Abstract
Despite the advent of effective antiretroviral therapy (ART), HIV-seropositive injection drug users (IDU) continue to suffer from elevated levels of morbidity and mortality. Evidence is needed to identify social- and structural-level barriers to effective ART. We investigated the impact of homelessness on plasma HIV RNA response among illicit drug users initiating ART in a setting with free and universal access to HIV care. We accessed data from a long-running prospective cohort of community-recruited IDU linked to comprehensive HIV clinical monitoring and ART dispensation records. Using Cox proportional hazards with recurrent events modeling, we estimated the independent effect of homelessness on time to plasma HIV viral load suppression. Between May 1996 and September 2009, 247 antiretroviral naïve individuals initiated ART and contributed 1755 person–years of follow-up. Among these individuals, the incidence density of plasma HIV RNA suppression less than 500 copies/mm3 was 56.7 (95% confidence interval [CI]: 46.9–66.0) per 100 person–years. In unadjusted analyses, homelessness was strongly associated with lower rates suppression (hazard ratio=0.56, 95% CI: 0.40–0.78, p=0.001), however, after adjustment for adherence this association was no longer significant (adjusted hazard ratio=0.79, 95% CI: 0.56–1.11, p=0.177). Homelessness poses a significant structural barrier to effective HIV treatment. However, since this relationship appears to be mediated by lower levels of ART adherence, interventions to improve adherence among members of this vulnerable population are needed.
Introduction
The development of combination antiretroviral therapy (ART) has resulted in dramatic reductions in HIV-related morbidity and mortality among many seropositive populations worldwide. Given appropriate levels of adherence, ART reliably suppresses plasma levels of HIV, delaying disease progression and death.1,2 Unfortunately, some seropositive groups have yet to enjoy the full benefits of ART. Studies among HIV-seropositive individuals who inject drugs (IDU) have identified how ongoing illicit drug use and other behavioral factors pose substantial barriers to effective ART.3,4 In comparison, however, the contribution of social and structural factors to treatment outcomes have yet to be well described.5
Homelessness, as well as living in poor or unstable housing conditions, has long been recognized as an important component of vulnerability for infection with HIV.6–8 In addition to poorer access to preventative health care,9 individuals who are homeless also suffer from high levels of mental illness,10 illicit drug use,11 incarceration,12 and violence,13 as well as seroprevalence of HIV many times higher than among comparable nonhomeless populations.6,14 For example, in a study involving over 1200 homeless and marginally housed adults in San Francisco, California, 187 (15.4%) tested seropositive for HIV; the population had high levels of incarceration (22%), sex trade involvement (33%), and unprotected sex (44%).14
While studies have illuminated the links between housing and the health of individuals who are infected with HIV,9,15–17 a number of important issues remain to be addressed. Most notably, while homelessness is a common experience for individuals who use illicit drugs and an important determinant of their health,18 we are unaware of any prospective analysis that has considered the independent effect of homelessness on outcomes from HIV treatment among IDU with access to free HIV care. While numerous studies have identified sub-optimal ART adherence among homeless individuals linked to various drug-using, social support, and structural exposures,19–23 existing evidence has commonly been derived from short-term studies, often cross-sectional, involving participants recruited from HIV treatment settings. A further issue is that, up to now, studies have been conducted in settings in which access to ART and/or medical care is neither free of charge or universal. In light of recent findings from our setting on plasma viral load (PVL) suppression among drug users24 and reports of lower levels of ART adherence among homeless individuals,25 in the current study we sought to use data from a long-running prospective cohort of drug users with free access to HIV care to assess the effect of homelessness on HIV treatment success. We hypothesize that the relationship between homelessness and virologic response is mediated by ART adherence.
Methods
To inform these analyses, we accessed data from the AIDS Care Cohort to evaluate Exposure to Survival Services (ACCESS), an ongoing prospective observational cohort of HIV-seropositive illicit drug users in Vancouver, Canada. Individuals were contacted and recruited into the cohort using methods described in detail previously.26–28 Briefly, we used snowball sampling and extensive street outreach beginning in 1996 focused in Vancouver's Downtown Eastside neighborhood (DTES). The DTES is a postindustrial area with a large and established open drug market and endemic levels of illicit drug use, poverty, poor housing status, and HIV infection.26 Using word-of-mouth, postering, and other methods, individuals were recruited from harm reduction services, single-room occupancy hotels, drug-use areas, and health care settings in the DTES. Information contained in the province-wide ART drug treatment program (described below) was not used to recruit participants. Contacted individuals are invited to participate in an ongoing study of HIV treatment and related issues among individuals who use drugs in the DTES.
Individuals were eligible to participate in ACCESS if they were aged 18 years or older, were HIV-seropositive, had used illicit drugs other than cannibinoids in the month prior to enrollment, and provided written informed consent. Participants were compensated $20 at each study visit. Following recruitment, individuals answered an interviewer-administered questionnaire, underwent an examination by a study nurse, and provided blood samples for analysis at baseline and every 6 months follow-up interview. The ACCESS study has been approved by the University of British Columbia/Providence Healthcare Research Ethics Board.
Sociodemographic information as well as data on drug-using, housing status, and other characteristics and exposures gathered at each interview was augmented with comprehensive information on HIV care and treatment outcomes from the British Columbia Centre for Excellence in HIV/AIDS (BCCfE). A province-wide centralized ART dispensary and HIV/AIDS clinical monitoring laboratory, also described previously,26–28 the BCCfE provides a complete prospective profile of CD4+ T-cell counts, HIV-1 RNA PVL, and exposure to specific antiretroviral agents for each participant. Although ACCESS study staff can assist participants with referrals to ancillary medical care, treatment for drug use and other social services, HIV clinical care, including antiretroviral prescribing, operates independently of all study activities.
The analytic sample for this study was derived from the larger ACCESS cohort. Specifically, we included individuals who were naïve to ART at recruitment and initiated HIV treatment during the study period. As well, to be included in these analyses, at least one observation of both CD4 cell count and PVL had to be completed within 12 months of ART initiation. The outcome of interest was suppression of PVL, or the date of the first of two consecutive observations less than 500 copies/mm3. The primary explanatory variable of interest was reporting homelessness, defined as living on the street with no fixed address, at any time in the 6-month period proceeding the follow-up interview.
To estimate the relationship between homelessness and PVL response, we also considered secondary explanatory variables we hypothesised may confound this relationship. These included demographic and socioeconomic characteristics such as age; gender (female versus male); Aboriginal ancestry (yes versus no); educational attainment (less than high school diploma versus high school diploma or higher); and legitimate employment in the previous 6 months (yes versus no). We used a three-level variable to describe illicit drug use: No illicit drug use (reference) versus any illicit drug use versus any injection drug use. We also considered any involvement in the sex trade in the previous 6 months (yes versus no); and any incarceration overnight or longer in the previous 6 months (yes versus no).
Clinical variables included were the HIV experience of the prescribing physician (as cited previously,29 less than 6 patients at initiation versus 6 patients or greater); the year of ART initiation; HIV RNA viral load at baseline (per log10); CD4 cell count (per 100 cells microliter). The CD4 cell count was a time-updated measure referring to the 6-month period prior to the interview date. If more than one observation was available, the average of all observations was used; if no observations were available, the most recent observation was used.
We also gathered information on adherence to prescribed antiretroviral therapy (ART). As in previous studies using this confidential pharmacy dispensation data provided by the BCCfE,28,30 we defined ART adherence in each 6-month period as the number of days ART was dispensed over the number of days an individual was eligible for ART; this proportion was dichotomized as greater than 95% versus 95% or less.30 We have previously demonstrated the clinical utility of this dichotomous variable and shown it reliably predicts virologic suppression31–33 and survival.28,30
As a first step, we examined the cohort characteristics at baseline, stratified by the number of suppression events over the study period (≥1 versus 0). To test for significant differences, we calculated Pearson's χ2 statistic; in instances in which at least one cell contained a count of 5 or less, Fisher's exact test statistic was calculated. Next, we used Cox proportional hazards regression of the time to viral suppression to estimate unadjusted relative hazards (RH) for the effect of homelessness and all secondary explanatory variables on time to viral load suppression. Homelessness, all behavioral variables, and CD4 cell count, were all considered as time-updated measures.
To estimate the independent effect of homelessness on time to viral suppression, we constructed a multivariate model using an adaptation of a method described previously by Greenland and colleagues.34,35 To start, we fit a full model including all explanatory variables, noting the value of the coefficient associated with homelessness. Using a manual stepwise approach, we then constructed reduced models, each with one secondary explanatory variable removed from the full set of secondary explanatory variables. Comparing the value of the coefficient for the primary explanatory variable in the full model and each of the reduced models, we removed the secondary variable corresponding to the smallest relative change in the coefficient for homelessness. We continued this iterative process until the maximum change of the value for homelessness from the full model exceeded 5%. The intent of this model building strategy is to retain secondary variables in the final multivariate model with greater relative influence on the relationship between homelessness and time to viral suppression. This technique has been used successfully by several authors to estimate the independent relationship between an outcome of interest and a selected explanatory variable.34,36,37
To test whether the relationship between homelessness and PVL suppression was mediated by differences in ART adherence, we performed a mediation analysis as defined by Baron and Kenny.38 Their technique involves fitting four regression models and observing a covariate of interest and associated p value in each. These regressions correspond to four paths describing the relationships between the three variables of interest: path a, the effect of homelessness on ART adherence; path b, the effect of ART adherence on viral suppression; path c, the effect of homelessness on viral suppression; and path c′, the effect of homelessness on viral suppression adjusted for the effect of ART adherence. Each path was also adjusted with the set of secondary covariates identified in the procedure described above to build the multivariate model.
Plasma HIV-1 RNA was measured using the Roche Amplicor Monitor assay (Roche Molecular Systems, Mississauga, Canada). All statistical analyses were completed using R v2.10.1 (R Foundation, Vienna, Austria).
Results
Between May 1996 and October 2009, 762 HIV-seropositive illicit drug users were recruited into the ACCESS study. Of these, 246 (34.3%) initiated ART during the study period. Six individuals (2.3%) were excluded on the basis of incomplete clinical monitoring data. In comparison to the participants included in the study, individuals excluded for incomplete clinical data were not more likely to report homelessness within 12 months of initiation (p>0.5). The analytic sample of 240 participants included 112 (46.7%) women and 111 (46.2%) individuals reporting Aboriginal ancestry.
Over the study period, the participants contributed 1755 person–years of follow-up, with a median follow-up time per participant of 46.5 months (interquartile range [IQR]: 5.9–87.1). Over the study period, 136 participants achieved at least one episode of viral suppression for an incidence density of 56.7 (95% confidence interval [CI]: 46.9–66.0) per 100 person–years. The baseline characteristics of the sample, stratified by HIV RNA viral load suppression over follow-up, are presented in Table 1.
Table 1.
Baseline Cohort Characteristics Stratified by HIV RNA Viral Load Suppression Over Follow-Up Among Injection Drug Users Initiating Therapy in Vancouver, Canada (n=240)
| Characteristic | No viral suppression 104 (43.3) | >0 viral suppression 136 (56.7) | Odds ratio | 95% Confidence interval | p Value |
|---|---|---|---|---|---|
| Homelessnessa | |||||
| No | 87 (83.7) | 112 (82.4) | 1.00 | ||
| Yes | 17 (16.3) | 24 (17.6) | 1.10 | 0.55–2.17 | 0.791 |
| Age | |||||
| Median (IQR) | 35.8 (28.2–43.3) | 37.2 (31.2–43.1) | 1.01 | 1.00–1.01 | 0.083 |
| Gender | |||||
| Male | 53 (51.0) | 75 (55.1) | 1.00 | ||
| Female | 51 (49.0) | 61 (44.9) | 0.85 | 0.51–1.41 | 0.520 |
| Aboriginal ancestry | |||||
| No | 57 (54.8) | 72 (52.9) | 1.00 | ||
| Yes | 47 (45.2) | 64 (47.1) | 1.08 | 0.65–1.80 | 0.774 |
| Educationa | |||||
| ≥ High school diploma | 62 (59.6) | 84 (61.8) | 1.00 | ||
| < High school diploma | 42 (40.4) | 52 (38.2) | 0.91 | 0.54–1.54 | 0.735 |
| Employmenta | |||||
| No | 102 (98.1) | 127 (93.3) | 1.00 | ||
| Yes | 2 (1.9) | 9 (6.6) | 3.61 | 0.76–17.10 | 0.085 |
| Illicit drug usea | |||||
| None | 9 (8.7) | 12 (8.8) | 1.00 | ||
| Any illicit drug use | 11 (10.6) | 15 (11.0) | 1.02 | 0.32–3.27 | 0.970 |
| Any injection drug | 84 (80.8) | 109 (80.1) | 0.97 | 0.39–2.42 | 0.953 |
| Sex trade participationa | |||||
| No | 83 (79.8) | 116 (85.3) | 1.00 | ||
| Yes | 21 (20.2) | 20 (14.7) | 0.68 | 0.35–1.34 | 0.263 |
| Incarcerationa | |||||
| No | 75 (72.1) | 111 (81.6) | 1.00 | ||
| Yes | 29 (27.9) | 25 (18.4) | 0.58 | 0.32–1.07 | 0.081 |
| HIV MD experience | |||||
| ≥6 patients | 87 (83.7) | 117 (86.0) | 1.00 | ||
| <6 patients | 17 (16.3) | 19 (14.0) | 0.83 | 0.41–1.69 | 0.610 |
| Year of ART initiation | |||||
| Median (IQR) | 1998 (1995–2001) | 1999 (1997–2001) | 1.00 | 0.98–1.01 | 0.662 |
| Plasma HIV-1 RNA | |||||
| Median (IQR) | 4.7 (4.3–5.1) | 3.6 (2.6–4.6) | 0.83 | 0.79–0.87 | <0.001 |
| CD4 cell count | |||||
| Median (IQR) | 2.7 (1.5–3.9) | 2.6 (1.7–3.5) | 1.01 | 0.98–1.04 | 0.709 |
Refers to the 6-month period prior to the baseline interview.
IQR, interquartile range; ART, antiretroviral therapy.
For participants, homelessness was common during the study period. At baseline, 41 (17.1%) individuals reported at least one instance of homelessness in the previous 6-month period. In the 2354 interviews over the study period, 248 (10.5%) contained a report of homelessness. Of 240 participants included in this analysis, 101 (42.1%) experienced at least one episode of homelessness since initiating ART.
Table 2 presents the unadjusted RH of time to viral suppression by all primary and explanatory factors. As shown, homelessness was inversely and significantly associated with time to suppression (RH=0.56, 95% CI: 0.40–0.78; p<0.001) as was incarceration in the previous six months (RH=0.65, 95% CI: 0.49–0.86, p=0.003). In the multivariate model, also shown in Table 2, homelessness was independently associated with a lower likelihood of achieving viral suppression following the initiation of treatment (adjusted hazard ratio [AHR]=0.60, 95% CI: 0.43–0.84, p=0.003) after adjustment for age, recent incarceration, the year of ART initiation, and baseline PVL.
Table 2.
Univariate and Multivariate Analyses of Factors Associated with Time to Plasma Viral Load Suppression Among 240 Injection Drug Users Initiating Antiretroviral Therapy in Vancouver, Canada
| Characteristic | HR | 95% CI | p Value | AHR | 95% CI | p Value |
|---|---|---|---|---|---|---|
| Homelessnessa | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 0.56 | 0.40–0.78 | <0.001 | 0.60 | 0.43–0.84 | 0.003 |
| Age | ||||||
| Per 10 years older | 1.03 | 1.02–1.04 | <0.001 | 1.02 | 1.01–1.03 | <0.001 |
| Gender | ||||||
| Male | 1.00 | |||||
| Female | 0.98 | 0.84–1.15 | 0.827 | |||
| Aboriginal ancestry | ||||||
| No | 1.00 | |||||
| Yes | 0.99 | 0.85–1.16 | 0.894 | |||
| Educationa | ||||||
| ≥ High school dip | 1.00 | |||||
| < High school dip | 0.89 | 0.76–1.05 | 0.170 | |||
| Employmenta | ||||||
| No | 1.00 | |||||
| Yes | 0.90 | 0.68–1.19 | 0.458 | |||
| Illicit drug usea | ||||||
| None | 1.00 | |||||
| Any illicit use | 0.91 | 0.70–1.19 | 0.502 | |||
| Any injection use | 0.94 | 0.77–1.16 | 0.586 | |||
| Sex trade participationa | ||||||
| No | 1.00 | |||||
| Yes | 0.77 | 0.58–1.04 | 0.090 | |||
| Incarcerationa | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 0.65 | 0.49–0.86 | 0.003 | 0.84 | 0.63–1.13 | 0.248 |
| HIV MD experienceb | ||||||
| ≥6 patients | 1.00 | |||||
| <6 patients | 0.96 | 0.76–1.21 | 0.720 | |||
| Year of ART initiationb | ||||||
| Per year increase | 1.11 | 1.07–1.15 | <0.001 | 1.07 | 1.03–1.11 | <0.001 |
| Baseline PVLb | ||||||
| Per log10 increase | 0.68 | 0.63–0.72 | <0.001 | 0.72 | 0.67–0.77 | <0.001 |
| CD4 cell counta | ||||||
| Per 100 cells | 1.08 | 1.05–1.12 | <0.001 | |||
Refers to the 6-month period prior to the interview.
Measured at baseline.
HR, hazard ratio; CI, confidence interval; AHR, adjusted hazard ratio; ART, antiretroviral therapy; PVL, plasma viral load.
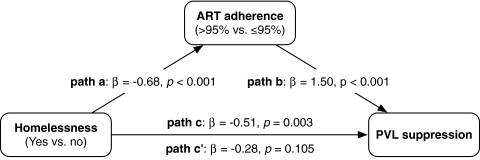
The results of the mediation analysis are detailed in Fig. 1. As described above, homelessness was independently and inversely associated with PVL suppression (path c, β=−0.51, p=0.003). Homelessness was also independently associated with ART adherence (path a, β=−0.68, p<0.001); ART adherence was also an independent predictor of a greater likelihood of PVL suppression (path b, β=1.50, p<0.001). Finally, when we considered the relationship between homelessness and PVL in the presence of ART adherence (path c′), there was no longer an association (β=−0.28, p=0.105).
FIG. 1.
Mediation effects for antiretroviral therapy (ART) adherence on the relationship between homelessness and plasma viral load (PVL) suppression among 240 HIV-infected injection drug users in Vancouver, Canada. Coefficient estimates adjusted for age, PVL at baseline, incarceration in the previous 6 months, and year of ART initiation.
Discussion
In this study, the first to our knowledge to prospectively evaluate the effects of homelessness on HIV treatment outcomes among individuals who use illicit drugs, we observed that failure to achieve PVL suppression after initiating ART was common. In almost half of the participants we did not observe two consecutive PVL measures indicating suppression following initiation of ART. While homelessness was independently associated with a lower rate of suppression, we found that the effect of homelessness on viral suppression was mediated by lower levels of adherence among homeless individuals.
Our finding of suboptimal ART adherence linked to homelessness echoes previous studies comparing homeless to housed individuals on ART.39–42 In their study of 113 current and former IDU recruited from a methadone clinic, Berg et al.39 found that lacking permanent and stable housing was significantly associated with worse ART adherence. Unfortunately, this study and others were conducted in areas without universal access to ART and did not include the nature of access to ART as an explanatory covariate. Thus, it is possible these analyses were unable to distinguish the effect of homelessness on adherence independent of the confounding influence of financial need. For example, a recent study of 125 HIV-seropositive homeless and marginally-housed individuals in San Francisco found that individuals on Medicare Part D—a new government-supported drug insurance plan that covers only a limited proportion of the cost of antiretrovirals—had six times higher odds of ART interruptions over the study period.43 Most individuals on Part D who discontinued cited drug cost as their primary barrier.43
By conducting our study in an area of universal access to HIV care, we have identified an apparent effect of homelessness on treatment outcomes independent of financial barriers. Several factors associated with poorer housing status and linked to suboptimal treatment outcomes, including food insufficiency,44 might account for this. We also suggest that several environmental aspects of homelessness, such as lacking a space to safely store medication, might have an effect, as could the need for homeless individuals to prioritize immediate survival over the secondary demands of medication adherence.45,46 Our results do not contradict previous authors45,47 who have observed that homeless individuals, given adequate adherence, can benefit from ART at levels similar to nonhomeless individuals.
Our findings support the development of measures to improve adherence among homeless individuals. For example, this might include providing housing assistance through increased access to low-barrier housing among active drug users. Considering the complex barriers to adherence that homeless individuals experience, there is a need to develop and expand comprehensive adherence support programs that cater to the specific needs of this population. A range of programs and services has emerged to fill this need for marginalized seropositive groups48–50 and they have been shown to increase adherence, retention, and viral load suppression.51,52
The finding of an independent effect of the housing environment on the success of ART for IDU has a number of implications for both the health of HIV seropositive individuals as well as efforts to control the ongoing pandemic. First, it bears out the observations from other settings that providing housing can be an important structural intervention to support the health of vulnerable HIV-seropositive individuals. An ethnographic survey of women living with HIV/AIDS in four U.S. cities46 as well as the study by Aidala et al.15 from New York State revealed how individuals who possess stable housing are better able to concentrate on meeting the demands of treatment. Similarly, results from a recent randomized clinical trial providing housing to homeless and marginally housed individuals with HIV saw significant improvements in CD4 cell count and the proportion of individuals with undetectable viral load.53 However, housing is only one structural barrier to effective treatment, especially in areas without universal access to free care. For example, in a large study of survival among individuals in the San Francisco AIDS Registry, while homelessness at baseline was a significant risk factor for death, a greater risk was faced by individuals with no health insurance as compared to individuals with private or public support.54 Thus, in settings without universal access to ART, provision of housing might have limited benefit on treatment outcomes. Finally, with increasing recognition of the important role both individual- and community-level viral loads play in HIV transmission dynamics,55–57 housing interventions effective at improving adherence and treatment outcomes might contribute to lowered incidence of HIV infection among vulnerable populations in our setting and others.58
Our study has limitations. First, although the cohort was recruited through community outreach, it was not randomly recruited and thus might not be representative of all HIV-seropositive drug users in this setting or others. Second, numerous studies have identified mental illness as a predictor of HIV treatment adherence and outcomes.59 A consistent measure of mental health was not included as part of the study instrument. We recognize that this could be a source of residual confounding. Future studies should seek to assess the combined role of homelessness and mental health co-morbidities to assess how they are associated with viral suppression in this population.
To conclude, we analyzed patterns of homelessness and response to ART using data from a long-running community-recruited prospective cohort of HIV-seropositive drug users in a setting of free and universal access to HIV care. We observed that both homelessness and failure to achieve at least one instance of PVL suppression was common this sample. In a multivariate model, homelessness was independently associated with lower rates of viral load suppression, although this relationship was not observed when ART adherence was considered simultaneously. Thus, our findings support the provision of enhanced services to support ART adherence including low-barrier housing among drug users initiating treatment as an intervention to overcome this structural barrier to effective ART and reduce elevated levels of HIV-related morbidity and mortality.
Acknowledgments
The authors thank the study participants for their contribution to the research as well as current and past researchers and staff. We would specifically like to thank Deborah Graham, Tricia Collingham, Caitlin Johnston, Steve Kain, and Calvin Lai for their research and administrative assistance. The study was supported by the US National Institutes of Health (R01DA021525) and the Canadian Institutes of Health Research (MOP-79297, RAA-79918). Thomas Kerr is supported by the Michael Smith Foundation for Health Research and the Canadian Institutes of Health Research (CIHR). M.-J. Milloy is supported by a doctoral research award from CIHR.
The ACCESS study was supported by the U.S. National Institutes of Health (R01DA021525) and the Canadian Institutes of Health Research (MOP-79297, RAA-79918).
Dr. Montaner has received educational grants from, served as an ad hoc adviser to or spoken at various events sponsored by Abbott Laboratories, Agouron Pharmaceuticals Inc., Boehringer Ingelheim Pharmaceuticals Inc., Borean Pharma AS, Bristol–Myers Squibb, DuPont Pharma, Gilead Sciences, GlaxoSmithKline, Hoffmann–La Roche, Immune Response Corporation, Incyte, Janssen–Ortho Inc., Kucera Pharmaceutical Company, Merck Frosst Laboratories, Pfizer Canada Inc., Sanofi Pasteur, Shire Biochem Inc., Tibotec Pharmaceuticals Ltd., and Trimeris Inc. Dr. Bangsbergh receives research support from Abbott Laboratories, Gilead Sciences, and Bristol-Myers Squibb.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hogg RS. Heath KV. Yip B, et al. Improved survival among HIV-infected individuals following initiation of antiretroviral therapy. JAMA. 1998;279:450–454. doi: 10.1001/jama.279.6.450. [DOI] [PubMed] [Google Scholar]
- 2.Porter K. Babiker A. Bhaskaran K. Darbyshire J. Pezzotti P. Walker AS. Determinants of survival following HIV-1 seroconversion after the introduction of HAART. Lancet. 2003;362:1267–1274. doi: 10.1016/s0140-6736(03)14570-9. [DOI] [PubMed] [Google Scholar]
- 3.Shannon K. Bright V. Duddy J. Tyndall MW. Access and utilization of HIV treatment and services among women sex workers in Vancouver's downtown eastside. J Urban Health. 2005;82:488–497. doi: 10.1093/jurban/jti076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stein MD. Rich JD. Maksad J, et al. Adherence to antiretroviral therapy among HIV-infected methadone patients: Effect of ongoing illicit drug use. Am J Drug Alcohol Abuse. 2000;26:195–205. doi: 10.1081/ada-100100600. [DOI] [PubMed] [Google Scholar]
- 5.Krüsi A. Wood E. Montaner J. Kerr T. Social and structural determinants of HAART access and adherence among injection drug users. Int J Drug Pol. 2010;21:4–9. doi: 10.1016/j.drugpo.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Allen DM. Lehman JS. Green TA. Lindegren ML. Onorato IM. Forrester W. HIV infection among homeless adults and runaway youth, United States, 1989–1992. Field Services Branch. AIDS. 1994;8:1593–1598. [PubMed] [Google Scholar]
- 7.Robertson MJ. Clark RA. Charlebois ED, et al. HIV seroprevalence among homeless and marginally housed adults in San Francisco. Am J Public Health. 2004;94:1207–1217. doi: 10.2105/ajph.94.7.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torres RA. Mani S. Altholz J. Brickner PW. Human immunodeficiency virus infection among homeless men in a New York City shelter. Association with Mycobacterium tuberculosis infection. Arch Intern Med. 1990;150:2030–2036. [PubMed] [Google Scholar]
- 9.Smith MY. Rapkin BD. Winkel G. Springer C. Chhabra R. Feldman IS. Housing status and health care service utilization among low-income persons with HIV/AIDS. J Gen Intern Med. 2000;15:731–738. doi: 10.1046/j.1525-1497.2000.91003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fichter MM. Quadflieg N. Prevalence of mental illness in homeless men in Munich, Germany: Results from a representative sample. Acta Psychiatr Scand. 2001;103:94–104. doi: 10.1034/j.1600-0447.2001.00217.x. [DOI] [PubMed] [Google Scholar]
- 11.Das-Douglas M. Colfax G. Moss AR. Bangsberg DR. Hahn JA. Tripling of methamphetamine/amphetamine use among homeless and marginally housed persons, 1996–2003. J Urban Health. 2008;85:239–249. doi: 10.1007/s11524-007-9249-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kushel MB. Hahn JA. Evans JL. Bangsberg DR. Moss AR. Revolving doors: Imprisonment among the homeless and marginally housed population. Am J Public Health. 2005;95:1747–1752. doi: 10.2105/AJPH.2005.065094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heslin KC. Robinson PL. Baker RS. Gelberg L. Community characteristics and violence against homeless women in Los Angeles County. J Health Care Poor Underserved. 2007;18:203–218. doi: 10.1353/hpu.2007.0011. [DOI] [PubMed] [Google Scholar]
- 14.Buchér JB. Thomas KM. Guzman D. Riley E. Dela Cruz N. Bangsberg DR. Community-based rapid HIV testing in homeless and marginally housed adults in San Francisco. HIV Med. 2007;8:28–31. doi: 10.1111/j.1468-1293.2007.00423.x. [DOI] [PubMed] [Google Scholar]
- 15.Aidala AA. Lee G. Abramson DM. Messeri P. Siegler A. Housing need, housing assistance, and connection to HIV medical care. AIDS Behav. 2007;11(6 Suppl):101–115. doi: 10.1007/s10461-007-9276-x. [DOI] [PubMed] [Google Scholar]
- 16.Leaver CA. Bargh G. Dunn JR. Hwang SW. The effects of housing status on health-related outcomes in people living with HIV: A systematic review of the literature. AIDS Behav. 2007;11(6 Suppl):85–100. doi: 10.1007/s10461-007-9246-3. [DOI] [PubMed] [Google Scholar]
- 17.Weiser SD. Frongillo EA. Ragland K. Hogg RS. Riley ED. Bangsberg DR. Food insecurity is associated with incomplete HIV RNA suppression among homeless and marginally housed HIV-infected individuals in San Francisco. J Gen Intern Med. 2009;24:14–20. doi: 10.1007/s11606-008-0824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galea S. Vlahov D. Social determinants and the health of drug users: Socioeconomic status, homelessness, and incarceration. Public Health Rep. 2002;117(Suppl 1):S135–145. [PMC free article] [PubMed] [Google Scholar]
- 19.Bangsberg DR. Hecht FM. Charlebois ED, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000;14:357–366. doi: 10.1097/00002030-200003100-00008. [DOI] [PubMed] [Google Scholar]
- 20.Knowlton AR. Arnsten JH. Eldred LJ, et al. Antiretroviral use among active injection-drug users: The role of patient-provider engagement and structural factors. AIDS Patient Care STDs. 2010;24:421–428. doi: 10.1089/apc.2009.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehavot K. Huh D. Walters KL. King KM. Andrasik MP. Simoni JM. Buffering effects of general and medication-specific social support on the association between substance use and HIV medication adherence. AIDS Patient Care STDs. 2011;25:181–189. doi: 10.1089/apc.2010.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moss A. Hahn J. Perry S, et al. Adherence to highly active antiretroviral therapy in the homeless population in San Francisco: A prospective study. Clin Infect Dis. 2004;39:1190–1198. doi: 10.1086/424008. [DOI] [PubMed] [Google Scholar]
- 23.Weiser SD. Bangsberg DR. Kegeles S. Ragland K. Kushel MB. Frongillo EA. Food insecurity among homeless and marginally housed individuals living with HIV/AIDS in San Francisco. AIDS Behav. 2009;13:841–848. doi: 10.1007/s10461-009-9597-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nolan S. Milloy MJ. Zhang R, et al. Adherence and plasma HIV RNA response to antiretroviral therapy among HIV-seropositive injection drug users in a Canadian setting. AIDS Care. doi: 10.1080/09540121.2010.543882. (in press). [DOI] [PubMed] [Google Scholar]
- 25.Palepu A. Milloy MJ. Kerr T. Zhang R. Wood E. Homelessness and adherence to antiretroviral therapy among a cohort of HIV-infected injection drug users. J Urban Health. 2011;88:545–555. doi: 10.1007/s11524-011-9562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strathdee SA. Palepu A. Cornelisse PG, et al. Barriers to use of free antiretroviral therapy in injection drug users. JAMA. 1998;280:547–549. doi: 10.1001/jama.280.6.547. [DOI] [PubMed] [Google Scholar]
- 27.Wood E. Hogg RS. Bonner S, et al. Staging for antiretroviral therapy among HIV-infected drug users. JAMA. 2004;292:1175–1177. doi: 10.1001/jama.292.10.1175-b. [DOI] [PubMed] [Google Scholar]
- 28.Wood E. Hogg RS. Lima VD, et al. Highly active antiretroviral therapy and survival in HIV-infected injection drug users. JAMA. 2008;300:550–554. doi: 10.1001/jama.300.5.550. [DOI] [PubMed] [Google Scholar]
- 29.Wood E. Hogg RS. Yip B. Harrigan PR. O'Shaughnessy MV. Montaner JS. Is there a baseline CD4 cell count that precludes a survival response to modern antiretroviral therapy? AIDS. 2003;17:711–720. doi: 10.1097/00002030-200303280-00009. [DOI] [PubMed] [Google Scholar]
- 30.Wood E. Hogg RS. Yip B. Harrigan PR. O'Shaughnessy MV. Montaner JS. Effect of medication adherence on survival of HIV-infected adults who start highly active antiretroviral therapy when the CD4+ cell count is 0.200 to 0.350×10(9) cells/L. Ann Intern Med. 2003;139:810–816. doi: 10.7326/0003-4819-139-10-200311180-00008. [DOI] [PubMed] [Google Scholar]
- 31.Low-Beer S. Yip B. O'Shaughnessy MV. Hogg RS. Montaner JS. Adherence to triple therapy and viral load response. J Acquir Immune Defic Syndr. 2000;23:360–361. doi: 10.1097/00126334-200004010-00016. [DOI] [PubMed] [Google Scholar]
- 32.Palepu A. Yip B. Miller C, et al. Factors associated with the response to antiretroviral therapy among HIV-infected patients with and without a history of injection drug use. AIDS. 2001;15:423–424. doi: 10.1097/00002030-200102160-00021. [DOI] [PubMed] [Google Scholar]
- 33.Wood E. Montaner JS. Yip B, et al. Adherence and plasma HIV RNA responses to highly active antiretroviral therapy among HIV-1 infected injection drug users. CMAJ. 2003;169:656–661. [PMC free article] [PubMed] [Google Scholar]
- 34.Maldonado G. Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 35.Rothman KJ. Greenland S. Modern Epidemiology. New York: Lippincott Williams & Wilkins; 1998. [Google Scholar]
- 36.Lima V. Fernandes K. Rachlis B. Druyts E. Montaner J. Hogg R. Migration adversely affects antiretroviral adherence in a population-based cohort of HIV/AIDS patients. Soc Sci Med. 2009;68:1044–1049. doi: 10.1016/j.socscimed.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 37.Marshall BD. Kerr T. Shoveller JA. Patterson TL. Buxton JA. Wood E. Homelessness and unstable housing associated with an increased risk of HIV and STI transmission among street-involved youth. Health Place. 2009;15:753–760. doi: 10.1016/j.healthplace.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baron RM. Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 39.Berg KM. Demas PA. Howard AA. Schoenbaum EE. Gourevitch MN. Arnsten JH. Gender differences in factors associated with adherence to antiretroviral therapy. J Gen Intern Med. 2004;19:1111–1117. doi: 10.1111/j.1525-1497.2004.30445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carballo E. Cadarso-Suárez C. Carrera I, et al. Assessing relationships between health-related quality of life and adherence to antiretroviral therapy. Qual Life Res. 2004;13:587–599. doi: 10.1023/B:QURE.0000021315.93360.8b. [DOI] [PubMed] [Google Scholar]
- 41.Kidder DP. Wolitski RJ. Campsmith ML. Nakamura GV. Health status, health care use, medication use, and medication adherence among homeless and housed people living with HIV/AIDS. Am J Public Health. 2007;97:2238–2245. doi: 10.2105/AJPH.2006.090209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parruti G. Manzoli L. Toro PM, et al. Long-term adherence to first-line highly active antiretroviral therapy in a hospital-based cohort: Predictors and impact on virologic response and relapse. AIDS Patient Care and STDs. 2006;20:48–56. doi: 10.1089/apc.2006.20.48. [DOI] [PubMed] [Google Scholar]
- 43.Das-Douglas M. Riley ED. Ragland K, et al. Implementation of the Medicare Part D Prescription Drug Benefit is associated with antiretroviral therapy interruptions. AIDS Behav. 2009;13:1–9. doi: 10.1007/s10461-008-9401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiser SD. Frongillo EA. Ragland K. Hogg RS. Riley ED. Bangsberg DR. Food insecurity is associated with incomplete HIV RNA suppression among homeless and marginally housed HIV-infected individuals in San Francisco. J Gen Intern Med. 2009;24:14–20. doi: 10.1007/s11606-008-0824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bangsberg D. Tulsky JP. Hecht FM. Moss AR. Protease inhibitors in the homeless. JAMA. 1997;278:63–65. [PubMed] [Google Scholar]
- 46.Scott A. Ellen J. Clum G. Leonard L. HIV and housing assistance in four U.S. cities: variations in local experience. AIDS Behav. 2007;11(6 Suppl):140–148. doi: 10.1007/s10461-007-9247-2. [DOI] [PubMed] [Google Scholar]
- 47.Waldrop-Valverde D. Valverde E. Homelessness and psychological distress as contributors to antiretroviral nonadherence in HIV-positive injecting drug users. AIDS Patient Care STDs. 2005;19:326–334. doi: 10.1089/apc.2005.19.326. [DOI] [PubMed] [Google Scholar]
- 48.Altice FL. Mezger JA. Hodges J, et al. Developing a directly administered antiretroviral therapy intervention for HIV-infected drug users: Implications for program replication. Clin Infect Dis. 2004;38(Suppl 5):S376–387. doi: 10.1086/421400. [DOI] [PubMed] [Google Scholar]
- 49.McCance-Katz EF. Gourevitch MN. Arnsten J. Sarlo J. Rainey P. Jatlow P. Modified directly observed therapy (MDOT) for injection drug users with HIV disease. Am J Addict. 2002;11:271–278. doi: 10.1080/10550490290088072. [DOI] [PubMed] [Google Scholar]
- 50.Garland WH. Wohl AR. Valencia R, et al. The acceptability of a directly-administered antiretroviral therapy (DAART) intervention among patients in public HIV clinics in Los Angeles, California. AIDS Care. 2007;19:159–167. doi: 10.1080/09540120600911428. [DOI] [PubMed] [Google Scholar]
- 51.Macalino GE. Hogan JW. Mitty JA, et al. A randomized clinical trial of community-based directly observed therapy as an adherence intervention for HAART among substance users. AIDS. 2007;21:1473–1477. doi: 10.1097/QAD.0b013e32811ebf68. [DOI] [PubMed] [Google Scholar]
- 52.Maru DS-R. Bruce RD. Walton M, et al. Initiation, adherence, and retention in a randomized controlled trial of directly administered antiretroviral therapy. AIDS Behav. 2008;12:284–293. doi: 10.1007/s10461-007-9336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolitski RJ. Kidder DP. Pals SL, et al. Randomized trial of the effects of housing assistance on the health and risk behaviors of homeless and unstably housed people living with HIV. AIDS Behav. 2010;14:493–503. doi: 10.1007/s10461-009-9643-x. [DOI] [PubMed] [Google Scholar]
- 54.Schwarcz SK. Hsu LC. Vittinghoff E. Vu A. Bamberger JD. Katz MH. Impact of housing on the survival of persons with AIDS. BMC Public Health. 2009;9:220. doi: 10.1186/1471-2458-9-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Attia S. Egger M. Muller M. Zwahlen M. Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: Systematic review and meta-analysis. AIDS. 2009;23:1397–1404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 56.Montaner JS. Hogg R. Wood E, et al. The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet. 2006;368:531–536. doi: 10.1016/S0140-6736(06)69162-9. [DOI] [PubMed] [Google Scholar]
- 57.Wood E. Kerr T. Marshall BD, et al. Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: Prospective cohort study. BMJ. 2009;338:b1649. doi: 10.1136/bmj.b1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aidala A. Cross JE. Stall R. Harre D. Sumartojo E. Housing status and HIV risk behaviors: Implications for prevention and policy. AIDS Behav. 2005;9:251–265. doi: 10.1007/s10461-005-9000-7. [DOI] [PubMed] [Google Scholar]
- 59.Chander G. Himelhoch S. Moore RD. Substance abuse and psychiatric disorders in HIV-positive patients: Epidemiology and impact on antiretroviral therapy. Drugs. 2006;66:769–789. doi: 10.2165/00003495-200666060-00004. [DOI] [PubMed] [Google Scholar]