Abstract
Complement, natural killer T (NKT) and natural killer (NK) cells play critical roles in the first line defense against pathogens. Functional roles for both C5a receptors, i.e. C5aR and C5L2 in sepsis have been demonstrated. However, the role of C5a in innate lymphocyte activation during sepsis remains elusive. Here, we show that naïve NKT and NK cells already express high levels of C5aR and minor levels of C5L2 mRNA, but no protein. Upon E. coli-induced sepsis, we found C5aR surface expression on subpopulations of NKT and NK cells suggesting rapid translation into C5aR protein upon bacterial encounter. Importantly, significantly increased survival in the absence of C5aR, NKT and NK cells, but not of C5L2, was associated with reduced IFN-γ and TNF-α serum levels. Sepsis induction in C5aR+/C5aR− mixed bone marrow chimeras identified cognate engagement of C5aR on NKT cells as an important factor for the recruitment of NKT cells. Further, we found synergistic interaction between C5aR and Toll-like receptors enhancing the production of TNF-α and IFN-γ from NKT and NK cells in co-cultures with DCs. Our results identify C5aR activation as a novel pathway driving detrimental effects of NKT and NK cells during early experimental sepsis.
Keywords: cytokines, C5a, C5L2, NKT cells, NK cells, sepsis, E. coli
Introduction
Activation of NKT and NK cells is observed upon infection with a broad range of pathogens including bacteria, viruses, parasites and fungi. Cytokines, particularly IL-12 and IL-18 released from toll-like receptor (TLR) activated APCs during infection contribute to the activation of both cell populations (1, 2). However, while NKT cells recognize endogenous mammalian or exogenous bacterial glycosphingolipids (GSLs) presented by CD1d on APCs (3) through their conserved semi-invariant mouse TCR, NK cell activation involves activating and inhibitory receptors that recognize “self”, “missing self” and “altered or stressed self” (4).
Although NKT and NK cells belong to distinct lineages and show differences in their mode of activation, they present striking similarities which include the ability to release massive amounts of cytokines with extreme celerity and without prior sensitization. Like NK cells, NKT cells constitutively express mRNA but not protein for IFN-γ, a hallmark of their poised effector stage (5). Unlike NK cells, however, NKT cells also produce TNF-α, IL-4 and IL-13 and the importance of Th1 and Th2 cytokines has been demonstrated in vivo, in conditions where NKT cells either improved or aggravated disease (6, 7). NKT-deficient CD1d- or Jα18- knockout mice suffer from impaired early IFN-γ secretion, correlating during some infections with reduced anti-microbial defense (6, 7), while ablation of NK cells impairs immune responses preferentially to Shigella flexneri and Mycobacterium tuberculosis (8, 9).
NKT cells interact with and possibly regulate NK cells during infections in multiple ways (7). NK cell activation contributes to the local and systemic inflammation during lipopolysaccharide (LPS)-induced shock (10), whereas NKT cells may even exhibit anti-inflammatory effects (11). The rapid cross-activation of NK cells upon NKT cell activation (12) implies that innate lymphocyte responses can augment each other dramatically under certain circumstances and in response to defined stimuli. However, the engagement of different receptors may contribute to the opposing effects of NKT cells in the respective models and may interfere with NK cell activation in various ways. Thus, these receptors and their respective effects on NKT and NK cells need to be identified and their mode of action on both cell populations delineated.
Sepsis is associated with a strong activation of the complement system and the generation of the anaphylatoxins (ATs) C3a and C5a in mice and humans(13). Both ATs exert their biologic functions through binding and activation of their cognate G-protein-coupled receptors, i.e. the C3a receptor (C3aR) and the C5a receptor (C5aR/CD88). C5a and its primary degradation product, C5adesArg, can further bind to another seven-transmembrane receptor, C5a receptor-like 2 (C5L2), which is uncoupled from G-proteins (13). In CLP-induced septic peritonitis, functional roles for both C5a receptors have been demonstrated (14). However, as this is a model involving intestinal flora, the effects of defined bacterial species on the expression of C5aR and C5L2 and the subsequent effects of C5aR and C5L2 expression on the control of bacterial infection and the release of cytokines remain unknown. Up to now, the detrimental effects of C5a in sepsis have mainly been attributed to the paralysis of neutrophils (15). However, other cell populations may be involved in C5a mediated effects. As innate lymphocytes are not only a major cellular source of various cytokines, but also shape the subsequent adaptive immune response we have assessed in this study the role of C5a in the activation of NK and NKT cells in an E. coli-induced sepsis model.
We observed that NKT and NK cells from naïve mice already express both C5a receptors, C5aR and C5L2 at the mRNA, but not at the protein level. In vivo exposure to E. coli resulted in the rapid surface expression of C5aR protein on subpopulations of NKT and NK cells, which was associated with an enhanced expression of the early activation marker CD69.
C5aR deficiency resulted in a decreased expression of Nkp46 on NK cells and in a reduced release of IFN-γ and TNF-α by NKT and NK cells along with an impaired recruitment of NKT and NK cells to the site of infection. Animal survival in response to E. coli-induced sepsis was significantly higher in the absence of C5aR, NKT and NK cells. Improved survival correlated with reduced IFN-γ and TNF-α levels suggesting that signaling through C5aR during sepsis contributes to the detrimental effects of NKT and NK cells. Sepsis induction in C5aR+/C5aR− mixed bone marrow chimeras identified cognate engagement of C5aR on NKT cells as a critical factor for NKT cell recruitment, while NK cell recruitment required preferentially C5aR engagement on DCs.
Materials and Methods
Mice
C57BL/6 mice (B6) were purchased from the Jackson laboratories. Ly5.1 (CD45.1+) and C5aR−/− (CD45.2+) mice were maintained in the animal facility of Cincinnati Children’s Hospital Medical Center. C5L2−/− mice were kindly provided by Dr. Craig Gerard (Harvard Medical School). All mice were raised in a specific pathogen-free environment according to the respective Institutional Animal Care and Use Committee guidelines.
Bacterial strains and live infection experiments
E. coli (ATCC 25922) were grown overnight in tryptic soy broth, diluted in fresh medium, grown for 8 h at 37°C (OD 0.5), washed and diluted in PBS. 1×109 E. coli CFUs were injected i.p. (100μl).
Purification of NKT and NK cells and costimulation assays
Lymphocyte preparations, cell staining and sorting with CD1d-αGalCer tetramers were performed as described (3, 16). Briefly, spleen cells were incubated with CD1d-αGC tetramers for 2 hrs at room temperature. Other mAbs, specific for either TCRβ, C5aR, or CD69 were then added and cells were incubated for 30 min on ice. Spleen cells, staining double positive for TCRβ and α-GalCer tetramer (NKT cells) or for Nkp46 and NK1.1 (NK cells) were purified using a FACSAria II (BD Bioscience) cell sorter resulting in purities > 98% and >95%, respectively. APCs were BM-derived GM-CSF (100 ng/ml, R&D Systems) cultured DCs (2.5×105/200μl well). Spleen cells and co-cultures of DCs with purified NKT or NK cells were stimulated with recombinant human C5a (100nM) (Sigma), 100ng/ml Pam3CSK4, poly IC, LPS, STA-FLA, FSL-1 or ssRNA-40 each, 108 heat-killed Listeria monocytogenes (HKLM) and 5μM ODN1826 according to the manufacturer’s instructions (InVivogen) for 48 hrs. Concentrations of IFN-γ and TNF-α in the supernatants were measured using the respective ELISA kits (BD Bioscience).
cDNA synthesis and RT-qPCR for mRNA expression
cDNA was synthesized using a first strand cDNA synthesis kit for RT-PCR (Roche, Indianapolis, IN) following the manufacturer’s instructions, with slight modifications where necessary. Quantitative real-time PCR for C5aR and C5L2 (FWD: 5′-CACACCACCAGCGAGTATTATG-3′; rev: 5′-AGCACAAGCAGGACTATCAGG-3′) was performed as described (17–18). The primers used for the analysis of TLR expression were as follows: TLR2 (FWD: 5′-AGCTCTGATGCCAGGCTCCGTTC-3′; rev: 5′-CTCG CTTAAGTGAAGAGTC AGGTGATGG-3′), TLR3 (Fwd: 5′-AACATTTGTGTCACT TGCTCATTCTCCC-3′, rev: 5′-TGTGTCTATTTCCTTGAA ACCAAGAATCCG-3′), TLR4 (FWD: 5′-TTCAGAACTTCAGTGGCTGGATTTA TCC-3′; rev: 5′-GAGGTCT AAGTGTCTCAG GCTGTTTGTTCC-3′), TLR7 (FWD: 5′-ATCAACCACATACCAAG CATCTCT CCAG- 3′; rev: 5′-TAGTGCCAAGGTCA AGAACTTCCAGC-3′), TLR1 (FWD: 5′-ACGTCCTATACCCATGTGGCAATGC TC-3′; rev: 5′-ACAACTTGATGTA TCG ACAAAGCCTTCAGAG-3′), TLR5 (FWD: 5′-TCTGTTCCACCAAGACAAGAA GA ATCTGC-3′; rev: 5′-AGTTCCTTGTGATG TCCACCGTCCAG-3′), APPCT-1F: 5′-GAATTCCGACATGACTCAGG-3′ and APPCT-1R: 5′-GTTCTGCTGCATCTTGG ACA-3′).
Mixed bone marrow (BM) radiation chimeras
A mixture of 5×106 CD45.1+ C5aR+/+ and 5×106 CD45.2+ C5aR−/− BM cells was injected i.v. into 7–12 week old CD45.1+ C5aR+/+ mice (B6 background) which had been 900 Rad-irradiated with a cesium source (Gammacell 40, Nordion Int. Inc. Ontario, Canada) one day before. Similarly, mixed BM chimeras generated from CD45.1+ C5aR+/+ and CD45.2+ C5aR−/− BM cells as well as CD45.1+ C5L2+/+ and CD45.2+ C5L2−/− BM cells were prepared. The cell reconstitution of the mixed BM chimeras was determined 6–8 weeks after BM injection by FACS analysis of blood samples using anti-CD45.1 and anti-CD45.2 antibodies.
Flow cytometry
CD1d-lipid tetramers were prepared as described (19). Anti-NK1.1, -Nkp46, -NKG2D, -Ly49H, -Ly49D, -CD69, -TCRβ, -CD45.1, -CD45.2 and -CD69 antibodies were purchased from eBioscience. Anti-C5aR (clone 20/70) and control Ig were obtained from Serotech. Cells were analyzed on an LSR II (BD Biosciences) with FlowJo software.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism (version 4.00). Statistical significance of the data was analyzed by either one-way Analysis Of Variance (one-way ANOVA), unpaired student’s t-test, Mantel-Cox test or Mann-Whitney test as indicated in the respective experiments. A sample size of at least 3 (n=3) was used for each group in a given experiment, and a p-value of less than 5% (p<0.05; *) or less than 1% (p<0.01, **) was considered as significant to accept the alternate hypothesis.
Results
Improved survival of C5aR- and NK-/NKT- cell deficient mice correlates with reduced IFN-γ and TNF-α serum levels
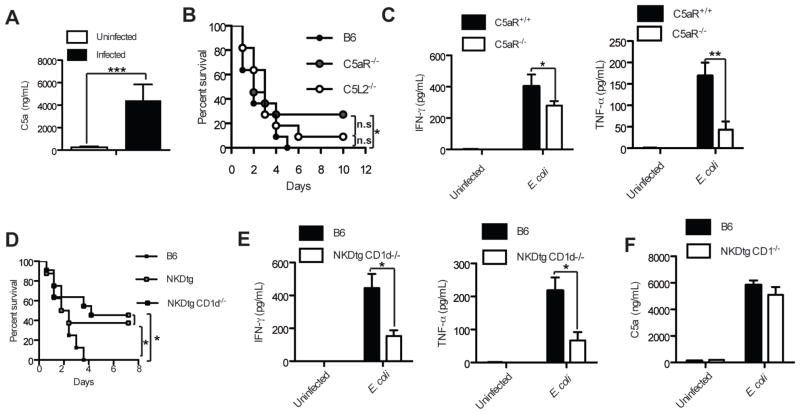
Complement factors including C5a are released during multiple inflammatory conditions. In order to investigate the release of C5a during E. coli - induced sepsis, we obtained plasma from mice infected i.p. with 1×109 E. coli CFUs and from uninfected control mice. Following an initial peak of C5a within the first minutes after infection (not shown), high C5a levels were detected one hour later (Fig. 1A). Genetic deletion of C5aR resulted in improved survival in response to E. coli from 0% to 30% (Fig. 1B) similar to what had been reported in a CLP-induced sepsis model (14). The absence of C5L2 improved survival only marginally (Fig. 1B). Improved survival in C5aR−/− mice correlated with reduced serum levels of cytokines including IFN-γ and TNF-α (Fig. 1C). A detrimental role has been attributed to both cytokines during sepsis (20). Further, NK and NKT cells along with myeloid cells are the major cellular sources for these two cytokines during the initial immune response (21). Genetic deletion of NK and NKT cells improved the survival of mice up to 50% (Fig. 1D), which was associated with decreased serum IFN-γ and TNF-α levels (Fig. 1E). The absence of NKT and NK cells did not affect C5a plasma levels (Fig. 1F) excluding a role of both cell populations in modulating the generation of C5a. Collectively, these data suggest that (i) NKT and NK cells play an important role in the pathogenesis of E. coli-mediated sepsis; and (ii) C5a contributes to the activation of NKT and NK cells and subsequent cytokine production during E. coli-mediated sepsis.
Figure 1.
Complement receptor C5aR, NK and NKT cells augment IFN-γ and TNF-α responses and decrease survival of mice during E. coli-sepsis. (A) Rapid release of C5a upon i.p. E. coli-infection. C5a levels were determined in plasma 1h after infection; n=6. (B) Absence of C5aR improves survival. The survival of B6, B6 C5L2−/− and B6 C5aR−/− mice was followed over time; n=11/group. (C) Decreased inflammatory serum cytokines in C5aR-deficient mice. Serum levels of IFN-γ and TNF-α from B6 mice were determined 4 h after infection; n=8–9/group. (D) Lack of NK and NKT cells improves survival. The survival of B6, NK cell deficient NKDtg and NK and NKT cells deficient NKDtg CD1d−/− mice was followed over time; n=13–14/group. (E) Decreased inflammatory serum cytokines in NKDtg and NKDtg CD1d−/− mice. Serum levels of IFN-γ and TNF-α from B6 and NKDtg CD1d−/− mice were determined 4h after infection; n=4/group. (F) Genetic deletion of NKT and NK cells does not affect the release of C5a. C5a levels from B6 and NKDtg CD1d−/− mice were determined in plasma 1h after infection; n=5/group. Statistical significance was calculated using either the student’ t-test (in A, C, E and F) or the Mantel-Cox test (in B and D). All experiments were either repeated either once or twice with similar results.
Differential regulation of C5aR and C5L2 expression in NKT and NK cells upon bacterial encounter
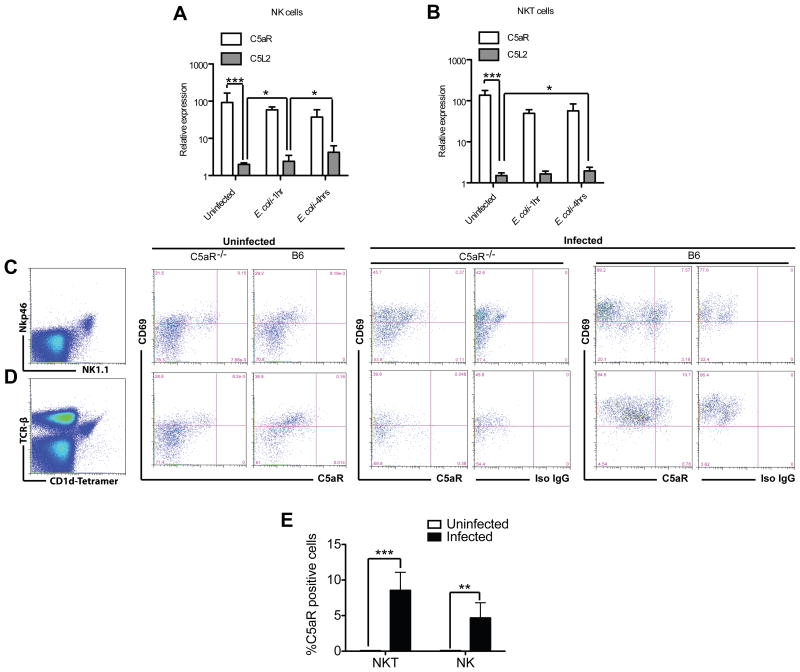
C5aR expression has been described on various cell populations including α/β and γ/δ T cells (22, 23). When we assessed C5aR and C5L2 expression on splenic NKT and NK cells from uninfected mice, we detected already multiple C5aR mRNA but only few C5L2 copies (Fig. 2, A and B). Surprisingly, although C5aR preferentially modulated the survival rates (Fig. 1B), C5aR mRNA levels remained unchanged upon exposure to E. coli, whereas C5L2 mRNA copy numbers slightly, but significantly increased.
Figure 2.
Regulation of C5aR expression on NK and NKT cells by infection. (A+B) NKT and NK cells express higher levels of C5aR mRNA than of C5L2 mRNA. Total RNA of TCRβ+ CD1d-tetramer+ NKT (A) and Nkp46+ NK1.1+ NK (B) cells (purity >98%) from naive mice was analyzed for their C5aR and C5L2 expression profile by RT-PCR as well as one and four hrs after E. coli-infection (one out of two experiments containing three individual mice each). Note the 10–50 time higher C5aR copy numbers in uninfected and infected mice despite the lack of regulation of C5aR expression. Statistical significance was calculated using ANOVA. The experiment was repeated once with similar results. (C-D) C5aR protein is expressed on the cell surface 24h after infection. Analysis of cell surface C5aR expression on NKT (C) and NK cells (D) by flow cytometry. Shown is a typical expression profile from one uninfected and one infected C5aR−/− and one wt B6 mouse out of 4. (E) Frequency of C5aR+ NKT and NK cells in uninfected and E. coli–infected mice (n=4). The experiment was repeated once with similar results.
As the survival of the mice was improved in the absence of C5aR, NKT and NK cells and as substantial more C5aR than C5L2 mRNA was detected in NKT and NK cells, C5a likely acts on NKT and NK cells preferentially via the engagement of C5aR. Therefore, we tested the regulation of C5aR protein expression upon infection by flow cytometry. Gating on CD1d-αGalCer tetramer+ TCRβ+ (Fig. 2C) and NK1.1+ Nkp46+ (Fig. 2D) cells in the spleen, we observed that only very few, if any NKT or NK cells expressed C5aR protein in naïve, uninfected mice (Fig. 2C–E). However, the number of C5aR positive NKT and NK cells increased 24h after infection (Fig. 2C–E), indicating that C5aR mRNA is rapidly translated into C5aR protein.
C5aR deficiency reduces the activation and the recruitment of NKT and NK cells in response to E. coli
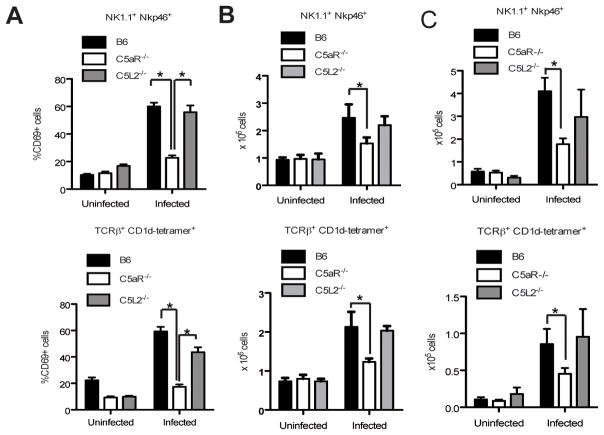
As shown above, the number of NKT and NK cells responding to C5a increases during infection. In order to investigate if C5aR plays a role in the activation of both cell populations, we tested the expression of CD69, an early activation marker, on NKT and NK cells from wild type (wt), C5aR−/− and C5L2−/− mice four hours after infection (Fig. 3A). As expected, CD69 was upregulated on NK (Fig. 3A, upper panel) and NKT cells (Fig. 3A, lower panel) from infected wt mice. In contrast, CD69 upregulation was impaired on NKT and NK cells from infected C5aR−/− mice, whereas C5L2 deletion had no impact on NKT and NK cell activation (Fig. 3A), as evidenced by CD69 expression levels similar to the ones observed in wt mice. The observation that C5aR is critical for early NKT and NK cell activation and the fact that C5aR expression was comparable between NKT and NK cells from wt and C5L2−/− mice (6.51±5.17% vs 8.84±6.83% for NKT and 4.86±3.71% vs 4.22±3.31% for NK cells in infected mice) suggested that NKT and NK cell activation by C5a is predominantly regulated through the engagement of C5aR and not C5L2, at least in the initial response phase to E. coli.
Figure 3.
C5aR but not C5L2 drives the early activation and recruitment of NKT and NK cells. (A) Analysis of CD69 expression on NK (upper panel) and NKT cells (lower panel) in the spleen performed four hrs after infection; n=9/group. The experiment was repeated three times with similar results. (B) Analysis of NK (upper panel) and NKT (lower panel) cell recruitment to the spleen performed 20 hrs after infection; n=4/group. The experiment was repeated once with similar results. (C) Two days after i.p. E. coli-infection, absolute NK (upper panel) and NKT (lower panel) cell numbers within the peritoneal cavity were determined; n=7/group. The experiment was repeated once with similar results. Statistical significance in (A-C) was calculated using ANOVA.
As C5a is a well-known chemoattractant for leukocytes (13), we next evaluated the numbers of NKT and NK cells in the spleens, livers, peritoneal cavities and the blood of uninfected and infected wt, C5aR−/− and C5L2−/− mice. While C5aR deficiency did not affect NKT and NK cell numbers in naïve mice, we found markedly reduced numbers of NKT and NK cells in the spleen (Fig. 3B) and the peritoneal cavity (Fig. 3C) of C5aR−/−, but not of C5L2−/− mice 20 and 48 hrs after infection when compared with wt mice. Although there was a tendency that C5L2−/− mice had a slight defect in recruiting NK cells (Fig. 3, B and C), we concluded that C5aR preferentially regulates the recruitment of NKT and NK cells to the site of infection in response to C5a.
Cognate engagement of C5aR activates NKT cells during E. coli-sepsis
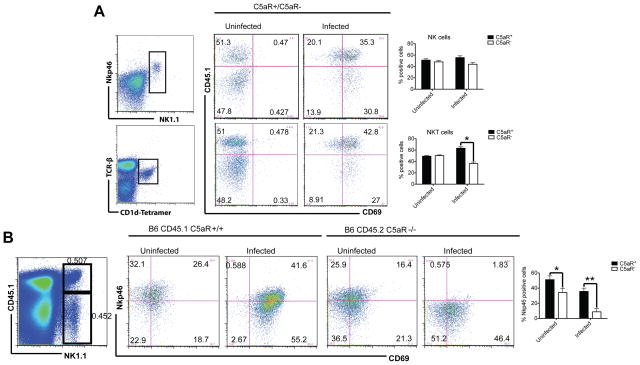
To test if cognate C5a-C5aR interactions are required for NKT and NK cell activation, we reconstituted irradiated B6 CD45.1 mice with a mixture of B6 CD45.1+ C5aR+/+ and B6 CD45.2+ C5aR−/− BMs. These mixed BM chimeras expressed similar numbers of CD45.2 (47.2±4.9) and CD45.1(52.1±6.8) positive cells as determined by FACS analysis in the peripheral blood. Using CD45.1 and CD45.2 to distinguish cells of C5aR+/+ and C5aR−/− origin and knowing that NKT and NK cell numbers accumulate in the spleen after infection, we observed significantly increased percentages of C5aR+ CD45.1+ NKT cells when the BM chimeras were exposed to E. coli 12 hrs before (Fig. 4A), suggesting that direct cognate engagement of C5aR on NKT cells affects NKT cell recruitment. Although there was a tendency that more C5aR+ NK cells were recruited, the effect was not statistically significant. Bystander effects through NK - DC interactions may play an important role here (see below). No significant differences in the percentages of NKT cells were detected in C5L2+/C5L2− mixed BM chimeras (data not show).
Figure 4.
C5aR engagement during E. coli sepsis directly activates NKT cells. (A+B) Irradiated B6 CD45.1 mice were reconstituted with a 1:1 mixture of C5aR-deficient CD45.2 and C5aR-sufficient CD45.1 BM cells or reconstituted with a 1:1 mixture of C5L2-deficient CD45.2 and C5L2-sufficient CD45.1 BM cells (not shown). Representative FACS dot plots of NK and NKT cells in the spleen 12 hours after infection are displayed. The expression of CD69 (A) and of NKp46 (B) was measured on NKT (gated on TCRβ+ CD1d-tetramer+) and NK (Nkp46+ NK1.1+) cells using CD45.1 specific antibodies to assign their origin to wt or C5aR-deficient cells. Graphs shown on the right in (A) depict the frequencies of CD45.1+C5aR+ (black bars) and CD45.2+C5aR−(white bars) NK cells (upper panel) and NKT cells (lower panel). The graph shown on the right in (B) depicts the frequency of Nkp46+ expression within the CD45.1+C5aR+ and the CD45.2+C5aR− NK cells (n=4).
In order to test if additional receptors are regulated on NKT and NK cells upon C5aR engagement, we evaluated a range of activating and inhibitory NK cell receptors including NKG2D, Ly49H, Nkp46 and Ly49D that are expressed on both cell populations. Nkp46 has been described as a killer receptor (24) critical for the elimination of influenza virus (25) and the production of IFN-γ. Nkp46 expression was significantly reduced on NK cells from C5aR−/−, but not from wt mice (Fig. 4B) after infection correlating with a reduced systemic IFN-γ production in C5aR−/− mice (Fig. 1, C and E). Thus, next to various bystander effects of sepsis-mediated immune activation, direct cognate C5a/C5aR interaction contributes to the activation of NKT and NK cells and thus, to the detrimental effects of NKT and NK cells during sepsis.
Engagement of TLRs and C5aR induces synergistic innate lymphocyte responses
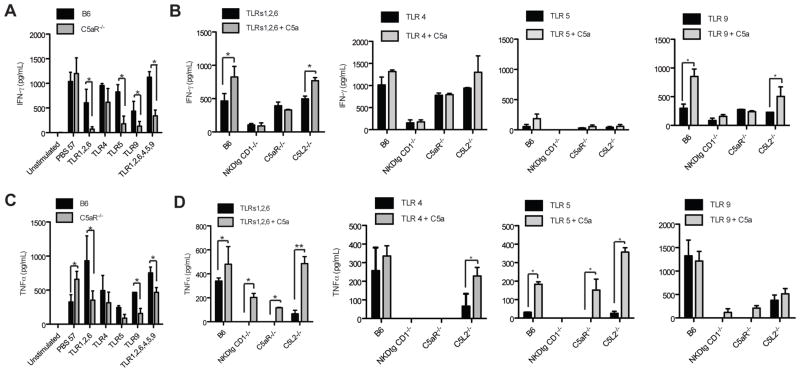
Cross-talk between C5aR and different TLRs on APCs has been described (26). However, the interaction between α-GalCer, the prototypical NKT cell TCR ligand and C5a has not been evaluated. To assess the direct functional impact of C5aR expression on NKT and NK cells, we tested the cytokine production from spleen cells of E.coli-infected B6 and C5aR−/− mice in response to different TLR ligands and the α-GalCer homologue, PBS-57 (27). Stimulation of spleen cells with PBS-57 or with different TLR ligands resulted in IFN-γ and TNF-α production (Fig. 5, A and C). Importantly, IFN-γ production was significantly reduced in the absence of C5aR in response to ligands for TLR 5, TLR 9 and the combination of TLRs 1/2/6 or TLRs 1/2/6, 4, 5 and 9 (Fig. 5A). However, C5aR deficiency did not affect LPS-induced IFN-γ (Fig. 5A). TNF-α production was also significantly reduced, preferentially following stimulation with ligands for TLRs 1/2/6, TLRs 1/2/6, 4, 5, 9 or TLR 9 (Fig. 5C). In contrast, we observed enhanced TNF-α production in C5aR−/− spleen cells in response to PBS-57 suggesting that C5a suppresses TCR-mediated induction of TNF-α from NKT cells. Thus, our data indicate that C5a may exert a dual role in NKT cell activation: it suppresses cognate antigen mediated NKT cell activation, but synergizes with TLRs to drive the production of the pro-inflammatory cytokines TNF-α and IFN-γ.
Figure 5.
Administration of C5a enhances TLR-driven cytokine release during the early septic response. (A-D) C5a and TLRs act synergistically on early NKT and NK cell activation. Statistical significance was calculated using ANOVA. The experiment was repeated twice with similar results. Spleen cells were pooled from two individual mice; one experiment out of two is displayed. (A+C) Spleen cells obtained from B6 and C5aR−/− mice infected 24 hrs before with E. coli were stimulated with the indicated TLR ligands. (B+D) Release of cytokines by spleen cells from naïve mice of the indicated strains was determined by ELISA. Spleen cells were stimulated with the indicated TLR ligands and C5a.
To directly test the contribution of NKT and NK cells to the TLR and C5a-induced cytokine production, we stimulated spleen cells from C5aR−/−, NKT-/NK-cell double-deficient and wt mice with different TLR ligands in the presence or absence of C5a (Fig. 5, B and D). The ligation of TLRs 1/2/6, TLR 4, TLR 5 and TLR 9 resulted in IFN-γ and TNF-α production from wt mice. C5a stimulation enhanced the TLR1/2/6 and TLR9-driven production of IFN-γ (Fig. 5D) as well as the TLR 1/2/6 and TLR 5-driven production of TNF-α from wt mice (Fig. 5D). Surprisingly, the engagement of C5aR was essential for the LPS-mediated production of TNF-α in spleen cells similar to what we observed with NKT/NK-double-deficient cells. As LPS can trigger the release of TNF-α preferentially by myeloid cells that constitute only a minor population in the spleen, C5aR engagement affects other cellular sources of TNF-α including NKT cells that fail to promote TNF-α production by LPS stimulated myeloid cells. Altogether, NKT-/NK-double-deficient spleen cells produced only minor amounts of IFN-γ and TNF-α upon in vitro stimulation with TLR ligands in the presence or absence of C5a suggesting that both innate lymphocyte populations are the major sources for IFN-γ and TNF-α in the early stages of sepsis.
C5a receptor signaling is critical for TLR-driven TNF-α production from NKT cells and for IFN-γ production resulting from NK and DC cell cross-talk
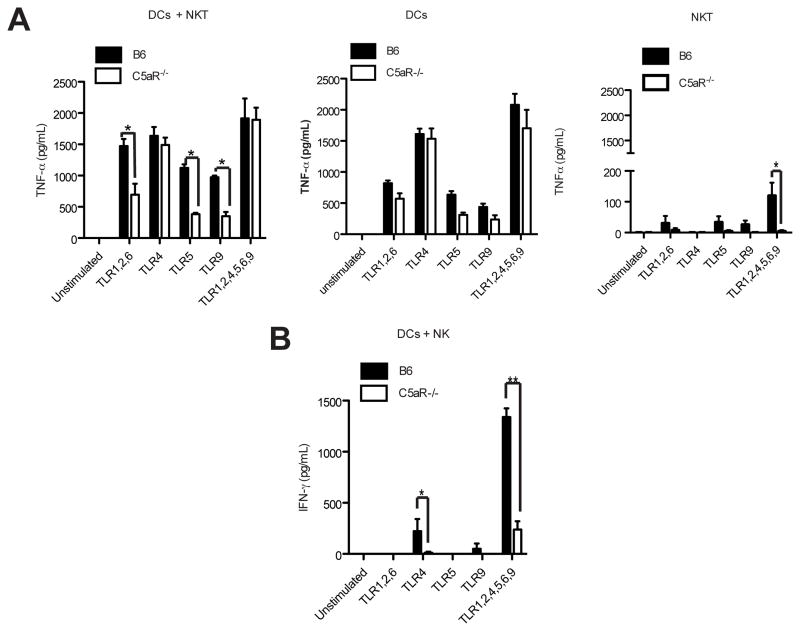
Importantly, several other innate immune cells including DCs can produce TNF-α in response to TLR and C5aR stimulation. Further, cross-talk between NKT cells and DCs can affect the production of this cytokine. The comparison of monocultures of BM-derived DCs with co-cultures of DCs and purified NKT cells from infected, but not from uninfected mice suggests a synergism between NKT cells and BM-DCs for TNF-α production through combined C5aR engagement and TLRs 1/2/6, TLR 5 or TLR 9 ligation (Fig. 6A). Albeit significantly lower than in the co-cultures with BM-DCs, NKT cells alone produced TNF-α in response to selected combinations TLR ligands (Fig. 6A, right panel) correlating with the expression of TLRs 5, 6 and 7 along with TLR 3 as determined by RT-PCR (not shown). Importantly, this TLR-driven TNF-α production was almost completely absent in cells from C5aR-deficient mice suggesting that crosstalk between TLRs and C5aR is critical for genuine TNF-α production from NKT cells. Further, IFN-γ responses could be induced in NKT cells in response to all TLR ligand combinations tested that were reduced in the absence of C5aR (not shown). In contrast to PBS-57-mediated IFN-γ production, which was a direct effect of the NKT cell TCR engagement, TLR induced IFN-γ was due to the release of IL-12 and IL-18 in response to TLR engagement on DCs (not shown).
Figure 6.
Crosstalk of C5aR and defined TLRs on DCs and NKT as well as DCs and NK cells drives synergistic TNF-α and IFN-γ production. (A+B) C5aR and TLR-mediated cross-talk between DCs and NKT (A, left panel) cells promotes early TNF-α production whereas cross-talk between DCs and NK cells (B) drives IFN-γ production in early E. coli-mediated sepsis. Sorted NKT cells (purity >98%), NK cells (purity >95%) as well as BM-derived DCs from infected B6 or C5aR−/− were exposed to the indicated TLR ligands. Cytokine release was determined 48 h later by ELISA. Monocultures of DCs (A, middle panel) and of NKT cells (A, right panel) produced TNF-α. In contrast, neither monocultures of BM-derived DCs nor of purified NK cells produced IFN-γ (not shown). Statistically significant differences were determined using ANOVA. The experiment was repeated once with similar results.
It has been reported that NK cells from naïve mice do not respond to TLR9 ligation (28). Similarly, we found no IFN-γ production in monocultures of BM-DCs or NK cells in response to TLR activation (data not shown). In contrast, IFN-γ responses could be induced in co-cultures of BM-DCs with purified NK cells from infected mice in response to TLR 4 alone or in response to a combination of all tested TLR ligands (Fig. 6B). This TLR-induced IFN-γ production by NK cells was almost completely abolished in the absence of C5aR, suggesting that DCs drive NK cell activation upon C5aR engagement.
Discussion
Our data suggest a synergism for cytokine production by NKT and NK cells when synergistically activated by defined TLR ligands in combination with C5aR. The NKT and NK cell-mediated “cytokine storm” in this context likely contributes to the impaired survival during sepsis. While C5aR engagement induces various bystander effects on NK cells through activation of DCs, cognate engagement of C5aR has a more prominent role on NKT than on NK cell recruitment. Thus, NKT cells may guide NK cells to follow them to the site of infection. This novel mechanism of complement-driven NKT cell activation with unique cytokine profiles may provide an explanation why NKT cells can exert suppressive as well as stimulatory properties (6, 7). Depending on whether TLRs are ligated in the context of complement activation, NKT cells may exert distinct immune-modulatory effects on NK cells due to an altered magnitude of cytokine production.
While TLR and C5aR mediated signals act synergistically on NKT cells, administration of α-GalCer appears to have the opposite effect for NKT cell mediated TNF-α production. Thus, C5a-mediated signals may contribute to the activation and recruitment of NKT cells to the site of infection in an environment where multiple TLRs are ligated, while the engagement of the NKT cell TCR by GSL antigens may promote the attachment of NKT cells to the site of pathogen persistence. In this context, it has been reported that NKT cells crawl along liver sinusoids under physiologic conditions and rapidly arrest once they detect GSL antigens like α-GalCer (29). Similar mechanisms may apply for NK cells; NKG2D, for example, may detect infected NKG2D ligand-expressing cells and consequently lead to the arrest of NK cells at the site of infection. This may also explain why most of the NK cell receptors on NKT and NK cells are not regulated upon C5aR ligation. However, during sepsis, various receptors on NKT and NK cells will be engaged including the ones for C5a and the sequence and hierarchy of these interactions need to be delineated in future studies. Although our data indicate that the absence of C5aR increased the amount of TNF-α released in spleen cell cultures stimulated with α-GalCer, it remains to be determined if endogenous GSLs that are available at the site of infection in higher numbers than in uninfected tissues (30) can arrest NKT cells at a septic focus when various other stimuli are around. In fact, endogenous GSL antigens exhibit much lower stimulatory capacity than α-GalCer (3, 7). Our data suggest that only C5aR activation directly triggers NKT and NK cell activation; however, the influence of the second C5a receptor, C5L2 needs to be evaluated in more detail during sepsis. The increase of TNF-α in spleens cells in C5aR-deficient mice in response to combined C5a and TLR5 or TLR1/2/6 engagement suggests synergistic effects between C5L2 and TLRs in our model (Fig. 5D). Similarly, recent studies have uncovered critical roles of C5L2 in inflammatory diseases such as CLP-mediated septic peritonitis (14) and allergic asthma (18). In fact, C5L2-deficient mice showed increased survival in a model of CLP-induced mid-grade septic peritonitis. The minor survival of C5L2-deficient mice that we found in E. coli-mediated sepsis may result from the distinct experimental model. C5L2 is likely not to serve solely as a decoy receptor, as initially thought. C5L2 has been shown to affect C5aR signaling via the β-arrestin pathway in neutrophils demonstrating that both receptors can be linked independent of G-protein-coupled pathways (31). Further elucidation of the connection between the two receptors for C5a is important to better understand the role of C5a at different phases of sepsis and to therapeutically target them.
In summary, this is to our knowledge the first report showing the expression of the two C5a receptors on NK and NKT cells and the requirement of C5aR engagement on NKT and NK cells for host survival under septic conditions. We show that C5aR signaling not only regulates TLR but also TCR-mediated cytokine production from NKT cells and the cross-talk with DCs in sepsis highlighting the crucial role of C5a in disease pathogenesis. Thus, targeting of C5a will not only affect neutrophil dysfunction, cell apoptosis, tissue factor expression and cardiomyopathy (32), but also the function of innate lymphoyctes in sepsis.
Acknowledgments
This work was in part supported by SFB/TR22 (project A21) to JK. JM is supported by the Lupus Research Institute, in parts by PHS grant P30 DK078392, by the IZKF, DFG grant MA 2621/2-1 and by NIDDK grant R01DK084054.
We thank Paul B. Savage for providing PBS-57 and the NIH Tetramer Facility for providing α-GalCer CD1d-tetramers. We also thank Dr. Wayne Yokoyama for providing NKDtg mice.
Abbreviations
- B6
C57BL/6
- DC
dendritic cell
- APC
antigen presenting cell
- NKT
natural killer T cell
- NK
natural killer cell
- C5aR
complement receptor C5a
- C5L2
C5a receptor-like 2
- TLR
toll like receptor
- GSL
glycosphingolipid, BM, bone marrow
- TCR
T cell receptor
- AT
anaphlylatoxin
- i.p
intraperitoneal
Footnotes
The authors have no conflicting financial interests.
References
- 1.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d- restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 2.Chan SH, Perussia B, Gupta JW, Kobayashi M, Pospisil M, Young HA, Wolf SF, Young D, Clark SC, Trinchieri G. Induction of interferon gamma production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J Exp Med. 1991;173:869–879. doi: 10.1084/jem.173.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, 3rd, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, Hoebe K, Schneewin O, Walker D, Beutler B, Teyton L, Savage PB, Bendelac A. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 4.Colucci F, DiSanto JP, Leibson PJ. Natural killer cell activation in mice and men: different triggers for similar weapons? Nat Immunol. 2002;3:807–813. doi: 10.1038/ni0902-807. [DOI] [PubMed] [Google Scholar]
- 5.Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, Kronenberg M, Locksley RM. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198:1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 7.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 8.Le-Barillec K, Magalhaes JG, Corcuff E, Thuizat A, Sansonetti PJ, Phalipon A, DiSanto JP. Roles for T and NK cells in the innate immune response to Shigella flexneri. J Immunol. 2005;175:1735–1740. doi: 10.4049/jimmunol.175.3.1735. [DOI] [PubMed] [Google Scholar]
- 9.Vankayalapati R, Garg A, Porgador A, Griffith DE, Klucar P, Safi H, Girard WM, Cosman D, Spies T, Barnes PF. Role of NK cell-activating receptors and their ligands in the lysis of mononuclear phagocytes infected with an intracellular bacterium. J Immunol. 2005;175:4611–4617. doi: 10.4049/jimmunol.175.7.4611. [DOI] [PubMed] [Google Scholar]
- 10.Emoto M, Miyamoto M, Yoshizawa I, Emoto Y, Schaible UE, Kita E, Kaufmann SH. Critical role of NK cells rather than V alpha 14(+)NKT cells in lipopolysaccharide-induced lethal shock in mice. J Immunol. 2002;169:1426–1432. doi: 10.4049/jimmunol.169.3.1426. [DOI] [PubMed] [Google Scholar]
- 11.Sireci G, La Manna MP, Di Sano C, Di Liberto D, Porcelli SA, Kronenberg M, Dieli F, Salerno A. Pivotal advance: alpha-galactosylceramide induces protection against lipopolysaccharide-induced shock. J Leukoc Biol. 2007;81:607–622. doi: 10.1189/jlb.0506298. [DOI] [PubMed] [Google Scholar]
- 12.Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, Bendelac A. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–4650. [PubMed] [Google Scholar]
- 13.Klos A, Tenner AJ, Johswich KO, Ager RR, Reis ES, Köhl J. The role of the anaphylatoxins in health and disease. Mol Immunol. 2009;46:2753–2766. doi: 10.1016/j.molimm.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rittirsch D, Flierl MA, Nadeau BA, Day DE, Huber-Lang M, Mackay CR, Zetoune FS, Gerard NP, Cianflone K, Köhl J, Gerard C, Sarma JV, Ward PA. Functional roles for C5a receptors in sepsis. Nat Med. 2008;14:551–557. doi: 10.1038/nm1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riedemann NC, Guo RF, Bernacki KD, Reuben JS, Laudes IJ, Neff TA, Gao H, Speyer C, Sarma VJ, Zetoune FS, Ward PA. Regulation by C5a of neutrophil activation during sepsis. Immunity. 2003;19:193–202. doi: 10.1016/s1074-7613(03)00206-1. [DOI] [PubMed] [Google Scholar]
- 16.Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human valpha24 natural killer T cells. J Exp Med. 2002;195:637–641. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, I, Lewkowich P, Köhl G, Clark JR, Wills-Karp M, Köhl J. A protective role for C5a in the development of allergic asthma associated with altered levels of B7-H1 and B7-DC on plasmacytoid dendritic cells. J Immunol. 2009;182:5123–5130. doi: 10.4049/jimmunol.0804276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Schmudde I, Laumonnier Y, Pandey MK, Clark JR, König P, Gerard NP, Gerard C, Wills-Karp M, Köhl J. A critical role for C5L2 in the pathogenesis of experimental allergic asthma. J Immunol. 2010;185:6741–6752. doi: 10.4049/jimmunol.1000892. [DOI] [PubMed] [Google Scholar]
- 19.Benlagha K, Weiss A, Beavis A, Teyton L, Bendelac A. In vivo identification of glycolipid antigen specific T cells using fluorescent CD1d tetramers. J Exp Med. 2000;191:1895–1903. doi: 10.1084/jem.191.11.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozmen L, Pericin M, Hakimi J, Chizzonite RA, Wysocka M, Trinchieri G, Gately M, Garotta G. Interleukin 12, interferon gamma, and tumor necrosis factor alpha are the key cytokines of the generalized Shwartzman reaction. J Exp Med. 1994;180:907–915. doi: 10.1084/jem.180.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogasawara K, Takeda K, Hashimoto W, Satoh M, Okuyama R, Yanai N, Obinata M, Kumagai K, Takada H, Hiraide H, Seki S. Involvement of NK1+ T cells and their IFN-gamma production in the generalized Shwartzman reaction. J Immunol. 1998;160:3522–3527. [PubMed] [Google Scholar]
- 22.Strainic MG, Liu J, Huang D, An F, Lalli PN, Muqim N, Shapiro VS, Dubyak GR, Heeger PS, Medof ME. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28:425–435. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu R, Wang R, Han G, Wang J, Chen G, Wang L, Li X, Guo R, Shen B, Li Y. Complement C5a regulates IL-17 by affecting the crosstalk between DC and gammadelta T cells in CLP-induced sepsis. Eur J Immunol. 2010;40:1079–1088. doi: 10.1002/eji.200940015. [DOI] [PubMed] [Google Scholar]
- 24.Orr MT, Lanier LL. Natural killer cell education and tolerance. Cell. 2010;142:847–856. doi: 10.1016/j.cell.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gazit R, Gruda R, Elboim M, Arnon TI, Katz G, Achdout H, Hanna J, Qimron U, Landau G, Greenbaum E, Zakay-Rones Z, Porgador A, Mandelboim O. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat Immunol. 2006;7:517–523. doi: 10.1038/ni1322. [DOI] [PubMed] [Google Scholar]
- 26.Hawlisch H, Belkaid Y, Baelder R, Hildeman D, Gerard C, Köhl J. C5a negatively regulates toll-like receptor 4-induced immune responses. Immunity. 2005;22:415–426. doi: 10.1016/j.immuni.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Goff RD, Zhou D, Mattner J, Sullivan BA, Khurana A, Cantu C, 3rd, Ravkov EV, Ibegbu CC, Altman JD, Teyton L, Bendelac A, Savage PB. A modified alpha-galactosyl ceramide for staining and stimulating natural killer T cells. J Immunol Methods. 2006;312:34–39. doi: 10.1016/j.jim.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Krug A, French AR, Barchet W, Fischer JA, Dzionek A, Pingel JT, Orihuela MM, Akira S, Yokoyama WM, Colonna M. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–119. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, Dustin ML, Littman DR. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3:e113. doi: 10.1371/journal.pbio.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darmoise A, Teneberg S, Bouzonville L, Brady RO, Beck M, Kaufmann SH, Winau F. Lysosomal alpha-galactosidase controls the generation of self lipid antigens for natural killer T cells. Immunity. 2010;33:216–228. doi: 10.1016/j.immuni.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bamberg CE, Mackay CR, Lee H, Zahra D, Jackson J, Lim YS, Whitfeld PL, Craig S, Corsini E, Lu B, Gerard C, Gerard NP. The C5a receptor (C5aR) C5L2 is a modulator of C5aR-mediated signal transduction. J Biol Chem. 2010;285:7633–7644. doi: 10.1074/jbc.M109.092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 2008;8:776–787. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]