Abstract
Mitochondrial morphological and structural changes play a role in several cellular processes, including apoptosis. We recently reported that mitochondrial elongation is also critical to sustain cell viability during macroautophagy. During macroautophagy unopposed mitochondrial fusion leads to organelle elongation both in vitro and in vivo. Longer mitochondria are protected from being degraded and possess more cristae where activity of the ATP synthase is increased, optimizing ATP production in times of nutrient restriction.
Key words: mitochondria, autophagy, fusion, cAMP, PKA, DRP-1, cell death
Mitochondria are central organelles in energy conversion and in the regulation of several signaling cascades. The functions that they play during macroautophagy, a catabolic process that allows the recycling of intracellular components under conditions of nutrient depletion, have just started to be unveiled. Mitochondria-derived reactive oxygen species contribute to the amplification of autophagy. Moreover, mitochondria can participate in the formation of the autophagosomal membrane, in a process that depends on the tethering of mitochondria to the endoplasmic reticulum. However, changes in mitochondrial morphology during induction of macroautophagy have been greatly overlooked. While we know that mitophagy, i.e., the selective degradation of mitochondria, is preceded by mitochondrial fragmentation associated with dysfunction of the organelle, whether mitochondrial morphology changes when macroautophagy is induced is completely unclear. Do mitochondria fragment in order to be engulfed and degraded? Would not degradation of mitochondria early during macroautophagy be paradoxical given their functions in energy conversion and even in biogenesis of the autophagosome?
When we recently set out to address these questions, we unexpectedly found that mitochondria elongate early during induction of autophagy. In cell culture, mitochondrial elongation is observed as soon as 30 min after starvation and maintained for 48 h. Mitochondrial elongation in response to starvation is observed in several transformed cell lines as well as in primary mouse hepatocytes. Moreover, knockdown of mTOR, another classical stimulus of autophagy, also results in mitochondrial elongation. Notably, elongation of the mitochondrial network upon induction of autophagy is also observed in vivo: elongated mitochondria are found in muscle and liver from mice fasted for 12 h.
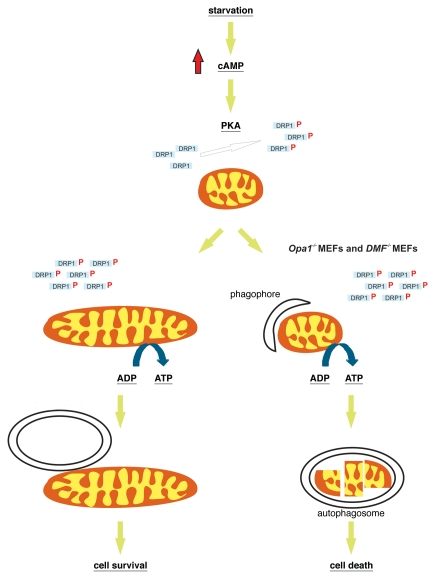
How does induction of autophagy trigger mitochondrial elongation? Starvation leads to an increase in cAMP levels and subsequent activation of PKA. Active PKA phosphorylates the cytosolic pro-fission protein Drp1 at Ser637. Once phosphorylated, DRP1 is retained in the cytosol and is unable to translocate to mitochondria, an essential step in mitochondrial fragmentation. Accordingly, during starvation, less Drp1 is associated with mitochondria that therefore can elongate as a result of unopposed fusion. Indeed, mouse embryonic fibroblasts (MEFs) lacking mitochondrial fusion proteins in the outer mitochondrial membrane, Mfn1 and Mfn2 (DMF-/- MEFs), or in the inner mitochondrial membrane, Opa1 (Opa1-/- MEFs), do not display mitochondrial elongation in response to starvation (Fig. 1).
Figure 1.
Mitochondrial elongation determines cell fate during starvation. The scheme illustrates the cascade of mitochondrial elongation induced by starvation and its role in cell survival. In cells that elongate mitochondria in response to starvation (left), the organelle is protected from degradation and ATP levels are maintained. When mitochondrial fusion is impaired (right), mitochondria are degraded while the remaining organelles consume cellular ATP, precipitating cell death.
Both starvation and mTOR silencing induce macroautophagy and the paradoxical elongation of mitochondria. Obviously, these findings raise many questions. Are mitochondria randomly targeted to the autophagosomes even if they are elongated? Does mitochondrial shape affect the rate of mitochondrial degradation during macroautophagy? To address these questions we followed the rate of degradation of mitochondrial proteins in cell models where mitochondria could or could not (Opa1-/- and DMF-/- MEFs) elongate. When fusion is impaired, mitochondria are degraded faster than when they can elongate in response to autophagic stimuli. Moreover, degradation of mitochondrial proteins can be pharmacologically blocked using an autophagy inhibitor. Thus, mitochondrial elongation during starvation protects mitochondria from autophagic degradation.
Our results raise the question of whether macroautophagy is strictly speaking a nonselective process. Degradation of organelles in response to starvation has been shown not to be a random process, but to occur in an orderly fashion. A simply steric limitation could explain why elongated mitochondria are excluded from autophagosomes. Another possibility is that elongated mitochondria lack the signal that targets them to autophagy. Interestingly, we found that during starvation short mitochondria, which lack mitochondrial fusion proteins, display a latent dysfunction. An appealing hypothesis would be that a signal responsible for targeting the organelle to autophagy, as, for instance, Parkin, re-localizes to these dysfunctional mitochondria, prompting their degradation.
Why should mitochondria be spared from macroautophagy? During nutrient deprivation, cells need to maximize energy production that under these conditions relies mainly on internal substrates, like amino acids resulting from protein catabolism. In eukaryotic cells this task is optimally performed by mitochondria. The energetic role of mitochondria is intimately linked to their ultrastructure: the invaginations of the inner membrane known as cristae increase the surface of the membrane where the electrochemical gradient is generated (thereby maximizing thermodynamic efficiency) and are also the site where ATP synthase can dimerize to work efficiently in converting the electrochemical gradient into ATP. Interestingly, the number of cristae per surface increases in the elongated mitochondria of autophagic cells, and this is accompanied by more dimers of the ATP synthase. Being able to optimize ATP production when cells have a limited access to substrates could have an impact on cell viability. Indeed, we found that cells unable to elongate mitochondria die faster in response to starvation than their wild-type counterparts (Fig. 1). As mentioned before, shorter mitochondria are not only less efficient in producing ATP, but they also display a latent dysfunction, consuming cytosolic ATP to maintain their membrane potential. Consumption of ATP leads to a bioenergetic crisis that contributes to the faster rate of cell death.
The discovery that mitochondria elongate during macroautophagy, and that this has a major impact on viability of the starving cells, illustrates another cellular process regulated by these organelles. Many more questions remain to be addressed: what is, for example, the physiological significance of mitochondrial elongation during nutrient depletion in vivo? Is this a stereotypical response of all tissues? How do individuals that present mutations in the mitochondrial-shaping machinery deal with neonatal starvation? Similarly, our results can contribute to clarify why heteroplasmic mitochondrial DNA (mtDNA) mutations are not efficiently removed by the mitophagy machinery: if mitochondria are still able to elongate, they could be spared from autophagic degradation. Given that the fusion proteins are GTPases amenable to drug treatment, one could envision the possibility of inducing efficient mitophagy by combining inhibition of fusion with limited starvation. Hopefully, this could be a possible treatment to ameliorate the load of mutated mtDNA in patients with mitochondrial diseases.
Punctum to: Gomes LC, Benedetto GD, Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol. 2011;13:589–598. doi: 10.1038/ncb2220.