Abstract
Intracranial self-stimulation (ICSS) is one procedure used to evaluate abuse liability of drugs. The mu opioid receptor agonist morphine is an acknowledged drug of abuse, and the present study examined factors that may influence expression of abuse-related morphine effects on ICSS in rats. Adult male rats were equipped with intracranial electrodes targeting the medial forebrain bundle, and 10 stimulus frequencies (56-158 Hz in 0.05 log increments) were available during each daily session under a continuous reinforcement schedule. The primary dependent variable was ICSS rate at each frequency. Under control conditions, ICSS rate increased with frequency. After acute morphine (1-10 mg/kg), rate-decreasing effects predominated at early pretreatment times (10-30 min), and rate-increasing effects predominated at later pretreatment times (100-180 min). Acute morphine effects dissipated after 300 min. Repeated morphine (3.2-18 mg/kg/day × 7 days at each dose) produced tolerance to rate-decreasing effects, enhanced expression of rate-increasing effects, and enhanced rate-dependency of morphine effects. Withdrawal from repeated morphine produced small but significant dose-dependent decreases in ICSS. These results show that the magnitude and valence of morphine effects on rates of ICSS in rats are strongly influenced by morphine dose and pretreatment time, history of morphine exposure, and baseline ICSS rate.
Keywords: Morphine, morphine addiction, morphine dependence, drug abuse, intracranial self-stimulation, rat
Introduction
Intracranial self-stimulation (ICSS) comprises a family of operant procedures in which responding is maintained by electrical stimuli delivered to target brain regions such as the medial forebrain bundle at the level of the lateral hypothalamus (Olds and Milner, 1954; Stellar and Stellar, 1985; Carlezon and Chartoff, 2007). One application of ICSS has been to generate stable baselines of schedule-controlled responding for use in evaluating abuse-related drug effects (Kornetsky and Esposito, 1979; Reid, 1987; Wise, 1998). Within this research tradition, facilitation of ICSS (indicated by increased rates of ICSS) is often interpreted as a rewarding drug effect that may contribute to, or be predictive of, abuse liability by that drug in humans. For example, amphetamine is representative of one class of abused drugs (i.e. indirect dopamine agonists) that reliably and robustly facilitate ICSS across a broad range of experimental conditions in rats (Esposito et al., 1980; Gallistel and Freyd, 1987; Pereira Do Carmo et al., 2009a).
Mu opioid agonists, including morphine, constitute another class of abused drugs that has been evaluated extensively in ICSS, but mu agonist effects on ICSS have been less consistent than effects produced by amphetamine-like stimulants. More specifically, mu agonist effects appear to be influenced by the particular type of procedure used to assess ICSS performance, as well as by other factors that include pretreatment time and extent of prior exposure to opioids. In the simplest type of ICSS procedure, responding produces electrical stimulation of a constant magnitude, and the primary dependent measure is the rate of responding or reinforcement. This approach can generate relatively constant rates of ICSS, and early studies using this approach revealed two major findings (Adams et al., 1972; Lorens and Mitchell, 1973; Koob et al., 1975; Reid, 1987). First, acute treatment with morphine or other mu agonists produced effects with a biphasic time course consisting of an initial decrease followed by a subsequent increase in rates of ICSS. Second, repeated or chronic treatment produced tolerance to initial rate-decreasing effects and earlier expression of rate-increasing effects. As appreciated by investigators using this constant-reinforcer-magnitude approach, expression of rate-decreasing or rate-increasing effects depended in part on baseline ICSS rates engendered by the selected reinforcer magnitude. High stimulus magnitudes maintained high ICSS rates preferentially sensitive to rate-decreasing effects, whereas lower stimulus magnitudes maintained lower ICSS rates preferentially sensitive to rate-increasing effects (Reid, 1987).
More recent studies have evaluated mu agonist effects using more sophisticated ICSS procedures, in which reinforcer magnitude is systematically manipulated during each experimental session by manipulating either the intensity or frequency of electrical stimulation. Although these procedures have the potential to efficiently assess drug effects on a wide range of ICSS rates maintained by a wide range of reinforcer magnitudes, the focus has been on threshold reinforcer magnitudes that maintain low rates or low probabilities of responding and are especially sensitive to rate-increasing effects linked to abuse liability. In accordance with this sensitivity, some studies reported rapid facilitation of ICSS after acute treatment with low mu agonist doses (Kornetsky and Esposito, 1979; Carlezon and Wise, 1993; Jha et al., 2004), although this finding has not always been obtained (Stratmann and Craft, 1997; Pereira Do Carmo et al., 2009b). Other studies have provided evidence to suggest that, in accordance with earlier studies using simpler procedures, facilitation of ICSS may be more robust later in the time course after acute mu agonist treatment or after chronic treatment (Carlezon and Wise, 1993; Craft et al., 2001; O'Neill and Todtenkopf, 2010). The main goal of this study was to further evaluate the role of dose, pretreatment time and regimen of repeated treatment as determinants of morphine effects on ICSS using a “frequency-rate” procedure, in which the frequency of the reinforcing electrical stimulus was varied to generate a wide range of response rates during each daily session. Data were submitted to rate-dependency analysis to assess the degree to which morphine effects on ICSS were determined by baseline rates of ICSS.
Methods
Subjects
Ten male Sprague-Dawley rats (Harlan, Frederick MD) weighing 310-350 g at the time of surgery were used. Rats were individually housed and were maintained on a 12h light/dark cycle, with lights on from 06.00 to 18.00h. Rats had free access to food and water except during testing. Animal maintenance and research were in compliance with NIH guidelines on care and use of animal subjects in research, and all animal-use protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
Assay of Intracranial Self-Stimulation (ICSS)
ICSS electrode implantation
Rats were anesthetized with isoflurane gas (2.5-3% in oxygen; Webster Veterinary, Phoenix, AZ) for implantation of stainless steel electrodes (Plastics One, Roanoke, VA). One pole (the cathode) of each bipolar electrode was 0.25 mm in diameter and covered with polyamide insulation except at the flattened tip, whereas the other pole (the anode) was 0.125 mm in diameter and uninsulated. The cathode was implanted in the left medial forebrain bundle at the level of the lateral hypothalamus (2.8 mm posterior to bregma, 1.7 mm lateral from midsaggital suture, and 7.8 mm below dura). The anode was wrapped around one of three skull screws to serve as the ground, and the skull screws and electrode assembly were secured to the skull with orthodontic resin. The animals were allowed to recover for at least 7 days prior to commencing ICSS training.
ICSS apparatus
Experiments were conducted in sound attenuating boxes that contained modular acrylic test chambers (29.2 × 30.5 × 24.1 cm) equipped with a response lever (4.5 cm wide, extended 2.0 cm through the center of one wall, 3 cm off the floor), stimulus lights (three lights colored red, yellow, and green, positioned 7.6cm directly above the response lever), a 2-W white house light, and an ICSS stimulator (Med Associates, St. Albans, VT). Electrodes were connected to the stimulator via bipolar cables and a swivel connector (Model SL2C, Plastics One, Roanoke, VA). The stimulator was controlled by a computer and software that also controlled all the programming parameters and data collection (Med Associates, St. Albans, VT).
Behavioral procedure
After initial shaping of lever-press responding, rats were trained under a continuous reinforcement schedule of brain stimulation using procedures similar to those described previously (Pereira Do Carmo et al., 2009a,b; Negus et al., 2010). During experimental sessions, each lever press resulted in the delivery of a 0.5-s train of square wave cathodal pulses (0.1-ms pulse duration), and stimulation was accompanied by the illumination of the stimulus lights over the lever. Responses during the 0.5-s stimulation period did not earn additional stimulation. During initial training sessions lasting 30 to 60 min, the frequency of stimulation was held constant at 158 Hz, and the stimulation intensity for each rat was adjusted gradually to the lowest value that would sustain a high rate of reinforcement (>30 stimulations/min). Once this criterion was met, frequency manipulations were introduced. Sessions involving frequency manipulations consisted of sequential 10-min components. During each component, a descending series of 10 current frequencies (158-56 Hz in 0.05 log increments) was presented, with a 60-s trial at each frequency. A frequency trial was initiated by a 5-s time out followed by a 5-s “priming” phase, during which animals received 5 non-contingent stimulations with a 0.5-s interval between each stimulation. This non-contingent stimulation was then followed by a 50-s “response” phase, during which responding produced electrical stimulation under the continuous reinforcement schedule. Training continued with presentation of up to three sequential components per day, and the current intensity was again adjusted at this stage of training until rats reliably responded for the first three to four frequency trials of all components for at least three consecutive days. This intensity was then held constant for the remainder of the study.
Acute dosing study
Once training was completed, subsequent studies examined (a) the time course of acute morphine doses, and (b) the effects of repeated morphine doses. Tests sessions to examine the time course of acute morphine doses consisted of multiple 10-min components identical to those described above. Each session began with three consecutive “baseline” components. The first baseline component was considered to be an acclimation component, and data from this component were discarded. Data from the second and third baseline components were used to calculate baseline parameters of frequency-rate curves for that session (see Data Analysis). Morphine (1.0-10.0 mg/kg) or its vehicle (saline) was administered immediately after the third baseline component. Subsequently, consecutive pairs of test components were initiated 10, 30, 100 and 300 min after morphine or vehicle treatment. Thus, ICSS performance was evaluated from 10-30, 30-50, 100-120, and 300-320 min after each treatment. Morphine doses were delivered in a mixed order across rats. Test sessions were typically conducted on Tuesdays and Fridays and were separated by at least three days. Training sessions consisting of three components were conducted on Mondays, Wednesdays, Thursdays, and occasionally, on Saturdays. In some cases, data from these training sessions were used to assess ICSS performance 24 h after high doses of morphine. In these cases, data from the second and third components of the training session were used as the “24 h time point” for data analysis.
Chronic dosing study
At the conclusion of the acute dosing study, the effects of repeated morphine were evaluated. During the first three days of this phase of the study, rats were exposed daily to three consecutive “baseline” components. Data from the first component each day were discarded, and data for the second and third components of each day were averaged to generate the baseline frequency-rate curve for comparison with subsequent chronic morphine effects (see Data Analysis). Immediately after the third baseline component on the third day, repeated morphine treatments were initiated for a period of 4 consecutive weeks. Rats were treated with 3.2 mg/kg/day morphine during week 1, 5.6 mg/kg/day morphine during week 2, 10 mg/kg/day morphine during week 3, and 18 mg/kg/day morphine during week 4. Morphine doses were administered daily as a single bolus injection at 1:30 pm, and 30 minutes after each morphine dose, rats were exposed to two consecutive ICSS test components. The following day, 23.5 h after each morphine dose (and immediately before the next morphine dose) rats were exposed to another three consecutive ICSS test components, and data from the second and third of these components were used for data analysis. Thus, ICSS performance was evaluated 30 min and 23.5 h after each morphine dose. After the last dose of 18 mg/kg/day morphine, morphine dosing was terminated, and three-component test sessions were conducted daily for an additional three days. Again, data from the second and third components of these sessions were used for data analysis to assess changes in ICSS during the first three days of withdrawal. The primary goal of this study was to quantify effects of chronic morphine treatment and withdrawal on ICSS. However, to provide some assessment of potential somatic withdrawal signs, rats were also weighed daily, cages were inspected for evidence of diarrhea, and animals were observed for signs of teeth chattering and wet-dog shakes immediately prior to each daily session.
Drug
Morphine sulfate was provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). All solutions were prepared in sterile water for subcutaneous injection.
Data analysis
The primary dependent variable in this ICSS procedure was the reinforcement rate in stimulations/min during each frequency trial. To normalize these data, raw reinforcement rates from each trial in each rat were converted to Percent Maximum Control Rate (%MCR) for that rat. During the acute dosing study, the maximum control rate was determined daily and was defined as the mean of the maximal rates observed in any frequency trial during the second and third “baseline” components for that day. For the repeated dosing study, the maximum control rate was determined prior to the initiation of repeated morphine dosing and was defined as the mean of the maximal rates observed during the second and third “baseline” components over a period of three consecutive days (six total baseline components). Thus, %MCR values for each trial were calculated as (Response Rate During a Frequency Trial ÷ Maximum Control Rate) × 100.
For the acute dosing study, data from each pair of consecutive test components at each time point after morphine injection were averaged and normalized to the maximum control rate for that day as discussed above. For the repeated dosing study, data from each pair of test components 30 minutes and 23.5 h after injection were averaged and normalized to the maximum control rate determined prior to the initiation of the study as discussed above. For statistical analysis, normalized data were compared by two-way ANOVA, with treatment time and ICSS frequency as the two factors. A significant ANOVA was followed by a Holm-Sidak post hoc test, and the criterion for significance was set at p<0.05.
To provide an additional summary of ICSS performance during the repeated dosing study, the total number of stimulations per component was calculated as the sum of stimulations delivered across all 10 frequency trials of each component. Test data were than normalized to baseline data using the equation % Baseline Stimulations per Component = (Mean Stimulations per Test Component ÷ Mean Stimulations per Baseline Component) × 100. Data were then averaged across rats in each experimental condition and compared by one-way ANOVA. A significant ANOVA was followed by the Dunnett post hoc test, and the criterion for significance was set a priori at p<0.05.
Finally, rate-dependent effects of morphine under selected conditions were examined by graphing log percent control ICSS rate after morphine treatment as a function of log control ICSS rate for each stimulation frequency. For the purposes of rate-dependency analysis, control ICSS rates at each brain stimulation frequency were defined as the mean number of stimulations obtained at each frequency after vehicle treatment for the acute studies, and during the baseline sessions before initiation of chronic morphine in the chronic studies. Percent control reinforcement rates after morphine were calculated as [(# stimulations after morphine/# control stimulations)*100] for each stimulation frequency. The analysis was applied to data collected (a) 30 and 100 min after acute treatment with 3.2, 5.6 and 10 mg/kg morphine, and (b) 30 min after the 7th daily treatment with chronic 3.2, 5.6 and 10 mg/kg morphine. The resulting rate-dependency plots were subjected to linear regression analysis using Prism 5 for Macintosh (GraphPad Software Inc., La Jolla, CA). Morphine effects were considered to be significantly rate-dependent if the 95% confidence limits of the slope did not include “0” and if p<0.05 for the regression.
Results
Effects of acute morphine
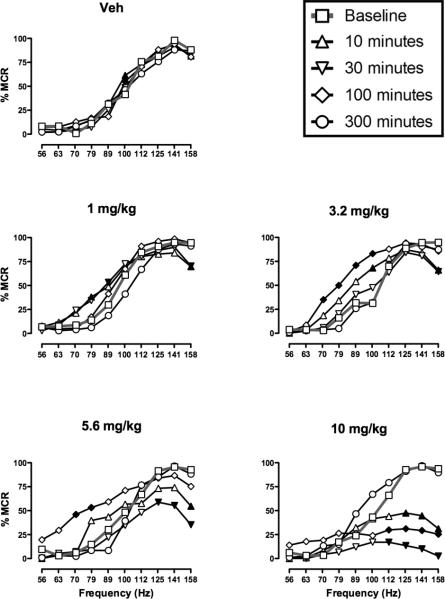
For each test session, a “baseline” ICSS frequency-rate curve was determined before testing to permit determination of the maximum control rate (MCR) for that session. During studies of acute morphine effects, the average MCR was 56.3 ± 13.0 stimulations/trial. Reinforcement rates for each rat during each frequency trial of a session were then normalized as a percentage of the MCR (%MCR) for that rat in that session, and the average baseline frequency-rate curves for each test are shown in Figure 1 (gray lines, open squares). Rats generally did not respond at frequencies of 56–79 Hz, and reinforcement rates increased across a frequency range of 112–158 Hz. Maximum reinforcement rates were usually observed at the highest stimulation frequencies. There were no statistically significant differences between baselines on different days (data not shown).
Fig 1.
Morphine pretreatment produced dose-, time- and frequency-dependent changes in ICSS. Horizonal axes: Frequency of electrical brain stimulation in hertz (log scale). Vertical axes: ICSS rate expressed as percent maximum control rate (%MCR). Each panel shows ICSS frequency-rate curves determined before (Baseline) or at various times after (10-300 min) treatment with vehicle or morphine (1.0-10 mg/kg). Filled symbols indicate frequencies at which ICSS rates were significantly lower or higher than baseline as determined by the Holm-Sidak post hoc test following a significant analysis of variance (p<0.05). ANOVA results were as follows: Vehicle: significant main effect of frequency [F(9,45)=44.0; p<0.001], significant main effect of time [F(4,20)=3.5; p=0.025], no significant frequency×time interaction [F(36,180)=1.4; NS]; 1 mg/kg morphine: significant main effect of frequency [F(9,45)=148.0; p<0.001], no significant main effect of time [F(4,20)=1.6; NS], significant frequency×time interaction [F(36,180)=3.0; p<0.001]; 3.2 mg/kg morphine: significant main effect of frequency [F(9,45)=98.7; p<0.001], significant main effect of time [F(4,20)=3.9; p<0.02], significant frequency×time interaction [F(36,180)=2.5; p<0.001]; 5.6 mg/kg morphine: significant main effect of frequency [F(9,45)=88.1; p<0.001], significant main effect of time [F(4,20)=4.2; p<0.02], significant frequency×time interaction [F(36,180)=4.0; p<0.001]; 10 mg/kg : significant main effect of frequency [F(9,45)=45.4; p<0.001], significant main effect of time [F(4,20)=4.0; p<0.02], significant frequency×time interaction [F(36,180)=9.0; p<0.001]. All points show mean data for 6 rats, and error bars are omitted for clarity.
Figure 1 also shows the time course of effects produced by vehicle and morphine (1.0-10 mg/kg), and detailed statistical results are provided in the figure legend. Vehicle injection had little effect on ICSS frequency-rate curves, producing significant but only small increases in reinforcement rates at 100 Hz 10 and 100 min after treatment. Morphine produced dose-, time-and frequency-dependent changes in ICSS. At earlier time points (10-30 min), the predominant effect of morphine was a dose-dependent decrease in reinforcement rates maintained by high frequencies of brain stimulation (112-158 Hz). These rate-decreasing effects peaked at 30 min, and the highest dose of 10 mg/kg morphine nearly eliminated responding at 30 min. In addition to these predominant rate-decreasing effects at 10 and 30 min, low doses of 1.0 and 3.2 mg/kg morphine also produced significant but small increases in reinforcement rates at some lower frequencies. After 100 min, the rate-decreasing effects of morphine had dissipated for all but the highest morphine dose, and doses of 3.2 and 5.6 mg/kg morphine produced significant and robust rate-increasing effects at intermediate frequencies of brain stimulation (71-100 Hz), resulting in leftward shifts in frequency-rate curves relative to baseline. After 300 min, none of the morphine doses produced effects significantly different from baseline.
Overall, these results suggested that morphine produced rate-decreasing effects with a relatively short duration of action and rate-increasing effects with a longer duration of action. Data with 10 mg/kg morphine suggested that a period of predominant rate-increasing effects may have been missed between 100 and 300 min. To evaluate this possibility, effects of morphine were determined 180 min after administration of 10 mg/kg morphine in a separate group of 3 rats (Fig. 2). These results confirmed that 10 mg/kg produced a robust facilitation of ICSS at this time point.
Fig 2.
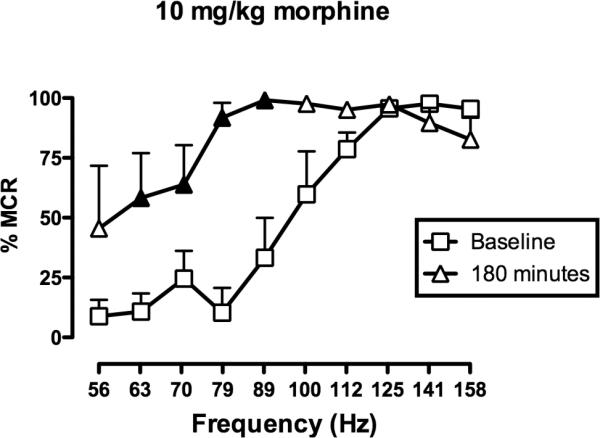
10 mg/kg morphine facilitated ICSS after 180 minutes. Horizonal axis: Frequency of electrical brain stimulation in hertz (log scale). Vertical axis: ICSS rate expressed as percent maximum control rate (%MCR). Filled symbols indicate frequencies at which ICSS rates were significantly higher than baseline as determined by the Holm-Sidak post hoc test following a significant analysis of variance (p<0.05). ANOVA revealed a significant main effect of frequency [F(9,18)=17.3; p<0.001], no significant effect of treatment [F(1,2)=9.9; p=0.09], but a significant frequency×treatment interaction [F(9,18)=4.0; p<0.01]. All points show mean data for 3 rats, and error bars are omitted for clarity.
Effects of chronic morphine
Figure 3 shows effects of chronic morphine on ICSS. Each panel shows the baseline frequency-rate curve determined prior to chronic treatment together with the frequency-rate curves determined 30 min after morphine on the first and seventh day of treatment with each morphine dose (3.2-18 mg/kg/day). Thus, chronic studies evaluated morphine effects at a time (30 min) when initial acute studies revealed primarily rate-decreasing effects of morphine (see above).
Fig 3.

Effects of chronic morphine on ICSS. Horizonal axes: Frequency of electrical brain stimulation in hertz (log scale). Vertical axes: ICSS rate expressed as percent maximum control rate (%MCR). Rats were treated for 28 consecutive days with an ascending sequences of four morphine doses (3.2, 5.6, 10 and 18 mg/kg/day). Each dose was administered for 7 days, and ICSS frequency-rate curves were determined 30 minutes after each injection. Each panel shows frequency-rate data obtained prior to chronic morphine (baseline) and on the 1st and 7th days of treatment with each morphine dose. Filled symbols indicate frequencies at which ICSS rates were significantly lower or higher than baseline, and asterisks indicate frequencies at which ICSS rates on Day 7 were significantly higher than rates on Day 1, as determined by the Holm-Sidak post hoc test following a significant analysis of variance (p<0.05). ANOVA results were as follows: 3.2 morphine: significant main effect of frequency [F(9,36)=28.2; p<0.001], significant main effect of day [F(2,8)=5.3; p<0.05], significant frequency×day interaction [F(18,72)=4.4; p<0.001]; 5.6 morphine: significant main effect of frequency [F(9,36)=18.5; p<0.001], significant main affect of day [F(2,8)=4.5; p<0.05], significant frequency×day interaction [F(18,72)=4.5; p<0.001]; 10 morphine: significant main effect of frequency [F(9,36)=25.2; p<0.001], no significant main affect of day [F(2,8)=3.0; NS], and a significant frequency×day interaction [F(18,72)=3.2; p<0.001]; 18 morphine: significant main effect of frequency [F(9,36)=27.6; p<0.001], no significant main affect of day [F(2,8)=2.2; NS], and a significant frequency×day interaction [F(18,72)=5.9; p<0.001]. All points show mean data for 5 rats, and error bars are omitted for clarity.
Prior to chronic treatment, the maximum control rate was 54.0±14.1 stimulations/trial, and the baseline frequency-rate curve was similar to that described above. The lower doses of 3.2 and 5.6 mg/kg morphine produced only rate-increasing effects and leftward shifts in frequency-rate curves relative to baseline on both Day 1 and Day 7 of treatment. In general, there was little difference in the effects of morphine on Days 1 and 7, although for the initial dose of 3.2 mg/kg morphine, response rates were slightly but significantly higher at frequencies of 70 and 112 Hz on Day 7 than on Day 1. The higher two doses of 10 and 18 mg/kg morphine produced only rate-decreasing effects on the first day of treatment, and these rate-decreasing effects were greater for 18 than for 10 mg/kg morphine. However, after 7 days of treatment, these rate-decreasing effects were no longer apparent, and both doses produced only rate-increasing effects.
To assess the impact of morphine abstinence and spontaneous morphine withdrawal on ICSS, frequency-rate curves were also determined before each daily morphine injection (i.e. 23.5 h after the injection on the previous day). This period of abstinence was associated with a dose-dependent decrease in ICSS. For example, figure 4 (left panel) shows summary data for ICSS 23.5 h after the last injection of each dose, and during the three days after termination of treatment with the highest dose of 18 mg/kg/day morphine. After 3.2 and 5.6 mg/kg morphine, there were slight but nonsignificant decreases in total number of stimulations delivered. This effect, however, was significant after 10 and 18 mg/kg daily morphine administration. Figure 4 (right panel) shows the frequency-rate curve 23.5 h after the last dose of 18 mg/kg morphine. ICSS recovered completely back to baseline levels within three days after termination of treatment with 18 mg/kg morphine. Rats were also observed for signs of somatic withdrawal prior to daily ICSS sessions and for three days after termination of chronic morphine. Chronic morphine produced a dose-dependent decrease in body weight. From a mean±SEM starting weight before chronic treatment of 454.2±15.7 g, morphine produced mean±SEM body weight losses of 1.6±1.1, 2.3±1.1, 4.4±0.9 and 7.2±1.0% after 3.2, 5.6, 10 and 18 mg/kg morphine, respectively. However, this decrease in body weight could not confidently be attributed to withdrawal as distinct from a direct morphine effect, and other somatic signs of opioid withdrawal (diarrhea, teeth chattering and wet-dog shakes) were not observed.
Fig 4.

Effects of morphine abstinence on ICSS. The left panel shows the total number of stimulations per component expressed as a percent of baseline stimulations per component 23.5 hours after the last injection of each dose and during three days after the last dose of 18 mg/kg morphine. Horizonal axis: Dose of morphine (mg/kg) prior to abstinence. Vertical axis: Percent baseline number of stimulations per component. One way ANOVA indicated a significant main effect of abstinence condition [F(6,24)=3.13; p<0.025]. Asterisks indicate conditions under which total number of stimulations was significantly lower than baseline (100%), as determined by the Dunnett's post hoc test. The right panel shows the baseline frequency-rate curve and the frequency-rate curve determined on the day after the last dose of 18 mg/kg morphine. Horizontal axis: frequency of brain stimulation in hertz (log scale). Vertical axis: ICSS rate expressed as percent maximum control response rate (%MCR). Two-way ANOVA indicated a significant main effect of frequency [F(9,36)=57.1; p<0.001], significant main effect of day [F(1,4)=8.3; p<0.05], but no significant frequency×day interaction [F(9,36=1.3; NS]. Filled symbols indicate frequencies at which reinforcement rates were significantly lower than baseline as determined by the Holm-Sidak post hoc test. All bars and symbols show mean data from five rats, and error bars in the right panel show SEM.
Rate-dependent effects of morphine on ICSS
Figure 5 shows the degree to which morphine effects on ICSS varied as a function of baseline ICSS rates. Data are shown for results obtained 30 min after acute or chronic treatment with 3.2, 5.6 and 10 mg/kg morphine to illustrate the breadth of changes in rate-dependency. Table 1 shows results of linear regression analysis applied to the rate-dependency plots shown in Figure 5, and also shows data obtained 100 min after acute administration of each dose. Overall, the extent of rate dependency was influenced by dose, pretreatment time and chronicity of treatment. Effects of the lowest dose of 3.2 mg/kg were not rate-dependent 30 or 100 min after acute administration, but effects were rate-dependent 30 min after the last dose of chronic treatment. Effects of the intermediate dose of 5.6 mg/kg morphine were not rate dependent 30 min after acute treatment, but became rate dependent 100 min after acute treatment and 30 min after the last dose of chronic treatment. Effects of the high dose of 10 mg/kg morphine were rate-dependent under all conditions; however, the nature of that rate dependency changed. Thus, acute 10 mg/kg morphine had little or no effect on low baseline rates of ICSS but decreased high baseline rates of ICSS. Conversely, after chronic administration, morphine increased low baseline rates of ICSS but had little effect on high baseline rates. Overall, morphine exposure produced either by the longer 100 min pretreatment time or by chronic treatment had the general effect of increasing the negative slope, correlation coefficient and statistical significance of rate-dependency plots and shifting these plots vertically upward. After chronic treatment, all morphine doses produced significant rate-dependent effects expressed as an increase in low baseline rates of ICSS and little change in high baseline rates of ICSS.
Figure 5.
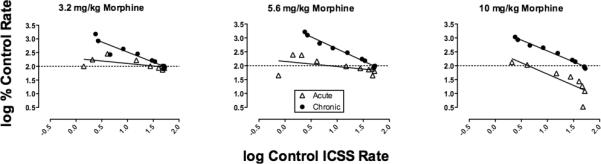
Rate dependency of morphine effects on ICSS. Horizonal axes: Log control ICSS rate (in units of stimulations/frequency trial) at each of the 10 frequencies of brain stimulation. Vertical axes: Log percent control ICSS rate observed 30 min after morphine treatment. Panels show effects of 3.2, 5.6 and 10 mg/kg morphine during the acute dosing phase of the study, and after the 7th daily dose during the chronic dosing phase of the study. All points show mean data for 5-6 rats.
| Morphine Dose (mg/kg) | Acute vs. chronic | Slope | 95% confidence interval | P value | R2 |
|---|---|---|---|---|---|
| 3.2 | Acute (30 min) | - 0.18 | - 0.40 to + 0.04 | 0.09 | 0.36 |
| Acute (100 min) | 0.04 | - 0.65 to + 0.73 | 0.89 | 0.00 | |
| Chronic* | - 0.73 | - 0.95 to - 0.52 | <0.01 | 0.89 | |
| 5.6 | Acute (30 min) | - 0.18 | - 0.45 to + 0.08 | 0.15 | 0.24 |
| Acute (100 min)* | - 0.60 | - 0.79 to - 0.42 | <0.01 | 0.88 | |
| Chronic* | - 0.87 | - 0.95 to - 0.79 | <0.01 | 0.99 | |
| 10 | Acute (30 min)* | - 0.81 | - 1.35 to - 0.28 | 0.01 | 0.70 |
| Acute (100 min)* | - 0.80 | - 0.92 to - 0.76 | <0.01 | 0.97 | |
| Chronic* | - 0.78 | - 0.86 to - 0.71 | <0.01 | 0.99 |
Discussion
This study examined morphine effects on intracranial self-stimulation (ICSS) in rats using a “frequency-rate” procedure, in which a wide range of ICSS rates was maintained by a wide range of brain stimulation frequencies during each daily session. There were three main findings. First, acute morphine produced time-dependent changes in ICSS, such that rate-decreasing effects predominated at earlier time points (10-30 min), whereas rate-increasing effects predominated at later time points (100-180 min). Second, repeated morphine produced tolerance to rate-decreasing effects and unmasked robust rate-increasing and rate-dependent effects 30 min after morphine administration. Finally, withdrawal from repeated morphine produced small but significant decreases in ICSS that recovered over the course of three days after withdrawal from the highest morphine dose. Taken together, these data indicate that morphine dose and pretreatment time, the history of morphine exposure, and baseline ICSS rate are critical determinants of both the magnitude and valence of morphine effects on ICSS.
Effects of acute morphine
The present results agree with previous studies in finding that acute morphine effects on ICSS can display a biphasic time course characterized by an initial decrease followed by a subsequent increase in rates of ICSS (Adams et al., 1972; Lorens and Mitchell, 1973; Bermudez-Rattoni et al., 1983; Reid, 1987). A similar biphasic time course was also produced by the mu agonist heroin (Koob et al., 1975). In these earlier studies, baseline responding was maintained at relatively stable rates by constant intensities and frequencies of electrical stimulation. The present study extends these findings by using a “frequency-rate” procedure that generated a wide range of baseline response rates to systematically examine the rate-dependency of acute morphine effects. This procedure confirmed that early rate-decreasing effects were greatest for high baseline response rates maintained by high frequencies of stimulation, whereas later rate-increasing effects were most prominent for low to intermediate response rates maintained by low to intermediate frequencies of stimulation. Additional discussion of rate dependence is provided below.
In the present study, low morphine doses (1-3.2 mg/kg) produced small but significant increases in low ICSS rates during the first 30 min after morphine injection. This agrees with the finding that similarly low morphine doses at similarly short pretreatment times produced facilitation of ICSS in some (Kornetsky and Esposito, 1979; Carlezon and Wise, 1993; Jha et al., 2004) but not all (Stratmann and Craft, 1997; Pereira Do Carmo et al., 2009b) studies that focused on low baseline ICSS rates maintained by threshold frequencies or intensities of stimulation. However, any facilitation of ICSS observed early in the time course of low-dose acute morphine is weaker and less consistent than the more robust facilitation of ICSS observed later in the time course of larger morphine doses (present study; Easterling and Holtzman, 1997; O'Neill and Todtenkopf, 2010).
The biphasic time course of acute morphine effects on rates of ICSS is similar to the biphasic time course of morphine effects on rates of locomotor activity in rats (Vasko and Domino, 1978; Craft et al., 2006). For example, morphine (1–10 mg/kg sc) produced initial dose-dependent decreases (approximately 30–90 min post injection) followed by later increases (approximately 2–5 h post injection) in horizontal activity in male rats relative to their saline-treated controls (Craft et al., 2006). Moreover, the time course of morphine-induced depression of ICSS and locomotion corresponds to the time course of morphine-induced depression of some other behaviors, such as thermal nocifensive behaviors in tail-flick and hot-plate assays (Cicero et al., 1996, 1997), and a stimulation of pain-related behaviors may emerge after antinociceptive effects have dissipated (i.e. opioid-induced hyperalgesia) (Chu et al., 2008). Taken together, these findings provide evidence of relatively broad behavioral depressant effects of acute morphine that may be followed by later behavioral stimulant effects. Factors that underlie the emergence of morphine-induced stimulant effects are not known. One possibility is that acute tolerance develops to behavioral depressant effects, thereby unmasking behavioral stimulant effects. Data presented below from repeated-dosing studies support this possibility.
Effects of chronic morphine
In the present study, repeated morphine treatment reduced the initial rate-decreasing effects of morphine while producing earlier expression of rate-increasing effects. The onset of tolerance to rate-decreasing effects of morphine appeared to be rapid. Informal analysis of data obtained during the acute dosing phase of the study did not reveal a systematic effect of dose order on rate-increasing vs. rate-decreasing morphine effects (data not shown); however, ICSS was not significantly altered 30 min after 3.2 mg/kg morphine during the acute phase of the study but was significantly facilitated 30 min after administration of this same dose on Day 1 of the chronic dosing phase of the study. This suggests that morphine exposure associated with the acute dosing phase of the study was sufficient to produce some degree of tolerance to the rate-decreasing effects of morphine, and the extent of this tolerance became more pronounced during the chronic dosing phase. This agrees with effects of repeated morphine from studies that used simpler ICSS procedures in which relatively constant baseline response rates were maintained by constant magnitudes of brain stimulation (Lorens and Mitchell, 1973; Reid, 1987). For example, Lorens and Mitchell (1973) administered morphine doses of 5, 10 or 20 mg/kg daily for five days in rats trained to respond for a single magnitude of brain stimulation. On the first day of treatment, all three doses produced initial rate-decreasing effects followed by later rate-increasing effects. However, as early as the third day of treatment, doses of 5 and 10 mg/kg morphine ceased to produce rate-decreasing effects, and rate-increasing effects occurred with an earlier onset and similar duration of action. The present results also agree with studies using more sophisticated procedures that showed reductions in initial rate-decreasing effects and/or increased expression of rate-increasing effects during repeated morphine treatment (Carlezon and Wise, 1993; Easterling and Holtzman, 1997; Craft et al., 2001). The present study adds to this literature by evaluating effects of a broad range of morphine doses on responding maintained across a broad range of ICSS rates. Finally, the reduction in morphine-induced rate-decreasing effects produced by repeated morphine in assays of ICSS is qualitatively similar to the rapid reduction in morphine-induced rate-decreasing effects that develops in assays of locomotor activity (Babbini and Davis, 1972; Vasko and Domino, 1978; Smith et al., 2009).
A parsimonious interpretation of these data is that morphine effects on ICSS reflect an integration of rate-decreasing and rate-increasing effects, and that tolerance to rate-decreasing effects results in an unmasking of rate-increasing effects. The mechanisms responsible for this tolerance are not known and may include processes of pharmacodynamic or behavioral tolerance (Smith, 1979; Negus et al., 2010; Foltin, 2010). For example, it is well-established that tolerance can develop at different rates to different morphine effects, and this differential tolerance to behavioral effects is accompanied by differential tolerance to intracellular signaling in different mu opioid receptor populations in brain. Thus, chronic morphine treatment selectively decreased mu agonist-stimulated G-protein phosphorylation in brainstem nuclei (including dorsal raphe nucleus and locus coeruleus), but not in forebrain structures (including nucleus accumbens and amygdala) thought to contribute to the stimulant effects of opioids (Sim et al., 1996). Overall, mu receptor populations mediating abuse-related facilitation of ICSS may be more resistant to tolerance during chronic opioid exposure than mu receptor populations mediating rate suppression. Increased expression of morphine-induced stimulant effects may involve not only a resistance to tolerance but also a sensitization of neural circuits that mediate these effects. For example, a regimen of repeated morphine administration similar to that used here increased expression of the GluR1 subunit of AMPA glutamate receptors in rat ventral tegmental area (VTA), an effect that could increase sensitivity of VTA dopaminergic neurons to glutamatergic inputs (Fitzgerald et al., 1996).
In addition to producing tolerance to morphine-induced rate-decreasing effects, repeated morphine also produced dependence as indicated by dose-dependent decreases in ICSS during spontaneous morphine withdrawal. These findings agree with other studies reporting reductions in ICSS during spontaneous or antagonist-precipitated morphine withdrawal (Schaefer and Michael, 1983; Easterling and Holtzman, 1997; Liu and Schulteis, 2004). The absence of clear somatic withdrawal signs in this study further suggests that ICSS may be more sensitive than commonly assessed somatic signs to the impact of opioid withdrawal.
Rate-dependency of morphine effects
A key finding of this study was that repeated morphine treatment increased the rate dependency of morphine effects on ICSS. Early in the time course after acute administration, morphine effects on ICSS displayed rate-dependence only insofar as the highest morphine dose (10 mg/kg) decreased high ICSS rates maintained by high frequencies of stimulation more than it decreased lower ICSS rates maintained by lower frequencies of stimulation. This generally agrees with the rate-dependence of acute morphine effects in previous studies using other schedules of reinforcement and other consequent stimuli. For example, morphine primarily decreased food-maintained response rates maintained under different schedules of reinforcement in rats, and these effects were rate-dependent insofar as high rates were decreased more than low rates (Thompson et al., 1970). Acute morphine has been reported to increase low response rates maintained during the early segments of fixed-interval schedules or under a differential-reinforcement-of-low-rates-of-responding schedule (McMillan and Morse, 1967; Ford and Balster, 1976), although these rate-increasing effects may be dependent on the consequent stimulus (McKearney, 1980). In the present study, low doses of acute morphine also occasionally increased response rates, but these effects were not rate dependent and were most evident for intermediate ICSS rates maintained by intermediate frequencies of brain stimulation. Morphine exposure associated with longer pretreatment times or with repeated morphine treatment produced qualitative and quantitative changes in the rate dependency of morphine effects, increasing the correlation coefficients and slopes of rate-dependency plots and shifting these plots vertically upward such that morphine primarily increased low rates of ICSS rather than decreasing high rates of ICSS. In this regard, morphine effects after repeated morphine qualitatively resembled rate-dependent effects of CNS stimulants such as amphetamine (Sanger and Blackman, 1976; Pereira Do Carmo et al., 2009a). To our knowledge, this is the first study to report a change in rate-dependent effects of morphine or any other mu opioid receptor agonist produced by repeated drug treatment. Moreover, insofar as stimulant-like and rate-dependent facilitation of ICSS is predictive of abuse liability (Reid, 1987), these findings are consistent with the hypothesis that the abuse liability of morphine increases with repeated exposure (Negus 2006).
Acknowledgements
We appreciate the technical assistance of Ember Morrissey. This research was supported by grants R01-NS070715, and Jordan University of Science and Technology, Irbid, Jordan. The authors have no conflict of interest to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams WJ, Lorens SA, Mitchell CL. Morphine enhances lateral hypothalamic self-stimulation in the rat. Proc Soc Exp Biol Med. 1972;140:770–1. doi: 10.3181/00379727-140-36549. [DOI] [PubMed] [Google Scholar]
- Babbini M, Davis WM. Time-dose relationships for locomotor activity effects of morphine after acute or repeated treatment. Br J Pharmacol. 1972;46:213–24. doi: 10.1111/j.1476-5381.1972.tb06866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez-Rattoni F, Cruz-Morales S, Reid LD. Addictive agents and intracranial stimulation (ICS): novel antagonists and agonists of morphine and pressing for ICS. Pharmacol Biochem Behav. 1983;18:777–84. doi: 10.1016/0091-3057(83)90022-9. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2:2987–95. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Wise RA. Morphine-induced potentiation of brain stimulation reward is enhanced by MK-801. Brain Res. 1993;620:339–42. doi: 10.1016/0006-8993(93)90177-o. [DOI] [PubMed] [Google Scholar]
- Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain. 2008;24:479–96. doi: 10.1097/AJP.0b013e31816b2f43. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Nock B, Meyer ER. Gender-related differences in the antinociceptive properties of morphine. J Pharmacol Exp Ther. 1996;279:767–73. [PubMed] [Google Scholar]
- Cicero TJ, Nock B, Meyer ER. Sex-related differences in morphine's antinociceptive activity: relationship to serum and brain morphine concentrations. J Pharmacol Exp Ther. 1997;282:939–44. [PubMed] [Google Scholar]
- Craft RM, Stoffel EC, Stratmann JA. Effects of chronic morphine treatment on responding for intracranial stimulation in female versus male rats. Exp Clin Psychopharmacol. 2001;9:198–208. doi: 10.1037//1064-1297.9.2.198. [DOI] [PubMed] [Google Scholar]
- Craft RM, Clark JL, Hart SP, Pinckney MK. Sex differences in locomotor effects of morphine in the rat. Pharmacol Biochem Behav. 2006;85:850–8. doi: 10.1016/j.pbb.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterling KW, Holtzman SG. Intracranial self-stimulation in rats: sensitization to an opioid antagonist following acute or chronic treatment with mu opioid agonists. J Pharmacol Exp Ther. 1997;281:188–99. [PubMed] [Google Scholar]
- Esposito RU, Perry W, Kornetsky C. Effects of d-amphetamine and naloxone on brain stimulation reward. Psychopharmacology (Berl) 1980;69:187–91. doi: 10.1007/BF00427648. [DOI] [PubMed] [Google Scholar]
- Fitzgerald LW, Ortiz J, Hamedani AG, Nestler EJ. Drugs of abuse and stress increase the expression of GluR1 and NMDAR1 glutamate receptor subunits in the rat ventral tegmental area: common adaptations among cross-sensitizing agents. J Neurosci. 1996;16:274–82. doi: 10.1523/JNEUROSCI.16-01-00274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin R. Behavioral tolerance. In: Stolerman I, editor. Encyclopedia of Psychopharmacology. Springer-Verlag; Berlin: 2010. pp. 214–216. [Google Scholar]
- Ford RD, Balster RL. Schedule-controlled behavior in the morphine-dependent rat. Pharmacol Biochem Behav. 1976;4:569–73. doi: 10.1016/0091-3057(76)90199-4. [DOI] [PubMed] [Google Scholar]
- Gallistel CR, Freyd G. Quantitative determination of the effects of catecholaminergic agonists and antagonists on the rewarding efficacy of brain stimulation. Pharmacol Biochem Behav. 1987;26:731–41. doi: 10.1016/0091-3057(87)90605-8. [DOI] [PubMed] [Google Scholar]
- Jha SH, Knapp CM, Kornetsky C. Effects of morphine on brain-stimulation reward thresholds in young and aged rats. Pharmacol Biochem Behav. 2004;79:483–90. doi: 10.1016/j.pbb.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Koob GF, Spector NH, Meyerhoff JL. Effects of heroin on lever pressing for intracranial self-stimulation, food and water in the rat. Psychopharmacologia. 1975;42:231–4. doi: 10.1007/BF00421261. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU. Euphorigenic drugs: effects on the reward pathways of the brain. Fed Proc. 1979;38:2473–6. [PubMed] [Google Scholar]
- Liu J, Schulteis G. Brain reward deficits accompany naloxone-precipitated withdrawal from acute opioid dependence. Pharmacol Biochem Behav. 2004;79:101–8. doi: 10.1016/j.pbb.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Lorens SA, Mitchell CL. Influence of morphine on lateral hypothalamic self-stimulation in the rat. Psychopharmacologia. 1973;32:271–7. doi: 10.1007/BF00422149. [DOI] [PubMed] [Google Scholar]
- McKearney JW. Fixed ratio schedules of food presentation and stimulus shock termination: effects of d-amphetamine, morphine, and clozapine. Psychopharmacology (Berl) 1980;70:35–9. doi: 10.1007/BF00432367. [DOI] [PubMed] [Google Scholar]
- McMillan DE, Morse WH. Some effects of morphine and morphine antagonists on schedule-controlled behavior. J Pharmacol Exp Ther. 1967;157:175–84. [PubMed] [Google Scholar]
- Negus SS. Choice between heroin and food in non-dependent and heroin-dependent rhesus monkeys: Effects of naloxone, buprenorphine and methadone. J Pharmacol Exp Ther. 2006;317:711–23. doi: 10.1124/jpet.105.095380. [DOI] [PubMed] [Google Scholar]
- Negus SS, Selley DE, Sim-Selley LJ. Pharmacodynamic tolerance. In: Stolerman I, editor. Encyclopedia of Psychopharmacology. Springer-Verlag; Berlin: 2010. pp. 994–997. [Google Scholar]
- O'Neill KS, Todtenkopf MS. Using a rate-frequency curve method to assess the rewarding properties of morphine in the intracranial self-stimulation paradigm in rats. J Neurosci Methods. 2010;189:75–9. doi: 10.1016/j.jneumeth.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–7. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Pereira Do Carmo G, Folk JE, Rice KC, Chartoff E, Carlezon WA, Jr, Negus SS. The selective non-peptidic delta opioid agonist SNC80 does not facilitate intracranial self-stimulation in rats. Eur J Pharmacol. 2009a;604:58–65. doi: 10.1016/j.ejphar.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira Do Carmo G, Stevenson GW, Carlezon WA, Negus SS. Effects of pain- and analgesia-related manipulations on intracranial self-stimulation in rats: further studies on pain-depressed behavior. Pain. 2009b;144:170–7. doi: 10.1016/j.pain.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid LD. Tests involving pressing for intracranial stimulation as an early procedure for screening the likelihood of addiction of opioids and other drugs. In: Bozarth MJ, editor. Methods of Assessing the Reinforcing Properties of Abused Drugs. Springer-Verlag; Berlin: 1987. pp. 391–420. [Google Scholar]
- Sanger DJ, Blackman DE. Rate-dependent effects of drugs: a review of the literature. Pharmacol Biochem Behav. 1976;4:73–83. doi: 10.1016/0091-3057(76)90178-7. [DOI] [PubMed] [Google Scholar]
- Schaefer GJ, Michael RP. Morphine withdrawal produces differential effects on the rate of lever-pressing for brain self-stimulation in the hypothalamus and midbrain in rats. Pharmacol Biochem Behav. 1983;18:571–7. doi: 10.1016/0091-3057(83)90283-6. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Selley DE, Dworkin SI, Childers SR. Effects of chronic morphine administration on mu opioid receptor-stimulated [35S]GTPgammaS autoradiography in rat brain. J Neurosci. 1996;16:2684–92. doi: 10.1523/JNEUROSCI.16-08-02684.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD. Behavioral influences on tolerance to the effects of morphine on schedule-controlled behavior. Psychopharmacology. 1979;66:105–7. doi: 10.1007/BF00431998. [DOI] [PubMed] [Google Scholar]
- Smith MA, Greene-Naples JL, Lyle MA, Iordanou JC, Felder JN. The effects of repeated opioid administration on locomotor activity: I. Opposing actions of mu and kappa receptors. J Pharmacol Exp Ther. 2009;330:468–75. doi: 10.1124/jpet.108.150011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellar JR, Stellar E. The Neurobiology of Motivation and Reward. Springer-Verlag; New York: 1985. [Google Scholar]
- Stratmann JA, Craft RM. Intracranial self-stimulation in female and male rats: no sex differences using a rate-independent procedure. Drug Alcohol Depend. 1997;46:31–40. doi: 10.1016/s0376-8716(97)00040-9. [DOI] [PubMed] [Google Scholar]
- Thompson T, Trombley J, Luke D, Lott D. Effects of morphine on behavior maintained by four simple food-reinforcement schedules. Psychopharmacologia. 1970;17:182–92. doi: 10.1007/BF00402708. [DOI] [PubMed] [Google Scholar]
- Vasko MR, Domino EF. Tolerance development to the biphasic effects of morphine on locomotor activity and brain acetylcholine in the rat. J Pharmacol Exp Ther. 1978;207:848–58. [PubMed] [Google Scholar]
- Wise RA. Drug-activation of brain reward pathways. Drug Alcohol Depend. 1998;51:13–22. doi: 10.1016/s0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]