Abstract
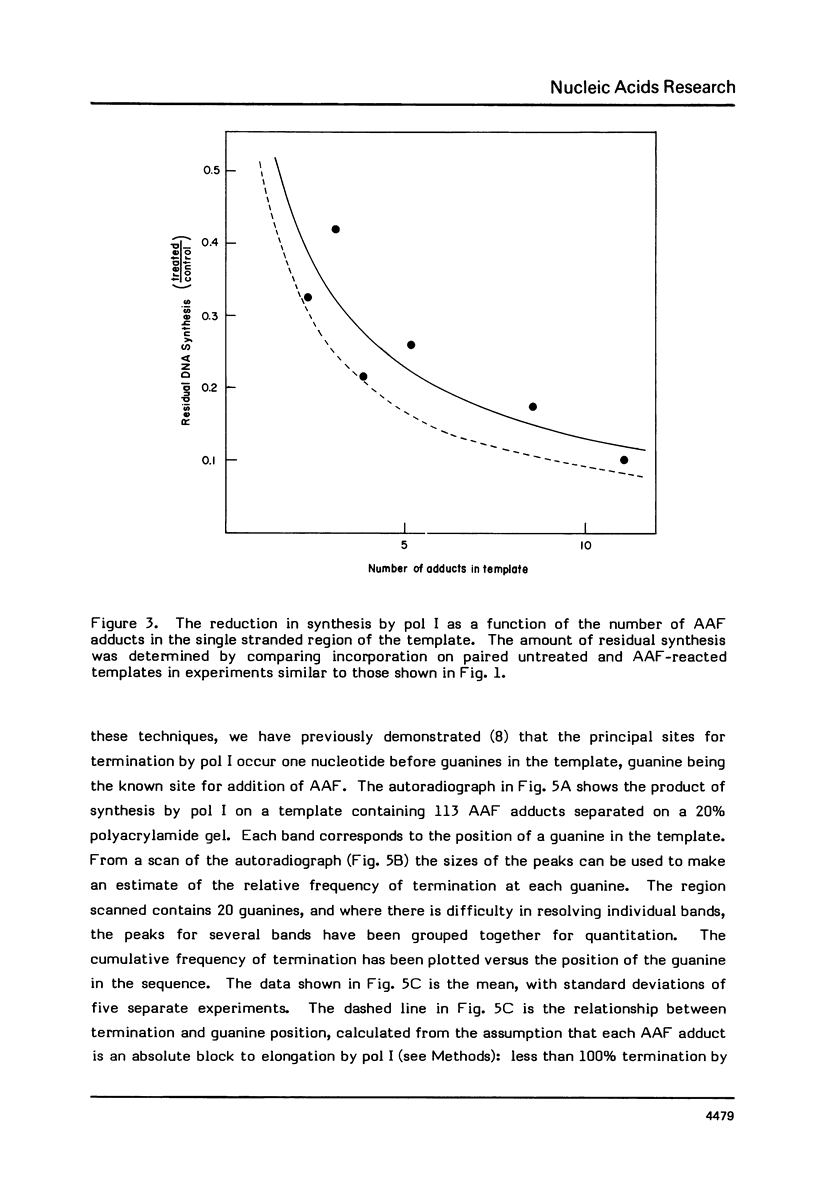
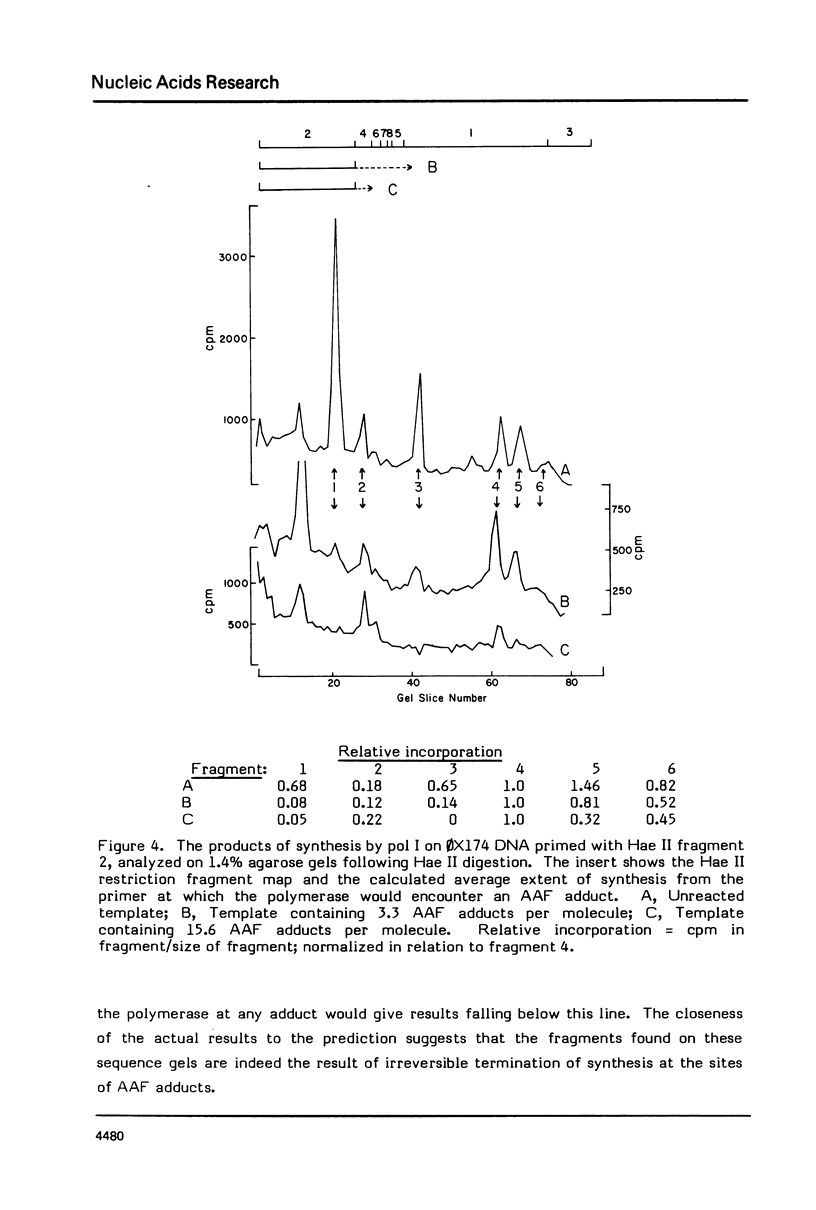
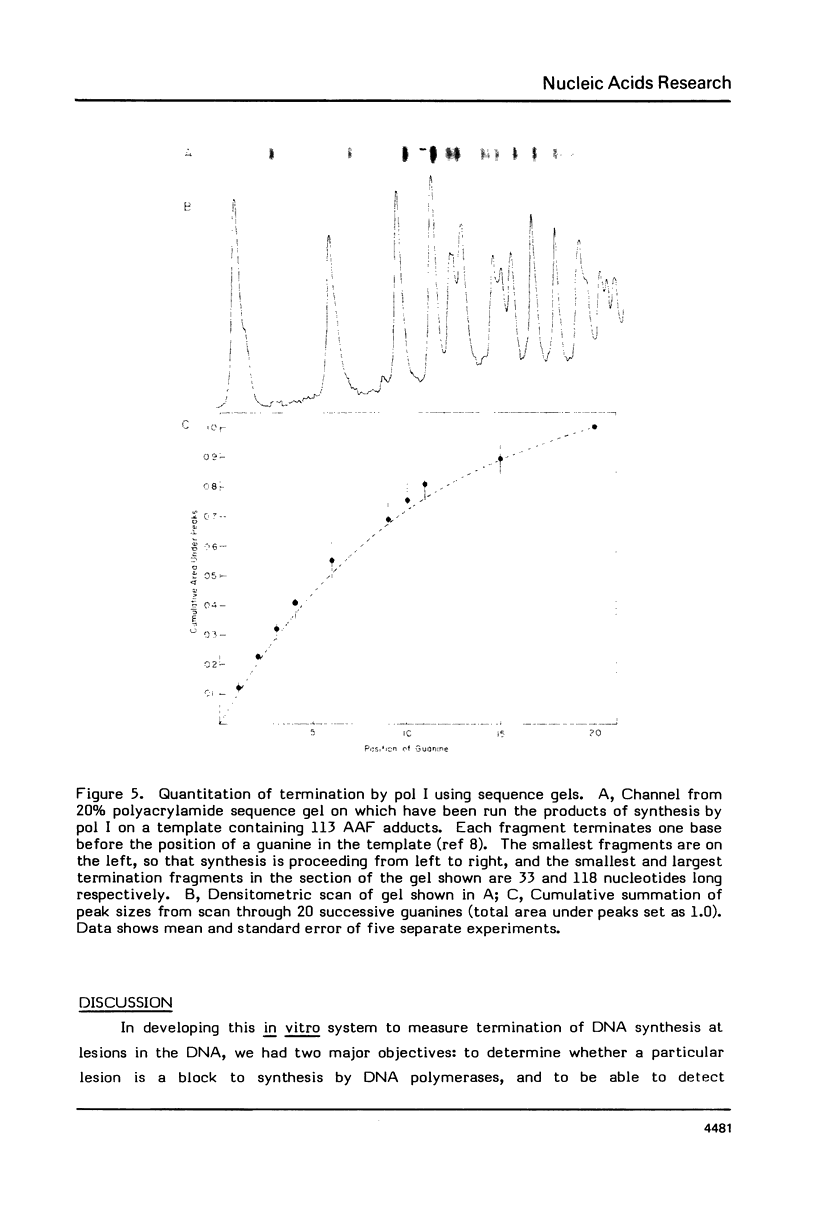
DNA synthesis catalyzed by E. coli polymerases I or III is inhibited on templates containing N-acetoxy-acetylaminofluorene-reacted adducts. Termination of synthesis occurs just before the site of the adduct. Synthesis on 0X174 templates primed with restriction fragments and treated with AAAF can be visualized on DNA sequencing gels. Comparison of the amounts of the different newly synthesized fragments with those calculated from the probability of termination as determined from the average number of adducts per molecule shows that synthesis terminates, rather than stutters, at each adduct. This method may be useful for detecting the bypass of lesions.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOLLUM F. J., SETLOW R. B. Ultraviolet inactivation of DNA primer activity. I. Effects of different wavelengths and doses. Biochim Biophys Acta. 1963 Apr 30;68:599–607. doi: 10.1016/0006-3002(63)90189-6. [DOI] [PubMed] [Google Scholar]
- Coulian M. Initiation of the replication of single-stranded DNA by Escherichia coli DNA polymerase. Cold Spring Harb Symp Quant Biol. 1968;33:11–20. doi: 10.1101/sqb.1968.033.01.006. [DOI] [PubMed] [Google Scholar]
- Drake J. W., Baltz R. H. The biochemistry of mutagenesis. Annu Rev Biochem. 1976;45:11–37. doi: 10.1146/annurev.bi.45.070176.000303. [DOI] [PubMed] [Google Scholar]
- Eisenberg S., Harbers B., Hours C., Denhardt D. T. The mechanism of replication of phiX174 DNA. XII. Non-random location of gaps in nascent phiX174 RF II DNA. J Mol Biol. 1975 Nov 25;99(1):107–123. doi: 10.1016/s0022-2836(75)80162-8. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Rupp W. D., Wilkins B. M., Cole R. S. DNA replication and recombination after UV irradiation. Cold Spring Harb Symp Quant Biol. 1968;33:195–207. doi: 10.1101/sqb.1968.033.01.023. [DOI] [PubMed] [Google Scholar]
- Hsu W. T., Lin E. J., Harvey R. G., Weiss S. B. Mechanism of phage phiX174 DNA inactivation by benzo(a)pyrene-7,8-dihydrodiol-9,10-epoxide. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3335–3339. doi: 10.1073/pnas.74.8.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapuler A. M., Michelson A. M. The reaction of the carcinogen N-acetoxy-2-acetyl-aminofluorene with DNA and other polynucleotides and its stereochemical implications. Biochim Biophys Acta. 1971 Mar 25;232(3):436–450. doi: 10.1016/0005-2787(71)90598-3. [DOI] [PubMed] [Google Scholar]
- Kriek E., Miller J. A., Juhl U., Miller E. C. 8-(N-2-fluorenylacetamido)guanosine, an arylamidation reaction product of guanosine and the carcinogen N-acetoxy-N-2-fluorenylacetamide in neutral solution. Biochemistry. 1967 Jan;6(1):177–182. doi: 10.1021/bi00853a029. [DOI] [PubMed] [Google Scholar]
- McHenry C. S., Crow W. DNA polymerase III of Escherichia coli. Purification and identification of subunits. J Biol Chem. 1979 Mar 10;254(5):1748–1753. [PubMed] [Google Scholar]
- Millette R. L., Fink L. M. The effect of modification of T7 DNA by the carcinogen N-1-acetylaminofluorene: termination of transcription in vitro. Biochemistry. 1975 Apr 8;14(7):1426–1432. doi: 10.1021/bi00678a012. [DOI] [PubMed] [Google Scholar]
- Moore P., Strauss B. S. Sites of inhibition of in vitro DNA synthesis in carcinogen- and UV-treated phi X174 DNA. Nature. 1979 Apr 12;278(5705):664–666. doi: 10.1038/278664a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani G., Boiteux S., Radman M. Mechanism of ultraviolet-induced mutagenesis: extent and fidelity of in vitro DNA synthesis on irradiated templates. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3037–3041. doi: 10.1073/pnas.75.7.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weymouth L. A., Loeb L. A. Mutagenesis during in vitro DNA synthesis. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1924–1928. doi: 10.1073/pnas.75.4.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H., Pulkrabek P., Grunberger D., Weinstein I. B. Differential excision from DNA of the C-8 and N2 guanosine adducts of N-acetyl-2-aminofluorene by single strand-specific endonucleases. Cancer Res. 1977 Oct;37(10):3756–3760. [PubMed] [Google Scholar]