Abstract
The prevalence of smoking in HIV-infected subjects is high. As a smoking cessation aid, varenicline (Champix®, Pfizer, Saint-Laurent, QC, Canada or Chantix®, Pfizer, Mission, KS) has not been previously evaluated in HIV-infected smokers. In this multicenter pilot open label study, varenicline 1.0 mg was used twice daily for 12 weeks with dose titration in the first week. Adverse events (AEs) during the treatment period were recorded. Changes from baseline in laboratory tests, vital signs, daily cigarette consumption, nicotine dependence, and withdrawal were measured through week 24. Self-reported abstinence was validated by serum cotinine at week 12. We enrolled 36 subjects with a mean of 29 pack-years of smoking and a minimum of 4 cigarettes per day. All but 1 were male, 33 (92%) were white. The most frequently reported AEs were nausea (33%), abnormal dreams (31%), affect lability (19%), and insomnia (19%). Six (17%) subjects discontinued varenicline due to AEs. No grade 3/4 laboratory abnormalities or serious AEs occurred during the study. There was no significant change in HIV viral load. CD4 counts increased by 69 cells/mm3 (p=0.001) at week 24. Serum cotinine-verified 4-week continuous abstinence rate through weeks 9–12 was 42% (95% confidence interval [CI]: 26–58%). AEs and abstinence rates were comparable to those in published randomized controlled trials conducted in generally healthy HIV-negative smokers. Varenicline was safe and appears effective among HIV-infected smokers in this exploratory study, although AEs were common. The most common AE was nausea, with no adverse effect on HIV treatment outcome. Close monitoring of liver enzymes and blood pressure is recommended for HIV-positive smokers taking varenicline.
Introduction
With modern anti-retroviral therapies (ART), there is a greatly increased chance of long-term survival and a decreased chance of opportunistic infections in HIV-infected persons.1,2 The majority of HIV deaths in developed countries are now caused by cancer and by heart, lung, and liver disease.3,4 Smoking is the most common and important modifiable risk factor for these diseases. Smoking prevalence of 50–70% or higher in HIV-infected subjects has been reported previously,3,5–8 compared with less than 20% in the general Canadian adult population.9 Quitting smoking can improve symptom burden and the quality of life,10 and decrease the incidence of cardiovascular diseases8 in HIV-positive people. After appropriate initiation of ART, smoking cessation may be the single most important intervention for improving long-term survival and quality of life in HIV-infected people. However, there are limited data regarding the efficacy of smoking cessation in the context of HIV, despite a few successful smoking cessation programs in HIV-infected people.11,12
In February 2007, Health Canada approved varenicline tartrate (Champix® in Canada and many countries [Saint-Laurent, QC, Canada], or Chantix® in the United States [Pfizer, Mission, KS]) as a new smoking cessation aid in adults, to be used in conjunction with smoking cessation counseling. Varenicline acts as a partial agonist at α4β2 nicotinic acetylcholine receptors in the brain, reducing nicotine craving. A previous review showed that varenicline is more effective than bupropion (Zyban®, GlaxoSmithKline, Philadelphia, PA), nicotine replacement therapy, or placebo.13 The most frequently reported adverse event (AE) of varenicline was nausea, which was generally mild or moderate. Dose titration over the first week helps minimize nausea. As a new promising smoking cessation aid, varenicline had not yet been tested in HIV-infected subjects prior to our study.
The study protocol was approved by Hospital Research Ethics Boards (REBs) at the Hamilton Health Sciences, Hamilton, Ontario, Canada, and at Windsor Regional Hospital, Windsor, Ontario, Canada. Health Canada issued a “No objection letter.” All participants gave written consent.
Methods
Study design, setting, and participants
We designed a multicenter open-label study, conducted at two regional HIV clinics in Hamilton and Windsor, Ontario, Canada, between October 2008 and July 2010. Subjects satisfied all of the following five inclusion criteria: (1) age 19–64 years; (2) smokes one or more cigarettes per day; (3) has attempted to quit at least once previously; (4) no abstinence period of greater than 3 months during the past year; and (5) weight 45–125 kg. Subjects who had any one of the following seven conditions were excluded: (1) pregnant or nursing women; (2) allergy to varenicline; (3) cancer or transplant; (4) epilepsy, on antiepileptic drugs, on antidepressants, or antipsychotic drugs; (5) significant cardiovascular disease, uncontrolled hypertension, or cataract; (6) nausea, irritable bowel, or other significant gastrointestinal symptoms; or (7) currently on nicotine replacement therapy. In addition, if the subject was not on ART, a CD4 T-lymphocyte count of at least 350 cells/mm3 was required, and subjects for whom the physician was planning to start ART within the next 6 months were excluded. If the subject was on ART, a CD4 T-lymphocyte count of over 200 cells/mm3 was required, with undetectable viral load (less than 50 copies per milliliter) for the past 6 months, and he/she should be judged by the physician to not require a change in ART in the next 6 months. Potential eligible subjects were referred to research staff by their physician or were approached by our trained research staff during routine clinical visits. After consenting, all the subjects underwent an electrocardiography to rule out signs of past cardiovascular disease.
Study intervention and follow-up
We titrated the dosage of varenicline in the first week: 0.5 mg once daily for days 1–3, then 0.5 mg twice daily for days 4–7, followed by 1.0 mg twice daily from day 8 until week 12. We offered each subject a package of reading materials, including educational materials from the Ontario Canadian Cancer Society (“Thinking about quitting?” “Quit. You Have It in You”; “What's your poison?”), Hamilton Public Health Services (“How You Can Manage Your Nicotine Withdrawal Symptoms”) and the Ontario Lung Association (“Tips to Help You Quit!”). Counseling was delivered to the subject by a physician or a trained counselor, in accordance with a U.S. clinical practice guideline on treating tobacco use and dependence.14 A target quit date was set 8–14 days after starting varenicline.
We followed subjects weekly during the 12-week treatment period and biweekly during the 12-week nontreatment period in Hamilton. In Windsor, we followed subjects weekly during the first 4 weeks, then at weeks 8, 12, 16, and 24. At both sites, four clinical visits were scheduled at baseline, weeks 4, 12, and 24 when self-administered questionnaire, physical examination, and blood work were obtained. Fifteen (in Hamilton) or five (in Windsor) follow-up visits were scheduled over the 24-week study by telephone, e-mail, or personal interview to collect information on smoking and AEs and to deliver brief counseling. Subjects who discontinued varenicline before week 12 were still followed according to the original schedule, or information was collected by medical chart review if they declined further participation.
Safety assessment
AEs were collected through regular follow-up and patient report. We asked about AEs starting with a defined open-ended question: “Since the last follow-up, have you had any new symptoms?” We then assessed AE severity, duration, date of onset, action taken, and the suspected relationship to varenicline.15–17 All the AEs were self-reported. An SAE was judged by the subject's physician and by the principal investigator.
Blood work was measured at each clinical visit, including: complete blood count, creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), CD4 T-lymphocyte count, and plasma HIV viral load (Chiron 3.0 bDNA assay). Abnormalities in laboratory results were graded according to the Division of AIDS (DAIDS) AEs scale.18 In addition, for subjects with elevated AST or ALT at baseline, grading was based on changes relative to baseline rather than upper limit of normal. Grade numbers 1–4 represent mild, moderate, severe, and potentially life-threatening abnormality, respectively.18,19
Weight and blood pressure were measured at baseline, weeks 12, and 24.
Measurement of effectiveness, nicotine dependence, and withdrawal
The primary outcome for effectiveness was measured by the serum cotinine-verified 4-week continuous abstinence rate (4W-CAR) from weeks 9–12. Abstinence from smoking was defined as no smoking, not even a puff, for at least 7 consecutive days prior to each follow-up. Self-reported abstinence at week 12 was validated by serum cotinine concentrations below 3 ng/mL.20 Cotinine was quantified by liquid chromatography-mass spectrometry.21 Secondary effectiveness estimates included: serum cotinine-verified continuous abstinence rate (CAR) through weeks 9–24; self-reported 7-day point prevalence of abstinence (7D-PP) at each follow-up; and changes in daily cigarette consumption.
The Fagerström Test for Nicotine Dependence (FTND) questionnaire22–24 was self-administrated at baseline for all subjects and at subsequent clinical visits for subjects who still smoked. FTND scores of 0–2 indicate very low nicotine dependence, 3–4, low; 5, medium; 6–7, high; and 8–10, very high.24 The Minnesota Nicotine Withdrawal Scale (MNWS) questionnaire25–27 was self-administrated at each clinical visit for all the subjects, measuring five subdomains of nicotine withdrawal symptoms: negative affect (including depressed mood, irritability/frustration/anger, anxiety/nervousness, and difficulty concentrating), with subscore ranging from 0–16; insomnia (including difficulty going to sleep and staying asleep), with subscore ranging from 0–8; restlessness ranging from 0–4; increased appetite from 0–4, and craving from 0–4. In each subdomain, higher scores indicated more severe nicotine withdrawal symptoms.27
Statistical analysis
This study was an exploratory investigation using a pre–post design. The sample size of 36 was determined based on feasibility considerations. Demographic and baseline characteristics were summarized using descriptive statistics reported as mean (standard deviation [SD]) for continuous variables and count (percent) for categorical variables. Proportions were calculated as the number of events divided by the total number of subjects, and expressed with 95% confidence intervals (CIs). The change from baseline to follow-up visit was reported as mean (standard error [SE]) and analyzed by general linear models allowing for repeated measures. Repeated measured proportions were analyzed using generalized estimating equations. Missing data were replaced by the data from last follow-up visit. All statistical tests were two-sided with an α of 0.05. We did not adjust the overall level of significance for multiple testing as the analyses were primarily exploratory. We used PASW Statistics 18 (SPSS Inc., Chicago, IL) to analyze data.
In addition, we found randomized controlled trials (RCTs) conducted in HIV-negative generally healthy smokers from the published literature, identified RCTs with at least one treatment arm using varenicline at the same dosage and duration as in our study, and compared our results to the pooled published results. Pooled estimation from external comparison studies was weighted by the variance in each study. We used one sample t tests to compare the results in our study to pooled estimates.
Results
Demographic and baseline information
We recruited 36 subjects, including 20 subjects from Hamilton and 16 from Windsor (Table 1). Subjects were similar between the two sites, with mean (SD) age of 46 (8) years old. The majorities were male, white, on ART, and had viral load less than 50 copies per milliliter. Mean (SD) CD4 T-lymphocyte count was 601 (291) cells/mm3. Subjects had smoked an average of 29 (22) pack-years, with minimum 4 cigarettes per day and 23 (64%) having medium, high or very high levels of nicotine dependence by FTND score. Thirty (83%) subjects had previously quit smoking for more than 7 days with a mean (SD) of 2.3 (2.5) previous quit attempts.
Table 1.
Demographic and Baseline Information
| Total, n=36 | Hamilton, n=20 | Windsor, n=16 | |
|---|---|---|---|
| Age, mean (SD) years | 46 (8) | 46 (7) | 46 (9) |
| Male, n (%) | 35 (97) | 20 (100) | 15 (94) |
| White, n (%) | 33 (92) | 19 (95) | 14 (88) |
| Years living with HIV, mean (SD) | 11 (7) | 11 (7) | 12 (7) |
| ART use, n (%) | 31 (86) | 15 (75) | 16 (100) |
| CD4, mean (SD) cells/mm3 | 601 (291) | 605 (295) | 595 (297) |
| Suppressed viral load, n (%) | 29 (81) | 14 (70) | 15 (94) |
| Log10 viral load if detectable, mean (SD) | 3.92 (0.75) | 3.85 (0.80) | 4.31 (-) |
| Cigarettes per day, mean (SD) | 19 (10) | 18 (8) | 20 (13) |
| Number of years smoked, mean (SD) | 29 (8) | 28 (7) | 31 (10) |
| Pack-years of smoking, mean (SD) | 29 (22) | 25 (14) | 34 (29) |
| FTND score, mean (SD) | 5.4 (2.2) | 5.7 (1.8) | 5.1 (2.6) |
| Medium/high/very high FTND score, n (%) | 23 (64) | 14 (70) | 9 (56) |
| Previous quit attempts of more than 7 days, mean (SD) | 2.3 (2.5) | 2.9 (2.5) | 1.7 (2.3) |
p values comparing two sites (not shown) were obtained by one-way analysis of variance (ANOVA) for continuous variables and by χ2 test for categorical variables. Fisher's exact test was used if the number in any cell was less than 10. All p values were more than 0.05. Suppressed viral load, viral load less than 50 copies per millilitre. FTND scores of 0–2 indicate very low nicotine dependence; 3–4, low; 5, medium; 6–7, high; and 8–10, very high.24
SD, standard deviation; ART, anti-retroviral therapy; FTND, Fagerström Test for Nicotine Dependence.
Safety and tolerability
Incidence and severity of AEs
Twenty-eight (78%) subjects reported AEs during the treatment period (Table 2). The most frequently reported AE was nausea, reported by one third of subjects including 2 (6%) who reported it as severe; followed by abnormal dreams (31%); affect lability (irritability, mood swing, agitation, or anger; 19%), and insomnia (19%). Other AEs were generally reported as trivial, mild, moderate, or marked. The incidence rate of abnormal dreams was 29% (5/17) in those on efavirenz versus 32% (6/19) in those not on efavirenz (p=1.000). No SAEs occurred during the drug treatment period. A 61-year-old white man, who had smoked for 45 years at 10 cigarettes a day, was diagnosed with lung cancer at week 22. Because of the long latency of lung cancer, we did not consider this event to be related to varenicline.
Table 2.
Adverse Events During Treatment (n=36)
| Total reported, n (%) | Severe or intolerable, n (%) | |
|---|---|---|
| Any AEs | 28 (78) | 12 (33) |
| Nausea | 12 (33) | 2 (6) |
| Abnormal dreams | 11 (31) | 2 (6) |
| Affect labilitya | 7 (19) | 3 (8) |
| Insomnia | 7 (19) | 2 (6) |
| Headache | 5 (14) | 2 (6) |
| Gastroesophageal reflux disease | 4 (11) | 2 (6) |
| Abdominal pain | 4 (11) | 1 (3) |
| Nightmares | 3 (8) | 2 (6) |
| Sleep disorder | 3 (8) | 1 (3) |
| Weight gain | 3 (8) | 1 (3) |
| Vomiting | 3 (8) | 0 |
| Constipation | 3 (8) | 0 |
| Taste perversion | 3 (8) | 0 |
| Fatigue | 3 (8) | 0 |
| Depressed mood | 2 (6) | 1 (3) |
| Smell perversion | 2 (6) | 1 (3) |
| Gas | 2 (6) | 0 |
| Sweat | 2 (6) | 0 |
| Increased appetite | 1 (3) | 1 (3) |
| Dry mouth | 1 (3) | 0 |
| Dizziness | 1 (3) | 0 |
| Hot flush | 1 (3) | 0 |
| Muscle pain | 1 (3) | 0 |
Affect lability, included irritability, mood swing, agitation, and anger.
AEs, adverse events.
Subjects' responses to AEs and discontinuation of varenicline
Four (11.1%) subjects decreased their dose of varenicline because of AEs during the treatment period, including 3 (8%) subjects with nausea. Only 1 subject completed the entire 12-week treatment at the decreased dose, whereas the remaining 3 subjects discontinued varenicline before week 12. Nine (25%) subjects took medications for AEs during the treatment period, and 1 (3%) visited his doctor for pneumonia as well as for a sleeping disorder. No subject was hospitalized because of AEs. In total, 10 (28%) subjects discontinued varenicline before week 12, and 6 (17%) of these discontinuations were due to AEs, including 4 (11%) cases of nausea, 1 (3%) of anger, and 1 (3%) of insomnia. Of these 10 subjects, 3 (8%) withdrew from the study.
We found seven published RCTs conducted in generally healthy smokers, with at least one arm using varenicline 1.0 mg twice a day for 12 weeks, serving as our external comparison studies.28–34 Incidence rates in overall AE and nausea in our study were comparable to those in external comparison studies (Table 3), however, we had a significantly higher incidence rate of abnormal dreams (31% versus 9%, p<0.001) and a higher discontinuation rate (28% versus 13%, p=0.011).
Table 3.
Comparison of Varenicline Adverse Events and Effectiveness Rates % (95% CI) Between the Current and Previously Published Studies
| Study | Total n | Any AE | Nausea | Abnormal dreams | Discontinuation | 4W-CAR weeks 9–12 | CAR weeks 9–24 |
|---|---|---|---|---|---|---|---|
| Current study | 36 | 78 (64, 91) | 33 (18, 49) | 31 (16, 46) | 28 (13, 42) | 42 (26, 58) | 28 (13, 42) |
| Wang et al., 200928 | 165 | 77 (71, 83) | 29 (22, 36) | 4 (1, 7) | 3 (0, 6) | 50 (42, 58) | 38 (31, 46) |
| Aubin et al., 200829 | 376 | 85 (81, 88) | 37 (32, 42) | 12 (8, 15) | 17 (13, 21) | 56 (51, 61) | 32 (28, 37) |
| Nakamura et al., 200730 | 156 | 80 (74, 86) | 24 (18, 31) | — | 8 (4, 13) | 65 (57, 74) | 38 (29, 46) |
| Tsai et al., 200731 | 126 | 87 (81, 92) | 44 (35, 52) | 6 (2, 10) | — | 60 (51, 68) | 47 (38, 56) |
| Oncken et al., 200632 | 130 | 79 (72, 86) | 35 (27, 43) | 19 (13, 26) | 23 (16, 31) | 55 (46, 63) | — |
| Jorenby et al., 200633 | 344 | — | 29 (25, 34) | 13 (10, 17) | 24 (20, 29) | 44 (39, 49) | 30 (25, 34) |
| Gonzales et al., 200634 | 352 | 79 (75, 83) | 28 (23, 33) | 10 (7, 14) | 26 (21, 30) | 44 (39, 49) | 30 (25, 34) |
| Pooled studya | 82 (80, 84) | 32 (29, 34) | 9 (8, 11) | 13 (11, 14) | 51 (49, 54) | 33 (31, 36) | |
| p value | 0.545 | 0.819 | <0.001 | 0.011 | 0.248 | 0.495 |
Pooled study: studies 28–34, all of them were conducted in non-HIV generally healthy smokers, and only the arm using varenicline 1.0 mg twice daily for 12 weeks was included. Estimations in pooled study were weighted by the variance in each study. p values were calculated by one sample t-test, where the proportions in our study were compared to that in pooled study.
AE, adverse event; 4W-CAR, 4-week continuous abstinence rate; CAR, continuous abstinence rate.
CD4-positive T-lymphocyte count
CD4 counts increased at week 24 by a mean (SE) of 69 (20) cells/mm3 (p=0.001, Table 4). The change was neither associated with study site, age, HIV infection category, smoking status or discontinuation of varenicline before week 12, nor with suppressed viral load or ART use.
Table 4.
Effects on Immune System, Fagerström Test for Nicotine Dependence Score, Minnesota Nicotine Withdrawal Scale, and Daily Cigarette Consumption
| |
|
Change,amean (SE) |
||
|---|---|---|---|---|
| Variable | Baseline, mean (SD) | Week 4 | Week 12 | Week 24 |
| CD4 count, cells/mm3, n=36 | 601 (291) | −2 (22) | 20 (23) | 69 (20)b |
| Log10 viral load if detectable, n=6 | 3.79 (0.73) | −0.20 (0.17) | −0.39 (0.25) | −0.64 (0.42) |
| FTDN score, n=17 | 5.5 (2.3) | −3.1 (0.6)c | −1.6 (0.5)b | −2.1 (0.6)b |
| MNWS, n=36 | ||||
| Negative affect | 3.4 (3.3) | −0.4 (0.6) | −0.5 (0.7) | 0.1 (0.7) |
| Insomnia | 3.1 (2.7) | −0.2 (0.5) | −0.9 (0.4)d | 0.4 (0.5) |
| Restlessness | 1.2 (1.2) | −0.1 (0.2) | −0.2 (0.2) | 0 (0.2) |
| Increased appetite | 0.9 (1.2) | 0.5 (0.2)d | 0.3 (0.2) | 0.2 (0.3) |
| Craving | 2.6 (1.0) | −1.1 (0.2)c | −1.2 (0.3)c | −1.1 (0.2)c |
| Cigarettes per day, n=17 | 19 (11) | −11 (2)c | −7 (2)b | −5 (2)d |
Change, calculated by comparing to baseline.
p<0.01.
p<0.001.
p<0.05.
FTND scores of 0–2 indicate very low nicotine dependence; 3–4, low; 5, medium; 6–7, high; and 8–10 very high.24 Negative affect includes depressed mood, irritability/frustration/anger, anxiety/nervousness and difficulty concentrating, with subscore ranging 0–16; insomnia includes difficulty going to sleep and staying asleep, with subscore ranging 0–8; restlessness ranging 0–4; increased appetite 0–4, and craving 0–4. In each subdomain, higher scores indicated more severe nicotine withdrawal symptoms.27
FTDN, Fagerström Test for Nicotine Dependence; MNWS, Minnesota Nicotine Withdrawal Scale; SD, standard deviation; SE, standard error.
Plasma HIV viral load
All 29 (80.6%) subjects with suppressed viral load at baseline were still undetectable at each clinical visit. In addition, 1 subject started ART after completing 12-week varenicline treatment and had undetectable viral load at week 24. Log10 HIV viral load did not vary for 6 subjects who always had a detectable HIV viral load throughout the study.
Other laboratory tests
The changes in white blood cell (WBC) count, platelet count, and AST were not significantly different during the study. For hemoglobin and creatinine, although the changes were significantly different at some visits, the changes were considered within normal range at all visits. Mean (SD) baseline ALT was 32 (20) IU/L, the mean (SE) change was significantly different at week 12 by 10 (4) (p=0.011) and at week 24 by 8 (3) IU/L (p=0.004), respectively. All the ALT elevations were grade 1 at all follow-up visits: mild elevation in 2 (6%) subjects at week 4, in 6 (17%) subjects at week 12, and in 7 (19%) subjects at week 24, respectively, versus at baseline when 4 (11%) subjects had mild and 1 (3%) had moderate ALT elevations.
Blood pressure changes
Baseline mean (SD) blood pressure was 123 (15)/76 (9) mm Hg. Systolic blood pressure did not change significantly during the study, whereas diastolic blood pressure increased by a mean (SE) of 6 (1) mm Hg (p<0.001) at week 12 and 4 (2) mmHg (p=0.049) at week 24, respectively. Eight (22%) subjects had elevated diastolic blood pressure resulting in hypertension, including 6 (17%) at week 12 and 4 (11%) at week 24. No grade 3/4 abnormality in diastolic blood pressure occurred.
Weight changes
Subjects gained a significant amount of weight by a mean (SE) of 1.7 (0.6) kg at week 12 (p=0.007) and 2.8 (0.7) kg at week 24 (p<0.001). Weight changes were not associated with study site, or with smoking status.
Effectiveness
Continuous abstinence rates (CARs)
Serum cotinine-confirmed 4W-CAR (95% CI) through weeks 9–12 was 42% (26%, 58%), and CAR through weeks 9–24 was 28% (13%, 42%; Table 3). Both abstinence rates were comparable to those in previously published trials (Table 3).
Self-reported seven-day point prevalence (7D-PP) of abstinence
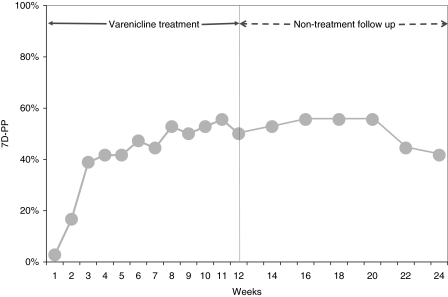
The 7D-PP increased markedly to 39% in the first 3 weeks, was 50% at week 12 and 42% at the end of study at week 24 (Fig. 1). Twenty (56%) subjects quit for at least 7 days at least once during the 12-week treatment period and 23 (64%) subjects did so during the entire 24-week study.
FIG. 1.
Self-reported seven-day point prevalence (7D-PP) of abstinence at each follow-up. 7D-PP at each follow-up was defined as the number of subjects who self-reported to be continuously abstinent from smoking for the 7 days preceding follow-up divided by the total number of subjects who participated in the study at baseline. Weekly follow-up was taken during 12-week varenicline treatment period and biweekly follow up was taken during nontreatment period until week 24.
Effect on daily cigarette consumption and FTND score
Seventeen (47%) subjects never quit, although they cut down significantly, with mean (SE) decrease of 11 (2), 7 (2), and 5 (2) cigarettes per day at weeks 4, 12, and 24, respectively (Table 4). Those continuous smokers also had significantly decreased FTND scores at each visit.
Effect on MNWS score
The changes in MNWS subscores of negative affect, restlessness, and increased appetite were not significantly different (Table 4). For insomnia, the change was significant by week 12 only. For craving, the subscore significantly decreased at each visit.
Discussion
In this study, we found that stable HIV-positive subjects were able to take varenicline for 12 weeks. Side effects were common but generally tolerable, and varenicline had no adverse effects on HIV control. Indeed, CD4 T-lymphocyte counts improved during the study. We had no direct comparison in our study; however, we compared our results with that in HIV-negative generally healthy smokers from published RCTs with one arm using varenicline 1.0 mg twice a day for 12 weeks, and found our rates comparable in overall incidence rates of any adverse events, and of nausea. However, the incidence rate of abnormal dreams was significantly higher in our study, and our discontinuation rate was also somewhat higher. While side effects were common, they were not necessarily bothersome. AEs such as weight gain or abnormal dreams were desirable to some subjects. Conversely, HIV-positive subjects are often questioned regarding abnormal dreams as part of their routine HIV care since certain commonly used HIV medications such as efavirenz frequently cause abnormal dreams. Our study subjects might be more aware of this side effect compared to HIV-negative subjects, and HIV-infected smokers as a whole might report more abnormal dreams than their HIV-negative counterparts, reflecting potential ascertainment bias. However, in HIV-infected smokers, we found no association between self-reported abnormal dreams and the use of efavirenz, indicating that varenicline itself is likely the cause of abnormal dreams in HIV-positive subjects. The higher incidence of reported abnormal dreams and the higher discontinuation rate in our study suggest precautions for using varenicline in HIV-infected smokers, and questioning about these side effects at follow-up visits. In addition, a recent published meta-analysis including 14 RCTs conducted in non-HIV smokers showed varenicline was associated with an elevated odds ratio of 1.72 (95% CI: 1.09–2.71) for any ischemic or arrhythmic cardiovascular event.35 Given the existing concerns of ART-related cardiovascular events,6,36,37 precautions and close monitor of serious cardiovascular AEs are needed in HIV-infected smokers using varenicline.
Changes in laboratory tests and blood pressure verified that varenicline generally was safe, without significant changes or grade 3/4 abnormalities during the study in hemoglobin, WBC, platelet, creatinine, AST, and systolic blood pressure. However, we found mild ALT elevations during the study. We also detected small but statistically significant changes in diastolic blood pressure, which were not associated with weight change. On the other hand, in three external comparison studies we used, only one case of ALT elevation was reported respectively,28,30,31 and no significant change in blood pressure was reported in any external comparison study. Although inconclusive, due to limitations in our study, our results suggest that HIV-positive patients taking varenicline may benefit from monitoring of their liver enzymes and blood pressure.
In addition to estimating AEs, we designed our study to examine the safety and tolerability of varenicline in the context of HIV infection. We detected no adverse effect of varenicline on HIV control. Viral loads did not change. However, we found a statistically significant and clinically important improvement in CD4 T-lymphocyte counts by week 24 of follow-up. By comparison, a previous study showed that CD4 count significantly increased by 93–151 cells/mm3 within 6 months after highly active antiretroviral therapy initiation, then slowed down to 22–36 cells/mm3 per year through the first 4 years, and then leveled off.38 In this context, the CD4 increase in our study (69 cells/mm3 in 24 weeks) is potentially important, and was not associated with either smoking status or with use of ART. However, as this was not an anticipated finding, it could be due to chance and must be interpreted with caution. Nevertheless, larger cohort studies should examine the long-term effects of varenicline and of smoking cessation on CD4 lymphocyte counts, as an immunologic benefit would argue for an even greater emphasis on smoking cessation in HIV care.
The serum cotinine-verified 4W-CAR through weeks 9–12 in our study was similar to the pooled estimate in the literature (Table 3), as well as the verified CAR through weeks 9–24. The comparable efficacy of varenicline in our study is also supported by the significantly decreased daily cigarette consumption, FTND scores and MNWS craving subscores in our study subjects (Table 4) and in the literature.33,34 However, due to small sample size and lack of control group, the effectiveness of varenicline in our study should be interpreted with caution. Moreover, we enrolled daily smokers with more than one cigarette per day, versus more than 10 cigarettes a day in six external comparison studies28,30–34 and more than 15 cigarettes a day in one external comparison study.29 A lower daily cigarette consumption is associated with higher odds of quitting,39 although in our study neither the abstinence at week 12 or 24 was associated with baseline cigarettes per day.
Our study has important limitations. We had a small sample size in this open label, nonrandomized study. Thus, we can provide estimates of side effects and of effectiveness, but with fairly wide confidence intervals. We were not able to differentiate AEs of varenicline from nicotine withdrawal symptoms without a placebo control group. We studied only stable subjects, who were not on antidepressant or antipsychotic medication, with a high mean (SD) baseline CD4 count of 601 (291) cells/mm3, and 81% of our subjects had undetectable viral load. Thus, we cannot generalize to acutely ill or to more severely immunocompromised HIV patients, or those on treatment for mental illness, in whom side effects or efficacy could be different. We only verified self-reported smoking status at week 12, and could have slightly overestimated the effectiveness of varenicline. However, we classified all invalidated subjects as smokers when we calculated 4W-CAR through weeks 9–12 and CAR through weeks 9–24, thereby minimizing any possible overestimation.
In summary, varenicline was as safe in HIV-infected smokers as in HIV-negative smokers, although AEs were common and occasionally resulted in drug cessation. Monitoring of side effects such as nausea, abnormal dreams, and cardiovascular AEs, clinical parameters such as liver enzymes, blood pressure, and weight are recommended until there is a greater experience with varenicline in HIV-infected patients. In addition, effectiveness of varenicline in this exploratory study was comparable to the HIV-negative smokers from previously published trials. We conclude that varenicline appears to be a safe and effective adjunct for smoking cessation among HIV-infected patients, together with appropriate counseling, and encourage further studies to evaluate efficacy of varenicline and immunologic benefit of smoking cessation on CD4 lymphocyte counts in this vulnerable population.
Acknowledgments
Presented in part at the annual research conference of Ontario HIV Treatment Network, Toronto, November 15–16, 2010.
Pfizer Canada Inc. sponsored this investigator-initiated study. Canadian Institutes of Health Research provided a stipend to Q.C. during her Ph.D. studies. Dr. Christine Lee, Dr. Shariq Haider, Dr. Philippe El-Helou, Dr. Atreyi Mukherji, and Dr. Kevin Woodward helped in recruiting subjects, delivering brief counseling, and performing physical examinations. Alisa Koop, Gita Sobhi, and Karen Currie helped with drug dispensing. The Canadian Cancer Society (Ontario), Hamilton Public Health Services and the Ontario Lung Association provided educational materials. We thank all these people and organizations, as well as our study subjects, for their support and help.
Pfizer Canada funded this investigator-initiated study. Pfizer Canada did not have any involvement in study design, data collection, data analysis, or manuscript writing. The authors are editorially independent from the funding body. None of the authors has commercial associations (consultancies, stock ownership, equity interest, or patent/licensing arrangements) that might pose a conflict of interest in connection with the study.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Lima V. Hogg R. Harrigan P, et al. Continued improvement in survival among HIV-infected individuals with newer forms of highly active antiretroviral therapy. AIDS. 2007;21:685–692. doi: 10.1097/QAD.0b013e32802ef30c. [DOI] [PubMed] [Google Scholar]
- 2.The Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: A collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krentz H. Kliewer G. Gill M. Changing mortality rates and causes of death for HIV-infected individuals living in Southern Alberta, Canada from 1984 to 2003. HIV Med. 2005;6:99–106. doi: 10.1111/j.1468-1293.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 4.Sackoff JE. Hanna DB. Pfeiffer MR. Torian LV. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145:397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- 5.Benard A. Bonnet F. Tessier J, et al. Tobacco addiction and HIV infection: Toward the implementation of cessation programs. ANRS CO3 Aquitaine Cohort. AIDS Patient Care STDs. 2007;21:458–468. doi: 10.1089/apc.2006.0142. [DOI] [PubMed] [Google Scholar]
- 6.Friis-Møller N. Reiss P. Sabin C, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 7.Tesoriero J. Gieryic S. Carrascal A. Lavigne H. Smoking among HIV positive New Yorkers: Prevalence, frequency, and opportunities for cessation. AIDS Behav. 2010;14:824–835. doi: 10.1007/s10461-008-9449-2. [DOI] [PubMed] [Google Scholar]
- 8.Petoumenos K. Worm S. Reiss P, et al. Rates of cardiovascular disease following smoking cessation in patients with HIV infection: Results from the D:A:D study. HIV Med. 2011;12:412–421. doi: 10.1111/j.1468-1293.2010.00901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Health Canada. Overview of Historical Data. 1999–2009. www.hc-sc.gc.ca/hc-ps/tobac-tabac/research-recherche/stat/_ctums-esutc_2009/ann-histo-eng.php. [Oct 28;2010 ]. www.hc-sc.gc.ca/hc-ps/tobac-tabac/research-recherche/stat/_ctums-esutc_2009/ann-histo-eng.php Updated September 27, 2010.
- 10.Vidrine D. Arduino RC. Gritz E. The effects of smoking abstinence on symptom burden and quality of life among persons living with HIV/AIDS. AIDS Patient Care STDs. 2007;21:659–666. doi: 10.1089/apc.2007.0022. [DOI] [PubMed] [Google Scholar]
- 11.Drach L. Holbert T. Maher J. Fox V. Schubert S. Saddler L. Integrating smoking cessation into HIV care. AIDS Patient Care STDs. 2010;24:139–140. doi: 10.1089/apc.2009.0274. [DOI] [PubMed] [Google Scholar]
- 12.Zwiebel MA. Hughes V. Smoking Cessation Efforts in One New York City HIV Clinic. J Assoc Nurses AIDS Care. 2010;21:11–15. doi: 10.1016/j.jana.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Eisenberg M. Filion K. Yavin D, et al. Pharmacotherapies for smoking cessation: A meta-analysis of randomized controlled trials. CMAJ. 2008;179:135–144. doi: 10.1503/cmaj.070256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Treating Tobacco Use and Dependence. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services; Jun, 2000. [Google Scholar]
- 15.Wisniewski SR. Rush AJ. Balasubramani GK. Trivedi MH. Nierenberg AA. Self-rated global measure of the frequency, intensity, and burden of side effects. J Psychiatr Pract. 2006;12:71–79. doi: 10.1097/00131746-200603000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Bent S. Padula A. Avins AL. Brief communication: Better ways to question patients about adverse medical events: A randomized, controlled trial. Ann Intern Med. 2006;144:257–261. doi: 10.7326/0003-4819-144-4-200602210-00007. [DOI] [PubMed] [Google Scholar]
- 17.Ioannidis JPA. Mulrow CD. Goodman SN. Adverse events: The more you search, the more you find. Ann Intern Med. 2006;144:298–300. doi: 10.7326/0003-4819-144-4-200602210-00013. [DOI] [PubMed] [Google Scholar]
- 18.DAIDS (Division of Acquired Immunodeficiency Syndrome, National Institute of Allergy and Infectious Diseases) Table for Grading the Severity of Adult and Pediatric Adverse Events. Dec, 2004. www.ucdmc.ucdavis.edu/clinicaltrials/documents/DAIDS_AE_GradingTable_FinalDec2004.pdf. [Sep 20;2011 ]. www.ucdmc.ucdavis.edu/clinicaltrials/documents/DAIDS_AE_GradingTable_FinalDec2004.pdf
- 19.Kontorinis N. Dieterich D. Hepatotoxicity of antiretroviral therapy. AIDS Rev. 2003;5:36–43. [PubMed] [Google Scholar]
- 20.Benowitz NL. Bernert NT. Caraballo RS. Holiday DB. Wang JT. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States Between 1999 and 2004. Am J Epidemiol. 2009;169:236–248. doi: 10.1093/aje/kwn301. [DOI] [PubMed] [Google Scholar]
- 21.Zeidler J. Gauthier D. APCI LC-MS/MS analysis of a polar base using normal phase chromatography on a fluorinated column: Quantitation of cotinine in plasma; Presented at: The 53rd ASMS Conference on Mass Spectrometry and Allied Topics; San Antonio, TX. 2005. [Google Scholar]
- 22.Fagerström K. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- 23.Fagerström K. Schneider NG. Measuring nicotine dependence: A review of the Fagerstrom Tolerance Questionnaire. J Behav Med. 1989;12:159–182. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- 24.Heatherton T. Kozlowski L. Frecker R. Fagerström K. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 25.Hughes JR. Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 26.Hughes J. Tobacco withdrawal in self-quitters. J Consult Clin Psychol. 1992;60:689–697. doi: 10.1037//0022-006x.60.5.689. [DOI] [PubMed] [Google Scholar]
- 27.Cappelleri J. Bushmakin A. Baker C. Merikle E. Olufade A. Gilbert D. Revealing the multidimensional framework of the Minnesota nicotine withdrawal scale. Curr Med Res Opin. 2005;21:749–760. doi: 10.1185/030079905X43712. [DOI] [PubMed] [Google Scholar]
- 28.Wang C. Xiao D. Chan K. Pothirat C. Garza D. Davies S. Varenicline for smoking cessation: A placebo-controlled, randomized study. Respirology. 2009;14:384–392. doi: 10.1111/j.1440-1843.2008.01476.x. [DOI] [PubMed] [Google Scholar]
- 29.Aubin HJ. Bobak A. Britton JR, et al. Varenicline versus transdermal nicotine patch for smoking cessation: Results from a randomised open-label trial. Thorax. 2008;63:717–724. doi: 10.1136/thx.2007.090647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura M. Oshima A. Fujimoto Y. Maruyama N. Ishibashi T. Reeves K. Efficacy and tolerability of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, in a 12-week, randomized, placebo-controlled, dose-response study with 40-week follow-up for smoking cessation in Japanese smokers. Clin Ther. 2007;29:1040–1056. doi: 10.1016/j.clinthera.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 31.Tsai S. Cho H. Cheng H, et al. A randomized, placebo-controlled trial of varenicline, a selective α4β2 nicotinic acetylcholine receptor partial agonist, as a new therapy for smoking cessation in Asian smokers. Clin Ther. 2007;29:1027–1039. doi: 10.1016/j.clinthera.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Oncken C. Gonzales D. Nides M, et al. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Intern Med. 2006;166:1571–1577. doi: 10.1001/archinte.166.15.1571. [DOI] [PubMed] [Google Scholar]
- 33.Jorenby DE. Hays JT. Rigotti NA, et al. Efficacy of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: A randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 34.Gonzales D. Rennard SI. Nides M, et al. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation. A randomized controlled trial. JAMA. 2006;296:7–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 35.Singh S. Loke Y. Spangler J. Furberg C. Risk of serious adverse cardiovascular events associated with varenicline: A systematic review and meta-analysis. CMAJ. 2011;183:1359–1366. doi: 10.1503/cmaj.110218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hulten E. Mitchell J. Scally J. Gibbs B. Villines T. HIV positivity, protease inhibitor exposure and subclinical atherosclerosis: A systematic review and meta-analysis of observational studies. Heart. 2009;95:1826–1835. doi: 10.1136/hrt.2009.177774. [DOI] [PubMed] [Google Scholar]
- 37.Obel N. Farkas D. Kronborg G, et al. Abacavir and risk of myocardial infarction in HIV-infected patients on highly active antiretroviral therapy: A population-based nationwide cohort study. HIV Med. 2010;11:130–136. doi: 10.1111/j.1468-1293.2009.00751.x. [DOI] [PubMed] [Google Scholar]
- 38.Lifson AR. Krantz EM. Eberly LE, et al. Long-term CD4+ lymphocyte response following HAART initiation in a U.S. military prospective cohort. AIDS Res Ther. 2011;8:2. doi: 10.1186/1742-6405-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hyland A. Li Q. Bauer J. Giovino G. Steger C. Cummings K. Predictors of cessation in a cohort of current and former smokers followed over 13 years. Nicotine Tob Res. 2004;6(Suppl 3):S363–S369. doi: 10.1080/14622200412331320761. [DOI] [PubMed] [Google Scholar]