Abstract
Apoptotic cell death is an intricate and highly regulated process. To initiate apoptosis, the protein BIM binds to a hitherto unrecognized site on the BAX protein to trigger permeabilization of the outer mitochondrial membrane.
Cell death in animals mainly proceeds through the programmed process of apoptosis. In vertebrates, this usually occurs by means of a pathway involving the mitochondrion, a cellular organelle1. Following damaging cellular stress, the outer mitochondrial membrane becomes permeable, and factors are released into the cytoplasm that precipitate apoptosis. The pro-apoptotic effector proteins of the BCL-2 family — BAK and BAX — are responsible for permeabilizing the membrane, yet there is little structural information on the main protein–protein interactions necessary to promote this mitochondrial event. On page 1076 of this issue, Walensky, Tjandra and colleagues2 present an intriguing structural analysis of the inter actions between BAX and one of its activators, revealing an unsuspected binding site.
Proteins of the BCL-2 family control the integrity of the outer mitochondrial membrane and are divided into three subfamilies: anti-apoptotic proteins, pro-apoptotic effectors and BH3-only proteins (so called because, of the four evolutionarily conserved BCL-2-homology (BH) domains, only BH3 is present in these proteins)3. Anti-apoptotic BCL-2 proteins prevent apoptosis by inhibiting permeabilization of the membrane, whereas the BH3-only proteins promote it. When BAK and BAX are activated, they ‘homo-oligomerize’ — that is, they form complexes of either BAX-only or BAK-only molecules. These oligomers assemble in the outer mitochondrial membrane, causing its disruption. Proteins localized between the outer and inner membranes, such as cytochrome c, can then diffuse into the cytosol. Cytochrome c and other released proteins cooperate with cytosolic factors, leading to the activation of caspase enzymes that are responsible for the features of apoptosis. But even without caspase activation, permeabilized outer mitochondrial membranes can be sufficiently catastrophic to cause cell death.
The BH3-only proteins promote permeabilization of the outer mitochondrial membrane by regulating the other two subfamilies. These proteins bind to, and thereby inhibit, the anti-apoptotic BCL-2 proteins. Also, at least two BH3-only proteins — BID and BIM — directly activate BAK and/or BAX, allowing them to insert in the outer mitochondrial membrane, oligomerize there and permeabilize the membrane (BID and BIM are therefore referred to as direct activators). Although considerable structural and biochemical data4 have revealed how the BH3 domain of BH3-only proteins tightly binds to and inhibits the anti-apoptotic BCL-2 proteins, until now very little was understood about how the BH3 domains of BID or BIM induce the permeabilization activity of BAK and BAX.
Walensky and colleagues5,6 showed previously that BH3-domain peptides from BID and BIM can be ‘stapled’ into an α-helical conformation called SAHB (stabilized α-helices of BCL-2 domains), which can bind to and activate BAX. Now, using a combination of structural techniques, the authors2 provide the first glimpse of how the SAHB peptide of BIM interacts with BAX.
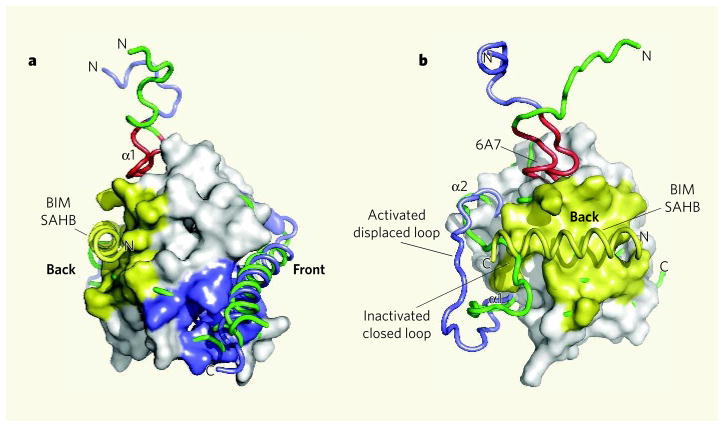
If BAX behaved like the structurally similar anti-apoptotic proteins, such as BCL-xL or MCL-1, then BIM SAHB would bind to the conventional hydrophobic BH3-binding pocket at the ‘front’ of the BAX protein (Fig. 1a). However, unexpectedly, BIM SAHB stabs BAX in the ‘back’. Consequently, the region of BAX adjacent to its amino-terminal α-helix (α1) swings outwards (Fig. 1b). Other conformational changes not seen in this structure must also occur, as the authors find that an amino-terminal motif in α1 (dubbed 6A7) becomes exposed when BAX monomers are activated by BIM SAHB.
Figure 1. Activation of the BAX protein by the BIM SAHB domain.
a, The structure of BAX based on a previous study11 is shown with BIM SAHB binding at a site on the ‘back’ of the molecule (yellow). This contrasts with the expected binding of a BH3-domain helix in the conventional (at least for anti-apoptotic proteins) hydrophobic BH pocket located at the ‘front’ of the molecule (blue). b, Walensky, Tjandra and colleagues2 find that conformational changes accompany the binding of a BIM SAHB peptide to BAX. The chain containing α-helix 1 (α1) and the loop between α1 and α2 is shown before (green) and after (blue) binding of BIM SAHB. The BAX amino-terminal region 6A7, which becomes exposed on association with BIM SAHB, is shown in red. (Images prepared by T. Moldoveanu, St Jude Children’s Research Hospital.)
In agreement with these results, a previous biochemical study7 suggested that cytosolic p53 — a direct activator that does not belong to the BCL-2 family — binds to a similar region in BAK to that described for the BIM-SAHB–BAX interaction. However, the findings contrast with another study8 showing that the BH3 domain of BID binds to BAK in the conventional hydrophobic BH3-binding pocket. As we will see, these observations are not necessarily incompatible.
A milestone in understanding proteins of the BCL-2 family will be to unravel how BAK and BAX form homo-oligomers, because this process seems to be essential for the permeabilization of the outer mitochondrial membrane. Earlier this year, researchers investigating BAK provided some notable clues. Using biochemical approaches, those authors showed9 that, during BID-induced BAK activation, BAK exposes its BH3 domain, probably revealing a groove corresponding to the hydrophobic BH pocket of the anti-apoptotic proteins at the front of the molecule. Two activated BAK molecules with their BH3 regions exposed then seem to interact with each other’s grooves to form a BAK dimer.
Mutational analysis of BAX supports similar interactions in this molecule10. By inference, we propose that the binding of BIM SAHB to BAX results in a conformational change that constrains the front of BAX. The carboxy-terminal tail of BAX, which normally resides in the front hydrophobic BH pocket, pops out, as, presumably, does the region containing the BH3 domain. Two such BAX molecules can then form ‘nose-to-nose’ dimers (Fig. 2a). If this model is correct, BAX with mutations in its BH3 region or in crucial residues in its hydrophobic groove would still bind to BIM SAHB but would not form oligomers or cause permeabilization of the outer mitochondrial membrane. Indeed, such mutations in BAX destroy its function10, but whether BIM SAHB would still bind remains unknown.
Figure 2. Possible consequences of BIM SAHB binding to BAX.
a, On the basis of the latest structural information2, we speculate that the binding of BIM SAHB to the ‘back’ of BAX, and the ensuing conformational changes, constrain the ‘front’ of the molecule, including the hydrophobic BH pocket, causing the carboxy-terminal region (not shown) and α-helix 4 containing the BH3 domain (green) to move out of the BH pocket and create a groove. Two such BAX molecules could then dimerize ‘nose-to-nose’ through interactions between the BH3 domain and the hydrophobic BH groove. b, Interaction at an additional, undefined interface might result in the formation of BAX oligomers and so permeabilization of the outer mitochondrial membrane. On BAX activation, the inducer of activation — in this case BIM SAHB, but presumably any activator — might dissociate to allow higher-order oligomer formation.
A problem with the idea of nose-to-nose dimer formation is that BAK and BAX form higher-order homo-oligomers that seem to be required for their apoptotic function. It is possible that there is at least one more inter action surface on BAX and BAK that mediates homo-oligomerization; and it is tempting to speculate that this surface is exposed when α1 is displaced by BIM SAHB (and presumably by the full-length BIM protein, or any other direct activator). As BID and BIM are not found in oligomers of BAX or BAK, homo-oligomerization at this surface might then displace the direct-activator protein (Fig. 2b). Interactions of the BID or BIM BH3 domain with the revealed BH pocket might also help to stabilize this intermediate structure and facilitate oligomerization at the nose-to-nose interface. If so, there might actually be two interaction sites between BAX (or BAK) and their activators, one at the back and one at the front. We do not know the conformations of the activated (dimerized or oligomerized) forms of BAK or BAX, nor can we reasonably model the nose-to-nose dimers at this point. But the pieces are starting to come together as we puzzle out how these killers do the dastardly deed of triggering apoptosis.
References
- 1.Green DR. Cell. 2005;121:671–674. doi: 10.1016/j.cell.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Gavathiotis E, et al. Nature. 2008;455:1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chipuk JE, Green DR. Trends Cell Biol. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Youle RJ, Strasser A. Nature Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 5.Walensky LD, et al. Science. 2004;305:1466–1470. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walensky LD, et al. Mol Cell. 2006;24:199–210. doi: 10.1016/j.molcel.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Pietsch EC, et al. J Biol Chem. 2008;283:21294–21304. doi: 10.1074/jbc.M710539200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moldoveanu T, et al. Mol Cell. 2006;24:677–688. doi: 10.1016/j.molcel.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Dewson G, et al. Mol Cell. 2008;30:369–380. doi: 10.1016/j.molcel.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 10.George NM, Evans JJ, Luo X. Genes Dev. 2007;21:1937–1948. doi: 10.1101/gad.1553607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki M, Youle RJ, Tjandra N. Cell. 2000;103:645–654. doi: 10.1016/s0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]