Abstract
Otoacoustic emission (OAE) amplitude can be reduced by acoustic stimulation. This effect is produced by the medial olivocochlear (MOC) reflex. Past studies have shown that the MOC reflex is related to listening in noise and attention. In the present study, the relationship between strength of the contralateral MOC reflex and masked threshold was investigated in 19 adults. Detection thresholds were determined for a 1000-Hz, 300-ms tone presented simultaneously with one repetition of a 300-ms masker in an ongoing train of 300-ms masker bursts at 600-ms intervals. Three masking conditions were tested: 1) broadband noise 2) a fixed-frequency 4-tone complex masker and 3) a random-frequency 4-tone complex masker. Broadband noise was expected to produce energetic masking and the tonal maskers were expected to produce informational masking in some listeners. DPOAEs were recorded at fine frequency interval from 500 to 4000 Hz, with and without contralateral acoustic stimulation. MOC reflex strength was estimated as a reduction in baseline level and a shift in frequency of DPOAE fine-structure maxima near 1000-Hz. MOC reflex and psychophysical testing were completed in separate sessions. Individuals with poorer thresholds in broadband noise and in random-frequency maskers were found to have stronger MOC reflexes.
Keywords: Medial olivocochlear (MOC) reflex, energetic masking, informational masking
1.0 Introduction
The medial olivocochlear (MOC) efferent loop is thought to have a direct effect on cochlear function (e.g., Fex, 1967; Francis & Nadol, 1993; Mountain, 1980; reviewed by Thiers, Burgess & Nadol, 2002). Neurons in the superior olivary complex receive afferent input via the cochlear nucleus and project to both the ipsilateral and contralateral ears through the crossed and uncrossed olivocochlear bundle, respectively (e.g., Guinan et al., 2010). By hyperpolarizing outer hair cells, the MOC system reduces cochlear amplifier gain, decreasing the output of the cochlea.
In humans, MOC effects on the cochlear response can be investigated using otoacoustic emissions (OAE). Early studies showed that contralateral acoustic stimulation (CAS) presented simultaneously with the OAE-evoking stimulus, altered the level of the OAE (e.g., Collet et al., 1990; Puel & Rebillard, 1990). An ipsilateral MOC elicitor can also alter the OAE but introduces confounds (Guinan, 2006). The contralateral MOC (MOC) reflex is mediated by the uncrossed efferent fibers from the medial olivary complex (Liberman & Brown, 1986; Robertson & Gummer, 1985).
It is thought that the MOC reflex increases the effective signal-to-noise ratio in the auditory nerve response, thus improving perception in noise. This effect is known as “MOC unmasking” (Guinan, 2006; 2010; Kujawa & Liberman, 2001). Physiological evidence in animals (e.g., Kawase, Delgutte, & Liberman, 1993; Winslow & Sachs, 1988) has shown that MOC activity improves the auditory nerve's response to signals by reducing the response to a noisy background, effectively shifting the dynamic range of hearing (e.g., Dolan & Nutall, 1988; Kawase, Delgutte & Liberman, 1993; Kujawa & Liberman, 2001).
Psychophysical studies have linked the MOC reflex to “overshoot”, the elevated threshold observed when the probe is presented at the onset of a simultaneous masker, compared to the case when the probe is presented some time after the onset of a simultaneous masker (Keefe et al., 2010; Schmidt & Zwicker, 1991; Zwicker, 1965). The magnitude of the MOC reflex has also been linked to attention (Froehlich et al., 1993; Garinis et al., 2011; Giard et al., 1994; Harkrider et al., 2009; Perrot et al., 2005) and auditory training (de Boer & Thornton, 2008). However, these studies only indirectly address the question of whether this reflex improves hearing in noise generally.
Three approaches have been used to show a more direct connection between hearing in noise and the MOC effect. One approach has been to show that presenting a sound that would be expected to activate the MOC reflex also improves hearing in noise (Giraud et al., 1997; Kumar & Vanaja, 2004; Micheyl & Collet, 1996; Zeng et al., 2000). These studies demonstrated that the magnitude of improvement in perception with CAS was correlated with the magnitude of the MOC reflex; individuals who showed greater CAS-induced improvements in hearing, also had stronger MOC reflexes. One problem of interpretation in such studies is the possibility of centrally mediated unmasking effects. Although the cited experiments used uncorrelated noise to avoid a binaural masking level difference, interaural level differences can shift the perceived intracranial position of the image evoked by binaural uncorrelated noise (Hartmann & Constan, 2002). This could lead to a release from masking.
The role of the MOC reflex in auditory perception has also been tested by examining the psychoacoustical performance of patients who have had their efferent fibers severed during vestibular neurectomy (VNT) (Giraud et al., 1997; Scharf et al., 1994; 1997; Zeng et al., 2000). Two of these studies reported that speech recognition in noise was poorer in the operated ear than in the unoperated ear of VNT patients (Giraud et al, 1997; Zeng et al., 2000), although Zeng et al. noted that peripheral hearing loss in the operated ear might have contributed to this effect. Scharf et al. reported that neither tone detection in quiet nor tone-in-noise detection was affected by VNT (but see Tan et al., 2008). Forward masked intensity discrimination at mid levels, overshoot, intensity discrimination in noise and the detection of unexpected frequencies were affected by the VNT surgery, but other psychoacoustical measures appeared to be unaffected (Scharf et al., 1997; Zeng et al., 2000; Zwicker, 1965). Giraud et al. (1997) also found that speech recognition did not improve with CAS nor was OAE level altered. Thus, these studies provide somewhat inconsistent evidence that disruption of the MOC reflex is associated with hearing-in-noise deficits. It is possible that the OCB had only partially been transected in some cases.
Finally, several studies of adults with normal hearing have examined the relationship between the absolute level of psychoacoustic performance in noise and the magnitude of the MOC reflex, reasoning that people with stronger reflexes should be better at processing signals in noise. The results have also been inconsistent. Micheyl and Collet (1996) reported that individuals with stronger MOC reflexes had better thresholds for a 2000-Hz tone in dichotic noise; no relationship was found for a 1000-Hz tone. Bhagat and Carter (2010) reported stronger MOC reflexes in individuals who had better 1000-Hz thresholds; no relationship was found for a 2000-Hz tone. The opposite result has also been reported, as Micheyl and colleagues (1995) found that individuals with stronger MOC reflexes had poorer thresholds for a three-tone complex in noise. Recently Wagner et al. (2008) failed to detect any relationship between MOC reflex strength and speech recognition in noise.
The present study sought to clarify the relationship between the contralaterally evoked MOC reflex and masked sensitivity in normal-hearing adult listeners. As in the previous studies, the relationship between MOC reflex strength and threshold for a tone in noise was examined. However, threshold was also examined in several masking conditions. Masking of a tone by a broadband noise results from the interaction of the tone and masker at the auditory periphery. This is referred to as energetic masking. Masking also occurs in conditions where alterations in cochlear gain would have little effect on threshold. For example, for many listeners masking occurs when the frequencies in a masker change randomly from presentation to presentation even when there is little or no spectral overlap between the signal and masker. This is called informational masking (Neff & Green, 1987; Pollack, 1975). Recent studies indicate that informational masking of a tone by a tonal complex—with no components near the target tone frequency— is possible, even when the masker frequencies do not vary (Bonino & Leibold, 2008; Leibold & Werner, 2006; Leibold et al., 2009).
Informational masking reflects a failure of selective listening, rather than limited spectral resolution. As noted above, Scharf et al. (1997) found that, unlike normal-hearing subjects, surgical VNT patients did not detect expected frequencies better than unexpected frequencies in the operated ear. This suggests a deficit in selective listening. Tan et al. (2008) argued that the advantage of expected over unexpected sounds in normal-hearing listeners (tested in the typical psychoacoustic “probe-signal” paradigm) results from an enhancement of activity at the expected signal frequency, mediated by MOC activity. If the MOC reflex plays a role in selective listening, then it may be associated with informational masking; hence, variation in the strength of the MOC reflex could at least partially account for the observed between-subject variability in this type of masking. To address this possibility, the present study assessed the relationship between several indices of MOC reflex strength and masked sensitivity in both energetic and informational masking conditions.
2.0 Material and Methods
2.1 Overview
The relationship between the strength of the MOC effect and masked sensitivity was investigated by taking psychophysical and physiologic measurements from the same subjects. Detection of a 1000-Hz target tone was measured in broadband noise (BBN), a 4-tone complex of fixed frequencies more than an auditory-filter width away from 1000-Hz (FIXED) and a 4-tone complex of randomly varying frequencies more than an auditory-filter width away from 1000-Hz (RANDOM). Energetic masking would be the dominant influence with the BBN masker, while informational masking would be the dominant influence with the tonal maskers. The MOC reflex was activated with BBN presented in the contralateral ear while the 2f1-f2 distortion product otoacoustic emission (DPOAE) level was monitored ipsilaterally. Testing was completed in two 60-minute sessions.
2.2 Subjects
Nineteen adult subjects completed both psychophysical and contralateral MOC reflex tests. The final sample included 13 females and 6 males with a mean age of 24 yr (range= 20-30 yr). Some subjects did not provide all of the MOC measures for reasons detailed below, but each of the final correlative analyses included 10-19 subjects. The right ear was tested in 11 subjects and the left ear, in 8 subjects. The test ear was matched for OAE and psychophysical testing. Subjects were recruited through the Communication Studies Participant Pool at the University of Washington. Participants reported normal hearing, no history of noise exposure and less than 2 years of musical training. All subjects had thresholds better than 20 dB HL from 250 to 8000-Hz on audiometric screening and normal middle ear function as assessed by screening tympanometry. Peak admittance of at least 0.2 mmhos at a pressure between 200 and +50 daPa was required to pass the tympanometric screening.
The middle-ear muscle reflex (MEMR) threshold was measured using the Interacoustics AT235 middle-ear analyzer with a BBN elicitor. The Interacoustics instrument is a research device that measures middle-ear response in terms of energy reflectance and uses a maximum likelihood method to estimate a threshold. This device has been shown to produce MEMR thresholds 10-12 dB lower, on average, than typical clinical instrumentation (Feeney et al., 2003). Contralateral MEMR thresholds ranged from 59 to 94 dB SPL, averaging 78.6 dB SPL. Two subjects had MEMR thresholds of 59-60 dB SPL, near the level of CAS used to elicit the MOC reflex. Although we cannot rule out some contribution from the MEMR in these two subjects, the contralateral MEMR threshold was not significantly correlated with any index of MOC reflex strength (lowest p = 0.38).
2.3 Psychophysical Testing
2.3.1 Stimuli
The target tone was a 1000-Hz pure tone, 300 ms in duration with a 10 ms rise/fall time. The maskers were also 300 ms in duration with a 10 ms rise/fall time and an overall level of 60 dB SPL. When the target tone was presented, it was synchronized with one presentation of the masker. The BBN masker was band-pass filtered with cutoff frequencies of 300 and 3000-Hz. The RANDOM masker contained four tones with frequencies randomly chosen from frequencies ranging between 300 to 3000-Hz, excluding frequencies 920 to 1080-Hz to reduce the amount of energetic masking. The minimum spacing between components was 50-Hz. The FIXED masker contained four tones. Four different FIXED maskers were used; the specific frequencies in each are listed in Table 1. Leibold et al. (2010) showed that many subjects demonstrated informational masking with these four fixed-frequency maskers, even though very little energetic masking would be predicted based on the masker excitation patterns (Moore et al., 1997).
Table 1.
Frequency components of the fixed-frequency maskers (Hz)
| Masker 1 | Masker 2 | Masker 3 | Masker 4 |
|---|---|---|---|
| 447 | 308 | 311 | 512 |
| 1870 | 411 | 1758 | 521 |
| 2276 | 1638 | 2246 | 1914 |
| 2716 | 2350 | 2805 | 2430 |
All stimuli were calibrated in a Zwislocki coupler. The signal and masker were attenuated separately using two programmable attenuators (TDT & PA5), mixed and fed to a headphone buffer (TDT HB7). Stimuli were presented through an Etymotic ER-1 insert earphone. Thresholds were estimated in a double-walled sound booth.
2.3.2 Procedures
Masked thresholds were measured adaptively using a go/no-go procedure: Subjects listened to repeating masker bursts and responded when they heard the target tone presented with the masker. The go/no-go procedure was used, because it involves a high degree of uncertainty about the timing of the target tone, a characteristic of everyday listening. In addition, because the go/no-go procedure is typically used in psychophysical studies of infants, comparison between the adults and infants will be possible in future studies. The masker was repeated at 600 ms intervals throughout the session. The subjects were instructed to raise their hand when they “heard the sound that makes the light come on”. An observer outside the booth initiated test trials at irregular intervals, ranging from 4 to 24 s, and recorded listener responses for all listeners. The same observer was used for all testing. On a test trial, the computer presented either a “target-tone” or a “no-target-tone” trial, randomly determined. For threshold estimation, the two trial types were equally probable. On a target-tone trial the 1000-Hz target tone was presented simultaneously with one burst of the masker. On a no-target-tone trial the masker was presented alone. A light came on whenever a target tone was correctly detected; no feedback was provided otherwise.
Sessions consisted of two training phases and one testing phase. In both training phases, the target tone was presented at 70 dB SPL. The probability of a tone trial in training phase I was 0.80, and the listener received visual feedback after each target-tone trial, whether or not a response was recorded. Training phase I ended when the listener responded correctly on 4 of 5 consecutive trials, including at least one no-target-tone trial. In training phase II, the probability of target-tone and no-target-tone trials was 0.50. Training phase II ended when the listener achieved at least a 0.80 hit rate and at most a 0.20 false-alarm rate on the last 5 target-tone trials and the last 5 no-target-tone trials, respectively. Subjects only completed training for the initial masking condition. A programming error made it necessary to schedule an additional session to complete the RANDOM condition for 14 subjects. These subjects were retrained in that condition. The thresholds of the subjects who returned for testing in the RANDOM condition did not differ from those of the other subjects.
During the test phase, the probabilities of target-tone and no-target-tone trials were equal. In every nine trials, a “probe” trial was administered randomly at 70 dB SPL to ensure that the listener was on task. A threshold was only included in the analyses if the response rate on no-target-tone trials was at most 0.40 and on probe trials was at least 0.67.
Detection thresholds were estimated adaptively using a one-up, two-down algorithm (Levitt, 1971). The starting level of the target-tone was 10 dB above the expected threshold value, based on previous research and pilot testing. Thresholds were taken as the average of the last 6 of 8 reversals. The order of conditions was randomized for each subject, with the exception noted above. Unmasked thresholds for a 1000-Hz tone were determined at the end of testing.
2.4 MOC reflex measures
Participants were tested at the University of Washington in a satellite OAE laboratory established by the third author for the period of data acquisition. Throughout DPOAE testing, subjects were awake and sat quietly in a single-walled booth in an enclosed room. The output of the ear canal microphone was monitored on a visual display to detect excessive movement and pause or restart sweeps if necessary.
2.4.1 DPOAE
Hardware and software replicated those previously described in published work (Abdala et al., 2009, Dhar et al., 2002: Long et al., 2008; Talmadge et al., 1999). Signal generation and recordings were controlled by custom software (developed by C. Talmadge) and run on an Apple Macintosh laptop computer via a MOTU (Mark of the Unicorn, Cambridge, MA) 828 MK II. Primary tones, f1 and f2, were logarithmically swept via ER2 (Etymotic Research, Elk Grove, Village, IL) insert transducer at 8 s/octave for a total of 8 sweeps (24 s) in each condition. DPOAE recordings were made for frequencies between 500 and 4000 Hz using stimulus levels of 65 (L1) and 55 (L2) dB SPL and a constant stimulus frequency ratio (f2/f1) of 1.22. The output of the ER-10B+ microphone was pre-amplified and then passed through an analog high-pass filter with a 300-Hz cutoff frequency before being digitized by the MOTU and stored on the hard drive.
Stimulus tones approximately calibrated at the plane of the eardrum were delivered by compensating for the depth of probe insertion (Siegel, 2009). Insertion depth was estimated by normalizing the slow chirp response between 0.2 and 20 kHz to that recorded in a 50 ft long copper tube with an internal diameter of 7.9 mm (approximately that of the adult ear canal). The pressure response recorded for a similar insertion depth in a standard ear simulator (Bruel and Kjaer 4157: Naerum, Denmark) was used to compensate the driving voltage to the earphones in order to deliver the desired SPL at the plane of the eardrum.
2.4.2 DPOAE Contralateral Inhibition
Contralateral broadband noise was presented through an ER-2 insert transducer at 55, 60, 65 and 70 dB SPL; only results with a 60 dB SPL elicitor are considered here. This level was chosen because it has shown to be an effective elicitor of the MOC reflex in adults, and is generally unlikely to elicit a MEMR (Guinan, 2006). The broadband MOC reflex elicitor was turned on 1 s before the onset of the primary tones. The no-CAS and +CAS DPOAE sweeps were interleaved throughout the test protocol in pairs, with 2 s between sweeps, until a total of 8 sweeps was collected in each condition.
2.4.3 DPOAE data analyses
All analyses were conducted on the averaged DPOAE. DPOAE level and phase estimates were obtained using a least-squares-fit algorithm (LSF) (Long et al., 2008), yielding estimates every 2-4 Hz in the 1000-Hz frequency range and every 6-9 Hz around the 3000-Hz range for a total of ~ 500 individual data points. The noise floor was estimated after phase-inverting every alternate sweep window. In this implementation of the LSF technique, models for the stimulus tones and DPOAE of interest are created. Signal components are then fitted to these models to minimize the sum of squared errors between the model and the data.
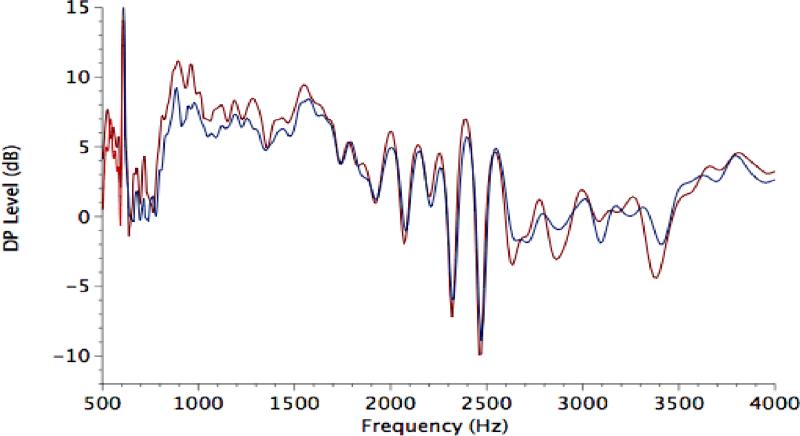
DPOAEs measured in the ear canal are the vector combination of two components, termed here distortion and reflection, each with a distinct source and generation mechanism (Shera & Guinan, 1999; Talmadge et al., 1999). DPOAE fine structure, as shown in Figure 1, reflects the destructive and constructive interference between these two components in the ear canal. The maxima or peaks in fine structure reflect frequencies at which the two components are adding constructively (in phase). Minima occur when the two components combine while out of phase. Although CAS has been reported to produce increases in DPOAE level rather than decreases (e.g., Abdala et al., 1999; Kim et al., 2002), these episodes of enhancement can result from differential effects of the noise elicitor on the reflection component. When the two components are out of phase, BBN can produce a release from cancellation and a subsequent artifactual increase in DPOAE level (Abdala et al., 2009; Deeter et al., 2009). In this study, measurements were made only at fine-structure maxima to avoid this confound.
Figure 1.
DPOAE fine structure in the no-CAS (red) and +CAS (blue) conditions for one adult subject.
Data were treated with a cleaning regimen prior to analysis. As part of this procedure, the median of every three consecutive points was calculated and compared to the noise estimate at the corresponding frequency to determine SNR, though individual values (not median) were used in all analyses. If SNR was less than 6 dB, the point was eliminated. The 6 dB SNR was used to avoid eliminating points at a minimum, which would prevent the accurate quantification of DPOAE fine structure depth and spacing. However, adult DPOAE data recorded with moderate-to high level primary tones in cooperative adults using the swept-tone and LSF methodology is characterized by SNRs well above this minimum criterion, in the range of 27- 32 dB (Abdala et al., 2011). Additionally the effect of CAS on DPOAE amplitude was measured at frequencies corresponding to fine-structure maxima only (Fig. 1) where the SNR is optimal.
In addition to recording only at fine structure maxima to ensure ensuring that components were mainly in-phase, we also only included MOC reflex data that were negative, reflecting MOC-induced inhibition. Less than 5% of observations showed level enhancement and were eliminated. This process was conducted to ensure that DPOAE component mixing, and associated release of cancellation, did not contaminate our indices of MOC strength.
Fine-structure peaks were identified using an automatic algorithm described in Abdala et al. (2011), then visually checked to eliminate any obvious classification errors. Two principal indices of MOC reflex strength were examined: (1) MOC reflex (MOCR), DPOAE amplitude in the no-CAS condition subtracted from amplitude in the +CAS condition at fine-structure peaks only. A normalized metric, (MOC reflex as a fraction of individual baseline amplitude) was also calculated. Because the normalized metric failed to show different trends from the MOCR dB index, it was not included in the analysis. (2) MOC shift (MOCS), the shift in fine-structure peak frequency (Hz) with no-CAS compared to +CAS. MOCS indirectly reflects the impact of CAS on DPOAE phase. As noted above, only DPOAE reductions in level and upward shifts in peak frequency were considered here. MOCR values were negative reflecting a reduction in level; MOCS values were positive, indicating a shift to higher frequencies.
MOCR was calculated only at the individual fine-structure maxima closest to 1000-Hz, the probe frequency used for psychophysical testing. The two peaks nearest in frequency to the probe, both above (MOCRHF) and below (MOCRLF) 1000-Hz were included. MOCS was analyzed in the 1/3-octave bands centered at 891-Hz (MOCS891) and at 1122-Hz (MOCS1122). These two frequency regions were chosen to limit the number of statistical comparisons and because previous work has shown the strongest MOC effect around the probe frequency used for psychophysical testing (Francis & Guinan, 2010).
3.0 Results
3.1 Psychophysical Results
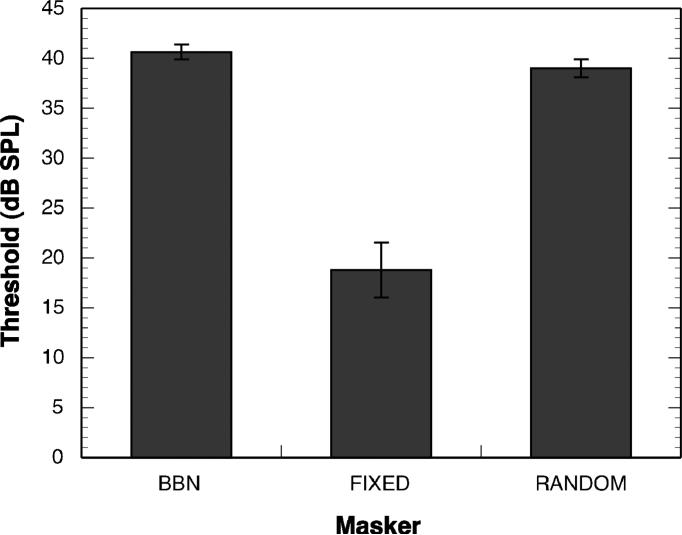
Mean thresholds are displayed for each masking condition in Figure 2. The mean thresholds are consistent with those reported in previous studies (e.g., Leibold & Werner, 2006; Leibold et al., 2010). Masked thresholds in the FIXED condition were lower than those in the BBN masker condition, as expected. The between-subject variability in masked threshold in the FIXED condition was high. The FIXED thresholds, on average, were about 10 dB higher than the unmasked threshold for the 1000-Hz tone, about 5 dB SPL, suggesting that informational masking occurred in this condition for at least some subjects. Mean threshold in the RANDOM condition was nearly as high as that in the BBN condition.
Figure 2.
Mean masked thresholds for broadband noise (BBN), fixed 4-tone complex (FIXED) and random 4-tone complex (RANDOM). Error bars represent one standard error of the mean.
Thresholds were not strongly interrelated. The BBN threshold was significantly correlated with the FIXED threshold [r (18) = 0.46, p = 0.02], but not significantly correlated with the RANDOM threshold [r (14) = 0.19, p = 0.24]. FIXED and RANDOM thresholds were not significantly correlated [r (15) = -0.08, p = 0.38].
3.2 DPOAE and MOC reflex
3.2.1 DPOAE fine structure
Mean DPOAE amplitude was typical for young adult subjects, ranging from 8 to 16 dB depending on frequency. The most robust DPOAEs were observed in the range of 1000-2000 Hz. Figure 1 shows a typical example of DPOAE level fine-structure from one subject with and without CAS presented. Measures of fine-structure spacing, depth and prevalence were also comparable to past reports in young adults (Reuter & Hammershoi, 2007; Abdala et al., 2009). One to two fine-structure periods were observed per 1/3 octave interval, spacing was approximately 100 Hz around 1500-Hz and 225-Hz around 4000-Hz (f/Δf = 10-15). Depth varied from 4-6 dB with deeper fine structure at the higher center frequencies. Thus, the subjects in this study produced DPOAE consistent with a normal, healthy peripheral auditory system and consistent with previous reports.
3.2.2 MOC effects
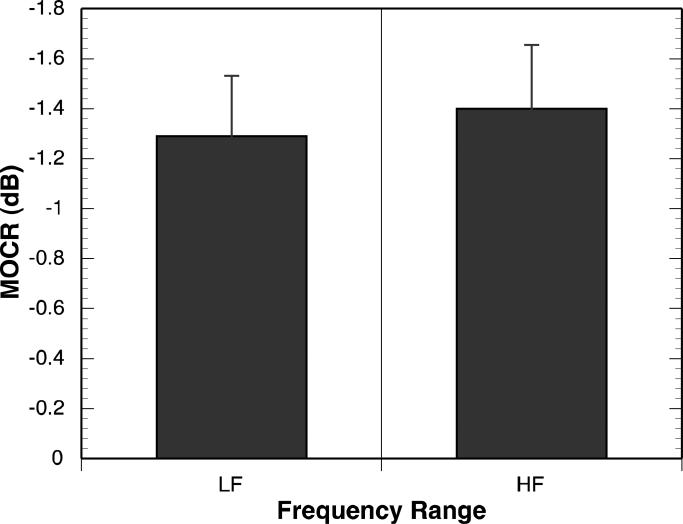
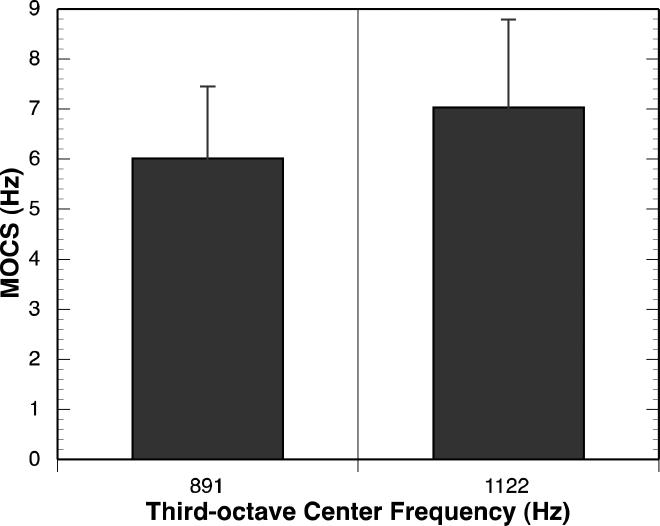
Overall, a 1.5 to 1.75 dB MOC reflex in the low- to mid-frequencies, reflecting an average of approximately 15% reduction in baseline DPOAE amplitude, was observed when the MOC reflex was elicited with 60 dB SPL BBN in the contralateral ear. MOCR ranged from 0.34 to 1.78 dB. MOCS showed shifts in fine-structure peak frequency ranging from 6 to 12 Hz across frequency. Fine-structure peaks shifted toward higher frequencies in over 90% of observations. These MOC reflex results are consistent with reports in independent groups of adults tested with a similar paradigm (e.g., Abdala et al., 2009; Deeter et al., 2009). Figure 3 displays mean MOCRLF and MOCRHF. Figure 4 displays mean MOCS891 and MOCS1122. MOC strength did not differ between the frequencies considered here. MOCRHF was significantly correlated with MOCRLF (r (11)=0.82, p <.001), but MOCS891 was not significantly correlated with MOCS1122(r (8)= 0.34, p = 0.46).
Figure 3.
Mean MOCR for single points measured below (MOCRLF) and above (MOCRHF) 1000-Hz. Error bars represent one standard error of the mean.
Figure 4.
Mean MOCS in two frequency ranges. Error bars represent one standard error of the mean.
3.3 MOCR and Masked Threshold
Prior to calculating the correlations between the measures of MOC reflex strength and masked thresholds, multivariate outliers were identified (hadimvo, Stata v11.1; Hadi, 1994) and excluded from the analyses. Hadimvo iteratively orders observations according to their distance from the multivariate center of the dataset to determine whether a set of data points reliably falls more than a criterion distance from the center. Of 120 data points in the correlational analysis, 1 was identified as an outlier, in the RANDOM threshold X MOCS1122 correlation. Some DPOAE measures were not available for all subjects who had completed psychophysical testing. If a measure was available for a subject, their data were included in the analysis of that measure, even if the subject had other missing data. Correlations were only calculated if at least 10 data points were available. Each of the MOC measures was considered as one “analysis” and the alpha level was Bonferroni adjusted so that the analysis-wise alpha level was 0.05. Six correlations were performed between MOCR and masked threshold (MOCRLF, MOCRHF X 3 maskers); hence, the alpha level for each correlation was set at 0.05/6 = 0.008. Five correlations were possible for MOCS, so the alpha level was set at 0.01 for those correlations.
Table 2 shows the correlations between masked thresholds and MOCR and MOCS. For BBN thresholds, the correlation with MOCRLF would have been significant with an alpha level of 0.05, but it was not significant using adjusted alpha levels. MOCS891 was significantly correlated with BBN thresholds. None of the correlations between FIXED thresholds and the measures of MOC strength were significant. The correlation between RANDOM threshold and MOCRLF was significant and of notable magnitude. RANDOM thresholds were not significantly correlated with MOCRHF or MOCS1122. If the one outlier identified was included, the correlation between RANDOM threshold and MOCS1122 increased slightly but was still not statistically significant. Insufficient data prevented calculation of a correlation between RANDOM threshold and MOCS891.
Table 2.
Correlations of MOC strength indices MOCR and MOCS with masked thresholds in the BBN, FIXED and RANDOM masking conditions. Significant correlations are shown in bold type.
| MASKER | MOCR* | MOCS** | ||
|---|---|---|---|---|
| LF | HF | 891 Hz | 1122 Hz | |
| BBN | -0.51 13 0.04 |
-0.45 13 0.06 |
0.73
11 0.005 |
0.27 13 0.19 |
| FIXED | -0.04 13 0.45 |
0.20 13 0.26 |
0.33 11 0.16 |
0.57 13 0.02 |
| RANDOM |
-0.81
10 0.002 |
-0.25 10 0.24 |
0.31 10 0.19 |
|
Bonferroni-corrected significance level 0.008.
Bonferroni-corrected significance level 0.01.
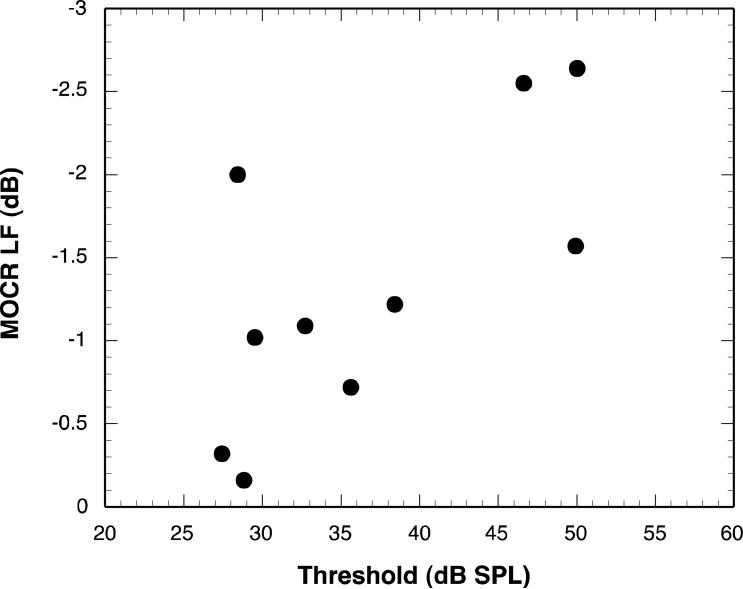
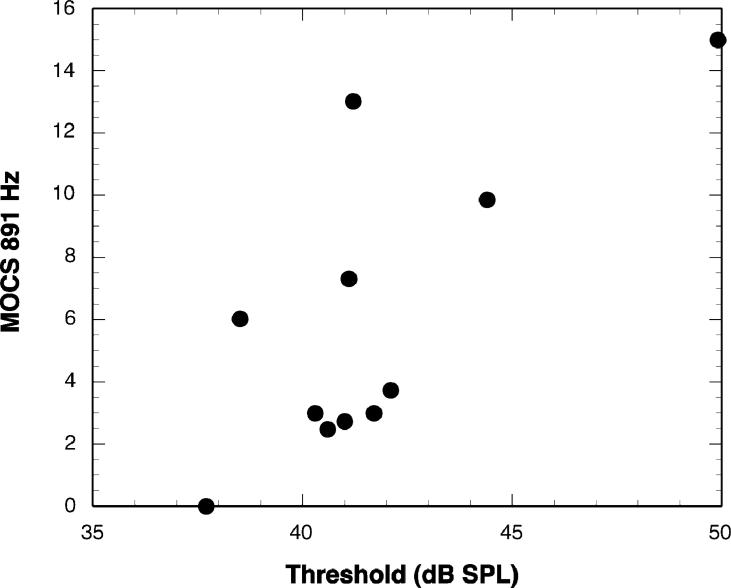
Contrary to expectation, MOCRLF was negatively correlated with threshold. Because MOCR is a negative number, indicating MOC-induced inhibition, the negative correlation means that subjects with a stronger MOC reflex had higher (i.e., poorer) RANDOM thresholds. The correlations between MOCR and BBN threshold, although not significant, are in the same direction. Likewise, the significant correlation between MOCS891 and BBN threshold is positive, indicating that subjects with the most marked shifts in DPOAE fine structure peak frequency had higher (i.e., poorer) BBN thresholds.
Figure 5 shows a scatterplot of MOCRLF as a function of threshold in the RANDOM masker condition. Note that the direction of the y-axis in that figure has been reversed. Figure 6 shows a scatterplot of MOCS891 as a function of threshold in the BBN masker condition. Although the data point at the far right corner of the plot may appear to be an outlier, it did not meet the statistical criterion for elimination.
Figure 5.
Scatterplot of MOCRLF as a function of masked threshold for the RANDOM condition.
Figure 6.
Scatterplot of MOCS891 as a function of masked thresholds for BBN condition.
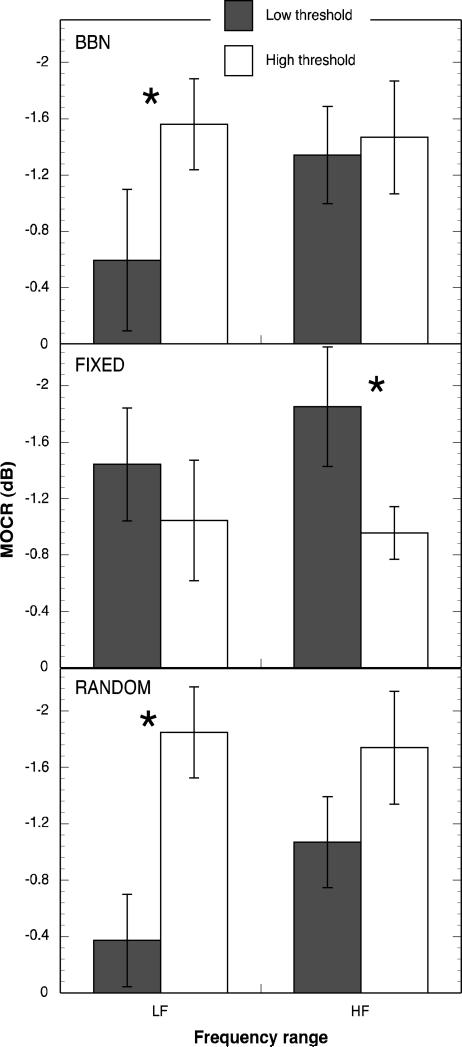
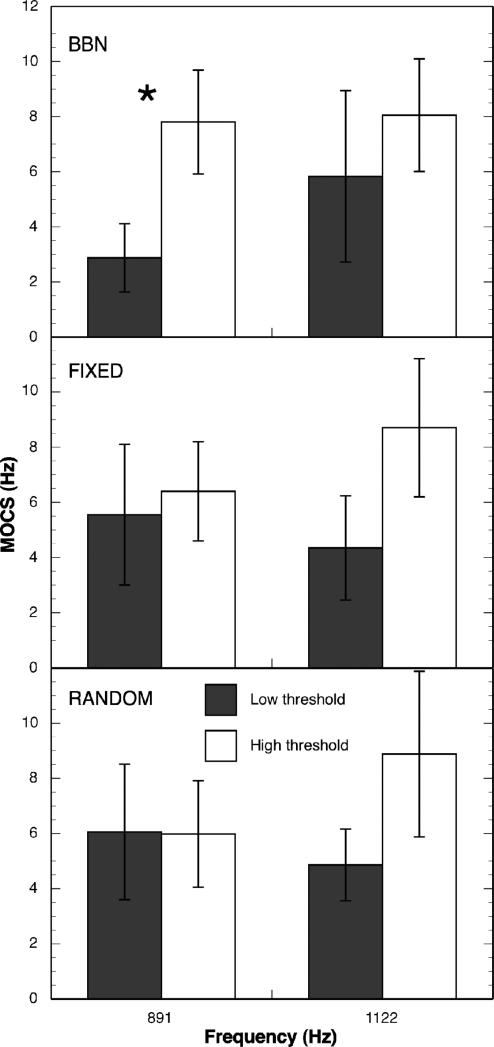
Correlations are sensitive to the distribution of values, and because the number of observations was relatively small, the correlations may be insensitive to the relationship between threshold and MOC effects. To check for this possibility, a median split analysis was also performed. MOCRLF, MOCRHF, MOCS891 and MOCS1122 were compared for subjects above and below the median threshold in each masker condition. Figure 7 shows mean MOCRLF and MOCRHF for the low- and high-threshold groups in each masker condition. For BBN and RANDOM maskers, the magnitude of MOCRLF and MOCRHF were greater for the high-threshold group than for the low-threshold group, although the difference was only significant for MOCRLF [t (12)= 3.34 p = 0.003], consistent with the correlation analysis. Figure 8 shows mean MOCS891 and MOCS1122 Hz for the low- and high-threshold groups in each masker condition. Note that in all cases except the RANDOM condition and MOCS891, the MOCS effect was stronger for the high-threshold group than for the low-threshold group. The difference is only statistically significant, however, at MOCS891 Hz for the BBN masker condition [t (9)= -1.82, p = 0.05] in agreement with the correlation analysis.
Figure 7.
Mean MOCRLF and MOCRHF for low-threshold and high-threshold groups in three masking conditions
Figure 8.
Mean MOCS891 and MOCS1122 for low- and high threshold groups in three masking conditions.
The single discrepancy between the correlation and the median-split analyses is for the FIXED masker condition, where a significant difference was seen in MOCRHF in the median-split analysis [t (12)= -1.93 p = 0.04]. However, for the FIXED masker condition, the low-threshold listeners had greater MOCR magnitude than the high-threshold listeners. This difference was statistically significant for MOCRHF. This is the only instance where a stronger MOC effect is associated with improved masked sensitivity.
4.0 Discussion
The goal of this investigation was to examine the relationship between the strength of contralaterally evoked MOC inhibition and masked threshold in energetic and informational masking conditions. The original hypothesis was that listeners with stronger MOC reflexes would exhibit better thresholds for masked tone detection. Contrary to expectation, the results indicate that adults with stronger MOC effects had higher masked thresholds in the broadband noise masking condition and in one random-frequency masker condition. A strong MOC effect was also associated with worse thresholds in BBN. Throughout the Discussion, we will refer to this relationship between MOC indices and masked thresholds as a negative correlation indicating a stronger MOC effect was associated with poorer masked sensitivity.
The results are less clear for thresholds in a fixed-frequency tonal masker. Both measures of MOC strength (MOCR and MOCS) tended to be stronger for low-threshold than for high-threshold listeners. The correlations were not statistically significant for MOCRHF. This is the only condition in which listeners with better sensitivity had stronger MOC reflexes.
The finding that stronger MOC reflexes were associated with poorer masked sensitivity seems to run counter to those reported in some previous studies. However, the majority of studies reporting that the MOC reflex improves masked sensitivity actually reported correlations between OAE-based inhibition and the associated change in detection rather than the absolute sensitivity to a signal in noise (Giraud et al., 1997; Micheyl & Collet, 1996; Kumar & Vanaja, 2004).
To our knowledge, there are four previous published studies that have reported correlations between contralateral MOC reflex strength and masked threshold in normal-hearing listeners with intact cochlear efferent projections. Two report that stronger MOC reflexes are associated with better masked sensitivity (Bhagat & Carter, 2010; Micheyl & Collet, 1996). One reports no relationship between MOC reflex strength and masked sensitivity (Wagner et al., 2008) and another reports that stronger MOC reflexes are associated with poorer masked sensitivity, as observed here (Micheyl et al., 1995).
The current study does differ from all the previous studies in its use of a high temporal-uncertainty psychophysical procedure. One speculation addressing the observed negative relationship is that good listeners maintained a high level of OCB activation (presumably to optimize efficiency) throughout contralateral MOC testing. Such listeners would minimize their no-CAS and +CAS differences and consequently appear to have small MOC reflexes. They would also have good measured thresholds, because they would benefit from the MOC-induced improvement in signal-to-noise ratio. In contrast, listeners who only activated the MOC near the onset of the probe might have poorer thresholds, owing to the delay in MOC activation.
At least three observations argue against this speculation. First, if some listeners kept their OCB activated throughout DPOAE recording, one might expect them to have lower DPOAE levels during no-CAS conditions. In fact, neither MOCR nor MOCS was significantly correlated with DPOAE level, in any frequency band, across listeners. Second, if listeners simply kept their OCB activated during psychophysical testing, it would be advantageous for detecting a tone under energetic masking conditions, but not necessarily under informational masking conditions, where signal-to-noise ratio is less important. Note also that a negative relationship was not observed when the masker was a tonal complex with fixed frequencies. Third, the present study is not the first to report the noted relationship between masked thresholds and the MOC effect. Micheyl et al. (1995) also report a negative relationship between the strength of the MOC reflex and masked threshold, even though their threshold estimates were made in a standard two-interval forced-choice procedure.
There are many methodological differences among the studies that have examined the link between MOC reflex strength and hearing in noise, including stimuli, OAE type and chosen MOC reflex metric. These variables are summarized for the four previously published studies and for the current study in Table 3. As is evident in the table, there is no obvious, single factor that distinguishes the studies reporting a positive relationship between the MOC reflex and masked threshold from those that report a negative relationship. Note, for example, that Bhagat and Carter (2010) used stimuli with the same duration and synchronous target-masker onset used here, but report a positive relationship between MOC reflex strength and masked threshold. Micheyl et al. (1995) and Micheyl and Collet (1996) both used shorter target sounds with target onset following masker onset, and yet report disparate results. Neither Bhagat and Carter nor the current study presented contralateral maskers while measuring psychoacoustic thresholds, yet Bhagat and Carter report a positive relationship, whereas a negative relationship was found here. Finally, one noted limitation of the present study (and all those that preceded it as noted in Table 3) is that the MOC reflex was not recorded as a task-dependent effect. The two activities –listening task and activating the MOC reflex- did not occur simultaneously or using a common masker/elicitor. This paradigm would perhaps reflect the most realistic probe of MOC effects on detection in noise.
Table 3.
Summary of methods from five studies of relationship between MOC reflex strength and masked threshold.
| Study | Subjects1 (n, gender; age) | Psychoacoustic Stimuli (target; masker; masker-target onset2) | Contralateral Noise3 (Threshold/MOC) | OAE Type4 | MOC Effect Metric | MOC-threshold relationship5 |
|---|---|---|---|---|---|---|
| Micheyl et al. (1995) | 10 F, 15 M; μ 22 yr SD 2.3 yr | 100 ms 3-tone complex; 400 ms BBN; +200 ms | Yes/Yes | TE | Difference in I/O function intercepts | Negative |
| Micheyl & Collet (1996) | 14 F, 16 M; μ 20 yr SD 1.09 yr | 100 ms tones; 400 ms BBN; +200 ms | Yes/Yes | TE | Stimulus attenuation producing equivalent reduction in OAE level | Positive |
| Wagner et al. (2008) | 30 F, 19 M; 19.7-41.7 yr | 5-word sentences; speech spectrum noise; noise continuous during sentence | Yes/Yes (soundfield) | DP | Measured at minima in fine structure | None |
| Bhagat & Carter (2010) | 14 F; 22-42 yr | 300-ms tone; 300-ms BBN; 0 ms | No/Yes | DP | Shift in I/O function compression threshold | Positive |
| Garinis et al. (2011) | 13 F, 6 M; 20-30 yr | 300-ms tone; 300-ms masker; 0 ms | No/Yes | DP | Change in level and phase at fine-structure maxima | Negative |
F = female; M = male; μ = mean; SD = standard deviation
onset time = masker onset time – target onset time
Noise presented during threshold measurement
TE = transient evoked OAE; DP = distortion product OAE
Positive = threshold lower for larger MOC effect; Negative = threshold higher for larger MOC effect; None = no significant relationship
It is likely that the premise of all of these studies is based on an oversimplification of the mechanisms involved in extracting signals from noise and other maskers. Certainly MOC-mediated and non-MOC-mediated mechanisms are involved. Tan et al. (2008), for example, argue that an expected frequency tone preceded by a cue indicating the target frequency is more easily detected than unexpected frequency tones, because the MOC enhances the response to the cued frequency, while a central mechanism inhibits the response to unexpected frequencies. May et al. (2004) have shown that while cats initially had difficulty processing signals in noise following olivocochlear bundle (OCB) lesions, they recovered following long-term training. Thus, both central mechanisms and the MOC system can independently facilitate hearing in noise. Moreover, there are undoubtedly multiple mechanisms of each type in play. For example, the enhancement at the target frequency hypothesized to improve cued detection by Tan et al. (2008) would not be expected to be helpful in detecting a tone in noise if no cue is presented. However, a broad suppression of cochlear response would be helpful in the latter case. The mechanism assessed by eliciting the effect of contralateral MOC activation on the DPOAE may not be the same mechanism by which top-down influences on the MOC system are realized. It may even be that the ipsilateral and contralateral reflexes differ in some respects. Various central mechanisms—temporal, spectral, and binaural—are involved in separating a signal from noise as well. Last but not least, the MEMR will also be active under normal circumstances.
That different listeners develop different strategies, broadly defined, to extract signals from masking sounds is evident in the great intersubject variability observed in measures like informational masking. For example, Neff and Dethlefs (1995) reported that while some listeners exhibited essentially no masking, others exhibited as much as 50 dB of masking as a result of random variation in masker frequency. They also found that informational masking was resistant to training for periods as long as several months, although subsequent studies have shown that musically trained listeners are less susceptible to informational masking than those without musical training (Oxenham et al., 2003). Nonetheless, Neff and Dethlefs found that susceptibility to informational masking was unrelated to the ability to detect tones in broadband noise. In other words, individuals susceptible to informational masking use a different approach to separating signals from maskers than individuals who are not susceptible to informational masking, but whatever their approach is, it is effective in extracting a tone from noise. On the other hand, their approach is less effective when the masker frequencies vary.
Assume that listeners vary in their dependence on the mechanism gauged by the MOC reflex, for extracting signals from noise; and that listeners who depend on that mechanism have stronger MOC reflexes than those who do not. Listeners who do not depend on this underlying mechanism as heavily may depend on more central processes, or on a mechanism that is not linked to the contralateral MOC reflex. The varied results of the studies that have examined the relationship between MOC reflex strength and masked sensitivity may suggest that dependence on this process or mechanism is helpful for detecting masked sounds in some situations. Positive relationships between MOC reflex strength and masked thresholds will be observed in those situations. In other situations the MOC reflex mechanism (as assayed by an OAE protocol) is maladaptive for detection of masked sounds. Negative relationships between MOC reflex strength and masked thresholds will be observed in those situations. In still other situations, that MOC mechanism and other mechanisms may be equally effective for extracting targets from maskers, and no relationship between the MOC reflex and masked sensitivity will be found.
The results of the studies summarized in Table 3 may help to identify the various types of listening situations. While Table 3 does not suggest any obvious factors, one possible difference between the studies reporting positive effects and those reporting negative effects, is that in the former (Micheyl & Collet, 1996; Bhagat & Carter, 2010) listeners would have been able to focus, for want of a better term, narrowly on a single frequency region. The signals would have been presented at predictable and relatively short intervals. In the Micheyl et al. (1995) study, the target sound was a 3-tone complex with frequency components spaced over an octave. Although trained listeners are able to focus simultaneously on multiple frequency regions (e.g., Buus et al., 1986; Schlauch & Hafter, 1991), it is likely that Micheyl et al.'s listeners did not master this skill in the short period over which they were tested and were listening somewhat broadly across frequency. In the current study, listeners detected a single tone, but the tone frequency was not cued on each trial and listeners would have to remember the tone's frequency over intervals as long as 24 seconds between trials, with the tone level near threshold when it was presented. Thus, listeners could be uncertain about the precise target frequency and as a result might listen over a somewhat broader frequency region than they would if the target frequency were well specified. The fixed-frequency masker may have provided a frequency referent for the target that the other maskers did not, so that the relationship between MOC reflex strength and threshold was actually positive. This speculation leads to the prediction that the MOC reflex-related mechanism is helpful in detection when the target is highly frequency specific, but not when it is broader in bandwidth or of uncertain frequency.
5.0 Conclusion
The present study shows that a stronger contralateral MOC inhibition effect is associated with poorer monaural masked sensitivity in broadband noise and random-frequency tonal maskers in normal-hearing adults tested in a condition with high temporal uncertainty. These results and those of previous studies of the same type suggest that the utility of MOC reflex-related mechanisms in extracting signals from noise may vary with the stimulus and the listening conditions in a complex way. Additional parametric studies may clarify this issue. An especially helpful approach would be to measure MOC activity while subjects are performing various sorts of detections tasks.
Acknowledgments
Supported in part by research grants R01 DC000396, R01 DC003552, T32 DC005361 and P30 DC004661 from the National Institutes of Health. The authors would like to express their gratitude to Ashley L. Flad for data analysis and Ping Luo for programming contributions.
References
- Abdala C, Dhar S, Kalluri R. Level dependence of distortion product otoacoustic emission phase is attributed to component mixing. J. Acoust. Soc. Am. 2011 doi: 10.1121/1.3573992. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdala CA, Mishra SK, Williams TL. Considering distortion product otoacoustic emission fine structure in measurement of the medial olivocochlear reflex. J. Acoust. Soc. Am. 2009;125(3):1584–1594. doi: 10.1121/1.3068442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdala C, Ma E, Sininger YS. Maturation of the medial efferent system function in humans. J. Acoust. Soc. Am. 1999;105(4):2392–2402. doi: 10.1121/1.426844. [DOI] [PubMed] [Google Scholar]
- Bhagat SP, Carter PH. Efferent-induced change in human cochlear compression and its influence on masking of tones. Neurosci. Let. 2010;485(2):94–7. doi: 10.1016/j.neulet.2010.08.069. [DOI] [PubMed] [Google Scholar]
- Bonino AY, Leibold LJ. The effect of signal-temporal uncertainty on detection in bursts of noise or a random-frequency complex. J. Acoust. Soc. Am. 2008;124(5):321–7. doi: 10.1121/1.2993745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buus S, Schorer E, Florentine M, Zwicker E. Decision rules in detection of simple and complex tones. J. Acoust. Soc. Am. 1986;80:1646–1657. doi: 10.1121/1.394329. [DOI] [PubMed] [Google Scholar]
- Collet L, Kemp DT, Veuillet E, Duclaux R, Moulin A, Morgon A. Effect of contralateral auditory stimuli on active cochlear micro-mechanical properties in human subjects. Hear. Res. 1990;43:251–261. doi: 10.1016/0378-5955(90)90232-e. [DOI] [PubMed] [Google Scholar]
- Deeter R, Abel R, Calandruccio L, Dhar S. Contralateral acoustic stimulation alters the magnitude and phase of distortion product otoacoustic emissions. J. Acoust. Soc. Am. 2009;126(5):2413–2424. doi: 10.1121/1.3224716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer J, Thornton AR. Neural correlates of perceptual learning in the auditory brainstem: efferent activity predicts and reflects improvement at a speech-in-noise discrimination task. J. Neurosci. 2008;28(19):4929–37. doi: 10.1523/JNEUROSCI.0902-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar S, Talmadge CL, Long GR, Tubis A. Multiple internal reflections in the cochlea and their effect on DPOAE fune structure. J. Acoust. Soc. Am. 2002;112:2882–2897. doi: 10.1121/1.1516757. [DOI] [PubMed] [Google Scholar]
- Dolan DF, Nuttall AL. Masked cochlear whole- nerve response intensity functions altered by electrical stimulation of the crossed olivocochlear bundle. J. Acoust. Soc. Am. 1988;83:1081–1086. doi: 10.1121/1.396052. [DOI] [PubMed] [Google Scholar]
- Feeney MP, Keefe DH, Marryott LP. Contralateral acoustic reflex thresholds for tonal activators using wideband energy reflectance and admittance. J. Speech Lang. Hear. Res. 2003;46(1):128–136. doi: 10.1044/1092-4388(2003/010). [DOI] [PubMed] [Google Scholar]
- Fex J. Efferent inhibition in the cochlea related to hair-cell dc activity: study of postsynaptic activity of the crossed olivocochlear fibers in the cat. J. Acoust. Soc. Am. 1967;1(3):666–675. doi: 10.1121/1.1910395. [DOI] [PubMed] [Google Scholar]
- Francis NA, Guinan JJ. Acoustic stimulation of human medial olivocochlear efferents reduces stimulus-frequency and click-evoked otoacoustic emission delays: Implications for cochlear filter bandwidths. Hear. Res. 2010;267(1-2):36–45. doi: 10.1016/j.heares.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis HW, Nadol JB., Jr. Patterns of innervation of outer hair cells in a chimpanzee: I. Afferent endings and reciprocal synapses. Hear. Res. 1993;64:184–190. doi: 10.1016/0378-5955(93)90004-k. [DOI] [PubMed] [Google Scholar]
- Froehlich P, Collet L, Morgon A. Transiently evoked otoacoustic emission amplitudes change with changes of directed attention. Physiol. Beh. 1993;53:679–682. doi: 10.1016/0031-9384(93)90173-d. [DOI] [PubMed] [Google Scholar]
- Garinis AC, Glattke TG, Cone Barbara. The MOC reflex during active listening to speech. J. Speech Lang. Hear. Res. 2011 doi: 10.1044/1092-4388(2011/10-0223). In Press. [DOI] [PubMed] [Google Scholar]
- Giard MH, Collet L, Bouchet P, Pernier J. Auditory selective attention in the human cochlea. Brain Res. 1994;633:353–356. doi: 10.1016/0006-8993(94)91561-x. [DOI] [PubMed] [Google Scholar]
- Giraud AL, Garnier S, Micheyl C, Lina G, Chays A, Chery Croze S. Auditory efferents involved in speech- in-noise intelligibility. Neuroreport. 1997;8:1779–1783. doi: 10.1097/00001756-199705060-00042. [DOI] [PubMed] [Google Scholar]
- Guinan JJ. Cochlear efferent innervation and function. Curr. Opin. Otolaryngol. Head. Neck. Surg. 2010;18(5):447–453. doi: 10.1097/MOO.0b013e32833e05d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan JJ. Olivocochlear efferents: Anatomy, physiology, function, and the measurement of efferent effects in humans. Ear Hear. 2006;27:589–607. doi: 10.1097/01.aud.0000240507.83072.e7. [DOI] [PubMed] [Google Scholar]
- Hadi AS. A modification of a method for the detection of outliers in multivariate samples. J. Royal Stat. Soc. B-Methodol. 1994;56(2):393–396. [Google Scholar]
- Harkrider AW, Bowers CD. Evidence for a cortically mediated release from inhibition in the human cochlea. J. Am. Acad. Audiol. 2009;20(3):208–15. doi: 10.3766/jaaa.20.3.7. [DOI] [PubMed] [Google Scholar]
- Hartmann WM, Constan ZA. Interaural level differences and the level-meter model. J. Acoust. Soc. Am. 2002;112(3):1037–45. doi: 10.1121/1.1500759. [DOI] [PubMed] [Google Scholar]
- Kawase T, Delgutte B, Liberman MC. Antimasking effects of the olivocochlear reflex. II. Enhancement of auditory-nerve response to masked tones. J. Neurophysiol. 1993;70(6):2533–49. doi: 10.1152/jn.1993.70.6.2533. [DOI] [PubMed] [Google Scholar]
- Keefe DH, Fitzpatrick D, Liu YW, Sanford CA, Gorga MP. Wideband acoustic-reflex test in a test battery to predict middle-ear dysfunction. Hear. Res. 2010;263(1-2):52–65. doi: 10.1016/j.heares.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Frisina DR, Frisina RD. Effects of age on contralateral suppression of distortion-product otoacoustic emissions in human listeners with normal hearing. Audiol. Neurootol. 2002;7:348–357. doi: 10.1159/000066159. [DOI] [PubMed] [Google Scholar]
- Kujawa S, Liberman CM. Effects of olivocochlear feedback on distortion product otoacoustic emissions in guinea pig. J. Assoc. Res. Otolaryngol. 2001;2:268–278. doi: 10.1007/s101620010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar UA, Vanaja CS. Functioning of olivocochlear bundle and speech perception in noise. Ear Hear. 2004;25:142–146. doi: 10.1097/01.aud.0000120363.56591.e6. [DOI] [PubMed] [Google Scholar]
- Leibold LJ, Werner LA. Effect of masker-frequency variability on the detection performance of infants and adults. J. Acoust. Soc. Am. 2006;119:3960–3970. doi: 10.1121/1.2200150. [DOI] [PubMed] [Google Scholar]
- Leibold LJ, Bonino AY. Release from informational masking in children: effect of multiple signal bursts. J. Acoust. Soc. Am. 2009;125(4):2200–2208. doi: 10.1121/1.3087435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibold LJ, Hitchens JJ, Buss E, Neff DL. Excitation-based and informational masking of a tonal signal in four-tone masker. J. Acoust. Soc. Am. 2010;127(4):2441–2450. doi: 10.1121/1.3298588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J. Acoust. Soc. Am. 1971:467–477. [PubMed] [Google Scholar]
- Liberman MC. Response properties of cochlear efferent neurons: monaural vs. binaural stimulation and the effects of noise. J. Neurophysiol. 1988;60:1779–1798. doi: 10.1152/jn.1988.60.5.1779. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Brown MC. Physiology and anatomy of single olivocochlear neurons in the cat. Hear. Res. 1986;24:17–36. doi: 10.1016/0378-5955(86)90003-1. [DOI] [PubMed] [Google Scholar]
- Long GR, Talmadge CL, Lee J. Measuring distortion product otoacoustic emissions using continuously sweeping primaries. J. Acoust. Soc. Am. 2008;124:1613–1625. doi: 10.1121/1.2949505. [DOI] [PubMed] [Google Scholar]
- May BJ, Niparko JK. Behavioral studies of the olivocochlear efferent system. Arch. Otolaryngol. Head Neck Surg. 2004;130:660–664. doi: 10.1001/archotol.130.5.660. [DOI] [PubMed] [Google Scholar]
- Micheyl C, Morlet T, Giraud AL, Collet L, Morgon A. Contralateral suppression of evoked otoacoustic emissions and detection of a multi-tone complex in noise. Acta Oto-Laryngol. 1995;115(2):178–182. doi: 10.3109/00016489509139286. [DOI] [PubMed] [Google Scholar]
- Micheyl C, Collet L. Involvement of the olivocochlear bundle in the detection of tones in noise. J. Acoust. Soc. Am. 1996;99:1064–1610. doi: 10.1121/1.414734. [DOI] [PubMed] [Google Scholar]
- Moore BC, Glasberg BR, Baer T. A model for the prediction of thresholds, loudness, and partial loudness. J. Audio. Eng. Soc. 1997;45:224–237. [Google Scholar]
- Mountain DC. Changes in endolymphatic potential and crossed olivocochlear bundle stimulation after cochlear mechanics. Science. 1980;210(4465):71–72. doi: 10.1126/science.7414321. [DOI] [PubMed] [Google Scholar]
- Neff DL, Green DM. Masking produced by spectral uncertainty with multicomponent maskers. Percept. Psychophys. 1987;41(5):409–415. doi: 10.3758/bf03203033. [DOI] [PubMed] [Google Scholar]
- Neff DL, Dethlefs TM. Individual differences in simultaneous masking with random-frequency, multicomponent maskers. J. Acoust. Soc. Am. 1995;98(1):125–134. doi: 10.1121/1.413748. [DOI] [PubMed] [Google Scholar]
- Oxenham AJ, Fligor BJ, Mason CR, Kidd G. Informational masking and musical training. J. Acoust. Soc. Am. 2003;114(3):1543–1549. doi: 10.1121/1.1598197. [DOI] [PubMed] [Google Scholar]
- Perrot X, Ryvlin P, Isnard J, Guenot M, Catenoix H, Fischer C, Mauguiere F, Collet L. Evidence for corticofugal activity. Evidence for corticofugal modulation of peripheral auditory activity in humans. Cerebral Cortex. 2005;16(7):941–948. doi: 10.1093/cercor/bhj035. [DOI] [PubMed] [Google Scholar]
- Pollack I. Auditory informational masking. J. Acoust. Soc. Am. 1975;57(suppl.1):5. [Google Scholar]
- Puel JL, Rebillard Effects of contralateral sound stimulation on the distortion product 2f1-f2: Evidence that the medial efferent system is involved. J. Acoust. Soc. Am. 1990;87:1630–1635. doi: 10.1121/1.399410. [DOI] [PubMed] [Google Scholar]
- Reuter K, Hammershøi D. Distortion product otoacoustic emission fine structure analysis of 50 normal-hearing humans. J. Acoust. Soc. Am. 2007;120:270–279. doi: 10.1121/1.2205130. [DOI] [PubMed] [Google Scholar]
- Robertson D, Gummer M. Physiological and morpho- logical characterization of efferent neurons in the guinea pig cochlea. Hear. Res. 1985;20:63–77. doi: 10.1016/0378-5955(85)90059-0. [DOI] [PubMed] [Google Scholar]
- Schlauch RS, Hafter ER. Listening bandwidths and frequency uncertainty in pure-tone signal detection. J. Acoust. Soc. Am. 1991;90(3):1332–1339. doi: 10.1121/1.401925. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Zwicker E. The effect of masker spectral asymmetry on overshoot in simultaneous masking. J. Acoust. Soc. Am. 1991;89(3):1324–1330. doi: 10.1121/1.400656. [DOI] [PubMed] [Google Scholar]
- Scharf B, Magnan J, Chays A. On the role of the olivocochlear bundle in hearing: 16 case studies. Hear. Res. 1997;103:101–122. doi: 10.1016/s0378-5955(96)00168-2. [DOI] [PubMed] [Google Scholar]
- Scharf B, Magnan J, Collet L, Ulmer E, Chays A. On the role of the olivocochlear bundle in hearing: a case study. Hear. Res. 1994;75:11–26. doi: 10.1016/0378-5955(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Shera C, Guinan JJ. Evoked otoacoustic emissions arise by two fundamentally different mechanisms: A taxonomy for mammalian OAEs. J. Acoust. Soc. Am. 1999;105:782–798. doi: 10.1121/1.426948. [DOI] [PubMed] [Google Scholar]
- Siegel J. Estimating wideband eardrum sound levels in humans. Association for Research in Otolaryngology; Baltimore, MD: Feb 14-19th, 2009. [Google Scholar]
- Talmadge CL, Long GR, Tubis A, Dhar S. Experimental confirmation of the two-source interference model for the fine structure of distortion product otoacoustic emissions. J. Acoust. Soc. Am. 1999;105:275–292. doi: 10.1121/1.424584. [DOI] [PubMed] [Google Scholar]
- Tan MN, Robertson D, Hammond GR. Separate contributions of enhanced and suppressed sensitivity to the auditory attentional filter. Hear. Res. 2008;241(1-2):18–25. doi: 10.1016/j.heares.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Thiers FA, Burgess BJ, Nadol JB. Reciprocal innervation of outer hair cells in a human infant. J. Assoc. Res. Otolaryngol. 2002;3(3):269–278. doi: 10.1007/s101620020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W, Frey K, Heppelmann G, Plontke SK, Hanz-Peter Z. Speech-in-noise intelligibility does not correlate with efferent olivocochlear reflex in humans with normal hearing. Acta Oto-Laryngol. 2008;128:53–60. doi: 10.1080/00016480701361954. [DOI] [PubMed] [Google Scholar]
- Winslow RL, Sachs MB. Single-tone intensity discrimination based on auditory-nerve rate responses in backgrounds of quiet, noise, and with stimulation of crossed olivocochlear bundle. Hear. Res. 1988;35:165–190. doi: 10.1016/0378-5955(88)90116-5. [DOI] [PubMed] [Google Scholar]
- Zeng FG, Martino KM, Linthicum FH, Soli SD. Auditory research perception in vestibular neurectomy subjects. Hear. Res. 2000;142(1-2):102–112. doi: 10.1016/s0378-5955(00)00011-3. [DOI] [PubMed] [Google Scholar]
- Zwicker E. Temporal effects in simultaneous masking and loudness. J. Acoust. Soc. Am. 1965;38:132–142. doi: 10.1121/1.1909588. [DOI] [PubMed] [Google Scholar]