Abstract
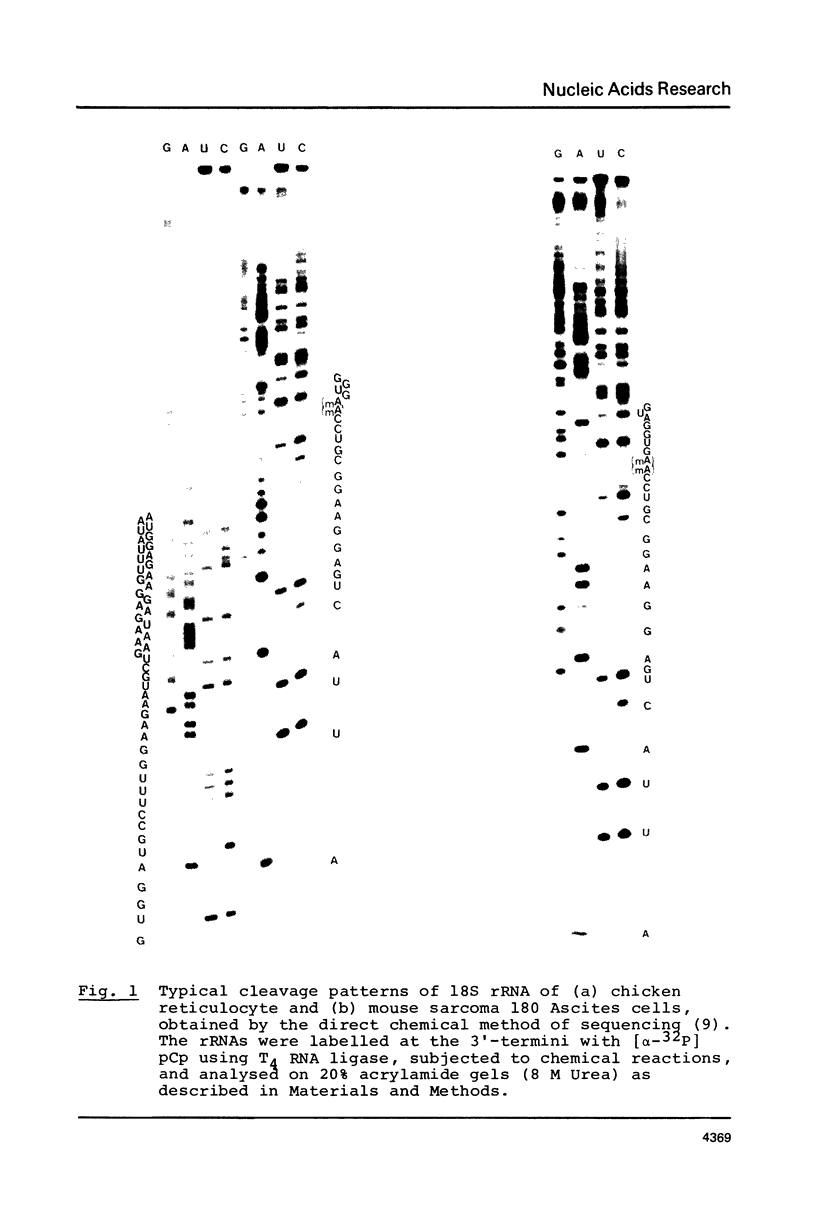
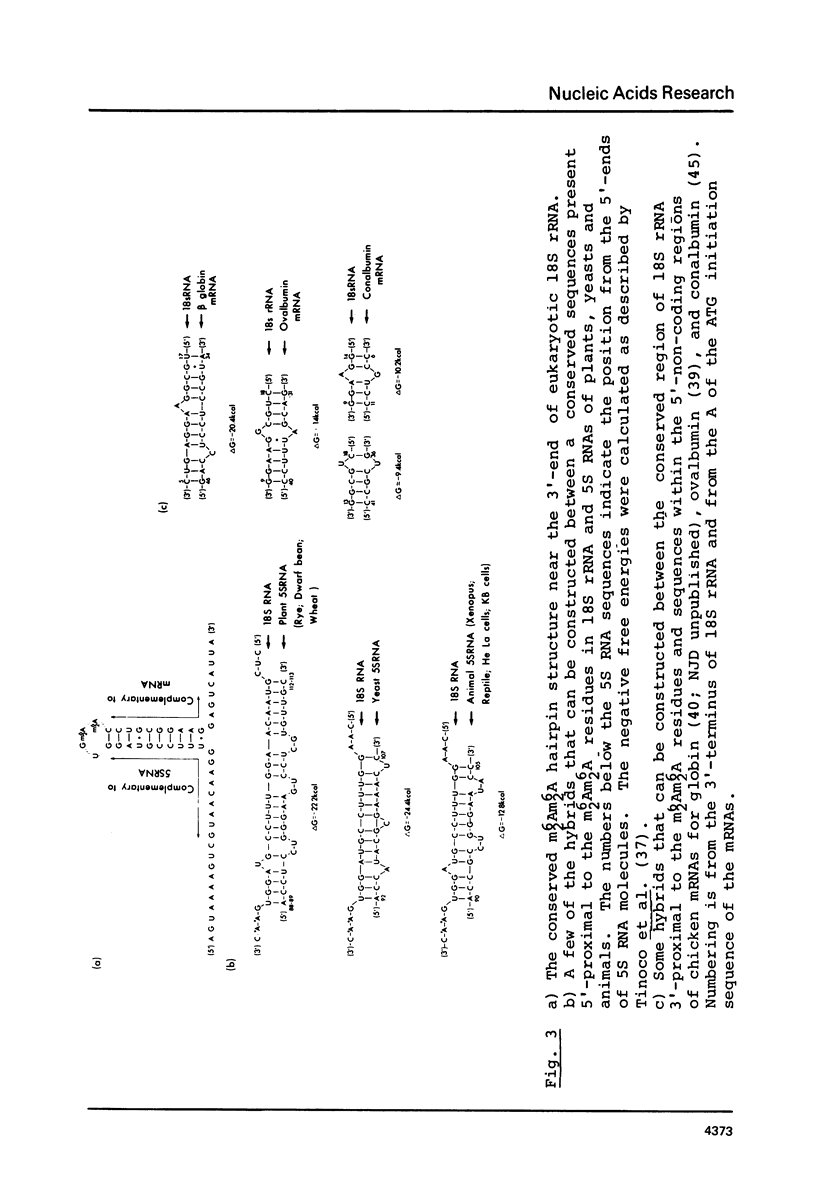
The 3'-terminal sequences of 18S rRNA from chicken reticulocyte, mouse sarcoma, rat liver, rabbit reticulocyte and barley embryo were determined by the direct chemical sequencing method. The regions sequenced show complete homology for the first 73 nucleotides. A sequence 5'-proximal to the m6(2)Am6(2)A residues that is complementary to eukaryotic 5S RNAs is totally conserved. This supports the hypothesis that base-paired interaction between 5S and 18S rRNA, which are present in the large and small ribosomal subunits respectively, may be involved in the reversible association of ribosomal subunits during protein synthesis.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akimenko N. M., Burckhardt G., Kadykov V. A., Avakian K. A., Evdokimov Y. M., Varshavsky Y. M. A compact from of methylated DNA is solutions containing poly (ethylene glycol). Nucleic Acids Res. 1977 Oct;4(10):3665–3676. doi: 10.1093/nar/4.10.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberty H., Raba M., Gross H. J. Isolation from rat liver and sequence of a RNA fragment containing 32 nucleotides from position 5 to 36 from the 3' end of ribosomal 18S RNA. Nucleic Acids Res. 1978 Feb;5(2):425–434. doi: 10.1093/nar/5.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad A. A. Cytoplasmic RNA from hen reticulocytes, mouse sarcoma 180 ascites cells, rat liver and barley embryos. Their preparation and purification by a standard procedure and characterization by polyacrylamide gel electrophoresis. Comp Biochem Physiol B. 1978;61(2):213–218. doi: 10.1016/0305-0491(78)90163-3. [DOI] [PubMed] [Google Scholar]
- Azad A. A., Deacon N. J. Base-paired interaction, in vitro, between hen globin 9S mRNA and eukaryotic ribosomal RNAs. Biochem Biophys Res Commun. 1979 Feb 14;86(3):568–576. doi: 10.1016/0006-291x(79)91751-0. [DOI] [PubMed] [Google Scholar]
- Azad A. A. Hybridization between 5S rRNA and 18S rRNA from barley embryos and mouse sarcoma 180 ascites cells. Biochem Biophys Res Commun. 1978 Jul 14;83(1):259–265. doi: 10.1016/0006-291x(78)90425-4. [DOI] [PubMed] [Google Scholar]
- Azad A. A. Intermolecular base-paired interaction between complementary sequences present near the 3' ends of 5S rRNA and 18S (16S) rRNA might be involved in the reversible association of ribosomal subunits. Nucleic Acids Res. 1979 Dec 11;7(7):1913–1929. doi: 10.1093/nar/7.7.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad A. A., Lane B. G. A possible role for 5 S rRNA as a bridge between ribosomal subunits. Can J Biochem. 1973 Dec;51(12):1669–1672. doi: 10.1139/o73-224. [DOI] [PubMed] [Google Scholar]
- Azad A. A., Lane B. G. Wheat embryo ribonucleates. II. 3'-Hydroxyl termini of the satellite, 18S, and 26S ribosomal ribonucleates. Can J Biochem. 1973 Aug;51(8):1195–1202. doi: 10.1139/o73-155. [DOI] [PubMed] [Google Scholar]
- Azad A. A., Lane B. G. Wheat-embryo ribonucleates. IV. Factors that influence the formation and stability of a complex between 5S rRNA and 18S rRNA. Can J Biochem. 1975 Mar;53(3):320–327. doi: 10.1139/o75-045. [DOI] [PubMed] [Google Scholar]
- Baan R. A., Hilbers C. W., Van Charldorp R., Van Leerdam E., Van Knippenberg P. H., Bosch L. High-resolution proton magnetic resonance study of the secondary structure of the 3'-terminal 49-nucleotide fragment of 16S rRNA from Escherichia coli. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1028–1031. doi: 10.1073/pnas.74.3.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baralle F. E., Brownlee G. G. AUG is the only recognisable signal sequence in the 5' non-coding regions of eukaryotic mRNA. Nature. 1978 Jul 6;274(5666):84–87. doi: 10.1038/274084a0. [DOI] [PubMed] [Google Scholar]
- Bowman C. M., Sidikaro J., Nomura M. Specific inactivation of ribosomes by colicin E3 in vitro and mechanism of immunity in colicinogenic cells. Nat New Biol. 1971 Dec 1;234(48):133–137. doi: 10.1038/newbio234133a0. [DOI] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. L., Smith M. The sequence of a region of bacteriophage phiX174 DNA coding for parts of genes A and B. J Mol Biol. 1977 Oct 15;116(1):1–28. doi: 10.1016/0022-2836(77)90115-2. [DOI] [PubMed] [Google Scholar]
- Bruce A. G., Uhlenbeck O. C. Reactions at the termini of tRNA with T4 RNA ligase. Nucleic Acids Res. 1978 Oct;5(10):3665–3677. doi: 10.1093/nar/5.10.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman N. M., Noller H. F. Protection of specific sites in 16 S RNA from chemical modification by association of 30 S and 50 S ribosomes. J Mol Biol. 1977 Jan 5;109(1):131–149. doi: 10.1016/s0022-2836(77)80049-1. [DOI] [PubMed] [Google Scholar]
- Cochet M., Gannon F., Hen R., Maroteaux L., Perrin F., Chambon P. Organization and sequence studies of the 17-piece chicken conalbumin gene. Nature. 1979 Dec 6;282(5739):567–574. doi: 10.1038/282567a0. [DOI] [PubMed] [Google Scholar]
- De Wachter R. Do eukaryotic mRNA 5' noncoding sequences base-pair with the 18 S ribosomal RNA 3' terminus? Nucleic Acids Res. 1979 Dec 11;7(7):2045–2054. doi: 10.1093/nar/7.7.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehresmann C., Stiegler P., Mackie G. A., Zimmermann R. A., Ebel J. P., Fellner P. Primary sequence of the 16S ribosomal RNA of Escherichia coli. Nucleic Acids Res. 1975 Feb;2(2):265–278. doi: 10.1093/nar/2.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J. D., von Hippel P. H. Effects of methylation on the stability of nucleic acid conformations: studies at the monomer level. Biochemistry. 1974 Sep 24;13(20):4143–4158. doi: 10.1021/bi00717a013. [DOI] [PubMed] [Google Scholar]
- Hagenbüchle O., Santer M., Steitz J. A., Mans R. J. Conservation of the primary structure at the 3' end of 18S rRNA from eucaryotic cells. Cell. 1978 Mar;13(3):551–563. doi: 10.1016/0092-8674(78)90328-8. [DOI] [PubMed] [Google Scholar]
- Khan M. S., Maden B. E. Nucleotide sequences within the ribosomal ribonucleic acids of HeLa cells, Xenopus laevis and chick embryo fibroblasts. J Mol Biol. 1976 Feb 25;101(2):235–254. doi: 10.1016/0022-2836(76)90375-2. [DOI] [PubMed] [Google Scholar]
- Kuebbing D., Liarakos C. D. Nucleotide sequence at the 5' end of ovalbumin messenger RNA from chicken. Nucleic Acids Res. 1978 Jul;5(7):2253–2266. doi: 10.1093/nar/5.7.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau R. Y., Kennedy T. D., Lane B. G. Wheat-embryo ribonucleates. III. Modified nucleotide constituents in each of the 5.8S, 18S and 26S ribonucleates. Can J Biochem. 1974 Dec;52(12):1110–1123. doi: 10.1139/o74-155. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J. L., Lane B. G. N-4-methyl-2'-O-methyl cytidine and other methyl-substituted nucleoside constituents of Escherichia coli ribosomal and soluble RNA. Biochim Biophys Acta. 1966 Jun 22;119(3):649–651. doi: 10.1016/0005-2787(66)90147-x. [DOI] [PubMed] [Google Scholar]
- Nichols J. L., Wijesinghe W. Identification of the 5S RNA binding site in intermolecular complexes of wheat embryo ribosomal 5S and 18S RNA. Can J Biochem. 1978 Jul;56(7):760–764. doi: 10.1139/o78-114. [DOI] [PubMed] [Google Scholar]
- Oakden K. M., Azad A. A., Lane B. G. Wheat embryo ribonucleates. VII. Rapid, efficient and selective formation of 5S-18S and 5.8S-26S hybrids in an aqueous solution of the four ribosomal polynucleotides, and the results of a search for the corresponding hybrids in wheat embryo ribosomes. Can J Biochem. 1977 Jan;55(1):99–109. doi: 10.1139/o77-016. [DOI] [PubMed] [Google Scholar]
- Oakden K. M., Lane B. G. Wheat embryo ribonucleates. VI. Comparison of the 3'-hydroxyl termini in 'rapidly labelled' RNA from metabolizing wheat embryos with the corresponding termini in ribosomal RNA from differentiating embryos of wheat, barley, corn and pea. Can J Biochem. 1976 Mar;54(3):261–271. doi: 10.1139/o76-039. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politz S. M., Glitz D. G. Ribosome structure: localization of N6,N6-dimethyladenosine by electron microscopy of a ribosome-antibody complex. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1468–1472. doi: 10.1073/pnas.74.4.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards R. I., Shine J., Ullrich A., Wells J. R., Goodman H. M. Molecular cloning and sequence analysis of adult chicken betal globin cDNA. Nucleic Acids Res. 1979 Nov 10;7(5):1137–1146. doi: 10.1093/nar/7.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samols D. R., Hagenbuchle O., Gage L. P. Homology of the 3' terminal sequences of the 18S rRNA of Bombyx mori and the 16S rRNA of Escherchia coli. Nucleic Acids Res. 1979 Nov 10;7(5):1109–1119. doi: 10.1093/nar/7.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Santer M., Shane S. Area of 16S ribonucleic acid at or near the interface between 30S and 50S ribosomes of Escherichia coli. J Bacteriol. 1977 May;130(2):900–910. doi: 10.1128/jb.130.2.900-910.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber J. P., Hsiung N., Cantor C. R. Fluorescence studies of the accessibility of the 3' ends of the ribosomal RNAs in Escherichia coli ribosomes and subunits. Nucleic Acids Res. 1979 Jan;6(1):181–193. doi: 10.1093/nar/6.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior B. W., Holland I. B. Effect of colicin E3 upon the 30S ribosomal subunit of Escherichia coli. Proc Natl Acad Sci U S A. 1971 May;68(5):959–963. doi: 10.1073/pnas.68.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J. A., Jakes K. How ribosomes select initiator regions in mRNA: base pair formation between the 3' terminus of 16S rRNA and the mRNA during initiation of protein synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4734–4738. doi: 10.1073/pnas.72.12.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H. Colicin E3 inhibits rabbit globin synthesis. FEBS Lett. 1978 May 1;89(1):121–125. doi: 10.1016/0014-5793(78)80536-5. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Turnowsky F., Drews J., Eich F., Högenauer G. In vitro inactivation of ascites ribosomes by colicin E 3. Biochem Biophys Res Commun. 1973 May 1;52(1):327–334. doi: 10.1016/0006-291x(73)90991-1. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Fox G. E., Zablen L., Uchida T., Bonen L., Pechman K., Lewis B. J., Stahl D. Conservation of primary structure in 16S ribosomal RNA. Nature. 1975 Mar 6;254(5495):83–86. doi: 10.1038/254083a0. [DOI] [PubMed] [Google Scholar]