Abstract
Motor vehicle crash injuries among the elderly are an important public health problem. We sought to determine if older individuals (65 years and older) had worse self-reported physical functioning and mental health status than younger adults (18–64 years) at 6 and 12 months post-injury, while controlling for pre-injury functional status, comorbidity, and injury severity. We used data from two sites of the Crash Injury Research and Engineering Network (CIREN) study. After exclusion based on missing Short Form-36 (SF-36) values, the final sample consisted of 579 CIREN cases; there were 500 individuals age 18–64 and 79 individuals (13.6%) age 65 or older. The outcome measures included the physical functioning scale (PFS), vitality scale (VS), and mental health scale (MHS) of the SF-36. The proportion of younger and older adults that had comorbidity was 17.6% and 54.4%, respectively. Multivariate linear regression models indicated that comorbidity, baseline PFS, and severe injury (Injury Severity Score [ISS] 25+ vs. ISS ≤ 8) were significantly associated with PFS scores at 6 months, but only comorbidity and baseline PFS were associated with PFS at 12 months. Multivariate models indicated that only pre-injury VS (p < .001) was associated with the VS at 6 months, but that both comorbidity (p < .01) and pre-injury VS (p < .001) were associated with VS at 12 months. MHS at 6 months was significantly associated with only the baseline MHS score, but both comorbidity and pre-injury MHS were associated with MHS at 12 months. There was no significant difference in the change in any of the SF-36 domains during the study year. Advanced age was not associated with lower self-reported health in any of the three SF-36 domains compared to younger age when pre-injury ISS and comorbidity were included in the model.
INTRODUCTION
The rapid aging of the US population has raised public health concern regarding motor vehicle crash (MVC) injuries among the elderly [Braver and Trempel, 2004]. Recent data suggest that the rates of fatal crash involvement in the older ages have decreased [Cheung, McCartt, & Braitman, 2008]. These trends highlight the need to understand other post-injury consequences such as decreased physical and mental health status [Fitzharris, Fildes, Charlton, et al., 2007]. However, most of the research in this area is wrought with methodological challenges including non-standardized outcome measures, and non-adjustment for potential confounders, such as pre-injury health status. Additionally, older age groups have not been typically involved in these studies, or age-group comparisons have not been reported [Ameratunga, Norton, Bennett, et al., 2004].
The existing literature suggests that declines in general health and functional status are significant and prolonged following MVC injuries. Many of these studies have examined these health outcomes using the Short Form-36 (SF-36) - a widely used and validated measure for assessing patients’ self-reported health status in multiple domains - allowing for comparisons across studies [Ware Jr & Sherbourne, 1992; MacKenzie, McCarthy, Ditunno, et al., 2002]. For example, Dischinger et al. (2005) and Read et al. (2004) have shown that lower extremity injuries (LEI) sustained during MVC negatively affect all domains of the SF-36 at 6- and 12-months post-injury. Further, MVC patients with LEI do not recovery as fully between 6- and 12-months as those with non-LEI [Dischinger, 2005].
Ameratunga et al. (2006) report that drivers admitted to hospital following MVC have a 10-fold increased chance of worse self-reported health (as indicted on the SF-36) at 18-months post-injury compared to drivers not injured in a MVC. Fitzharris et al. (2007) report that a group of adults (age 18–59) who were injured in traffic crashes had significant detriments in multiple domains of the SF-36 two months following the injuries, and these scores were still significantly lower at 8-months compared to the pre-injury period. These previously reported analyses, however, did not examine the effect of age on SF-36 domains following MVC. Thus, it is unknown whether the deficits in self-reported health status are the same in older adults who have been injured in MVCs. This is important because the physical functioning and vitality domains of the SF-36 have been used to assess aspects of the geriatric frailty syndrome, and poorer scores in these domains are associated with multiple negative outcomes including falls, disability, institutionalization, and mortality [Woods, LaCroix, Gray, et al., 2005].
The purpose of our study was to examine the differences in self-reported health, as measured in domains of the Short-Form-36 (SF-36), between young (ages 18–64) and old (age ≥65) individuals prior to a MVC injury and at 6- and 12-months post-injury using data from the Crash Injury Research and Engineering Network (CIREN) study. The SF-36 is a standardized and validated tool for assessing self-reported health status in multiple domains. We also sought to determine the independent effect of advanced age, comorbidity (the presence of 2 or more medical conditions), and the person’s pre-injury self-reported functional status on the respective post-injury outcomes. We hypothesized that older adults would have worse functioning than younger adults in the physical domains of the SF-36, but that the mental health domain would not be significantly different between the age groups, because older adults may exhibit more emotional resilience or self-efficacy [Carstensen, Pasupathi, Mayr, et al., 2000; Resnick, 1998]. We also hypothesized that advanced age would negatively influence post-MVC recovery so that we would observe steeper declines and less improvement in physical function domains of the SF-36.
METHODS
Data Sources
CIREN is a multicenter research network with detailed crash and injury data on serious MVC injuries. The National Highway Traffic Safety Administration (NHTSA) coordinates the enrollment of cases into the CIREN system. The CIREN database typically includes patients who sustained at least one injury yielding an Abbreviated Injury Scale (AIS) score ≥3 or two injuries of “medical significance,” yielding an AIS score ≥2, in a late-model vehicle (i.e., manufactured within 6–8 years before the crash). CIREN uses the 1995 Version of the AIS [Association for the Advancement of Automotive Medicine (AAAM), 1998]. For each enrolled case, the following data are obtained: medical data on injured occupants, crash scene data, and vehicle damage information. Injury information includes hospital discharge summaries and radiologic images and results narratives, operative reports, autopsy reports, injury photographs, and long-term psychosocial outcomes. It, therefore, represents an excellent source of data for understanding non-fatal outcomes among older, non-fatally injured, vehicle occupants.
The CIREN study has 8 sites, but we limited our study to data collected at only two sites, Baltimore and Seattle. We chose these sites based on the completeness of SF-36 data at the baseline and follow-up assessments. This study used CIREN case occupants that were 18 years old or older. The resulting dataset using these two centers included 725 CIREN cases (420 from Seattle and 332 from Baltimore). Since there were CIREN cases with missing values for some of the SF-36 measures, we took steps to refine this dataset to ensure that we were comparing the same group of patients at all time points. First, we excluded any cases with missing values for any of the three SF-36 scales, as it was essential to control for the pre-injury scores on these items. We also excluded any cases where data were missing for both the 6-month and 12-month follow-up scores on any of these three SF-36 scales.
Measures
Injury Severity
Injury Severity is based on the Injury Severity Score (ISS) [Baker, O'Neill, Haddon Jr., et al., 1974]. The ISS is a good measure of overall injury severity and the probability of survival from the injury. It is derived by summing the squares of the highest AIS scores of the three different body regions with the highest AIS scores [AAAM, 1998].
Comorbidity
We created a comorbidity index that was the sum of disease categories present at the injury hospital admission. Patients were considered to have comorbidity based on evidence of 2 or more disease classes present [Fried, Ferrucci, Darer, et al., 2004]. To determine the presence of disease, medical history information was retrieved from the patient, their proxy respondent, or the patient’s medical history. There were 16 disease categories considered including: obesity, cardiac, diabetes, gastrointestinal, psychiatric, immune suppressed, liver, malignancy, musculoskeletal, neurological, pulmonary, renal, hematological, general, and other. We did not specify the causes of the disease classes, but only indicated whether there was evidence for the overall disease class. For example, a history of either congestive heart failure or myocardial infarction would be evidence for cardiac disease, but we would not distinguish between a patient who had only one of these and a patient who had both.
Short Form-36 (SF-36)
Physical health, vitality, and mental health at baseline, and at 6 and 12 months post-MVC, were assessed by administering the Short Form-36 (SF-36) in an interview format (either in person at the in-hospital baseline assessment or by phone as soon as possible after discharge, and by phone at follow-up time points). The SF-36 refers to the previous four weeks; therefore, the baseline assessment queried the respondent regarding the four weeks prior to the injury, and the subsequent interviews related to the four weeks prior. The SF-36 is a widely used and validated tool to assess self-perceived health status [Ware, et al., 1992]. Its use has grown with the growing field of outcomes research [Ware and Kosinski, 2001], and it has been validated as an appropriate tool to administer to older adults in an interview setting [Lyons, Perry, & Littlepage, 1994]. The SF-36 consists of eight constructs covering three general aspects of health, namely, functional status, well-being, and overall evaluation of health [Andersen, Roos, Stanziano, et al. 2007]. Each scale is scored and then transformed to score between 0 and 100, with lower scores indicating lower levels of self-perceived health in these domains.
Based on our hypotheses, we elected to use three of the SF-36 scales. The physical functioning scale (PFS) is comprised of 10 items on the SF-36 that assess the extent of limitations individuals have in various physical tasks from vigorous activities, such as running, to less physically demanding tasks, such as climbing a flight of stairs or bathing. Scores closer to zero indicate that the person is “very limited in performing all physical activities” [Ware Jr, Kosinski, Bayliss, et al., 1995]. We elected to use only the PFS, although there are three other scales that assess functional status (social functioning, role limitations due to physical problems, and role limitations due to emotional problems), because the PFS measures only true physical detriments and would, therefore, not be biased by differential social roles between the young and the old age groups. Additionally, the PFS has been used to assess both the weakness and slowness components of the frailty syndrome in the Women’s Health Initiative – Observational Study (WHI-OS) [Woods, et al., 2005]. Scores of less than 75 on the PFS suggest the presence of these frailty markers.
The vitality scale (VS) of the SF-36 is comprised of 4 items that assess the respondent’s level of energy. The lowest scores on the VS indicate that the individual “feels worn out all of the time” [Ware, et al., 1995]. We included the VS because it addresses the issue of exhaustion, which as previously mentioned is a marker for frailty and is associated with the poor outcomes [Woods, et al., 2005]. Scores of less than 55 on the VS in the WHI-OS were determined to represent the threshold for exhaustion in post-menopausal women. The vitality scale, however, does overlap with mental health components because it is a more subjective measure of well-being (making the interpretation more complex), but it tends to concentrate more on the physical aspects of well-being [McHorney, Ware Jr., & Raczek, 1993].
The mental health scale (MHS) is comprised of 5 items that assess the individual’s level of nervousness/anxiety and depressive symptoms. The lowest scores on the MHS indicate “feelings of nervousness and depression” all of the time. We used the MHS because we had hypothesized that older adults, while they might be expected to have lower pre- and post-injury self-perceived physical functioning, would exhibit better self-perceived mental health due to better mental resilience or self-efficacy [Carstensen, et al., 2000; Resnick, 1998].
Statistical Analyses
Patient demographic and health characteristics were compared between the original dataset and the refined dataset to determine if the final sample would be imbalanced with respect to these characteristics. For the final dataset, the demographic and health characteristics were compared between age groups (< 65 and 65 or older) using Pearson’s chi-square statistics.
We examined self-reported scores on the physical functioning, mental health, and vitality scales of the Short Form-36 (SF-36) for all CIREN patients at baseline (referring to the 4 weeks prior to the injury), and at 6- and 12-months post-injury. Scores were analyzed based on a dichotomous categorization of age (those ≥65 versus those 18–64 years of age).
Bivariate analyses, using Student’s t-tests, were performed to ascertain the unadjusted effect of age group on outcome measures at 6 months and 12 months for each of 3 domains of the SF-36 questionnaire: physical functioning, vitality and mental health status. Bivariate analyses were also performed to examine the changes in the SF-36 values between baseline and 6-months and between baseline and 12-months across the age groups.
Separate linear regression models were run to examine the association between age group and outcome measure while adjusting for the following variables in each regression model: baseline results for the corresponding SF-36 domain, comorbidity, and Injury Severity Score (ISS) category (1–8, 9–15, 16–24 and 25 or more, indicating minor, mild, moderate, and severe injury severity, respectively). Interactions with age group were also examined to determine if the association of older subjects with outcome differed by number of co-morbidities or ISS grouping. For all analyses, a probability value below 0.05 was considered statistically significant.
RESULTS
Initially, there were 752 participants between the two CIREN centers with a mean age of 40.5 years; 104 (13.8%) participants were ≥65 years old. The proportion of younger and older adults with comorbidity was 16.2% and 56.7%, respectively. There was no significant difference in injury severity based on age group. After exclusion based on missing SF-36 values, the final sample consisted of 579 CIREN cases; there were 500 individuals age 18–64 and 79 individuals (13.6%) age 65 or older. The proportion of younger and older adults that had comorbidity was 17.6% and 54.4%, respectively.
The demographic, health, and injury characteristics of the sample are presented in Table 1. The mean age (standard deviation [sd]) of the entire final sample was 41.8 years (sd=18.4). The mean age (sd) of the two comparison groups, i.e. 18–64 years vs. ≥65 years was 36.6 (13.7) and 74.4 (7.2), respectively.
Table 1.
Health and Demographic Characteristics
| Variable (n, %) | Age 18–64 (n=500) | Age 65+ (n=79) | Total (n=579) | P-Value |
|---|---|---|---|---|
| CIREN Center | ||||
| Baltimore | 270 (87.1) | 40 (12.9) | 310 | 0.58 |
| Seattle | 230 (85.5) | 39 (14.5) | 269 | |
| Male (n, %) | 219 (44.9) | 35 (44.9) | 254 | 0.98 |
| White, non-hispanic (n, %) | 386 (79.6) | 72(92.3) | 458 | 0.007 |
| ISS (n, %) | ||||
| Minor (1–8) | 54 (10.8) | 13 (16.5) | 67 | 0.5 |
| Mild (9–15) | 212 (42.4) | 32 (40.5) | 244 | |
| Moderate (16–24) | 120 (24.0) | 16 (20.3) | 136 | |
| Severe (25+) | 114 (22.8) | 18 (22.8) | 132 | |
| Comorbidity (≥2 diseases) | ||||
| No | 412 (82.4) | 36 (45.6) | 448 | p<0.001 |
| Yes | 88 (17.6) | 43 (54.4) | 131 |
ISS = Injury Severity Score
P-values are based on Pearson Chi-square tests
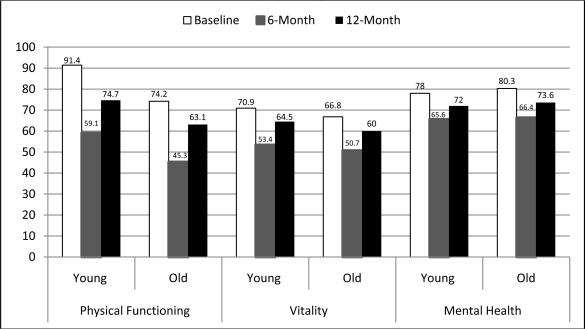
Univariate analyses comparing the SF-36 measures at baseline (pre-injury) and at 6- and 12-months post-injury are presented in Figure 1. These analyses indicated that, as expected, younger adults had significantly higher scores than older adults on the physical functioning scale (PFS) at all three time points (p < .05). Younger adults scored significantly higher on the vitality scale at baseline, but there was no significant difference at 6 months. The difference between young and old adults on the vitality scale approached significance at 12 months (p=.07). In accordance with our hypothesis, scores on the mental health scale (MHS) were higher at all time points in older adults, but these differences did not reach statistical significance.
Figure 1.
SF-36 Outcome Measures Stratified by Age Group
When we compare this sample’s scores on the PFS to the previously reported cut-offs used in the WHI-OS for evidenced weakness and slowness we see that the mean scores for the older age group began lower than this threshold and dropped significantly below the threshold at the follow-up time points. Interestingly, the younger age group’s mean score was significantly higher than the PFS threshold prior the injury, but was significantly below this threshold (59.1) at 6-months, and remained so, but to a lesser extent (74.7), at 12-months post-injury. The VS cut-off used as evidence for exhaustion in the WHI-OS was 55. Both age groups in this analysis had mean VS scores that were above this threshold prior to the injury. Both age groups dropped below the threshold at the 6-month time point (53.4 for the young and 50.7 for the older adults), but both age groups returned to VS levels above the threshold at 12-months post-injury.
Contrary to our hypothesis, there was no significant difference in the longitudinal change in any of the SF-36 scales examined between the younger and older age groups. The patterns of change, in fact, were remarkably similar in the two age groups between the 3 time points. Individuals in both age groups had a steep decline in self-reported functioning in the three domains between the time prior to the injury and 6-months post-injury. There was evidence of a recovery between 6- and 12-months, but the average score did not return to baseline values for any of these SF-36 domains. The difference is that the older adults began at a lower level.
When multivariate regression analyses were run to examine the differences in 6-month and 12-month SF-36 scores between young and old, while controlling for ISS, comorbidities, and pre-injury respective SF-36 measures, older age was no longer significantly associated with any of the outcome measures at either 6- or 12-months post-MVC. The interaction terms age*comorbidity and age*ISS were not significantly associated with the outcome measures in any of the models, indicating that the effects of comorbidity and ISS on SF-36 outcomes did not differ by age group. Therefore interaction terms were not included in the final models.
The results of the regression analyses are presented in Table 2. Comorbidity, baseline PFS, and severe injury (ISS 25+ vs. ISS ≤ 8) were significantly associated with PFS scores at 6 months, but only comorbidity and baseline PFS were associated with PFS at 12 months. Those with comorbidity had PFS scores that were 12.6 points lower at 6 months and 10.7 points lower at 12 months than those who had either none or 1 disease present. Additionally, those who were severely injured had PFS scores that were 9.6 points lower at 6 months compared to those with milder injuries. Multivariate models indicated that only pre-injury VS (p < .001) was associated with the VS at 6 months, but that both comorbidity (p < .01) and pre-injury VS (p < .001) were associated with VS at 12 months. Similar to VS, MHS at 6 months was significantly associated with only the baseline MHS score, but both comorbidity and pre-injury MHS were associated with MHS at 12 months.
Table 2.
Regression Analyses for SF-36 Outcomes at 6 and 12 Months Following Motor Vehicle Crash
| Physical Functioning Scale | Vitality Scale | Mental Health Scale | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 month | 12 month | 6 month | 12 month | 6 month | 12 month | |||||||
| Variable | Estimate | P-Value | Estimate | P-Value | Estimate | P-Value | Estimate | P-Value | Estimate | P-Value | Estimate | P-Value |
| Age | ||||||||||||
| 18–64 (ref) | ||||||||||||
| 65+ | 0.817 | 0.840 | 0.920 | 0.793 | 0.509 | 0.840 | –1.967 | 0.399 | 0.130 | 0.956 | 0.568 | 0.812 |
| Comorbid disease | ||||||||||||
| 0–1 (ref) | ||||||||||||
| 2+ | –12.638 | <.001 | –10.670 | <.001 | –1.775 | 0.410 | –4.108 | 0.036 | –1.366 | 0.487 | –3.937 | 0.044 |
| Baseline SF-36 scale* | 0.560 | <.001 | 0.655 | <.001 | 0.549 | <.001 | 0.499 | <.001 | 0.671 | <.001 | 0.689 | <.001 |
| ISS | ||||||||||||
| <8 (ref) | ||||||||||||
| 9–15 | –6.340 | 0.155 | –1.635 | 0.659 | –1.380 | 0.628 | 2.348 | 0.346 | –0.993 | 0.706 | 2.305 | 0.363 |
| 16–24 | –1.107 | 0.817 | 3.315 | 0.408 | –0.405 | 0.895 | 2.704 | 0.316 | –0.180 | 0.949 | 1.611 | 0.557 |
| 25+ | –9.605 | 0.045 | –3.558 | 0.375 | –3.657 | 0.235 | –0.245 | 0.928 | –3.729 | 0.188 | –1.139 | 0.677 |
Ref=referent; ISS = Injury Severity Scale; Estimate refers to the parameter estimate in multivariate linear regression models
Refers to the baseline value for the respective outcome measure in each analysis
DISCUSSION
Our study revealed that, as expected, the general health status (measured by the SF-36 subscales) of vehicular crash victims was lower among the elderly at baseline, 6 months, and 12 months, compared to younger adults. However, age ceased to be a significant indicator of outcomes when comorbidities and pre-injury health status were considered. When we examined 6- and 12-month general health status outcomes adjusting by baseline health status, injury severity and co-morbidities, age revealed no association with the outcomes. Pre-injury self-reported physical and mental health and comorbidities influenced the physical functional status at 6 and 12 months post-injury. Injury severity influenced the physical functional status at 6 months.
In this population, age itself is not a significant predictor of the potential for recovery when other age-associated conditions are considered. Thus, our results suggest the need for a method to assess biological age, or underlying frailty, which is a better indicator of a person’s ability to recover from an injury than is the person’s chronological age. Injury researchers examining age differences in outcomes should take into account the individual’s disease state and pre-injury functional status (based on self-reported SF-36 scale scores) as the main factors in predicting a person’s ability to recover their physical functioning following a vehicular injury.
Although a comparison of the SF-36 scores between our study population and the general population is outside the scope of this project, we note that the mean values for the PFS, VS, and MHS for males and females between the ages 65–74 are 69.4, 59.9, and 76.9, respectively [Ware, 1997]. The population norms for the same SF-36 scales among men and women age 75+ are 52.3, 50.4, and 74.0, respectively. Therefore, it appears that our sample of older MVC victims is above average for these specific domains of the SF-36 before the time of the injury, but that the mean values at 6 and 12 months post-injury are below the national standards. Since the CIREN study is not population-based it is not appropriate to compare these samples further, but we include the figures for a general reference.
The findings of our study also have clinical implications for the treatment of traumatic injuries in older MVC victims. After controlling for self-reported pre-injury physical and mental health functional status, we identified age differences in these domains following the MVC injury. These results might suggest that older patients require rehabilitation efforts that are focused more on physical domains of functioning, while younger individuals might require more support for mental health issues. However, this would require controlled prospective studies and a more representative sample.
Previous studies have illustrated that injuries sustained in MVC have significant prolonged effects on an individual’s functioning. We have shown that similar patterns exist for older adults, but that older adults begin at a lower level of self-perceived functional status and have more prevalent comorbidity, which influences the post-injury functioning. Of note, the trajectories of scores on the SF-36 values in this and other studies closely resemble that seen in the geriatric hip fracture literature. Following hip fracture, older adults exhibit a pattern of steep decline, (in two SF-36 scales included in a frailty assessment) in the two months following the injury, followed by a period of recovery between 2- and 12-months post-fracture that typically does not return to pre-fracture levels [Andersen, Miller, Shardell, et al., 2010]. In fact, this pattern is seen in multiple domains of functioning following hip fracture [Magaziner, Simonsick, Kashner, et al., 1990; Magaziner, Hawkes, Hebel, et al., 2000]. The study populations may, however, be believed to be different; that is, hip fracture patients represent those with underlying weakness, mobility and balance difficulties, and osteoporosis, while individuals that are injured in MVC (especially drivers) are presumably more active and mobile. Nevertheless, we see prolonged functional declines following non-fatal MVCs.
Limitations of the study are related to CIREN selection criteria and methodology. Being a convenience sample, the CIREN dataset may not represent the crashes and population as well as a population-based sample. Further, the sample is intended to reflect a population of moderately and severely injured occupants of a modern vehicular fleet. Milder injuries, especially among the frail or the elderly, could also result in significant decreases in functioning if the injuries compromise mobility (i.e. ankle fractures). Injuries resulting in mortality on admission are unlikely to have biased this sample, because they would not have baseline SF-36 scores and would thus be excluded from our study. Older adults, however, are more likely to die in the follow-up period and not have one or both SF-36 follow-up assessments. In the former case they would be included in this study, which may bias these analyses; but in the latter case they would be excluded based on missing consecutive follow-up assessments.
Our analysis was only able to examine markers of the frailty syndrome such as exhaustion and weakness. It would be informative to determine the prevalence of pre-injury frailty with a comprehensive frailty assessment, such as that operationalized in the Cardiovascular Health Study [Fried, Tangen, Walston, et al., 2001], which has become the most widely used frailty assessment. Additionally, simultaneously examining comorbidity and disability in a population of older adult MVC victims would help determine their independent association with post-injury recovery. It has been shown that these three geriatric conditions are highly prevalent in community-dwelling older adults and that they are independent entities despite frequent co-occurrence [Fried, et al., 2004]. Further studies should explore other biological or functional measures of frailty that could better assist in understanding recovery and tailoring treatments to individual needs.
CONCLUSION
Results of this study indicate that being older than 65 years is associated with worse self-reported physical functioning and vitality both before and after a motor vehicle crash, but not mental health status, when compared to younger MVC victims. Importantly, this study suggests that chronological age is not significantly associated with any of these self-reported measures following a crash when comorbidity, ISS, and pre-injury self-reported status are taken into account.
Acknowledgments
Dr. Andersen was supported by the National Institutes of Health training grant T32 GM075767. Data were collected as part of the National Highway Traffic Safety Administration sponsored Crash Injury Research and Engineering Network (CIREN) study. Gordon Smith, M.D., Professor, National Study Center for Trauma and EMS and the Department of Epidemiology, University of Maryland School of Medicine, reviewed the manuscript and gave editorial and content suggestions. We would also like to thank the Seattle CIREN study team.
REFERENCES
- Ameratunga SN, Norton RN, Bennett DA, Jackson RT. Risk of disability due to car crashes: A review of the literature and methodological issues. Injury. 2004;35(11):1116–1127. doi: 10.1016/j.injury.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Ameratunga SN, Norton RN, Connor JL, Robinson E, Civil I, Coverdale J, et al. A population-based cohort study of longer-term changes in health of car drivers involved in serious crashes. Annals of Emergency Medicine. 2006;48(6):729–736. doi: 10.1016/j.annemergmed.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Andersen D, Miller R, Shardell M, Ferrucci L, Magaziner J, Orwig D. ((submitted)). Transitions between frailty states among female hip fracture patients. Journal of the American Geriatrics Society [Google Scholar]
- Andersen DA, Roos BA, Stanziano DC, Gonzalez NM, Signorile JF. Walker use, but not falls, is associated with lower physical functioning and health of residents in an assisted-living environment. Clinical Interventions in Aging. 2007;2(1):123. doi: 10.2147/ciia.2007.2.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association for the Advancement of Automotive Medicine . The abbreviated injury scale, 1990 revision. Des Plaines, IL: 1998. [Google Scholar]
- Baker SP, O'Neill B, Haddon W, Jr, Long WB. The injury severity score: A method for describing patients with multiple injuries and evaluating emergency care. The Journal of Trauma: Injury, Infection, and Critical Care. 1974;14(3):187. [PubMed] [Google Scholar]
- Braver ER, Trempel RE. Are older drivers actually at higher risk of involvement in collisions resulting in deaths or non-fatal injuries among their passengers and other road users? Injury Prevention. 2004;10(1):27. doi: 10.1136/ip.2003.002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, Pasupathi M, Mayr U, Nesselroade JR. Emotional experience in everyday life across the adult life span. Journal of Personality and Social Psychology. 2000;79(4):644–655. [PubMed] [Google Scholar]
- Cheung I, McCartt AT, Braitman KA. Exploring the decline in older driver fatal crash involvement. Annals of Advances in Automotive Medicine. 2008;52:255–264. [PMC free article] [PubMed] [Google Scholar]
- Dischinger PC, Read KM, Kufera JA, Kerns TJ, Ho SM, Burch CA, et al. CIREN report: Consequences and costs of lower-extremity injuries. Washington, D.C: NHTSA; 2005. (Technical Report No. DOT HS 809 871) [PMC free article] [PubMed] [Google Scholar]
- Fitzharris M, Fildes B, Charlton J, Kossmann T. General health status and functional disability following injury in traffic crashes. Traffic Injury Prevention. 2007;8(3):309–320. doi: 10.1080/15389580701216533. [DOI] [PubMed] [Google Scholar]
- Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- Lyons RA, Perry IM, Littlepage BNC. Evidence for the validity of the short-form 36 questionnaire (SF-36) in an elderly population. Age and Ageing. 1994;23(3):182. doi: 10.1093/ageing/23.3.182. [DOI] [PubMed] [Google Scholar]
- MacKenzie EJ, McCarthy ML, Ditunno JF, Forrester-Staz C, Gruen GS, Marion DW, et al. Using the SF-36 for characterizing outcome after multiple trauma involving head injury. The Journal of Trauma: Injury, Infection, and Critical Care. 2002;52(3):527. doi: 10.1097/00005373-200203000-00018. [DOI] [PubMed] [Google Scholar]
- Magaziner J, Hawkes W, Hebel JR, Zimmerman SI, Fox KM, Dolan M, et al. Recovery from hip fracture in eight areas of function. Journals of Gerontology Series A: Biological and Medical Sciences. 2000;55(9):498–507. doi: 10.1093/gerona/55.9.m498. [DOI] [PubMed] [Google Scholar]
- Magaziner J, Simonsick EM, Kashner TM, Hebel JR, Kenzora JE. Predictors of functional recovery one year following hospital discharge for hip fracture: A prospective study. Journal of Gerontology. 1990;45(3):M101–7. doi: 10.1093/geronj/45.3.m101. [DOI] [PubMed] [Google Scholar]
- McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-item short-form health survey (SF-36): II. psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care. 1993;31(3):247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- Read KM, Kufera JA, Dischinger PC, Kerns TJ, Ho SM, Burgess AR, et al. Life-altering outcomes after lower extremity injury sustained in motor vehicle crashes. The Journal of Trauma. 2004;57(4):815. doi: 10.1097/01.ta.0000136289.15303.44. [DOI] [PubMed] [Google Scholar]
- Resnick B. Efficacy beliefs in geriatric rehabilitation. Journal of Gerontological Nursing. 1998;24(7):34–44. doi: 10.3928/0098-9134-19980701-08. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: Summary of results from the medical outcomes study. Medical Care. 1995;33(4) [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-ltem short-form health survey (SF-36): I. conceptual framework and item selection. Medical Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- Ware JE, Jr, Kosinski M. Interpreting SF-36 summary health measures: A response. Quality of Life Research. 2001;10(5):405–413. doi: 10.1023/a:1012588218728. [DOI] [PubMed] [Google Scholar]
- Ware JE., Jr . Boston; Massachusetts: 1997. SF-36 Health Survey: Manual and Interpretation Guide. [Google Scholar]
- Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, et al. Frailty: Emergence and consequences in women aged 65 and older in the women's health initiative. Journal of the American Geriatrics Society. 2005;53(8):1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]