC3R. Cysteine 274 and C354 are unique in hMC3R but C352 is conserved among MCRs. Our receptor function study shows that all of these mutations are expressed at cell surface. The expression levels of these mutations are similar to that of hMC3R wild type (Table 1). To determine the ligand binding affinity at these mutations, cells expressing these MC3R mutants were incubated with 125I-NDP-MSH and various concentrations of unlabeled NDP-MSH. As shown in Figure 5A, unlabeled NDP-MSH dose dependently displaces 125 I NDP-MSH binding at the mutated receptor C274S, C352S and C354S and NDP-MSH binding affinity at these mutated receptors were not significantly altered (Table 1). Furthermore, our results indicate that these mutations did not alter NDP-MSH induced cAMP production (Figure 5B). Their EC50 values are shown in Table 1.
Figure 5.
Effects of the hMC3R intracellular loop cysteine residue mutation on NDP-MSH binding affinity and potency. Panel A shows the NDP-MSH binding affinity at these mutants. Panel B shows the ability of NDP-MSH stimulated cAMP production at these mutants. Data points represent the mean ± SE of three independent experiments with duplicate wells.
Discussion
In this study, we systematically characterized the role of the MC3R endogenous cysteine residues on receptor function. Our results indicate that three extracellular cysteine residues, C305, C311 and C313 are crucial for receptor expression. Two TM residues C115 and C162 are important for ligand binding and signaling.
Almost all GPCRs contain a pair of conserved Cys residues in the first and second extracellular domains or a disulfide bridge in the second extracellular loop (EL2) to the third transmembrane domain. Disulfide bond between the extracellular cysteine residues of the GPCR plays an important role in maintaining receptor structure and function. These residues have been shown to form a receptor stabilizing disulfide bridge structure. Rhodopsin, muscarinic acetylcholine receptors and β2 -adrenergic receptors, are the best studied GPCRs with regard to the presence of an extracellular disulfide bond (4 5, 6, 7). In rhodopsin, this disulfide bond has been confirmed through chemical and crystallographic approaches which plays a key role in the formation of a properly folded, stable receptor[11]. In muscarinic acetylcholine receptors, this disulfide bond was confirmed by mutagenesis. In β-adrenergic receptor, even two disulfide bonds were identified within the extracellular loop which plays an important role in receptor function [13, 24]. MC3R belongs to GPCR family but MC3R structurally exhibits several intriguing anomalies, which together place it in a unique subset of type I GPCRs. These differences include a relatively short N-terminus (34 amino acids), the absence of conserved proline residues in transmembrane helices I and V, and the presence of a glycine residue in the TM VI. Most strikingly, MC3R also lacks cysteine residues required to make up a highly conserved disulfide bridge thought to connect TM3 with extracellular loop 2 in most GPCRs. Although MC3R lacks the canonical disulfide bond described above, it does appear to contain other conserved cysteine residues; MC3R has three cysteines in the third extracellular loop instead. Our results indicate that mutations of these cysteines C305S, C311S and C313S decreased receptor expression and function, suggesting that these residues are important for MC3R function. These results are consistent with that of other MCRs because these residues in MC3R conserved with other MCRs. Previous results indicate that cysteine residues, C267, C273 in hMC1R; C245, C251 in hMC2R and C271 and C277 in hMC4R, were identified to be important for receptor function and may be involved in disulfide bond [4, 26, 30]. The residues Cys311 and Cys313 in hMC3R may also be involved in forming a disulfide bond. All these results suggest that cysteine residues in the extracellular loop 3 of melanocortin receptor family play important roles in maintaining normal receptor structure and function.
Human MC3R contains nine cysteines in the transmembrane domains and all of them are conserved among MCRs. It was reported that mutation of C78G in hMC1R (similar to C115 in hMC3R) significantly decreased NDP-MSH binding affinity and potency but remained high alpha MSH binding affinity and potency [16]. However, in our study, mutation of C115S in hMC3R significantly decreased both NDP-MSH and alpha-MSH binding affinity and potency, implying that their roles in receptor function are different. Experiment from hMC4R showed that mutation of C130 (conserved residue for C162 in hMC3R) in hMC4R was expressed at cell surface and did not alter NDP-MSH binding affinity and potency [34]. Our results indicate that mutations of C162 in hMC3R decreased ligand binding affinity and potency, suggesting that this residue is important for ligand binding and signaling at hMC3R. Cysteine residue 162 is located in TM3 of hMC3R which is also conserved among MCRs. This residue is very close to receptor ionic binding pocket [6, 32, 34] and mutation of this residue may alter micro conformation of MC3R binding pocket and then interfere ligand binding, suggesting that the role of C162 in TM3 of hMC3R is different from that of hMC4R. Cysteine residues in TM5 and TM6 have been reported to play an important role in MC1R and MC2R [16]. However, our results indicate that the mutations of the cysteine residues in TM5 and TM6 of hMC3R did not alter receptor expression and function. Our results suggest that the role of cysteine residues in MC1R, MC2R, MC3R and MC4R are different. Furthermore, cysteines in the C terminus have previously been implicated in receptor signaling in B2-adrenergic receptor, human follicle-stimulating hormone receptor and glucagon-like peptide receptor [14, 21, 27, 29]. Similar to other GPCR, cysteine 315 in the C terminus of hMC1R and cysteine 319 in the C terminus of hMC4R were also identified to be involved in receptor signaling [16, 28]. Mutations of C215G in IL3 and C315G in C terminal of hMC1R abolished ligand mediated receptor response but remained ligand high affinity binding [16]. However, our results indicate that cysteine in the C terminus of hMC3R is not involved in NDP-MSH binding and signaling. It was also reported that mutations of some specific IL3 and C terminal residues of the hMC4R decreased ligand potency at hMC4R [31]. However, mutations of C274S in IL3 and C352S, C354S in C terminal in hMC3R did not alter ligand binding affinity and potency, suggesting that the pathway of hMC3R signaling may be different from that of the other MCRs.
Intracellular retention of MCR mutants is a common disease-causing defect. To allow for the rapid evaluation of cell surface expression of MC3R relative to total expression of the receptor in individual transiently transfected cells, we utilized flow cytometry to detect MC3R expression at cell surface. We constructed a chimeric receptor containing a N-terminal extracellular Flag epitope. This chimeric construct remains responsive to the natural agonist α-MSH with the same EC50 as the native receptor. The cell pellets were suspended in 100 μl of incubation buffer containing CY™3-conjugated Affinity Pure Donkey Anti-Mouse Ig G and allows for the detection of cell surface expression of the transfected receptor. By using this detection method, we demonstrate here that mutation C305A, C311A and C313A cause partial or complete intracellular retention of the receptor. Our results also suggest that intracellular retention of the mutated protein correlates with dysfunction of the receptor.
Disulfide bond between the extracellular cysteine residues of the GPCR plays an important role in maintaining receptor structure and function. To determine whether disulfide bond exists in GPCRs, the positively charged, cell-impermeant sulfhydryl-reactive reagent DTT was utilized to treat both WT receptor and the mutated receptors [20, 25]. If the important disulfide bonds exist, DTT treatment will break this bond which may alter receptor function. However, our results indicate that the expression of MC3R mutations C305S, C311S and C313S is very low and we cannot determine disulfide bond between C311 and C313 using DTT. Therefore, we can’t directly determine disulfide bond between C311 and C313 but from other MCR’s results, we may believe that these residues are also involved in receptor disulfide bond formation.
In summary, our results indicate most of the cysteine residues in MC3R are unnecessary for protein expression, ligand binding, and G-protein coupling. Only endogenous cysteine residues C115 and C162 in TM3 and residue C305, C311 and C313 in EXL3 in hMC3R are important for receptor function. Our results demonstrate that C305, C311 and C313 are involved in receptor expression while C115 and C162 are crucial for receptor function. These results provide important insights into the functional role of cysteines on MC3R expression and signaling.
Highlights.
In this study, we examined the role of cysteine residues in melanocortin-3 receptor on receptor function. We find that mutations, C305S, C311S, and C313S significantly decrease receptor expression but C115S and C162S decreased receptor function. We conclude that these five cysteine residues are crucial for receptor function.
Acknowledgments
This work has been supported by NIH Grants R03 HD047312-01A1 (Yang, Y-K).
Abbreviations
- MCR
Melanocortin receptor
- hMC3R
human melanocortin-3 receptor
- GPCR
G-protein coupled receptor
- α-MSH
α-melanocyte-stimulating hormone
- ASIP
Agouti-signaling protein
- TM
transmembrane domains
- IBMX
3-Isobutyl-methylxanthine
- PCR
Polymerase chain reaction
- FACs
Flow cytometry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bhargava SK, Sachdev HS, Fall CH, Osmond C, Lakshmy R, Barker DJ, et al. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med. 2004;350:865–75. doi: 10.1056/NEJMoa035698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butler AA, Cone RD. Knockout studies defining different roles for melanocortin receptors in energy homeostasis. Ann N Y Acad Sci. 2003;994:240–5. doi: 10.1111/j.1749-6632.2003.tb03186.x. [DOI] [PubMed] [Google Scholar]
- 3.Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, et al. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–21. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 4.Chai BX, Pogozheva ID, Lai YM, Li JY, Neubig RR, Mosberg HI, et al. Receptor-antagonist interactions in the complexes of agouti and agouti-related protein with human melanocortin 1 and 4 receptors. Biochemistry. 2005;44:3418–31. doi: 10.1021/bi0478704. [DOI] [PubMed] [Google Scholar]
- 5.Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 6.Chen M, Aprahamian CJ, Celik A, Georgeson KE, Garvey WT, Harmon CM, et al. Molecular characterization of human melanocortin-3 receptor ligand-receptor interaction. Biochemistry. 2006;45:1128–37. doi: 10.1021/bi0521792. [DOI] [PubMed] [Google Scholar]
- 7.Cone RD. The Central Melanocortin System and Energy Homeostasis. Trends Endocrinol Metab. 1999;10:211–6. doi: 10.1016/s1043-2760(99)00153-8. [DOI] [PubMed] [Google Scholar]
- 8.Cone RD, Cowley MA, Butler AA, Fan W, Marks DL, Low MJ. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord. 2001;25 (Suppl 5):S63–7. doi: 10.1038/sj.ijo.0801913. [DOI] [PubMed] [Google Scholar]
- 9.Cook JV, Eidne KA. An intramolecular disulfide bond between conserved extracellular cysteines in the gonadotropin-releasing hormone receptor is essential for binding and activation. Endocrinology. 1997;138:2800–6. doi: 10.1210/endo.138.7.5233. [DOI] [PubMed] [Google Scholar]
- 10.Damcott CM, Moffett SP, Feingold E, Barmada MM, Marshall JA, Hamman RF, et al. Genetic variation in fatty acid-binding protein-4 and peroxisome proliferator-activated receptor gamma interactively influence insulin sensitivity and body composition in males. Metabolism. 2004;53:303–9. doi: 10.1016/j.metabol.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Davidson FF, Loewen PC, Khorana HG. Structure and function in rhodopsin: replacement by alanine of cysteine residues 110 and 187, components of a conserved disulfide bond in rhodopsin, affects the light-activated metarhodopsin II state. Proc Natl Acad Sci U S A. 1994;91:4029–33. doi: 10.1073/pnas.91.9.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dekkers JC, Podolsky RH, Treiber FA, Barbeau P, Gutin B, Snieder H. Development of general and central obesity from childhood into early adulthood in African American and European American males and females with a family history of cardiovascular disease. Am J Clin Nutr. 2004;79:661–8. doi: 10.1093/ajcn/79.4.661. [DOI] [PubMed] [Google Scholar]
- 13.Dohlman HG, Caron MG, DeBlasi A, Frielle T, Lefkowitz RJ. Role of extracellular disulfide-bonded cysteines in the ligand binding function of the beta 2-adrenergic receptor. Biochemistry. 1990;29:2335–42. doi: 10.1021/bi00461a018. [DOI] [PubMed] [Google Scholar]
- 14.Eason MG, Jacinto MT, Theiss CT, Liggett SB. The palmitoylated cysteine of the cytoplasmic tail of alpha 2A-adrenergic receptors confers subtype-specific agonist-promoted downregulation. Proc Natl Acad Sci U S A. 1994;91:11178–82. doi: 10.1073/pnas.91.23.11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. Jama. 2002;288:1723–7. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 16.Frandberg PA, Doufexis M, Kapas S, Chhajlani V. Cysteine residues are involved in structure and function of melanocortin 1 receptor: Substitution of a cysteine residue in transmembrane segment two converts an agonist to antagonist. Biochem Biophys Res Commun. 2001;281:851–7. doi: 10.1006/bbrc.2001.4429. [DOI] [PubMed] [Google Scholar]
- 17.Geller F, Reichwald K, Dempfle A, Illig T, Vollmert C, Herpertz S, et al. Melanocortin-4 receptor gene variant I103 is negatively associated with obesity. Am J Hum Genet. 2004;74:572–81. doi: 10.1086/382490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holtkamp K, Konrad K, Muller B, Heussen N, Herpertz S, Herpertz-Dahlmann B, et al. Overweight and obesity in children with Attention-Deficit/Hyperactivity Disorder. Int J Obes Relat Metab Disord. 2004;28:685–9. doi: 10.1038/sj.ijo.0802623. [DOI] [PubMed] [Google Scholar]
- 19.Hotu S, Carter B, Watson P, Cutfield W, Cundy T. Increasing prevalence of type 2 diabetes in adolescents. J Paediatr Child Health. 2004;40:201–4. doi: 10.1111/j.1440-1754.2004.00337.x. [DOI] [PubMed] [Google Scholar]
- 20.Karlin A, Akabas MH, Czajkowski C, Kaufmann C, Stauffer D, Xu M. Structures involved in binding, gating, and conduction in nicotinic acetylcholine receptors. Ren Physiol Biochem. 1994;17:184–6. doi: 10.1159/000173814. [DOI] [PubMed] [Google Scholar]
- 21.Kobilka BK, Kobilka TS, Daniel K, Regan JW, Caron MG, Lefkowitz RJ. Chimeric alpha 2-,beta 2-adrenergic receptors: delineation of domains involved in effector coupling and ligand binding specificity. Science. 1988;240:1310–6. doi: 10.1126/science.2836950. [DOI] [PubMed] [Google Scholar]
- 22.Kowalski TJ. The future of genetic research on appetitive behavior. Appetite. 2004;42:11–4. doi: 10.1016/j.appet.2002.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Nammi S, Koka S, Chinnala KM, Boini KM. Obesity: An overview on its current perspectives and treatment options. Nutr J. 2004;3:3. doi: 10.1186/1475-2891-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Probst WC, Snyder LA, Schuster DI, Brosius J, Sealfon SC. Sequence alignment of the G-protein coupled receptor superfamily. DNA Cell Biol. 1992;11:1–20. doi: 10.1089/dna.1992.11.1. [DOI] [PubMed] [Google Scholar]
- 25.Stauffer DA, Karlin A. Electrostatic potential of the acetylcholine binding sites in the nicotinic receptor probed by reactions of binding-site cysteines with charged methanethiosulfonates. Biochemistry. 1994;33:6840–9. doi: 10.1021/bi00188a013. [DOI] [PubMed] [Google Scholar]
- 26.Tarnow P, Schoneberg T, Krude H, Gruters A, Biebermann H. Mutationally induced disulfide bond formation within the third extracellular loop causes melanocortin 4 receptor inactivation in patients with obesity. J Biol Chem. 2003;278:48666–73. doi: 10.1074/jbc.M309941200. [DOI] [PubMed] [Google Scholar]
- 27.Ulloa-Aguirre A, Uribe A, Zarinan T, Bustos-Jaimes I, Perez-Solis MA, Dias JA. Role of the intracellular domains of the human FSH receptor in G(alphaS) protein coupling and receptor expression. Mol Cell Endocrinol. 2006 doi: 10.1016/j.mce.2005.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.VanLeeuwen D, Steffey ME, Donahue C, Ho G, MacKenzie RG. Cell surface expression of the melanocortin-4 receptor is dependent on a C-terminal di-isoleucine sequence at codons 316/317. J Biol Chem. 2003;278:15935–40. doi: 10.1074/jbc.M211546200. [DOI] [PubMed] [Google Scholar]
- 29.Vazquez P, Roncero I, Blazquez E, Alvarez E. Substitution of the cysteine 438 residue in the cytoplasmic tail of the glucagon-like peptide-1 receptor alters signal transduction activity. J Endocrinol. 2005;185:35–44. doi: 10.1677/joe.1.06031. [DOI] [PubMed] [Google Scholar]
- 30.Yang Y, Chen M, Kesterson RA, Jr, Harmon CM. Structural insights into the role of the ACTH receptor cysteine residues on receptor function. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1120–6. doi: 10.1152/ajpregu.00240.2007. [DOI] [PubMed] [Google Scholar]
- 31.Yang Y, Chen M, Loux TJ, Georgeson KE, Harmon CM. Molecular mechanism of the intracellular segments of the melanocortin-4 receptor for NDP-MSH signaling. Biochemistry. 2005;44:6971–9. doi: 10.1021/bi047521+. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y, Dickinson C, Haskell-Luevano C, Gantz I. Molecular basis for the interaction of [Nle4,D-Phe7]melanocyte stimulating hormone with the human melanocortin-1 receptor. J Biol Chem. 1997;272:23000–10. doi: 10.1074/jbc.272.37.23000. [DOI] [PubMed] [Google Scholar]
- 33.Yang YK, Dickinson C, Lai YM, Li JY, Gantz I. Functional properties of an agouti signaling protein variant and characteristics of its cognate radioligand. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1877–86. doi: 10.1152/ajpregu.2001.281.6.R1877. [DOI] [PubMed] [Google Scholar]
- 34.Yang YK, Fong TM, Dickinson CJ, Mao C, Li JY, Tota MR, et al. Molecular determinants of ligand binding to the human melanocortin-4 receptor. Biochemistry. 2000;39:14900–11. doi: 10.1021/bi001684q. [DOI] [PubMed] [Google Scholar]
- 35.Yang YK, Ollmann MM, Wilson BD, Dickinson C, Yamada T, Barsh GS, et al. Effects of recombinant agouti-signaling protein on melanocortin action. Mol Endocrinol. 1997;11:274–80. doi: 10.1210/mend.11.3.9898. [DOI] [PubMed] [Google Scholar]
- 36.Yang YK, Thompson DA, Dickinson CJ, Wilken J, Barsh GS, Kent SB, et al. Characterization of Agouti-related protein binding to melanocortin receptors. Mol Endocrinol. 1999;13:148–55. doi: 10.1210/mend.13.1.0223. [DOI] [PubMed] [Google Scholar]