Abstract
Protein kinase Cη (PKCη) is highly abundant in T cells and is recruited to the immunological synapse that is formed between a T cell and a cognate antigen-presenting cell; however, its function in T cells is unknown. Here, we showed that PKCη was required for the activation of mature CD8+ T cells by stimulation through the T cell receptor. PKCη−/− T cells showed poor proliferation in response to stimulation by antigen as compared to wild-type T cells, a trait shared with T cells deficient in PKCθ, the most abundant PKC isoform in T cells, and the only PKC previously thought to have a specific role in T cell activation. In contrast, defective homeostatic proliferation, a function requiring recognition of self antigens, was only observed in PKCη- deficient T cells. PKCη was dispensable for the development of thymocytes; however, thymocytes from mice doubly deficient in PKCη and PKCθ exhibited poor positive selection, indicating some redundancy between the PKC isoforms. PKCη and PKCθ had opposing effects on relative numbers of CD4+ and CD8+ T cells, because PKCη−/− mice had a higher ratio of CD4+ to CD8+ T cells compared to that of wild-type mice, whereas PKCθ−/− mice had a lower ratio. In mice deficient in both PKC isoforms, the ratio of CD4+ to CD8+ T cells returned to normal. Together, these data suggest that whereas PKCη shares redundant roles with PKCθ in T cell biology, it also performs nonredundant functions that are important for homeostasis and activation of T cells.
INTRODUCTION
The protein kinase C (PKC) family of serine and threonine kinases includes 10 isoforms in human and mouse, which play important roles in signal transduction in different cellular systems, including regulating differentiation, cell motility, secretion, growth and death (1). Of the PKC isoforms found in T cells (α, δ, ε, η, and θ), only PKCθ is thought to have an important and specific role in T cell biology, where it is involved in costimulation of signal transduction in response to antigen recognition, leading to activation of transcription factors including NF-κB and to changes in gene transcription resulting in responses such as increased IL-2 secretion (2-5). However, PKCη gene expression is increased during positive selection (6, 7), and gene expression profiling has shown that PKCη messenger RNA (mRNA) abundance, like that of PKCθ, is higher in T cells than in other mouse or human cell types and organs (http://biogps.org/#goto=genereport&id=18755; gnf1m00727 and U133A 206099_at) which suggests that PKCη may play a role in T cell biology. Although a PKCη-deficient mouse exists, no immunological studies of these mice have been reported (8).
PKCθ is recruited to the immunological synapse, the area of contact that is formed between mature T cells and antigen-presenting cells (APCs), where it concentrates in the central region (9-11). In addition, PKCθ associates with the coreceptor CD28 in microclusters that contain the T cell antigen receptor (TCR), which move centripetally into the immunological synapse and are thought to be important in the costimulation of T cells (12). A fusion protein of PKCη and green fluorescent protein (GFP) is also recruited to the immunological synapse upon TCR stimulation, where it is localized diffusely over the whole region (13). Studies with PKCθ−/− mice revealed the involvement of PKCθ in multiple signaling pathways downstream of TCR stimulation (14-20). Given the importance of PKCθ in mature T cells, it was surprising that initial studies showed no defect in T cell development in PKCθ−/− mice (14, 16); however, another study found that PKCθ is indeed involved in thymocyte development, with PKCθ−/− mice showing a mild defect in positive selection (21). Redundancy between different PKC isoforms may obscure the importance of any individual PKC in T cell development and function in vivo.
Here, we confirmed that the abundance of PKCη mRNA is increased during the positive selection of thymocytes, and we found that PKCη, similar to PKCθ, was recruited to the immunological synapse. To further investigate the specific role of PKCη and the potential redundancy between PKCη and PKCθ in T cell development and mature T cell function, we generated mice deficient in Prkch, the gene encoding PKCη (PKCη−/− mice), and mice doubly deficient in Prkch and Prckq, which encodes PKCθ, to generate PKCη−/−PKCθ−/− double knockout (DKO) mice. We found that although PKCη shared redundant functions with PKCθ during T cell development, PKCη was required for normal functions of mature T cells, because PKCη−/− T cells were less well able to proliferate in response to antigen or to undergo homeostatic proliferation than were wild-type mice, which was likely a result of impaired intracellular calcium (Ca2+) signaling and nuclear translocation of the transcription factor nuclear factor κB (NF-κB), defects similar to those found in PKCθ−/− mice. In contrast to PKCη−/− and PKCθ−/− mice, DKO mice had a more severe defect in T cell development and more profound functional defects in mature T cells, as was revealed in multiple assays. Together, our data suggest that in addition to sharing redundant roles with PKCθ, PKCη has its own specific functions in T cell biology.
RESULTS
The abundance of PKCη mRNA is increased during positive selection of thymocytes
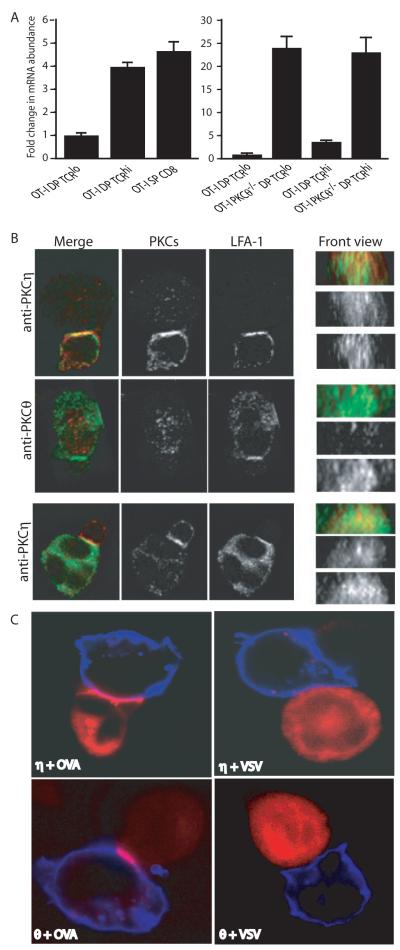
In a previous study, we showed that the abundance of PKCη mRNA is increased in pre-selection thymocytes when stimulated with an antibody against the Vβ chain of TCR (7). Independently, Hogquist and colleagues also found that PKCη mRNA abundance is increased during positive selection (6). Given the requirement for PKCθ for antigenic stimulation in mature T cells (9, 10, 14, 16), it was surprising that PKCη rather than PKCθ mRNA abundance was increased during positive selection (7). To investigate whether PKCη was involved in thymocyte positive selection in vivo, and to test whether PKCη might compensate for the loss of PKCθ, we measured PKCη mRNA amounts by real-time reverse transcription polymerase chain reaction (RT-PCR) assay. We found that PKCη mRNA was increased in abundance in late-stage CD4+ and CD8+ double-positive (DP) cells and in CD4+ single-positive (SP) and CD8+ SP cells, that is, in cells that had undergone positive selection (Fig. 1A, left). DP cells with a high abundance of TCR as assessed by flow cytometry (TCRhi DP cells) showed about a four-fold increase in PKCη mRNA abundance compared to that in TCRlo DPs, and this abundance stayed high in the SP cells. Because PKCθ is found in earlier developmental stages in thymocytes than PKCη, if there was redundancy between the PKCθ and PKCη isoforms, we would expect that PKCη would be produced earlier in cells in the absence of PKCθ. Indeed, we found that expression of the gene encoding PKCη was induced earlier and to a greater extent than normal in cells before positive selection (TCRlo DP cells) (Fig. 1A, right). These results suggested that PKCη might play a role in thymocyte development.
Fig. 1.
Analysis of PKCη abundance and localization. (A) PKCη mRNA is increased in abundance during positive selection. PKCη mRNA abundance was determined by real-time RT-PCR. Results are shown as fold change compared to the amount of PKCη mRNA in OT-I TCRlo cells. Data are representative of two experiments, each performed in duplicate. (B) PKCη recruitment to the immunological synapse in the absence of PKCθ. To avoid potential cross reactivity of PKC-specific antibodies, we used thymocytes and mature T cells from PKCθ−/− mice. After activation with superantigen-loaded LK35 cells, T cells or thymocytes were incubated with antibodies against LFA-1 and the PKC isoforms (anti-PKCθ and anti-PKCη), after which they were incubated with antisera against rabbit immunoglobulin (Ig). In the merged image panel, LFA-1 staining is shown in green, whereas PKC staining is in red. The larger cells are LK35 cells and the smaller cells are T cells. Top and middle panels show thymocytes and the bottom panels show mature T cells, with corresponding en face views shown on the right. Data are representative of at least 50 cell-cell conjugates of each type. (C) Recruitment of PKCη to the immunological synapse in live cells. OT-I T hybridomas transfected with plasmid encoding PKCη-RFP (η) or PKCθ-RFP (θ) were stimulated with EL4 cells that were pulsed with OVA or VSV peptides. PKC protein is shown in red, whereas EL4 cells are shown in blue. Images are representative of at least 20 cells collected from three experiments.
PKCη is recruited to the immunological synapse
Because the gene encoding PKCη was induced during normal positive selection and because it was expressed earlier and to a greater extent in PKCθ−/− thymocytes than in wild-type thymocytes, we tested whether PKCη, like PKCθ, was recruited to the immunological synapse in thymocytes. The recruitment of PKCθ to the synapse is important in signal transduction and is a hallmark of recognition of antigenic peptide presented by major histocompatibility complex (MHC). To avoid the possibility of cross-reaction between antibodies against PKCη and PKCθ, we first isolated thymocytes and mature T cells from PKCθ−/− mice and activated them with APCs that presented the superantigens staphylococcal enterotoxin A (SEA) and SEB, which react with more than 40% of the Vβ repertoire of TCRs in B6 mice (22). Superantigens like SEA and SEB bind to MHC class II molecules and are then able to activate T cells by binding to the TCR through recognition of Vβ sequences. PKCη was recruited to the immunological synapse in thymocytes and in mature T cells upon stimulation with SEA and SEB (Fig. 1B). In PKCθ-sufficient thymocytes, PKCθ, but not PKCη, was present at the immunological synapse of DP cells before selection (PKCη is not yet present in these cells) (fig. S1). In SP cells, both PKCθ and PKCη were found at the immunological synapse. In PKCθ−/− thymocytes, PKCη mRNA was detected in less mature DP cells than in wild-type thymus (Fig. 1A), and consequently PKCη was present in the immunological synapses of DP cells (fig. S2).
To visualize the recruitment of PKCη to the immunological synapse in live cells, we transfected OT-I hybridoma cells (23), that have a TCR that recognizes a peptide from ovalbumin (OVA) presented on the MHC class I-molecule H2-Kb, with plasmids encoding mouse PKCη fused to red fluorescent protein (PKCη-RFP) or PKCθ-RFP. We stimulated these cells with EL4 cells that had been incubated (“pulsed”) with different peptides. When the stimulatory OVA peptide was presented to the OT-I cells, both PKCη and PKCθ were recruited to the immunological synapse; however, when the nonstimulatory vesicular stomatitis virus (VSV) peptide was presented, no such recruitment took place, which demonstrated that the recruitment of PKCη to the immunological synapse was antigen-specific (Fig. 1C). Genes for GFP tagged human PKCη, θ and α proteins were transfected into human Jurkat T cells (fig. S3A). PKCη and θ, but not PKCα, were recruited to the immunological synapse during recognition of a superantigen ligand. Similarly, stimulation of an MHC class II-restricted T cell hybridoma with antigenic peptide-pulsed APC, resulted in concentration of transgenic fluorescent-tagged PKCη and θ, but not PKCδ at the synapse (fig. S3B). To compare the recruitment of PKCη and PKCθ directly, we cotransfected OT-I cells with plasmids encoding PKCη-RFP and PKCθ-GFP and imaged the fluorescent proteins during antigen recognition (fig. S4). Both PKCη and PKCθ were recruited to the immunological synapse; however, whereas PKCη remained in a diffuse pattern over the whole area of the synapse (Fig. 1, B and C), which was consistent with an earlier study (13), PKCθ was more centrally concentrated at the immunological synapse (Fig. 1, B and C), as was expected from previous work (9, 10).
PKCη is not required for positive or negative selection of thymocytes
To investigate the role of PKCη in a broader manner, we generated Prkch-deficient (PKCη−/−) mice (fig. S5). The thymi of PKCη−/− mice contained ~20% fewer cells than did the thymi of wild-type mice. When we analyzed thymocyte subsets in the thymi of PKCη−/− mice, we found that the numbers of double-negative (DN1) and DN4 cells were reduced compared to those in thymi from wild-type mice; however, the numbers of DN2 and DN3 cells were not altered (Fig. 2A). DN4 cells develop into CD4+CD8+ DP cells, after which they are subjected to positive and negative selection to generate self-MHC–restricted, self-antigen–tolerant mature T cells (24). The proportions of DP, CD4+ SP, and CD8+ SP subsets were similar in the thymi of PKCη−/− and wild-type mice, with slightly reduced cell numbers of DP subsets in the PKCη−/− mice (Fig. 2B). These results suggested that PKCη was not required for the positive selection of thymocytes.
Fig. 2.
Thymocyte development in PKCη−/− mice is normal. Thymocytes from age- and sex-matched wild-type (WT) (n = 8 mice) and PKCη−/− mice (ηKO, n = 8 mice) were analyzed for the presence of (A) CD4−CD8− (DN) subsets, (B) CD4+ and CD8+ (DP, SP) and DN subsets. Left: representative flow cytometry plots showing the mean percentages of each subset ± the standard deviation (SD). Right: mean total numbers of cells ± SD for each subset. P values were calculated by Student’s t test. *P ≤ 0.05; ***P ≤ 0.001. (C) Negative selection of PKCη−/− thymocytes is normal. T cells expressing Vβ5, Vβ11, or Vβ12, but not Vβ7, were deleted by endogenous mouse mammary tumor virus-encoded superantigens in mice that express I-E (H-2b/d) but not in mice lacking I-E (H-2b/b). PKCη−/− thymocytes bearing Vβ5, Vβ11, or Vβ12 were deleted similarly to wild-type thymocytes. Each dot represents a single mouse.
To investigate the role of PKCη in negative selection, we used a superantigen-mediated deletion model. Mouse mammary tumor proviruses encode superantigens (Mtv) that are presented by the MHC class II molecule I-E to T cells, resulting in the intrathymic deletion of cells bearing Vβ elements recognized by particular Mtv superantigens (22, 25). Because B6 (MHC haplotype H-2b) mice do not express I-E, we crossed the B6 PKCη−/− mice to B10.D2 (H-2d) mice, producing F1 H-2b/d mice that express I-Ed. In combination with the endogenous superantigens Mtv-8 and Mtv-9, which are carried by both B6 and B10.D2 cells, I-E causes the deletion of thymocytes that express the Vβ5, Vβ11, and Vβ12 chains of the TCR on their surface (22, 25). When we compared H-2b/b mice with H-2b/d PKCη+/+ mice, we found that a substantial number of T cells expressing Vβ5, Vβ11, and Vβ12 TCRs were deleted in the H-2b/d, I-E expressing F1 mice (Fig. 2C). Vβ7+ T cells do not recognize Mtv-8 and Mtv-9 superantigens and thus were not deleted in either mouse. Superantigen-mediated negative selection was as efficient in the H-2b/d PKCη−/− as PKCη+/+ mice, indicating that PKCη was dispensable for superantigen-mediated negative selection.
Increased number of memory phenotype T cells in PKCη-deficient mice
We observed that the lymph nodes of PKCη−/− mice were larger than those of wild-type mice (Fig. 3A). In the cervical area there were more and larger readily identifiable lymph nodes in PKCη−/− mice than in wild-type mice (Fig. 3A, left). When we isolated the axillar, brachial, and inguinal lymph nodes, we found that they were larger in PKCη−/− mice than in wild-type mice (Fig. 3A, right). Consistent with this finding, the number of lymph node cells was increased in the PKCη−/− mice compared to that in the wild-type mice (Fig. 3B). These data suggested that PKCη−/− mice may have a general over-production of lymphocytes rather than swollen lymph nodes (lymphadenopathy); however, the number of lymphocytes in the spleens of PKCη−/− mice was less than that of wild-type mice (Fig. 3B). In the lymph nodes, although there were only minor changes in the proportions of T cells or B cells or in the T cell subpopulations [CD8+, CD4+CD25− conventional CD4+ cells, or CD4+CD25+ regulatory T cells (Tregs)] (Fig. 3C), the absolute number of each cell type was substantially increased in the PKCη−/− mice compared to those in wild-type mice (Fig. 3D), whereas the numbers of each cell type were lower in the spleens of PKCη−/− mice than in those of wild-type mice. The proportion of cells with a memory or activated phenotype within the conventional CD4+CD25− population (CD44+) of cells was increased in the lymph nodes and the spleens of PKCη−/− mice compared to those in wild-type mice (Fig. 3, C and D). We obtained similar results when we used the transcription factor Foxp3, rather than the surface marker CD25, to define Tregs (fig. S6, A and B). The total numbers of lymph node and splenic lymphocyte subsets were very similar in wild type and PKCη−/− mice, which suggested that the lymphocytes of PKCη-deficient mice might preferentially home to the lymph nodes rather than to the spleen. The exception to this conservation in total lymphocyte number in the secondary lymphoid tissues was the decrease in the total number of naïve CD4+CD25− and the increase in the number of CD4+CD25− memory phenotype T cells in the PKCη−/− mice compared to those of wild-type mice, suggesting that the knockout cells may have responded to more self-stimulation in the periphery than the wild-type cells (26).
Fig. 3.
PKCη−/− mice have enlarged lymph nodes. The mice analyzed in Fig. 2 were examined to determine the phenotype of their peripheral T cells. (A) Lymph nodes from the cervical area were photographed and are shown on the left; arrows indicate readily identifiable lymph nodes. On the right are axillar, brachial, and inguinal lymph nodes that were isolated from mice and aligned for comparison. (B) Total lymphocyte numbers from six pooled axillar, brachial, and inguinal lymph nodes are shown on the left, whereas total lymphocyte numbers from spleens are shown on the right. (C) Representative flow cytometry plots of lymph nodes (LNs) and spleens are shown with the proportion of each cell type listed as the mean ± SD. (D) Absolute cell numbers of the indicated subsets are listed for lymph nodes and spleen. For cell numbers, P values calculated by Student’s t test are shown as: *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; NS, not significant, n=8 mice. Representative of 3 experiments.
PKCη-deficient T cells show impaired proliferation
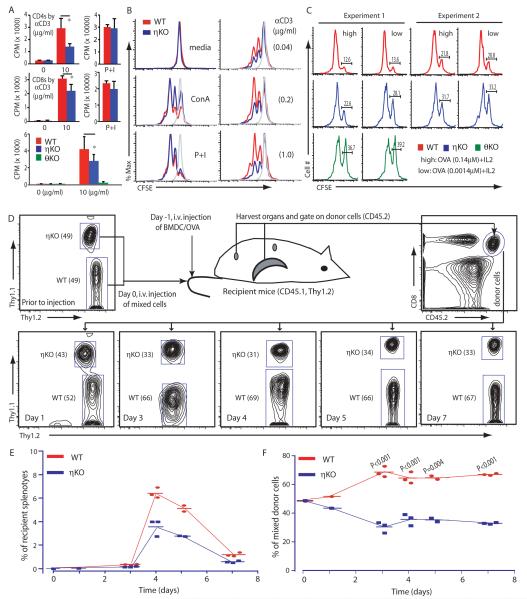
To test the proliferative response of PKCη−/− T cells, we purified naïve CD4+ or CD8+ T cells from lymph nodes, stimulated them through the TCR with various concentrations of antibody against CD3, and measured their proliferative responses. Naïve CD4+ and CD8+ T cells from PKCη−/− mice proliferated less well than did naïve CD4+ and CD8+ T cells from wild-type mice (Fig. 4A). Unlike PKCθ−/− CD8+ T cells whose proliferation was completely abrogated by loss of PKCθ, the proliferation of PKCη−/− CD8+ T cells was only moderately reduced by 30 to 50% (Fig. 4A). So that both cell types could experience an identical cytokine environment, we determined cell division by measuring the dilution of the cytoplasmic dye carboxyfluorescein succinimidyl ester (CFSE) with a mixture of PKCη−/− T cells (which had the surface marker CD45.2) and PKCη+/+ T cells (which had the surface marker CD45.1). CD8+ T cells from PKCη−/− mice proliferated less well than did PKCη+/+ cells in response to stimulation by antibody against CD3 or by crosslinking of T cell surface glycoproteins with the lectin concanavalin A (ConA) (Fig. 4B). We then performed a similar experiment with MHC class I–restricted OT-I TCR transgenic T cells that responded to different concentrations of OVA peptide presented by splenic APCs. In these experiments, PKCη−/− or PKCθ−/− T cells (which had the surface marker Thy1.2) were mixed with PKCη+/+ T cells (which had the surface marker Thy1.1). The PKCη−/− T cells proliferated more weakly than did the PKCη+/+ cells, but more strongly than did the PKCθ−/− cells (Fig. 4C).
Fig. 4.
Proliferation of PKCη−/− mature T cells is impaired. (A) Naïve T cells were stimulated with antibody against CD3 or with PMA and ionomycin (P+I) for 64 hours, and 3H-thymidine was added to the culture medium for the last 16 hours. Graph shows proliferation as mean counts per minute (CPM) of 3H incorporated into DNA ± SD of replicates (n ≥ 3). (B) Lymph node cells from WT (CD45.1+) and PKCη KO (CD45.2+) mice were mixed, labeled with CFSE, and stimulated as indicated for 48 hours. Dilution of CFSE was analyzed by flow cytometry after gating for CD45 allotype and CD8. (C) OTI TCR Tg WT (CD45.2+Thy1.1+) and PKCη−/− or PKCθ−/− (both CD45.2+Thy1.2+) naïve T cells were purified, mixed in a 1:1 ratio, labeled with CFSE, and stimulated with irradiated splenocytes (CD45.1+) that were pulsed with OVA peptide. After 48 hours, CFSE dilution was analyzed by flow cytometry. The percentages of undivided cells are indicated for two independent experiments. (D) Antigen-specific proliferation of PKCη−/− T cells is impaired in vivo. Experiments were designed as depicted in the cartoon. Flow cytometry plots show the proportions of WT and KO donor cells that were present at the indicated times. (E) Proportions of WT and PKCη KO donor cells per recipient mouse spleen. (F) Proportions of WT and PKCη KO cells within donor cell population. Each dot in (E) and (F) represents an individual mouse. *P ≤ 0.05. Data are representative of three (A) or four (B) and (D to F) independent experiments.
To investigate the proliferation of PKCη−/− T cells under more physiological conditions, we stimulated OT-I TCR transgenic PKCη−/− T cells in vivo with bone marrow–derived dendritic cells (BMDCs) that were loaded with OVA peptide. We purified wild-type (Thy1.1+CD45.2+) and PKCη−/− (Thy1.2+CD45.2+) OT-I T cells from lymph nodes, mixed the cells at a 1:1 ratio, and injected them into recipient mice that had been immunized with OVA-loaded BMDCs one day before the experiment (Fig. 4D). The proportional contribution of each donor cell type to the total population of cells was determined at various time points by flow cytometric analysis (Fig. 4D). Both types of donor cell proliferated in the recipient mice, reaching their peak numbers on day 4, after which they declined (Fig. 4E). However, although both types of donor cells proliferated with similar kinetics, their respective proliferative capacities were quite different. The proportions of PKCη+/+ and PKCη−/− T cells within the donor population started to diverge on day 1 (Fig. 4F). This difference in proportions increased over time, peaking at day 3, at which point the percentages of wild-type and PKCη−/− T cells were 69 versus 30%. This difference in cellular proportions was maintained until the end of the experiment. The differences in the proportion of PKCη+/+ T cells to PKCη−/− T cells could have occurred because of differential cell death between the populations. To test this possibility, we incubated donor cells with labeled Annexin V to detect cell surface phosphatidylserine (present on early apoptotic cells) and the DNA-intercalating dye 7-AAD to distinguish viable from dying cells. Both PKCη+/+ and PKCη−/− T cells had a small and roughly equal subset of dying cells (fig. S7), which suggested that differential cell death was unlikely to contribute to the difference in cellular proportions observed earlier (Fig. 4F). Collectively, these data indicate that the PKCη−/− CD8+ T cells had a defect in the proliferative response to TCR stimulation, although the defect that was observed in assays in vitro was milder than that seen in PKCθ−/− T cells.
Ca2+ and NF-κB signaling in PKCη-deficient T cells are defective
The proliferative defect in PKCη−/− T cells could be a result of insufficient production of the cytokine interleukin-2 (IL-2), a prosurvival factor for T cells, deficient synthesis of the α chain of the IL-2 receptor (IL-2Rα, also known as CD25), or both, as occurs in PKCθ−/− T cells (14, 16). In PKCη−/− CD8+ cells, the increase in the cell-surface abundance of CD25 was substantially reduced compared to that in wild-type CD8+ cells, similar to the reduction in PKCθ−/− T cells (16) (Fig. 5A), as was the increase in abundance of the early activation marker CD69 (Fig. 5B). Because CD4+ T cells are the main source of IL-2, we measured the amount of IL-2 secreted by PKCη−/− CD4+ T cells and found that it was slightly (but significantly) reduced compared to that of wild-type CD4+ T cells (fig. S8A).
Fig. 5.
Impaired signaling in mature PKCη−/− T cells. (A, B) Increased abundance of activation markers. Lymph node cells were activated by stimulation with antibody against CD3 for 16 hours, and the surface abundance of (A) CD25 and (B) CD69 was analyzed on CD8+ cells by flow cytometry. The gray line indicates the basal abundance of these markers on nonstimulated cells, whereas the color-hatched region represents stimulated cells. CD25+ and CD69+ cell populations are marked and the mean percentages ± SD are shown. The asterisk indicates statistically significant difference (by t test) between KO and WT mice (n = 3 mice); P = 0.05 for CD25 and P < 0.05 for CD69. (C and D) Analysis of Ca2+ signaling. Cells from WT (Thy1.1+) and PKCηKO (Thy1.2+) mice were mixed, loaded with Indo-1, and stimulated by crosslinking biotinylated antibody against CD3 (αCD3) with streptavidin (S.Av). Ca2+ flux was recorded by flow cytometry. (E and F) Imaging of the nuclear translocation of NF-κB p65 protein. Purified CD8+ T cells were stimulated with antibodies against CD3 (αCD3) and CD8 (αCD8) for 60 min, and the nuclear translocation of p65 was determined by microscopy as shown in (E), and summarized in (F). (G and H) Examination of the nuclear translocation of p65 by Western blotting analysis. Cells were stimulated as shown in (E and F), after which, cytosolic and nuclear extracts were prepared and subjected to Western blotting analysis (G). Normalized data are shown (H). Data are representative of two (A, B, G, and H) or 4 (C to F) experiments.
NF-κB and Ca2+ signaling pathways are defective in PKCθ−/− mice, whereas TCR-proximal and mitogen-activated protein kinase (MAPK) signaling pathways are intact (14, 16, 20). We therefore investigated these pathways to try to determine the potential mechanisms responsible for the defects that we observed in PKCη−/− T cells. To measure changes in Ca2+ flux, we mixed wild-type (Thy1.1+) and PKCη−/− (Thy1.2+) T cells, loaded them with the Ca2+-indicator dye Indo-1, and stimulated their TCRs by crosslinking prebound antibodies against CD3. In the PKCη-sufficient CD8+ T cells, we observed a transient peak of elevated Ca2+ followed by a sustained, gradually declining, Ca2+ signal. In PKCη−/− CD8+ T cells, the magnitude of the Ca2+ signal was reduced. This difference in Ca2+ signaling was not because of differential Indo-1 loading, because both cell types exhibited equivalent signals when stimulated with the Ca2+ ionophore ionomycin (Fig. 5C). Ca2+ signaling includes both the release of Ca2+ from endoplasmic reticulum (ER) stores and the subsequent induction of store-operated Ca2+ entry (SOCE) through channels in the plasma membrane (27). To identify at which stage Ca2+ signals were affected in PKCη−/− T cells, we measured Ca2+ flux in cells incubated with EGTA, which chelates extracellular Ca2+, thus enabling us to measure only ER-dependent Ca2+ release, and in cells that were subsequently replenished with extracellular Ca2+, thus enabling us to measure SOCE. These experiments showed no defect in ER-dependent Ca2+ release but did reveal a defect in SOCE in PKCη−/− T cells (Fig. 5D). We then directly compared Ca2+ signaling in PKCη−/− CD8+ T cells and PKCθ−/− CD8+ T cells and found a similar defect (fig. S8B).
The translocation of the NF-κB p65 subunit from the cytosol to the nucleus is a hallmark event in the NF-κB signaling cascade. We stimulated purified CD8+ T cells with crosslinked antibodies against CD3 and CD8 and imaged the nuclear and cytoplasmic localization of p65. We observed translocation of p65 to the nucleus of 40% of wild-type cells, but only in 22% of PKCθ−/− T cells, whereas only 15% of PKCη−/− T cells showed nuclear translocation of p65 (Fig. 5, E and F). This result was further confirmed by Western blotting analysis of cytosolic and nuclear extracts. In wild-type CD8+ T cells, nuclear p65 was increased in abundance for at least 60 min after stimulation, but in PKCη−/− T cells, the increase in the abundance of nuclear p65 ceased 20 min after stimulation (Fig. 5, G and H). The total amount of cellular p65 was not altered in PKCη−/− T cells (fig. S8C). Together, these results demonstrated that the NF-κB pathway was disrupted in the absence of PKCη at least as much as in the absence of PKCθ. TCR-proximal signaling and MAPK signaling pathways were normal in PKCη−/− T cells (fig. S8, D and E). Collectively, these results showed that PKCη−/− T cells were defective for Ca2+ and NF-κB signaling, which were also adversely affected in PKCθ−/− T cells.
DKO mice reveal isoform-specific and redundant roles for PKCη and PKCθ
The normal T cell development, but the defective Ca2+ and NF-κB signaling pathways, seen in PKCη−/− and PKCθ−/− mice, prompted us to investigate possible redundancy between PKCη and PKCθ in T cell development and function. To do this, we bred Prkcq−/−, Prckh−/− double knockout (DKO) mice. In the thymus, DKO mice had a more substantial reduction in the numbers of DN1 and DN4 thymocytes than were observed in either of the single knockout mice (Fig. 6, A and D). At the mature SP stage, although PKCθ−/− mice showed a mild defect in positive selection, the DKO mice had the most marked defect (Fig. 6, B and E). Thus, comparing the PKCθ−/− and DKO mice revealed a redundant role for PKCη in positive selection. This finding was confirmed when we analyzed the most mature population of cells in the thymus, the CD5hiTCRhi cells (Fig. 6C). No difference was noted between PKCη−/− and wild-type B6 mice, but the minor defect in thymocyte development in PKCθ−/− mice (21), was exacerbated by concomitant loss of PKCη (Fig. 6, C and F). Collectively, these findings demonstrated redundancy between PKCη and PKCθ; PKCη could substitute for PKCθ during positive selection, as was initially suggested by the increased abundance of PKCη and its recruitment to the immunological synapse in PKCθ−/− thymocytes (Fig. 1).
Fig. 6.
Comparison of thymic phenotypes in three PKC-deficient mouse strains. Three age- and sex-matched mice from each genotype were used in this analysis. Representative flow cytometry plots of (A) DN1 to DN4 stage thymocytes, (B) DN, DP, and SP stage thymocytes, and (C) the most mature CD5hiTCRhi thymocytes are shown with the proportions of each cell type listed as mean ± SD. Absolute cell numbers for each subset are listed in (D), (E), and (F) respectively. P values were calculated with the Student’s t test and are listed underneath as indicated. Data are representative of three experiments.
We performed a comprehensive analysis of the lymph nodes and spleens of B6 mice and the various PKC knockout strains (fig. S9). Although redundancy between PKCη and PKCθ was apparent in thymocyte development, we observed an isoform-specific phenotype in mature T cells in the lymph nodes. For example, DKO mice and normal B6 mice had the same total number of T cells, as well as similar numbers of CD4+ and CD8+ T cell subsets. In contrast, PKCη−/− and PKCθ−/− mice had the highest and lowest number of these subsets, respectively (fig. S9B). More evidence supporting an isoform-specific role for PKCη in the biology of peripheral T cells came from analysis of the ratio of CD4+ to CD8+ cells in wild-type B6 mice and PKC-deficient strains of mice (fig. S9E). The CD4:CD8 T cell ratio was highest in PKCη−/− mice (lymph nodes: 1.69 ± 0.29; spleen: 2.39 ± 0.47) and lowest in PKCθ−/− mice (lymph nodes: 1.04 ± 0.12; spleen: 1.31 ± 0.21), with wild-type mice exhibiting intermediate ratios (lymph nodes: 1.44 ± 0.31; spleen: 1.87 ± 0.53). The biased CD4:CD8 ratios seen in single PKC knockout mice were “corrected” in DKO mice (lymph nodes: 1.53 ± 0.34; spleen: 1.88 ± 0.39). Together, these results strengthen the notion that PKCη and PKCθ have both redundant and isoform-specific roles in T cell development.
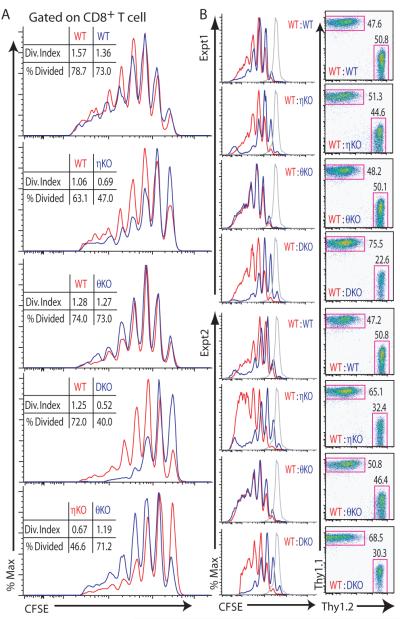
To determine whether the redundancy between PKCη and PKCθ was also manifested in T cell function, we measured the ability of T cells from the various PKC knockout mice to undergo homeostatic proliferation in vivo. T cell homeostatic proliferation occurs in response to a lymphopenic environment and is driven by TCR signaling (26). We sorted naïve T cells from wild-type (Thy1.1+) and the different PKC knockout mice (all Thy1.2+), mixed them at a 1:1 ratio, labeled them with CFSE, and injected them into sublethally irradiated recipient mice. One week later, we determined the CFSE staining of donor T cells by flow cytometry. We found that PKCη−/− CD8+ cells proliferated less well than did wild-type cells, with the most severe proliferation defect seen in DKO cells. PKCθ−/− cells showed no noticeable defect in proliferation compared to that of wild-type cells (Fig. 7A). That the homeostatic proliferation of DKO T cells was not completely abolished and that PKCη−/−, but not PKCθ−/−, CD8+ T cells had a noticeable proliferative defect was surprising. It was possible that the use of polyclonal donor T cells in these experiments obscured a defect in the PKC-deficient cells. Therefore, we repeated this experiment with MHC class I–restricted OT-I TCR transgenic T cells as the donor cells. The DKO cells proliferated very poorly compared to the wild-type cells in recipient mice (Fig. 7B, left). We also observed a proliferative defect in the PKCη−/− cells, but not in the PKCθ−/− cells, consistent with the earlier experiments. We also analyzed the proportions of wild type and PKC-deficient donor cells present in the recipient mice after one week. Although all of the cell combinations started at a 1:1 ratio, the proliferation of DKO cells was clearly outpaced by that of wild-type T cells (Fig. 7B, right). PKCη−/− cells also represented a reduced proportion of the cell population after a week, but the proportion of PKCθ−/− cells was similar to that of the wild-type cells. These changes matched the data from the CFSE-labeling experiments, demonstrating that PKCη, not PKCθ, played a major role in the homeostatic proliferation of CD8+ T cells. In addition to TCR signaling, cytokines such as IL-7 and IL-15 can also promote homeostatic proliferation; however, altered responsiveness to these cytokines during homeostatic proliferation seems unlikely in the case of PKCη−/− and DKO T cells, because the amounts of IL-7Rα (CD127) and IL-15R (CD122) on the surface of these cells was comparable to those of wild-type T cells (fig. S10).
Fig. 7.
Comparison of homeostatic proliferation in three PKC-deficient mouse strains. Homeostatic proliferation assays were performed with (A) naïve polyclonal T cells or (B) naïve OT-I TCR Tg T cells as donor cells. Insets in each histogram in (A) show the parameters of division index and the percentages of divided cells, as calculated with FlowJo software. The gray line in (B) shows undivided donor cells from nonirradiated mice. Results shown are one (A) or two (B) from four experiments.
DISCUSSION
The finding that the abundance of PKCη mRNA was increased during the positive selection of thymocytes (6, 7) was surprising given the widely accepted importance of PKCθ in T cell biology, in particular that PKCθ is recruited to the central region of the immunological synapse (9, 10). PKCη was also recruited to the immunological synapse during thymocyte development and in mature T cells. PKCη formed a diffuse pattern at the immunological synapse, such that although PKCη and PKCθ were co-recruited to the immunological synapse, PKCθ is concentrated at the central synapse, PKCη was not. Such different localization patterns might lead to different functions. This suggestion is consistent with our data showing that T cell activation involving immunological synapse formation (antigen-specific proliferation in response to APCs) was more severely affected in cells lacking PKCη than were responses that did not involve an immunological synapse, such as stimulation with antibody against CD3 or with a combination of PMA and ionomycin.
Knockout mice have been powerful tools in immunological studies, as evidenced by the studies of PKCθ in T cell biology (14, 16). In contrast, studies on PKCη have lagged behind. The first study of a PKCη knockout mouse was published several years ago, but lacked any immunological analysis (8). Until now, there has been no follow-up study of this or any other PKCη knockout mouse. In Table 1, we summarize the similarities and differences between PKCη−/−, PKCθ−/−, and the DKO mice based on this study and work from others (14, 16, 20, 21). That thymocyte development was normal in PKCη−/− mice was not unexpected. Initial reports on PKCθ−/− mice did not identify any defects in thymocyte development despite showing severely impaired mature T cell function (14, 16); however, a study reexamining thymocyte development in PKCθ−/− mice showed a rather mild deficiency (21), which is also in agreement with our observations here. Therefore, it is possible that the lack of obvious developmental or functional defects in any single PKC knockout mouse strain results from a redundancy between PKC isoforms, especially those members within the same subfamily. This is evidenced in our PKCη−/− PKCθ−/− DKO mice, in which positive selection was more severely impaired than in either single knockout mouse.
Table 1.
Comparison of PKCθ−/−, PKCη−/−, and DKO mice. IS, immunological synapse.
| PKCθ KO | PKCη KO# | DKO# | |
|---|---|---|---|
| Thymocytes | |||
| Positive selection | Mildly impaired# | Normal | Strongly impaired |
| Negative selection | Normal | Normal | ND |
| Mature T cells | |||
| CD4:CD8 T cell ratio | Lower than WT | Higher than WT | Equal to WT |
| Proliferation | |||
| to αCD3 in vitro | Severely impaired | Mildly impaired | ≈ PKCθ KO |
| to PMA/iono | Normal | Normal | ND |
| to antigen in vivo | Normal or Impaired | Impaired | ND |
| to antigen in vitro | Impaired | Impaired | ND |
| Homeostatic prolif. | |||
| Non-Tg | CD8 Normal | CD8 impaired | Strongly impaired |
| OT-I Tg | Normal# | Impaired | Strongly impaired |
| Signaling events | |||
| Ca2+ flux | Impaired | Impaired | ND |
| NF-κB translocation | Impaired | Impaired | ND |
| Recruitment to IS* | Recruited, central region | Recruited, diffuse pattern |
Not based on knockout mice.
Based on data obtained from this study. ND: not determined.
Although redundancy between different PKC isoforms obscures some roles of individual PKC isoforms, we did find clear individual roles for PKCη in this study. For example, a reduced homeostatic proliferation of CD8+ T cells (either polyclonal or OT-I TCR transgenic T cells) was seen only in PKCη−/− cells, but not in PKCθ−/− cells. Also, PKCη−/− mice had a higher ratio of CD4+ T cells to CD8+ T cells than did wild-type mice, whereas PKCθ−/− mice had a lower ratio. Knockout of both PKC isoforms resulted in a CD4:CD8 T cell ratio that was similar to that of wild-type mice. Previous work has shown that the ratio of CD4+ to CD8+ T cells is influenced by a number of factors during development (28-30); however, the effect of PKC knockout that we observed appeared to occur post-thymically, because SP thymocytes from the knockout mice did not show altered ratios of CD4+ to CD8+ T cells. Together, these results demonstrate PKCη-specific roles in T cells.
The enlarged lymph nodes seen in PKCη−/− mice relative to those of wild-type mice were also noted in PKCδ−/− animals (31), but were not observed in PKCθ−/− mice (14, 16). In PKCδ−/− mice, lymphadenopathy was associated with increased numbers of B cells as well as a marked increase in the ratio of B cells to T cells (31). However, the situation in PKCη−/− mice was more complicated. In the lymph nodes, the relative proportions of B cells and T cells were barely different (~4% difference), and thus were unlikely to account for the substantially increased cell numbers (40 to 50%) in the lymph nodes of the PKCη−/− mice compared to those of normal mice. In contrast, in the spleens of PKCη−/− mice, the numbers of B cells and T cells were reduced compared to those in spleens from wild-type mice. We speculate that the differences in lymphocyte numbers between the lymph nodes and spleens of the PKCη−/− mice may reflect altered lymphocyte homing, homeostasis, or both, and are worth further investigation. We found that Ca2+ flux and NF-κB translocation were impaired in PKCη−/− T cells, but that TCR-proximal tyrosine phosphorylation events and MAPK signaling pathways were intact. These signaling defects are similar to those of PKCθ−/− T cells. Unlike PKCθ, about which there are many in-depth studies on the molecular signaling machinery (15, 18-20), the investigation of PKCη is still at an early stage.
One puzzle raised in this and other studies is that there is an apparent discrepancy between the severe defect seen in PKCθ−/− T cells in studies in vitro and the mostly mild (if any) defect observed in studies in vivo. For example, the absence of PKCθ impairs the differentiation of T helper type 2 (TH2) cells but not that of TH1 cells, which results in reduced immune responses against Leishmania major (32). Similarly, PKCθ-deficiency results in impaired responses to Listeria monocytogenes infection (33), but not to impaired antiviral immune responses (34, 35). As we showed here, PKCθ−/− CD8+ T cells showed normal homeostatic proliferation, whereas this was impaired in PKCη−/− CD8+ T cells. Thus, the deficiencies seen in PKCη−/− T cells in vivo were more striking than those of PKCθ−/− T cells. It will be interesting to determine how PKCη−/− and DKO T cells behave in vivo in response to viral and bacterial infections.
We have demonstrated that PKCη has specific, as well as redundant (with PKCθ), functions in T cell biology, which are separated by developmental stage. Although redundant during T cell development, specific roles for PKCη in the responsiveness of CD8+ T cells were apparent in the periphery. Deficiencies in CD8+ T cell responses to pMHC may cause the increased CD4:CD8 T cell ratio in peripheral T cells that we observed in the PKCη−/− mice. In addition, PKCη was required to balance the distribution of lymphocytes between different lymphoid organs. Together, these factors point to the importance of PKCη in T cell biology.
MATERIALS AND METHODS
Mice
B6.PL-Thy1a/CyJ (Thy1.1+CD45.2+) and B6.SJL-Ptprca Pepcb/BoyJ (Thy1.2+CD45.1+) mice were purchased from Jackson Laboratories. C57BL/6 (Thy1.2+CD45.2+) mice were bred at The Scripps Research Institute (TSRI). OT-I and OT-I Tap−/− animals were obtained from S. Jameson and K. Hogquist (University of Minnesota, Minneapolis, MN), and Prkcq−/− mice (14) and OT-I Prkcq−/− were provided by A. Altman (La Jolla Institute for Allergy and Immunology). Prkch−/− mice were generated in this study. All experiments were performed in accordance with the guidelines of the Animal Care and Use Committee of TSRI.
Antibodies, cytokines, and reagents
Antibodies against CD3 (145-2C11), CD4 (RM4.4, RM4.5, GK1.5), CD8α (53-6.7), CD25 (PC61.5), CD62L (MEL14), CD44 (IM7), Vβ5 (MR9-6), Vβ6 (RR4-7), Vβ11 (CTVB11), and Vβ12 (CTVB12b), were obtained from eBioscience, Biolegend, or BD Biosciences. Antibodies against phosphorylated ERK1/2 (T202/Y204, clone 197G2), phosphorylated p38 MAPK (Thr180/Tyr182, clone 3DT), α-tubulin, and β-actin were from Cell Signaling Technology. Antibodies against p65, PKCη, and PKCθ were obtained from Santa Cruz Biotechnology. Recombinant IL-3, IL-6, and stem cell factor (SCF) were obtained from PeproTech. Granulocyte macrophage colony–stimulating factor (GM-CSF) was generated as described previously (36). Streptavidin was obtained from Jackson ImmunoResearch Laboratories. The GeneJammer transfection reagent was from Stratagene. The AmiconUltra centrifugal filter device (UFC810024) was obtained from Millipore. EasySep Mouse total T cell, CD4+, and CD8+ T Cell Negative Enrichment Kits were from StemCell Technologies. Indo-1 AM and CFDA-SE (CFSE) were from Molecular Probes (Invitrogen).
Real time RT-PCR
Total RNA from the thymi of 4- to 8-week old OT-I mice or from sorted subpopulations was isolated with the RNeasy Mini Kit (Qiagen). The RNA was treated with deoxyribonuclease I (DNAse I, Invitrogen) for 15 min at room temperature. The reaction was stopped by the addition of 25 mM EDTA, and the DNAse was inactivated by incubating the samples at 65°C for 10 min. RNA samples were used as templates to synthesize complementary DNA (cDNA) with the Superscript First Strand Synthesis System for RT-PCR (Invitrogen) according to the manufacturer’s recommendations. Oligonucleotide primers were designed with Primer Express 1.5 software (PE Applied Biosystems). The primer sequences are as follows: PKCη 5′ primer: 5′-CTCCAGACCGTCTGTTCTTTGTC-3′; 3′ primer: 5′-ATCTCCGCGGCGTAGAAA-3′, GenBank accession # D90242. Ribosomal protein 5′ primer: 5′-AGATGCAGCAGATCCGCAT-3′; 3′ primer: 5′-GGATGGCCTTGCGCA-3′, GenBank accession # BC011106. Real-time PCR reactions were performed on the ABI Prism 7700 Sequence Detection System with SYBR Green PCR Master Mix (PE Applied Biosystems). The PCR mix was optimized for SYBR Green reactions and contained SYBR Green 1 dye, AmpliTaqGold DNA polymerase, and dNTPs with dUTP. Each reaction was performed in a final volume of 25 μl and contained the SYBR Green PCR Master Mix, cDNA, and 150 nM of each primer. Two independent experiments were performed, and all reactions were performed in duplicate. A standard curve with a two-fold serial dilution of the standard cDNA was generated for the target gene as well as for the housekeeping gene ribosomal protein. cDNAs (20 ng/reaction) from the different cell populations were used as templates for real-time PCR. The standard curves were used to determine the relative concentrations of amplified transcripts. The amounts of PKCη mRNA were first normalized to those of the ribosomal protein mRNA. The normalized sample values were finally divided by the normalized amounts of PKCη mRNA in samples from OT-I TCRlo DP cells to obtain the fold-change in mRNA abundance between the different populations of DP thymocytes.
Generation of PKCη−/− mice
The strategy for generating PKCη knockout mice was as described previously (fig. S5) (37), except that all cloning materials, embryonic stem (ES) cell line (Bruce4), and founder mice used for breeding were on a B6 background. PKCθ knockout mice were described previously (14) and were maintained in TSRI animal facility by further breeding onto the B6 background. DKO mice were generated by breeding PKCη−/− and PKCθ−/− mice together.
T cell activation and proliferation assays
Purified naïve CD8+ T cells from mice were used in experiments as indicated in the text. Purification was performed with biotinylated antibodies in conjunction with magnetic beads conjugated with antibodies against biotin (BD Bioscience) or else cells were sorted with a flow cytometer. Cells were stimulated with plate-bound or soluble antibody against CD3 as indicated in the figures, and cells were incubated with antibodies against CD69, CD25, CD4, and CD8 for flow cytometric analysis. Thymidine incorporation assays were performed as described (14). For proliferation assays involving CFSE dilution, lymphocytes were incubated with CFSE (0.2 μM) at 37°C for 10 min, which was stopped by the addition of 10 volumes of cRPMI. After washing, cells were cultured with or without antibody against CD3 in cRPMI for 48 hours, or in the presence of irradiated B6.SJL-Ptprca Pepcb/BoyJ (CD45.1) splenocytes that had been pulsed with the indicated concentration of OVA peptide. T cell proliferation was determined by measuring the dilution of CFSE in cells by flow cytometry. To measure IL-2 production, CD4+ T cells were purified from lymph nodes with the EasySep Mouse CD4+ T Cell Enrichment Kit (StemCell), and 2 × 105 cells were seeded per well in a BioCoat T-Cell Activation Plate, which was pre-coated with antibody against CD3 (BD Biosciences). To stimulate cells, soluble antibody against CD28 was added to the cell culture medium to a final concentration of 1 μg/ml, and cells were incubated at 37°C in 5% CO2 for 48 hours. Culture supernatants were removed, and the concentration of IL-2 produced by the cells was measured with the mouse IL-2 enyme-linked immunosorbent assay (ELISA) Ready-SET-Go kit (eBioscience) according to the manufacturer’s instructions.
Ca2+ signaling
Ca2+ flux was performed as described previously (37, 38). Briefly, lymphocytes were isolated from wild-type mice (Thy1.1+ or Thy1.2+) and PKCη−/− mice (Thy1.2+). Cells from different genotypes were mixed at a 1:1 ratio. Cells were suspended at 2 to 5 × 106/ml in cRPMI [RPMI medium supplemented with 10% fetal calf serum (FCS), penicillin (100 U/ml), streptomycin (10 μg/ml), glutamine (292 μg/ml), 50 μM 2-mercaptoethanol, and 25 mM Hepes (pH 7.2-7.5)] and were incubated with 2 μM Indo-1 AM for 30 min at 37°C in 5% CO2. Cells were washed twice with cRPMI and resuspended in 0.5 ml of cRPMI or cHBSS [Hanks’ balanced salt solution (HBSS) supplemented with 1% FCS, 1 mM EGTA, 1 mM MgCl2, and 10 mM Hepes (pH 7.2-7.5)]. Cells were pre-warmed to 37°C before analysis and were kept at 37°C during event collection on a Becton-Dickinson LSR-II flow cytometer. To stimulate cells, reagents were added during the analysis as indicated in the figures, and maximal Ca2+ flux was obtained by adding ionomycin (500 ng/ml, Calbiochem). The mean fluorescence ratio was calculated with FlowJo software (TreeStar).
Western blotting analysis
CD8+ T cells were stimulated with crosslinking antibodies against CD3 and CD8 for the times indicated in the figures. Cell lysates were prepared, resolved by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and analyzed by Western blotting with the indicated antibodies, as described previously (37). To measure the nuclear translocation of NF-κB p65, cells were stimulated as described earlier, chilled on ice, and fractionated with the NE-PER kit (Pierce). Western blotting analysis of samples was performed with the Li-Cor Odyssey infrared imaging system. Normalization and calculation of fraction purity and the ratios of nuclear to cytosolic p65 abundance were performed according to Li-Cor protocols with p65, total protein, actin, and tubulin signals.
In vivo proliferation assays
Proliferation of OT-I TCR transgenic cells in response to stimulation with OVA peptide in vivo was measured as described previously (39). Briefly, OT-I TCR CD8+ T cells were purified with a CD8+ T cell Negative Enrichment kit (StemCell). Wild-type (Thy1.1+CD45.2+) and PKCη−/− (Thy1.2+CD45.2+) cells were mixed at a 1:1 ratio, and transferred into recipient B6.SJL mice (Thy1.2+CD45.1+). The recipient mice were intravenously injected with BMDCs that had been pulsed with OVA257-264 peptide one day before transfer of the T cells. At various time points, cells from the lymph nodes and spleens of recipient mice were collected, and the proportions and absolute cell numbers of donor cells were determined by flow cytometry. BMDC were cultured and induced to maturation as described previously (36, 39, 40). Briefly, on day 7 of BMDC culture, lipopolysaccharide (LPS, 100 ng/ml) was added overnight to induce maturation. BMDC maturity and phenotype were confirmed by flow cytometric analysis of the surface expression of CD11c, CD86, CD80, and MHC-II. The resultant cell population contained >90% CD11c+ MHC-IIhi BMDCs.
Homeostatic proliferation assays
Naïve T cells (CD62L+CD44−) were sorted from mice (all of whom were CD45.2+, but were either Thy1.1+ or Thy1.2+). Thy1.1+ or Thy1.2+ sorted cells were mixed in a 1:1 ratio, labeled with CFSE, and injected intravenously into recipient B6.SJL mice (Thy1.2+CD45.1+), which were sublethally irradiated (5.5 Gy) with a Gammacell 40 (MDS Nordion) before cell transfer. One week later, cells from the lymph nodes and spleens of recipient mice were collected and analyzed by flow cytometry.
Microscopy
To visualize the recruitment of PKC to the immunological synapse in live cells, we transfected T cell hybridomas with plasmid encoding fusion proteins of PKC isoforms and fluorescent proteins, as indicated in the text. The form of red fluorescent protein (RFP) used was tDimer2(12) (41). APCs were loaded with peptides or superantigens as indicated in the figures. T cell hybridomas and APCs were mixed together for various time points and were fixed before imaging. For time-lapse imaging of T cell–APC conjugates (fig. S4), T cell hybridomas and APCs were sequentially loaded into an FCS2 flow chamber FCS2 (Bioptechs) that was heated to 37°C. To visualize the recruitment of PKC to the immunological synapse in live primary T cells, we incubated 1 × 107 thymocytes with 2 × 107 EL4 cells that were pulsed with OVA peptide (10 mM) or with 2 × 107 LK35 B cell (class II+) tumor cells presenting the superantigens SEA (50 ng/ml) and SEB (10 μg/ml, Toxin Technology) in 96-well, round-bottomed plates for 30 or 60 min at 37°C. Thymocytes were then fixed with 4% paraformaldehyde (PFA) for 12 min at room temperature. To detect cell-surface markers, thymocytes were incubated with biotinylated antibodies against CD4, CD8, or LFA-1 (BD Bioscience), after which they were incubated with Alexa Fluor 488–conjugated streptavidin (Molecular Probes). For intracellular staining, cells were permeabilized with 0.2% saponin for 15 min at room temperature. Cells were then incubated with rabbit antibodies against the appropriate PKC isoform, after which they were incubated with Alexa Fluor 568– or Alexa Fluor 680–conjugated antibodies against rabbit antibodies (Molecular Probes). To visualize the nuclear translocation of NF-κB in primary T cells, we purified 2 × 106 CD8+ T cells from lymph nodes by magnetic negative selection and plated the cells on Lab-Tek II chambered cover glass coated with poly-D-lysine (Sigma). The cells were then stimulated with crosslinking antibodies against CD3 and CD8 for the times indicated in the figures. T cells were then fixed with 4% PFA for 12 min at room temperature. Cells were permeabilized with 0.3% Triton X-100 for 3 min at room temperature. Cells were then incubated with rabbit antibody against p65 (1 μg/ml), after which they were incubated with Alexa Fluor 488–conjugated secondary antibody against rabbit antibody (Molecular Probes). Nuclei were counterstained with the DNA-binding dye 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen). All images were captured with a Zeiss Axiovert 200 M inverted microscope. SlideBook software (Intelligent Imaging Innovation) was used for the capture as well as for deconvolution and image analysis, and ImageJ was used for image presentation.
Statistical analysis
Statistical differences were calculated with the mean difference hypothesis of Student’s two tailed t test assuming different variances and a confidence level of 95%. Calculations were performed with Microsoft Excel.
Supplementary Material
Acknowledgments
We thank A. Altman (La Jolla Institute for Allergy and Immunology), K. Hogquist and S. Jameson (University of Minnesota, Minneapolis), and D. Littman (Skirball Institute, New York) for mice and reagents, and the TSRI Mouse Genetics Core for help in making the knockout mice.
Funding: This work was supported by grants from NIH (GM048002 and GM065230 to N.R.J.G., OD006433 to H.C., and AI070845 to K.S.). N.N.-M. was supported by a fellowship from the Swiss National Science Foundation; J.C. was supported by a fellowship from the Spanish Ministerio de Innovacion y Ciencia (MICIIN); P.P.Y. was supported by NIH training grant T32HL07195; J.A.H.H. was supported by the Irving S. Sigal Fellowship of the American Chemical Society and by NIH training grant T32AI07244; and K.S. was supported by The Leukemia & Lymphoma Society Scholar Award 1440-11. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medicine or the National Institutes of Health. This is manuscript number 20858 from The Scripps Research Institute.
Footnotes
Author contributions: G.F. and N.R.J.G. designed the project; G.F. made the knockout mice with help from B.M.; G.F., J.H., A.L.G., F.L., S.F., H.C., J.A.A.H., and J.A. analyzed the mice; V.R., S.R. and K.S. performed or analyzed biochemical experiments; G.F., J.C., P.P.Y., and N.N.-M. performed imaging; N.N.-M. made the initial finding that PKCη abundance was increased during thymocyte development; and G.F. and N.R.J.G. wrote the manuscript.
Competing interests: The authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Dempsey EC, Newton AC, Mochly-Rosen D, Fields AP, Reyland ME, Insel PA, Messing RO. Protein kinase C isozymes and the regulation of diverse cell responses. Am J Physiol Lung Cell Mol Physiol. 2000;279:L429–438. doi: 10.1152/ajplung.2000.279.3.L429. [DOI] [PubMed] [Google Scholar]
- 2.Isakov N, Altman A. Protein kinase Cθ in T cell activation. Annu Rev Immunol. 2002;20:761–794. doi: 10.1146/annurev.immunol.20.100301.064807. [DOI] [PubMed] [Google Scholar]
- 3.Baier G. The PKC gene module: molecular biosystematics to resolve its T cell functions. Immunol Rev. 2003;192:64–79. doi: 10.1034/j.1600-065x.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi K, Altman A. Protein kinase C θ (PKCθ): a key player in T cell life and death. Pharmacol Res. 2007;55:537–544. doi: 10.1016/j.phrs.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manicassamy S, Gupta S, Sun Z. Selective function of PKC-θ in T cells. Cell Mol Immunol. 2006;3:263–270. [PubMed] [Google Scholar]
- 6.Mick VE, Starr TK, McCaughtry TM, McNeil LK, Hogquist KA. The regulated expression of a diverse set of genes during thymocyte positive selection in vivo. J Immunol. 2004;173:5434–5444. doi: 10.4049/jimmunol.173.9.5434. [DOI] [PubMed] [Google Scholar]
- 7.Niederberger N, Buehler LK, Ampudia J, Gascoigne NRJ. Thymocyte stimulation by anti-TCR-β, but not by anti-TCR-α, leads to induction of developmental transcription program. J Leukoc Biol. 2005;77:830–841. doi: 10.1189/jlb.1004608. [DOI] [PubMed] [Google Scholar]
- 8.Chida K, Hara T, Hirai T, Konishi C, Nakamura K, Nakao K, Aiba A, Katsuki M, Kuroki T. Disruption of protein kinase Cη results in impairment of wound healing and enhancement of tumor formation in mouse skin carcinogenesis. Cancer Res. 2003;63:2404–2408. [PubMed] [Google Scholar]
- 9.Monks CRF, Kupfer H, Tamir I, Barlow A, Kupfer A. Selective modulation of protein kinase C-θ during T-cell activation. Nature. 1997;385:83–86. doi: 10.1038/385083a0. [DOI] [PubMed] [Google Scholar]
- 10.Monks CRF, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 11.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 12.Yokosuka T, Kobayashi W, Sakata-Sogawa K, Takamatsu M, Hashimoto-Tane A, Dustin ML, Tokunaga M, Saito T. Spatiotemporal regulation of T cell costimulation by TCR-CD28 microclusters and protein kinase C θ translocation. Immunity. 2008;29:589–601. doi: 10.1016/j.immuni.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singleton KL, Roybal KT, Sun Y, Fu G, Gascoigne NRJ, van Oers NS, Wulfing C. Spatiotemporal patterning during T cell activation is highly diverse. Sci Signal. 2009;2:ra15. doi: 10.1126/scisignal.2000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun Z, Arendt CW, Ellmeier W, Schaeffer EM, Sunshine MJ, Gandhi L, Annes J, Petrzilka D, Kupfer A, Schwartzberg PL, Littman DR. PKC-θ is required for TCR-induced NF-kB activation in mature but not immature T lymphocytes. Nature. 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 15.Egawa T, Albrecht B, Favier B, Sunshine MJ, Mirchandani K, O’Brien W, Thome M, Littman DR. Requirement for CARMA1 in antigen receptor-induced NF-κB activation and lymphocyte proliferation. Curr Biol. 2003;13:1252–1258. doi: 10.1016/s0960-9822(03)00491-3. [DOI] [PubMed] [Google Scholar]
- 16.Pfeifhofer C, Kofler K, Gruber T, Tabrizi NG, Lutz C, Maly K, Leitges M, Baier G. Protein kinase Cθ affects Ca2+ mobilization and NFAT cell activation in primary mouse T cells. J Exp Med. 2003;197:1525–1535. doi: 10.1084/jem.20020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hara H, Bakal C, Wada T, Bouchard D, Rottapel R, Saito T, Penninger JM. The molecular adapter Carma1 controls entry of IκB kinase into the central immune synapse. J Exp Med. 2004;200:1167–1177. doi: 10.1084/jem.20032246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D, Matsumoto R, You Y, Che T, Lin XY, Gaffen SL, Lin X. CD3/CD28 costimulation-induced NF-κB activation is mediated by recruitment of protein kinase C-θ, Bcl10, and IκB kinase β to the immunological synapse through CARMA1. Mol Cell Biol. 2004;24:164–171. doi: 10.1128/MCB.24.1.164-171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roose JP, Mollenauer M, Gupta VA, Stone J, Weiss A. A diacylglycerol-protein kinase C-RasGRP1 pathway directs Ras activation upon antigen receptor stimulation of T cells. Mol Cell Biol. 2005;25:4426–4441. doi: 10.1128/MCB.25.11.4426-4441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manicassamy S, Sadim M, Ye RD, Sun Z. Differential roles of PKC-θ in the regulation of intracellular calcium concentration in primary T cells. J Mol Biol. 2006;355:347–359. doi: 10.1016/j.jmb.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 21.Morley SC, Weber KS, Kao H, Allen PM. Protein kinase C-θ is required for efficient positive selection. J Immunol. 2008;181:4696–4708. doi: 10.4049/jimmunol.181.7.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irwin MJ, Gascoigne NRJ. Interplay between superantigens and the immune system. J. Leukocyte Biol. 1993;54:495–503. doi: 10.1002/jlb.54.5.495. [DOI] [PubMed] [Google Scholar]
- 23.Yachi PP, Ampudia J, Gascoigne NRJ, Zal T. Nonstimulatory peptides contribute to antigen-induced CD8-T cell receptor interaction at the immunological synapse. Nat Immunol. 2005;6:785–792. doi: 10.1038/ni1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 25.Scherer MT, Ignatowicz L, Winslow GM, Kappler JW, Marrack P. Superantigens: Bacterial and viral proteins that manipulate the immune system. Annu. Rev. Cell Biol. 1993;9:101–128. doi: 10.1146/annurev.cb.09.110193.000533. [DOI] [PubMed] [Google Scholar]
- 26.Sprent J, Surh CD. Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat Immunol. 2011;12:478–484. doi: 10.1038/ni.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu KQ, Bunnell SC, Gurniak CB, Berg LJ. T cell receptor-initiated calcium release is uncoupled from capacitative calcium entry in Itk-deficient T cells. J. Exp. Med. 1998;187:1721–1727. doi: 10.1084/jem.187.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki H, Punt JA, Granger LG, Singer A. Asymmetric signaling requirements for thymocyte commitment to the CD4+ versus CD8+ T cell lineages: a new perspective on thymic commitment and selection. Immunity. 1995;2:413–425. doi: 10.1016/1074-7613(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 29.Corbella P, Moskophidis D, Spanopoulou E, Mamalaki C, Tolaini M, Itano A, Lans D, Baltimore D, Robey E, Kioussis D. Functional commitment to helper T cell lineage precedes positive selection and is independent of T cell receptor MHC specificity. Immunity. 1994;1:269–276. doi: 10.1016/1074-7613(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 30.Sim B-C, Aftahi N, Reilly C, Bogen B, Schwartz RH, Gascoigne NRJ, Lo D. Thymic skewing of the CD4/CD8 ratio maps with the T-cell receptor α-chain locus. Curr. Biol. 1998;8:701–704. doi: 10.1016/s0960-9822(98)70276-3. [DOI] [PubMed] [Google Scholar]
- 31.Mecklenbrauker I, Saijo K, Zheng NY, Leitges M, Tarakhovsky A. Protein kinase Cδ controls self-antigen-induced B-cell tolerance. Nature. 2002;416:860–865. doi: 10.1038/416860a. [DOI] [PubMed] [Google Scholar]
- 32.Marsland BJ, Soos TJ, Spath G, Littman DR, Kopf M. Protein kinase Cθ is critical for the development of in vivo T helper (Th)2 cell but not Th1 cell responses. J Exp Med. 2004;200:181–189. doi: 10.1084/jem.20032229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakowicz-Burkiewicz M, Nishanth G, Helmuth U, Drogemuller K, Busch DH, Utermohlen O, Naumann M, Deckert M, Schluter D. Protein kinase C-θ critically regulates the proliferation and survival of pathogen-specific T cells in murine listeriosis. J Immunol. 2008;180:5601–5612. doi: 10.4049/jimmunol.180.8.5601. [DOI] [PubMed] [Google Scholar]
- 34.Berg-Brown NN, Gronski MA, Jones RG, Elford AR, Deenick EK, Odermatt B, Littman DR, Ohashi PS. PKCθ signals activation versus tolerance in vivo. J Exp Med. 2004;199:743–752. doi: 10.1084/jem.20031022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marsland BJ, Nembrini C, Schmitz N, Abel B, Krautwald S, Bachmann MF, Kopf M. Innate signals compensate for the absence of PKC-θ during in vivo CD8+ T cell effector and memory responses. Proc Natl Acad Sci U S A. 2005;102:14374–14379. doi: 10.1073/pnas.0506250102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu G, Wijburg OL, Cameron PU, Price JD, Strugnell RA. Salmonella enterica Serovar Typhimurium infection of dendritic cells leads to functionally increased expression of the macrophage-derived chemokine. Infect Immun. 2005;73:1714–1722. doi: 10.1128/IAI.73.3.1714-1722.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu G, Vallee S, Rybakin V, McGuire MV, Ampudia J, Brockmeyer C, Salek M, Fallen PR, Hoerter JAH, Munshi A, Huang YH, Hu J, Fox HS, Sauer K, Acuto O, Gascoigne NRJ. Themis controls thymocyte selection through regulation of T cell antigen receptor-mediated signaling. Nat Immunol. 2009;10:848–856. doi: 10.1038/ni.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu G, Gascoigne NRJ. Multiplexed labeling of samples with cell tracking dyes facilitates rapid and accurate internally controlled calcium flux measurement by flow cytometry. J Immunol Methods. 2009;350:194–199. doi: 10.1016/j.jim.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krieg C, Boyman O, Fu YX, Kaye J. B and T lymphocyte attenuator regulates CD8+ T cell-intrinsic homeostasis and memory cell generation. Nat Immunol. 2007;8:162–171. doi: 10.1038/ni1418. [DOI] [PubMed] [Google Scholar]
- 40.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 41.Campbell RE, Tour O, Palmer AE, Steinbach PA, Baird GS, Zacharias DA, Tsien RY. A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. USA. 2002;99:7877–7882. doi: 10.1073/pnas.082243699. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.