Abstract
The Atacama Desert has long been considered a good Mars analogue for testing instrumentation for planetary exploration, but very few data (if any) have been reported about the geomicrobiology of its salt-rich subsurface. We performed a Mars analogue drilling campaign next to the Salar Grande (Atacama, Chile) in July 2009, and several cores and powder samples from up to 5 m deep were analyzed in situ with LDChip300 (a Life Detector Chip containing 300 antibodies). Here, we show the discovery of a hypersaline subsurface microbial habitat associated with halite-, nitrate-, and perchlorate-containing salts at 2 m deep. LDChip300 detected bacteria, archaea, and other biological material (DNA, exopolysaccharides, some peptides) from the analysis of less than 0.5 g of ground core sample. The results were supported by oligonucleotide microarray hybridization in the field and finally confirmed by molecular phylogenetic analysis and direct visualization of microbial cells bound to halite crystals in the laboratory. Geochemical analyses revealed a habitat with abundant hygroscopic salts like halite (up to 260 g kg−1) and perchlorate (41.13 μg g−1 maximum), which allow deliquescence events at low relative humidity. Thin liquid water films would permit microbes to proliferate by using detected organic acids like acetate (19.14 μg g−1) or formate (76.06 μg g−1) as electron donors, and sulfate (15875 μg g−1), nitrate (13490 μg g−1), or perchlorate as acceptors. Our results correlate with the discovery of similar hygroscopic salts and possible deliquescence processes on Mars, and open new search strategies for subsurface martian biota. The performance demonstrated by our LDChip300 validates this technology for planetary exploration, particularly for the search for life on Mars. Key Words: Atacama Desert—Life detection—Biosensor—Biopolymers—In situ measurement. Astrobiology 11, 969–996.
1. Introduction
The space science community agrees on the need to explore the martian subsurface for evidence of intact organic molecules (Kminek and Bada, 2006; Shkrob et al., 2010). In fact, ESA's ExoMars mission aims to search for life or its remains by analyzing samples taken from a drill hole of at least 2 m in depth (http://www.esa.int/SPECIALS/ExoMars/SEM10VLPQ5F_0.html ). Dartnell et al. (2007) suggested, after a series of simulation experiments on the effect of solar radiation on biological material (bacteria), that a minimum depth of 7.5 m would be needed to find viable cryopreserved cells.
Different robotic missions have shown that Mars is a salty planet with a volcanic basement (Murchie et al., 2009). Chlorides and bromides precipitated as cementing salts in eolian deposits (McLennan et al., 2005) or likely as sedimentary deposits that infilled shallow basins (Osterloo et al., 2008) due to a strong oversaturation of solutions that leached to the Mars surface under high evaporative rates. Chloride-bearing salts are excellent matrices for the preservation of biological remains (Fish et al., 2002; Stan-Lotter et al., 2006), and their hygroscopic properties can produce deliquescence events under low relative humidity (Davila et al., 2008). In fact, the high content of perchlorate (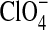 ) at the Phoenix lander site on Mars (Hecht et al., 2009) might promote the formation of stable liquid saline water on present-day Mars (Zorzano et al., 2009).
) at the Phoenix lander site on Mars (Hecht et al., 2009) might promote the formation of stable liquid saline water on present-day Mars (Zorzano et al., 2009).
The Atacama Desert is one of the most accurate terrestrial analogues for martian environments because it combines the formation of two key inorganic compounds: chlorides and perchlorates. Similar to events on Mars, harsh arid conditions promoted an extreme oversaturation of the ground and surface water solutions, which resulted in nearly exclusive precipitation of halite, the end member in evaporation from brines, with no other mineral phase (Chong-Díaz et al., 1999). The bounding regions of the Salar Grande (Cordillera de la Costa, región de Atacama, Chile) are characterized by saline subsoils associated with nitrate deposits that contain chlorides, sulfates, chlorates, chromates, iodates, and perchlorates. These salts are older and different from those of the more central region of the Salar Grande (Chong-Díaz et al., 1999).
Though the Atacama Desert has been studied for many years as a Mars analogue (Cameron, 1969; Cabrol et al., 2001; Glavin et al., 2004; Shafaat et al., 2005; Skelley et al., 2005), very few studies have focused on the microbiology or molecular biomarker content of the subsoil. Lester et al. (2007) described the microbiology from the surface to 15 cm deep, and Bobst et al. (2001) reported a 100 m drilling in the Salar de Atacama (23°S, 68°W) only for paleoclimatic studies. Gramain et al. (2011) described the archaeal community, identified via nested PCR and cultivation, that occupied a pure halite core up to 15 m deep from the Salar Grande. However, most of the microbiological and life-detection studies on the Atacama Desert have focused mainly on the surface or very close to it (Navarro-González et al., 2003; Lester et al., 2007; Weinstein et al., 2008). Cabrol et al. (2007) used a fluorescence detection system on board a rover (Zoë) to detect fluorescent signals from biological material (e.g., chlorophyll) along different transects. Piatek et al. (2007) used a combined orbital image analysis and a field rover with instrumentation aimed to examine the mineralogy, geomorphology, and chlorophyll potential of field sites on the surface. We did not find in the literature any systematic study that involved the search for life or traces of it or the microbiology of the Atacama subsurface below a few centimeters. Further, there have been no studies conducted in which techniques based on immunological biosensors were used.
Here, we present the results of the July 2009 astrobiological field campaign, AtacaMars2009, in which we tested a new in situ life-detection instrument designed for planetary exploration and study of the geomicrobiology of the Atacama subsurface at the west side of the Salar Grande. The key instrument was Signs Of LIfe Detector V3.0 (SOLID3) and its LDChip300 biosensor (a Life Detector Chip with 300 antibodies), which was designed to detect a broad range of molecular biomarkers with different specificities and sensitivities at low parts per billion levels for proteins and peptides or 104 to 105 cells per milliliter (Parro et al., 2005, 2008a, 2011a; Rivas et al., 2008; Parro, 2010). The LDChip300 biosensor contains antibodies against universal biomolecules like DNA, nucleotides, amino acids, and peptidoglycan, and includes antibodies that target general groups of bacteria (e.g., Gram-positive cells or Bacillus spp. spores) and bacterial strains from different phylogenetic groups and different extreme environments (it contains a relatively high redundancy of antibodies against environmental extracts and bacterial strains from the acidic sulfur- and iron-rich environment of Río Tinto in Spain). LDChip300 also contains some antibodies that target relevant metabolic pathways.
LDChip300 detected bacteria, archaea, and molecular biomarkers, all of which were especially abundant around 2 m deep. This suggests the presence of a microbial oasis in the Atacama subsurface. The results obtained in the field were exhaustively checked and validated in the laboratory with conventional techniques, which confirmed the presence of a new subsurface hypersaline environment. The mineralogical composition together with the extremely low liquid water availability suggests important analogies with some martian environments. Our LDChip may open a new era for life-detection systems in planetary exploration.
2. Materials and Methods
2.1. Logistics, base camp, and tasks
The campaign was carried out such that it was completed by July 31, 2009, wintertime in the region. A base camp for eight researchers and two assistants was set up at about 1 km distance from the drilling site (GPS coordinates: 20°58′7.30″S, 70°2′2.95″W) (Fig. 1A). The camp included a tent that was used as a field laboratory with space for three people and some equipment (a microcentrifuge, a water bath, a scanner, a handheld sonicator, tubes, and other materials and reactants). Drilling was usually done by two people, with the assistance of two more during the more complicated operations (removing the cores, changing the corers, etc). Three people were stationed permanently in the field laboratory processing and analyzing samples, one of whom performed geophysical studies and assisted in the lab.
FIG. 1.
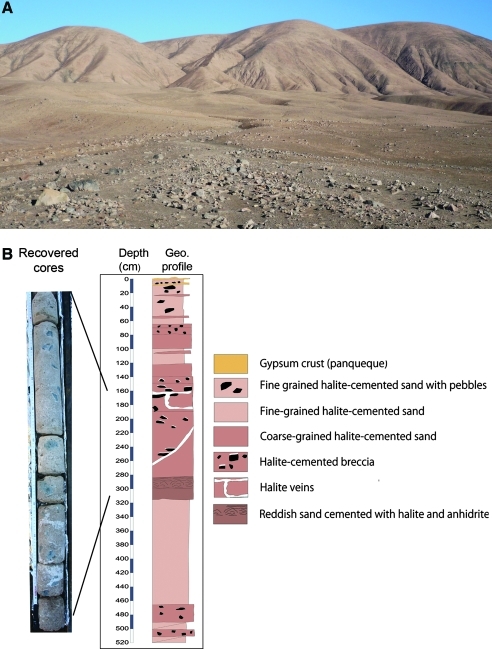
AtacaMars2009 drilling campaign. (A) The drilling site in one apron located at the base of a mountain to the west of the Salar Grande (Atacama Desert, Chile). (B) Geological profile of the core with the depth scale and a photograph of the collected core pieces (left), showing the white halite veins. Color images available online at www.liebertonline.com/ast
2.2. Drilling and sample collection
The apron at the base of a mountain of volcanic origin nearby the Salar Grande was chosen as a Mars analogue for the drilling and life-detection campaign (Fig. 1A). We measured temperature extremes of −3°C and 39°C at the drilling site during the campaign. The drilling was done with a CARDI EN 400, a 4.08–horse power gas-powered motor, and 85 mm diameter commercial off-the-shelf diamond-impregnated coring drill bits and rods. The drilling operations were difficult due to the limited power of the apparatus. To minimize cross contamination and assist drilling, compressed air was injected inside the corer to help transport chips up and out of the hole. No filtering devices were used to avoid potential contamination from the compressed air. If any contamination were to come from the compressed air, we would have detected it nearly uniformly in all core samples, which we did not. Rather, we obtained clearly differentiated patterns along the entire hole. Samples ranging from 500 g to 3 kg of this material were collected with a vacuum sampling system (a commercial aspirator with a new and sterile bag) and were labeled and stored for later analysis (Table 1). Samples were collected in plastic bags or wrapped in aluminum foil, and were then stored at room temperature (in the shade) inside closed boxes for as long as 2 h. The samples were then carried out to the field laboratory to be analyzed or further stored until transport to the laboratory in the Centro de Astrobiología, Madrid, Spain.
Table 1.
List of the Samples Collected during the Drilling Campaign
| Sample ID | Depth (cm) | Comments |
|---|---|---|
| 0 | 0–5 | Chips and powder |
| 5 | 5–10 | Chips and powder |
| 10 | 10–11 | Chips and powder |
| 20 | 20–25 | Chips and powder |
| 25 | 25–30 | Chips and powder |
| 30 | 30–33 | Chips and powder |
| 35 | 35–38 | Chips and powder |
| 40 | 39–40 | Chips and powder |
| 45 | 40–45 | Chips and powder |
| 60 | 60–68 | Chips and powder (hard crust) |
| 68 | 68–70 | Stones |
| 90 | 90–95 | Chips and powder (hard crust) |
| 100 | 100–104 | Chips and powder |
| 105 | 105–108 | Chips and powder |
| 110 | 110–112 | Chips and powder |
| 115 | 115–118 | Chips and powder |
| 120 | 120–122 | Chips and powder |
| 122 | 122–125 | Small core |
| 125 | 125–130 | Small core |
| 130 | 130–131 | Chips and powder |
| 131 | 131–134 | Small core |
| 135 | 135–138 | Small core |
| 140 | 140–142 | Chips and powder |
| 142 | 142–144 | Stones |
| 145 | 145–150 | Small core |
| 180 | 180–185 | Chips and powder |
| 185 | 185–200 | Chips and powder |
| 200 | 200–205 | Chips and powder |
| 216 | 216–220 | Chips and powder |
| 241 | 241–245 | Chips and powder |
| 250 | 250–255 | Chips and powder |
| 286 | 286–290 | Chips and powder |
| 296 | 296–305 | Chips and powder |
| 305 | 305–310 | Chips and powder |
| 330 | 330–335 | Chips and powder |
| 340 | 340–345 | Chips and powder |
| 350 | 350–355 | Chips and powder |
| 375 | 375–380 | Chips and powder |
| 384 | 384–388 | Chips and powder |
| 389 | 389–300 | Chips and powder |
| 391 | 391–395 | Chips and powder |
| 398 | 398–401 | Powder (white) |
| 404 | 404–408 | Chips and powder |
| 408 | 408–412 | Chips and powder |
| 431 | 431–433 | Chips and powder (gray) |
| 436 | 436–440 | Chips and powder |
| 447 | 447–450 | Chips and powder |
| 455 | 455–560 | Chips and powder |
| 460 | 460–465 | Chips and powder (hard crust) |
| 470 | 470–475 | Chips and powder |
| 489 | 489–499 | Chips and powder (gray-brown) |
| 507 | 507–508 | Chips and powder (gray crust) |
| 508 | 508–511 | Chips and powder |
2.3. Raman spectroscopy in the field
Intact cores were immediately photographed, analyzed with Raman spectroscopy, and then wrapped in aluminum foil to be transported to the laboratory in Spain. Raman spectra were taken in the field with a portable Avantes AvaSpec-2048TEC Raman spectrometer with 10 cm−1 spectral resolution, a 50 mW, 532 nm diode laser, and a fiber optic probe custom-made by InPhotonics according to these parameters. The Raman excitation source, made by PD-LD, is a “Volume Bragg Grating”–stabilized module, which consists of a stabilized laser diode and the associated electronics. This module provides a high-stability output around 532 nm, with a typical spectral line width of 0.08 nm.
2.4. Antibody production, purification, and labeling
The antibodies used in this research (see Table 2 in Appendix) were the result of several years of work aimed at the detection of molecular biomarkers for planetary exploration. At the time the present study began we had a collection of 300 antibodies, which constituted the so-called imnunosensor LDChip300. The criteria for antibody production and selection were explained previously by Parro et al. (2005), Parnell et al. (2007), Parro et al. (2008a), Rivas et al. (2008), and Parro et al. (2011a). Because we are developing an instrument for use on Mars, our choice of antibodies is guided by the types of molecular biomarkers found in organisms living on Earth under similar, Mars-like conditions. Consequently, LDChip300 contains antibodies raised against whole microbial cells (archaea and bacteria), extracellular polymers, environmental extracts from terrestrial analogues for Mars or Europa, proteins and peptides from well-conserved anaerobic metabolic pathways (nitrogen fixation, sulfate reduction, nitrate reduction, etc.), exopolysaccharides (EPS), and universal molecular biomarkers like DNA, amino acids, and other biomolecules (Table 2). All the antibodies were purified by Protein A affinity purification and fluorescently labeled as reported previously by Parro et al. (2005) and Rivas et al. (2008).
2.5. Design and construction of antibody microarrays
Antibody microarrays were constructed as described by Parro et al. (2005) and Rivas et al. (2008) with some modifications, as follows: (i) we used a commercial protein printing buffer 2×(Whatman, Schleicher & Schuell, Sandford, ME, USA) and 0.02% Tween 20 as a spotting solution; (ii) printing was done in a duplicate pattern on epoxy-activated glass slides (Arrayit Corp., Sunnyvale, CA, USA) with a MicroGrid II TAS arrayer (Biorobotics, Genomic Solutions, UK). Two microarray designs were performed: one for a manual procedure for the systematic analysis of all the samples with standard microscope slides (see Section 2.6.1) and another to test the automatic procedure with the SOLID3 instrument (Section 2.6.2). For the manual procedure, up to nine identical micoarrays (9 LDChip300) containing a duplicate spot pattern for the 300 antibodies were spotted on a microscope slide so that each LDChip300 fits with one of the nine flow cells in a MultiArray Analysis Module (MAAM) device (see Section 3.1; Parro, 2010).
To test SOLID3, the microarray was also designed and constructed with a MicroGrid II TAS arrayer (BioRobotics, Digilab, Holliston, MA, USA). Five LDChip300 replicas were printed in five parallel microarrays so that they fit into the five different flow cells on the SOLID3 Sample Analysis Unit (Parro et al., 2011a). Antibodies were printed at 1 mg mL−1 in protein printing buffer (Whatman, Schleicher & Schuell, Sandford, ME, USA) on a specially designed glass slide (dimensions 75×27×0.15 mm) activated with Superepoxy chemistry (custom made by Arrayit Corporation, CA, USA).
In all cases, after printing, the antibody microarrays were kept at room temperature until used. We verified that printed antibodies were stable in printing buffer for at least one year at room temperature and could be transported to the field with no significant loss of activity (data not shown). SOLID3 was run and operated as described previously (Parro et al., 2011a).
2.6. Sandwich microarray immunoassay (SMI) procedures
To analyze solid samples by SMI, the potential molecular biomarkers present in the sample have to be extracted into a liquid solution or suspension and then incubated in the presence of the capturing antibodies previously immobilized on the microarray. After a second incubation with fluorescent tracer antibodies, the positive antigen-antibody reactions are read by fluorescence emission either by CCD device or a scanner. In this campaign, we analyzed the samples by a manual procedure or automatically by using the SOLID3 instrument. Because SOLID3 is designed for single use for planetary exploration and it needs a clean room to load a new microarray chip, only five assays can be done. Therefore, the samples were assayed by manual procedure with use of the small and portable MAAM device for nine simultaneous analyses (see Section 3.1).
2.6.1. Manual SMI using the MAAM
Immediately before use, the nine microarrays containing printed slides were treated and washed with a BSA-containing solution to block all free binding sites on the slides and to remove the excess of non-covalently-bound antibodies. For that purpose, the slides were inverted and dropped onto a 0.5 M Tris-HCl pH 9, 5% (w/v) bovine serum albumin (BSA) solution for 2 min and then immersed into 0.5 M Tris-HCl pH 8, 2% BSA solution (TBSB, Tris buffered saline–BSA), with gentle agitation for 1 h. After drying with a short centrifugation, slides were ready for immediate use to analyze samples by a sandwich immunoassay. The samples to be analyzed were processed as follows: (i) up to 0.5 g of dust or ground core samples were sonicated in 1 mL of TBSTRR buffer (0.4 M Tris-HCl pH 8, 0.3 M NaCl, 0.1% Tween 20) by using a manual sonicator for 3×1 min cycles (Dr. Hielscher 50W DRH-UP50H sonicator, Hielscher Ultrasonics, Berlin, Germany); (ii) the sonicated samples were filtered through 20-micron nylon filters to remove sand and coarse material. Then 50 μL of the filtrate was injected into one of the nine flow cells of the MAAM device to put it in contact with LDChip300, where it was left to incubate at ambient temperature (20–30°C) for 1 h with mixing by pipetting every 15 min. To attain more homogeneous conditions between different microarray incubations, we avoided extreme temperatures (−1°C to 5°C during the night or up to 35–39°C during the day) by incubating during middle morning or late afternoon, when the ambient temperature in the field laboratory was in the range of 15–30°C. Then the microarrays were washed out by passing 3–5 mL of the same buffer through the flow cells and evacuating the last volume by pipetting air. The positive antibody-antigen reactions were revealed by incubation with 50 μL of a cocktail containing 300 fluorescent antibodies at a final concentration of approximately 100 μg mL−1 for the whole mixture and an average around 0.4 μg mL−1 of each antibody. After 1 h incubation at ambient temperature, the microarrays were washed out again to remove the excess fluorescent cocktail; the slide was separated from the MAAM, rinsed by dipping into a 50 mL falcon tube filled with distilled water, and finally dried by a short centrifugation in a small and portable microcentrifuge designed for slides (Arrayit Corp.). Finally, the slides were analyzed in the field laboratory by illuminating them with 635 nm light and imaging their fluorescent emission at 650 nm with a GenePix 4100A scanner. The images were quantitatively analyzed in the field by using GenePix Pro software (Genomic Solutions) installed on a laptop computer. A blank was always run in parallel with only buffer as an “antigenic” sample and then revealed with the same fluorescent antibody or antibody cocktail as the real samples. The final fluorescence intensity (F) for each antibody spot was calculated with the equation F=(Fsample−Fblank−3Favcontrol spots), where F is the fluorescent intensity at 635 nm minus the local background as quantified by the software (GenePix Pro) and where Fsample is the total fluorescence signal of the sample, Fblank the total fluorescence signal of the blank, and Favcontrol spots the average fluorescent signal of the control spots. Said control spots are located in different parts of the microarray and consist of BSA, only buffer, and others corresponding to the pre-immune antisera (more than 50). Because, theoretically, they should not exhibit any fluorescent signal, we use them as a baseline for fluorescence. We always subtract 2–3 times the Favcontrol spots as a stringency cutoff to minimize false positives. Those spots having obvious defects (missing or very tiny spots, an artifact due to a bad wash or dust in the array) or those duplicated spots whose standard deviation was 0.2 times higher than the mean were not considered for quantification.
2.6.2. Automatic SMI in SOLID3
SOLID3 is the third prototype of the SOLID (Signs Of Life Detector) instrument concept devoted to the detection of molecular biomarkers in planetary exploration (see Section 3.1). It is an analytical instrument, based on antibody microarray technology, that can detect a broad range of molecular-sized compounds, from the amino acid size level to whole cells, with sensitivities at 1–2 ppb (ng mL−1) for biomolecules and 104 to 105 cells mL−1 (Fernández-Calvo et al., 2006; Rivas et al., 2008; Parro et al., 2011a). SOLID3 is currently in Technology Readiness Level (TRL) 5–6, although some of the internal elements are in the TRL 9 stage (Parro et al., 2011a). The instrument accepts both solid (dust or ground rock or ice) and liquid samples. The limit of sample volume ranges from 0.5–1 cm3 to 2.5 cm3 (mL) at final processing volume (including the extraction buffer supplied by the instrument). SOLID3 is able to perform both sandwich and competitive immunoassays and consists of two separate functional units: a Sample Preparation Unit (SPU) for 10 different extractions by ultrasonication, and a Sample Analysis Unit (SAU) for fluorescent immunoassays. The SAU consists of five different flow cells, with an antibody microarray LDChip300 in each one. It is also equipped with an exclusive optical package and a CCD device for fluorescence detection.
After loading up to 0.5 g of dust or ground core sample into one of the homogenizing chambers of SOLID3's SPU, the instrument was set to run autonomously as described in Parro et al. (2011a). After being started, SOLID3 adds 2 mL of extraction-incubation buffer, TBSTRR, to the homogenizing chamber. A sonicator horn pushes a ring membrane forward, hermetically closing the chamber, and advances to the sonication position. After four cycles of sonication, the linear actuator pushes the sample forward through a filtering system (15 μm pore size). The filtrate is injected into the SAU, floods one of the flow cells, and contacts one of the LDChip300 antibody microarrays. An internal recirculation circuit allows the sample to be re-circulated for up to 1 h in order to enhance the reaction kinetics between antigens and spotted antibodies. After the incubation time, the remaining sample is discarded into the waste deposit, and the microarray cell is washed out with the incubation buffer to remove the nonbound sample. Then the buffer is injected into the auxiliary chambers that are pre-loaded with a cocktail of 300 lyophilized fluorescent antibodies. The buffer dissolves the antibodies and floods the microarray chamber, where it is re-circulated for up to 1 h. An additional wash removes the excess of fluorescent antibodies and leaves the microarray ready for fluorescent detection by excitation with a laser beam via total internal reflection through the glass support, which acts as a waveguide. The fluorescent signal is captured by a highly sensitive CCD detector and stored as a Flexible Image Transport System (i.e.,*.fits) image file that can be processed and analyzed by conventional microarray software as described in Section 2.6.1. One of the microarrays is incubated with only the buffer (blank) and revealed with fluorescent antibodies. The blank image is used as a baseline to calculate spot intensities in the tested sample, as described in Section 2.6.1.
2.7. Environmental DNA extraction and PCR amplification
Total DNA was extracted from 2 g of sample (powder or ground cores) by using the commercial DNA EZNA soil DNA kit (Omega Bio-Tek, Inc., Norcross, GA, USA) with some modification: after cell lysis, the extract was partially desalinated by using Amicon 30 filters (Millipore) following the manufacturer's protocol. We used the obtained DNA for simultaneous fluorescent labeling and PCR amplification by using the bacterial universal primers for 16S rRNA gene 16SF as forward (positions 8–27 of Escherichia coli 16S rRNA, 5′-AGAGTTTAGTCATGGCTCA) and 16SR as reverse (positions 1057–1074, 5′-CACGAGCTGACGACAGCCG). The reactants were previously set up in a gelified mixture (Biotools S.A, Madrid, Spain) containing the buffer, the nucleotide precursors, the fluorescent nucleotide Cy3-dUTP, the primers, and the enzyme as follows: 10×buffer (5.0 μL), 50 mM MgCl2 (3.0 μL), 10 mM dACTPs (0.5 μL), 1 mM dTTP (6.25 μL), 2 nm/μL Cy3-dUTP (5.0 μL), forward primer 10 mM 16SF (1.0 μL), reverse primer 10 mM 16SR (1.0 μL), Taq-platinum 5U/μL (Invitrogen) (1.0 μL), and distilled H20 (27 μL). The gelification process was carried out by Biotools S.A. (Madrid, Spain). The samples prepared in this way can be stored for long periods at ambient temperature. Additionally, we prepared PCR mixtures without fluorescent nucleotides: 10×Buffer (5.0 μL), MgCl2 50 mM (1.5 μL), dNTPs 10 mM (1.0 μL), primer 16SF 10 mM (1.0 μL), primer 16SR 10 mM (1.0 μL), Taq-platinum 5U/ul (1.0 μL), and dH20 (40 μL). Gelified samples were reconstituted by adding distilled nuclease-free water and the template DNA. For simultaneous labeling and PCR amplification, the field thermocycler was programmed as follows: 95°C, 5 min; 10×(95°C 20 s, 50°C 30 s, 68°C 60 s); 25×(95°C 20 s, 48°C 30 s, 68°C 60 s+5 s per cycle); 68°C 10 min; 4°C.
2.8. Oligonucleotide microarray hybridization in the field laboratory
Oligonucleotide microarrays containing specific probes for prokaryotic microorganisms, like our previously reported prokaryotic acidophile microarray (PAM), were printed and treated as described by Garrido et al. (2008). This microarray was specially developed for the detection of prokaryotic acidophiles in several field campaigns to the extremely acidic environment of Río Tinto (Huelva, Spain), but it also contains specific oligonucleotide probes for the 16S and 23S rRNA genes from other universal groups: Alpha-, Beta-, Gamma-, and Deltaproteobacteria, as well as Firmicutes (low GC content), high-GC-content Gram-positive bacteria, Archaea, and so on. Fluorescently labeled 16S rRNA gene amplicons were checked by agarose gel electrophoresis then purified with Qiagen PCR purification kit columns (Qiagen, CA, USA) and set to hybridize in HibIt hybridization buffer (Arrayit Corp.) at 50°C for 12 h in a water bath. They were then washed and scanned for fluorescence as previously described (Garrido et al., 2008). Because the probes for Beta- and Gammaproteobacteria correspond to sequences from the 23S rRNA gene and we used only the amplicon from 16S rRNA gene for fluorescent labeling, we could not detect these types of bacteria in hybridization with the oligonucleotide microarray.
2.9. Determination of total protein and sugar content in the field laboratory
Total protein and sugar content in the samples was determined in the field laboratory as follows: 1 g of sample (powder) was subjected to 3×1 min ultrasonication cycles in 2 mL of distilled water with 1–2 min stops by using a handheld sonicator (Dr. Hielscher 50W DRH-UP50H sonicator, Hielscher Ultrasonics, Berlin, Germany). Samples were centrifuged at 2000g to sediment the mineral particles, and the supernatants were directly assayed for protein concentration by using the bicinchoninic acid protein assay reagent (Pierce, Rockford, IL, USA) (Smith et al., 1985) and sugar content as described by Dubois et al. (1956), respectively. A NanoDrop (NanoDrop Int.) instrument was used for spectrophotometric measurements.
2.10. Agarose gel electrophoresis
For DNA fractionation and visualization a pre-stained agarose gel and a portable FlashGel System (Cambrex, East Rutherford, NJ, USA) were used and run following the manufacturer's recommendations. A personal digital camera (Panasonic) was used to take pictures of the gel.
2.11. Phylogenetic analysis by cloning and sequencing of the bacterial 16S rRNA gene
The extracted environmental DNA was used as template for PCR amplification of the bacterial 16S rRNA gene with the primer pair 16SF-16SR. The product from this reaction was used as template for a second PCR amplification with primers 63F (positions 43–63, 5′CAGGCCTAACACATGCAAGTC) and 907R (positions 906–926, 5′-CCGTCAATTCMTTTGAGTTT). The amplicon was cloned into vector pTOPO-TA (Invitrogen) and sequenced from both ends in an ABI 3710 automatic sequencer (PE Biosystems, General Electrics). Sequences were assembled with the package Phred-Phrap-Consed, with use of a Perl wrapper that automatically clustered and processed the chromatograms corresponding to individual clones (Ewing et al., 1998; Gordon, 2004). Putative chimeras were discarded after screening the assembled clone sequences with Bellerophon (Huber et al., 2004). The remaining clone sequences were assigned to taxonomical categories with use of the Ribosomal Database Project (RDP) web server (Cole et al., 2009). Complementary taxonomical information was obtained by comparing the clone sequences against the NCBI NR database with BLAST (Altschul et al., 1997). The alignments generated by RDP were used as input for Mothur application (Schloss et al., 2009) to calculate distance matrices ignoring terminal gaps, cluster clone sequences into operative taxonomical units (OTUs), generate rarefaction curves, identify OTUs shared across samples, and calculate the Ace and Chao1 estimators for species richness and the Simpson Index (D) for community diversity.
2.12. Phylogenetic analysis by cloning and sequencing of the archaeal 16S rRNA gene
For archaeal 16S rRNA gene amplification, two rounds of PCR amplification were performed with the universal archaeal primer pair 21F-958R (Delong, 1992) for the first round and the specific pair for the domain Archaea ARC344F-ARC915R (Stahl and Amann, 1991) for the second round. The thermocycler was programmed as follows: 94°C, 7 min; 30×(94°C, 1 min; 56°C, 1 min); 72°C, 7 min; 4°C for the first round, and 94°C, 4 min; 10×(94°C, 1 min; 68°C−1°C per cycle, 1 min; 72°C, 1.5 min); 20×(94°C, 1 min; 60°C, 1 min; 72°C , 1.5 min); 72°C, 30 min; 4°C, for the second round. We used the Go-Taq Green master mix and the GoTaq polymerase (Promega Corp., WI, USA) in a 50 μL final reaction volume.
2.13. DAPI staining
To visualize cells with optical microscopy, the microorganisms from the ground samples were separated from the inorganic and humic fractions with physical dispersion techniques, such as low- and high-speed centrifugation. Two 5 g aliquots of the samples analyzed by DAPI were resuspended in 50 mL of extraction buffer (1.5 M NaCl, 0.15% Tween 20, 10 mM Tris pH8, and 100 mM EDTA), then incubated for 2 h at room temperature in a roller and subsequently centrifuged at 2000g for 2 min. The pellet, which consisted mainly of minerals and coarse material, was discarded, and the supernatant was centrifuged at 10,000g for 10 min. The resulting new pellet was washed in 300 μL of the extraction buffer and centrifuged at 15000g for 5 min. The supernatant was discarded, and a few cells were collected with a sterile loop-spreader from the upper section of the wet pellet, spread on a microscope slide, left to dry, and incubated with 5 μL of 1 μg mL−1 DAPI-blue stain solution for 1 min. The slide was rinsed with water and then with 80% ethanol to remove the non-specific-bound DAPI stains. Cells were visualized in an Olympus Bx40 microscope at ×100 augmentations. Images were captured with an Olympus DP70 camera. Additionally, small pieces of core samples were subjected to a quick stain with DAPI in an Eppendorf tube, washed with buffer, and observed directly under the microscope. Under these conditions, we visualized cells with flagellar movement.
2.14. Biochemical extractions
Extracts were prepared from 20 to 40 g of different samples with GuHCl buffer (4 M guanidine-hydrochloride, 0.5 M EDTA, 0.5 M Tris pH 7.4) following the procedure described by Tuross and Stathoplos (1993). The final extracts were resuspended in sterile distilled water, dialyzed (>1200 Da cutoff) against water, and finally lyophilized. These extracts were used for the identification of amino acids by gas chromatography–mass spectrometry (see Section 2.15).
2.15. Determination of amino acids
For the identification of amino acids, the samples were first hydrolyzed with 6 M HCl at 110°C for 24 h and then freeze-dried to remove water, HCl, and any volatile organics. The hydrolyzed samples were analyzed, in a way similar to that described by Ruiz-Bermejo et al. (2007), by high-pressure liquid chromatography after derivatization with phenylisothiocyanate (Thermo-Fischer) with use of the following conditions: Solvent A: 50 mM NH4OAc buffer, pH 6.5; Solvent B: 100 mM NH4OAc-CH3CN (50:50), pH 6.5; 0 min 0% B (100% A), 45 min 70% B (30% A), 46 min 70% B (30% A), 48 min 100% B (0% A). The flow was 2 mL min−1, the column was thermostated at 52°C, and the chromatogram registered at 254 nm. High-pressure liquid chromatography analyses were carried out on a Surveyor (ThermoFinnigan, Scientific Instruments Services, Ringoes, NJ, USA) with a photodiode array detector with a Kromasil 100 C18 5 μm 25×0.46 column. The identifications of the amino acids were verified by comparing the retention times and UV absorbance spectra with external standards, purchased from Sigma-Aldrich and Fluka (Sigma-Aldrich, St. Louis, MO, USA).
2.16. Determination of total organic carbon
Up to 2 g of sample were dried by incubating at 110°C for 24 h. Total organic carbon was estimated by the weight of sample lost after a second incubation at 550°C for 3 h.
2.17. Testing the deliquescence properties of the drilled samples
To test the capacity of the collected samples to absorb and deliquesce atmospheric water vapor, we proceeded as follows: 2 g of samples was exhaustively dried at 110°C for 24 h (until no further weight loss was measurable); then they were stored in identical 100 mL glass beakers covered by aluminum foil (ajar) in a cold room at 4°C and below 75% relative humidity. Over the next 20 days, the samples were weighted daily. The water-weight gain was plotted as the percentage of the initial dried weight. Deliquescence, defined as the process by which a substance absorbs moisture from the atmosphere until it dissolves in the absorbed water and forms a solution, was clearly visible to the naked eye. Some of the samples even showed liquid water accumulating at the bottom of the beaker after only 5 days.
2.18. Ion chromatography analysis
Samples (10 g) were sonicated (3×1 min cycles) in 20 mL of water, and the mineral particles were removed by centrifugation. The supernatants were collected and loaded into a Metrohm 861 Advanced compact ion chromatographer IC (Metrohm AG, Herisau, Switzerland) undiluted or at dilution values of either 50% or 20%. For all the anions, except perchlorate, the column Metrosep A supp 7-250 was used with 3.6 mM sodium carbonate (NaCO3) as eluent. For perchlorate measurements, we used a Metrosep A supp 4-250 column with 1.9 mM Na2CO3, 1.9 mM NaHCO3, 25% (w/v) acetonitrile as eluent. The pH of the water solutions was measured with an inoLab pH meter (WTW, GmbH & Co. KG, Weilheim, Germany) after 24 h of solution stabilization.
2.19. Scanning electron microscopy (SEM)
Scanning electron micrographs were made with gold-coated pieces of core samples by using a JEOL JSM-5600 LV instrument (JEOL, Tokyo, Japan). To examine the micromorphology and mineralogy, it was operated at acceleration voltages of 10–20 kV and 2–3 kV. The phase compositions were measured by using energy-dispersive X-ray (INCA X-Sight, Oxford), with acceleration voltages of 10 kV for minerals and 2 kV for microorganisms.
3. Results
3.1. LDChip300 detected in situ microbial biomarkers in the Atacama subsurface
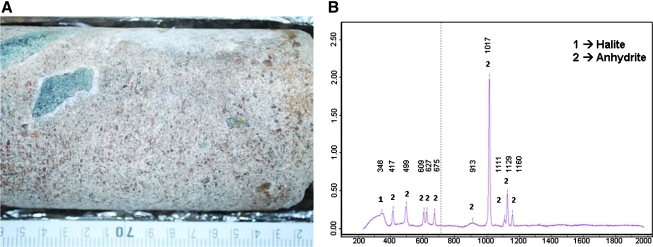
A 1-week duration drilling campaign was carried out in the apron at the base of a mountain to the west of Salar Grande (Materials and Methods; Fig. 1). We drilled a 5.2 m deep hole (reported here) and another one of 75 cm to the east of Salar Grande (not shown). More than 40 samples that contained the chips and came up and out of the hole were collected. Additionally, several intact core pieces from 160 to 310 cm deep were also recovered (Fig. 1B). This 1.5 m of solid core corresponded to several hard layers of solid material. The rest of the core mostly corresponded to soft or weakly cemented material, except approximately 1 m that we lost at the bottom of the hole (from approx. 4 to 5.20 m) when we finished the drilling operations. The loss of this core was due to a failure in the clamping system in the corer tip. The geological profile of the whole core showed thin gypsum crusts at the upper parts followed by fine-grained halite-cemented sand layers with and without pebbles (Fig. 1B). The recovered strongly compacted cores contained halite-cemented breccia and halite veins. These samples were analyzed with a field-portable Raman spectrometer rendering spectra with peaks identified as halite and anhydrite (CaSO4) crystals (Fig. 2).
FIG. 2.
Raman spectroscopy identified anhydrite and halite in the field. (A) Detailed image of one of the cores used for Raman analysis. (B) A characteristic Raman spectrum showing halite (NaCl) and the other abundant mineral, anhydrite (CaSO4). Color images available online at www.liebertonline.com/ast
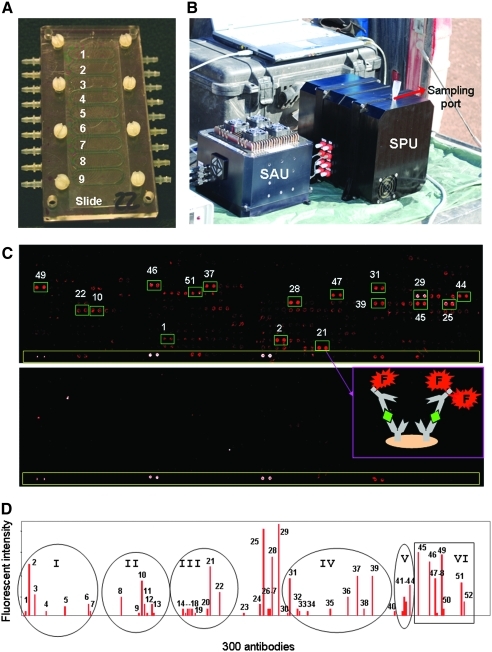
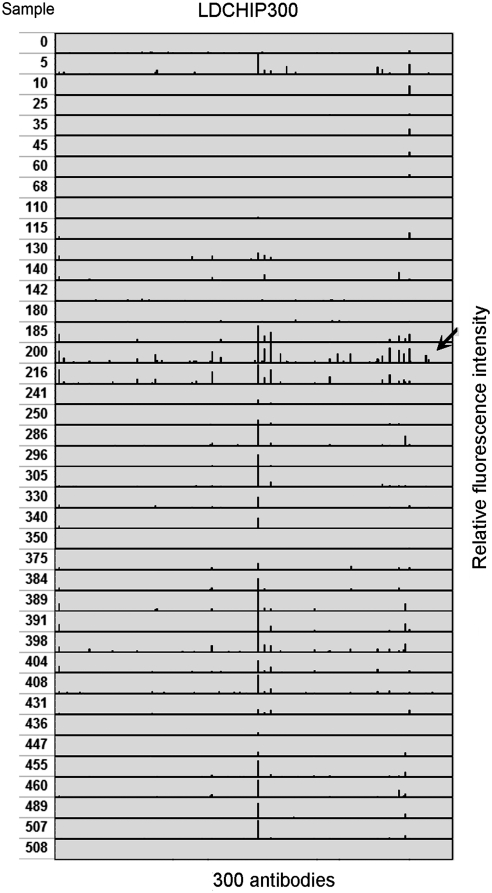
Forty powder- and chip-containing samples recovered from the core (from 0 to −5 m) were analyzed in the field by fluorescent sandwich antibody microarray immunoassay with LDChip300 (Figs. 3 and 4; see Materials and Methods). The fluorescent signal of each of the 300 antibodies (Fig. 3) was quantified and plotted, which generated an immunogram from each sample (Fig. 4). Positive reactions were identified with several antibodies in several samples. Especially significant were those taken at depths around 2 m (samples 185, 200, and 216), where positive reactions corresponded to antibodies raised against environmental biochemical extracts, microbial extracts, and biological compounds like DNA, EPS, or lipoteichoic acid polymers from Gram-positive bacteria, and others (Fig. 3 and Table 3). Among the microorganisms, we detected Gram-negative Alphaproteobacteria (weak signal from an Acetobacteraceae) and Gammaproteobacteria (Acidithiobacillus, Halothiobacillus, and Pseudomonadaceae), Gram-positive Actinobacteria and Firmicutes (Bacillales), Bacteroidetes, and the archaeon Halorubrum spp. The LDChip results confirm that complex polymers (EPS and lipoteichoic acids) were present in the analyzed samples, which, in turn, constitutes direct evidence of viable life or the remains of recently viable life with abundances especially high at ∼2 m. A strong fluorescent signal was obtained with an anti-steroid antibody in most of the samples below 2 m. We hypothesize that organomineral complexes bearing structurally similar compounds produced either by bacteria or fungi may have been responsible for such a high signal. A likely candidate was ergosterol, a triterpene lipid that forms part of the fungal cell membranes and functions like cholesterol in animal cells (Weete et al., 2010). To verify the authenticity of the antigen-antibody reactions, some samples were heated over a flame in sterile aluminum foil for 10 min in the field laboratory and then analyzed with LDChip300. Most (at least 90%) of the fluorescent signals disappeared from the immunograms, which indicates that the signals detected in the biochip were indeed a consequence of a sandwich immunoassay against organic (most probably biological) complex polymers.
FIG. 3.
LDChip300 detected microbes and polymeric biological remains at 2 m deep under the Atacama surface. (A) A picture showing the nine flow cells for nine simultaneous LDChip300 assays in the MAAM device (see Section 2.6.1). (B) A picture of the SOLID3 instrument in the field, showing its two functional units: the Sample Preparation Unit (SPU) and the Sample Analysis Unit (SAU) (See Section 2.6.2). (C) LDChip300 image obtained with sample 200 (top) indicating some of the spots with the highest fluorescent signals. The cartoon shows how capturing antibodies (Y forms) bind biological polymers (rhombi) and how they are sandwiched by fluorescent (F) tracer antibodies. The image obtained with the negative control (bottom) was used as a baseline (blank) to calculate spot intensities in the test samples. Long yellow rectangles encompass a fluorescent spot gradient. (D) Immunogram showing the fluorescence intensity of the positive spots. The peak numbers, antibody name, and immunogen can be seen in Table 3 (see also Table 2). Circles and squares indicate the main antibody clusters: (I) antibodies against whole extracts from sediments, biofilms, and water from Río Tinto rich in Gammaproteobacteria; (II) Gammaproteobacteria (Halothiobacillus spp., Acidithiobacillus spp.); (III) Desulfosporosinus, Pseudomonas, Salinibacter; (IV) different peptides and proteins; (V) cell wall polymeric components like lipoteichoic acids, lipidA, or peptidoglycan; (VI) nitroaromatic compounds and nucleotide derivatives. Color images available online at www.liebertonline.com/ast
FIG. 4.
LDChip300 immunoprofiling identified a new habitat in the Atacama subsurface. Ordered immunograms obtained after analyzing samples from different depths with LDChip300. The arrow indicates sample 200, collected at 2 m deep. Note that the most abundant and intense fluorescent signals are in and around this sample (see Fig. 3 and Table 3 for details). See also text for explanation.
Table 3.
LDChip300-Detected Microbial Compounds at 2 m Deep
|
LDChip300 |
|
|
||
|---|---|---|---|---|
| Phylum/group | No. Antibody | Immunogen (See also Table 2) | PAM (16 S) | 16S rRNA seq. |
| Gamma-proteobacteria | 1 IA1C1 | 3.2 damp water cellular fraction (Gamma, Nitrospira) | Gammaproteobacteria | |
| 2 IA1S1 | 3.2 damp water supernatant | Vibrionaceae | ||
| 3 IA2S1 | Green filaments EPS (Eukaryotes, Gamma …) | Xanthomonadaceae | ||
| 4 IC1C1 | 3.2 beach cells (Nitrospira, Gamma) | |||
| 5 IC6C1 | 3.1 beach under-crusts cells | |||
| 6 ID1C1 | Jarosite Nac. Cells (Gamma, Nitrospira, others) | |||
| 7 ID1C3 | Jarosite Nac. Sonicated Cells after EDTA | |||
| 8 IVE3C1 | Acidithiobacillus ferrooxidans intact cells | |||
| 9 IVE6C1 | A. caldus | |||
| 10 IVE7C1 | Halothiobacillus neapolitanus | |||
| 12 IVF2C1 | Shewanella gelidimarina (marine broth 15°C) | |||
| 13 IVF2S2 | S. gelidimarina (Alteromonadaceae) | |||
| 21 IVI1C1 | Pseudomonas putida (Pseudomonadaceae) | Pseudomonadaceae | ||
| 23 IVI6C3 | Azotobacter vinelandii (Pseudomonadaceae) | |||
| Alphaproteobacteria | 14 IVG1C1 | Acidocella aminolytica (Acetobacteraceae) | ||
| 15 IVG2C1 | Acidiphilium spp. | |||
| Actinobacteria | 11 IVE8C1 | Acidimicrobium ferrooxidans | HGC_G+ | Actinobacteria |
| Firmicutes | 20 IVI19C1 | Desulfosporosinus meridiei (Clostridiales. G+SRB) | LGCa_G+ | Firmicutes |
| 28 Lmo | Listeria monocitogenes (Bacillales, Listeriaceae) | LGCb_G+ | Bacillaceae | |
| 26 G+Bact | Gram+ LTA (crossreacts Bacillales) | LGCc_G+ | Paenibacillaceae | |
| 19 IVH1C1 | Bacillus subtilis spores (Bacillaceae) | Lactobacillaceae | ||
| 43 LTA | LTA, L. monocitogenes (Bacillales, Listeriaceae) | Leuconostaceae | ||
| Acidobacteria | 16 IVG3C1 | Acidobacterium capsulatum (Acidobacteria) | ||
| Deinococcus | 17 IVG4C1 | Thermus scotoductus (Deinococci-Thermales) | ||
| 18 IVG4C2 | T. scotoductus | |||
| Bacteroidetes | 22 IVI21S1 | Salinibacter ruber PR1 (Bacteroidetes) | ||
| Euryarchaeota | 24 IVJ8C1 | Halorubrum spp. (Halobacteriaceae) | Halorubrum spp. | |
| Epsilonproteobac. | 27 Hpyl | Helicobacter pylori (Gram-negative epsilon div.) | ||
| Nucleic acids and derivatives | 29 dsDNA(hu) | dsDNA (Human plasma) | ||
| 46 cGMP(bio) | cGMP-2′-succi-BSA | |||
| 49 cGMP(ups) | cGMP-BSA | |||
| 50 GMP_N | GMP-BSA | |||
| 51 cAMP_sh | cAMP-BSA | |||
| 52 Thiamine | Thiamine-BSA | |||
| Cell wall | 41 EPS_SP | EPS Santa Pola (Alicante, Spain) | ||
| 42 Lectin | Lectin potato specific for NAG-NAM oligomers | |||
| 44 LipidA | LipidA (monoclonal Ab, clon 43) | |||
| 25 Cortisol | Cortisol-17-BSA | |||
| 47 Digoxin | Digoxin (monoclonal Ab, mouse) | |||
| Xenobiotics | 45 Atrazine | Atrazine-BSA | ||
| 48 DNP | Dinitrophenol-BSA | |||
| Peptides | 30 ApsA | Adenylylsulfate reductase peptide (Desulfovibrio desulfuricans) | ||
| 31 ASB_prot | Bacterial ATPsynthase F1 subunit Alpha (prot) | |||
| 32 Beta-Glu | Beta-glucanase (E. coli) | |||
| 33 CcdA | CcdA peptide (Cytochrome biogenesys. Geobacter) | |||
| 34 DhnA | DhnA (Dehydrin) peptide (Nostoc) | |||
| 35 Hsp70 | Chaperon Hsp70 (M. tuberculosis) | |||
| 36 ModA | Molibdate transport peptide (Leptospirillum ferrooxidans) | |||
| 37 NifH2 | NifH nitrogenase peptide (L. ferrooxidans) | |||
| 38 NorB | NorB protein (Nitrobacter hamburgensis) | |||
| 39 Pfu_fer | Ferritin (Pyrococcus furiosus) protein | |||
| 40 Stv | Streptavidin | |||
List of the antibodies showing positive reaction in LDChip300 with sample 200 and comparison to the results obtained with oligonucleotide microarray (PAM) in the field, and by cloning and sequencing of the 16S rRNA gene in the laboratory.
Column No. indicates the number of each of the peaks of the immunogram in Fig. 3. The column PAM (16S) indicates the group of bacteria detected after fluorescent hybridization of bacterial 16S rRNA gene in the field: HGC_G+, high-GC-content Gram-positive bacteria; LGCa,b,c_G+, low-GC-content groups a, b, and c of Gram-positive Firmicutes (see Fig. 5). Alpha, Beta, Gamma: division of proteobacteria. LTA, lipoteichoic acids. EPS, Exopolysaccharides. dsDNA, double-stranded DNA. cGMP and cAMP, cyclic guanine and adenine monophosphate nucleotides, respectively.
3.2. Biochemical and molecular analyses in the field supported the LDChip300 results
We successfully extracted DNA from two out of eight samples assayed in the field: one from the surface (sample 0) and the other from a depth of 2 m (sample 200). The last one also had the strongest molecular biomarker signals obtained with LDChip300 (Figs. 3 and 4). The failure in obtaining DNA from the other samples may have been due to a lower number of cells. The obtained DNA was checked spectrophotometrically and by using PCR amplification of the 16S rRNA gene with bacterial and archaeal universal primers (See Sections 2.11 and 2.12, Materials and Methods). Additionally, the PCR amplicon from sample 200 was fluorescently labeled and used to hybridize to an oligonucleotide microarray designed for the analysis of prokaryotic diversity (Garrido et al., 2008). The results further confirm the presence of bacteria, mainly from the Gram-positive low-GC-content Firmicutes group, as well as from the high-GC-content Gram-positive bacteria (Fig. 5 and Table 3). Total protein and sugar contents were also determined in the field laboratory, showing a peak in the samples around 2 m deep (Fig. 6), which is in agreement with LDChip300 and DNA microarray analyses, although sugars still remain high to the bottom of the hole. All these data support and validate the in situ LDChip300 results and are consistent with the presence of a subsurface microbial habitat.
FIG. 5.
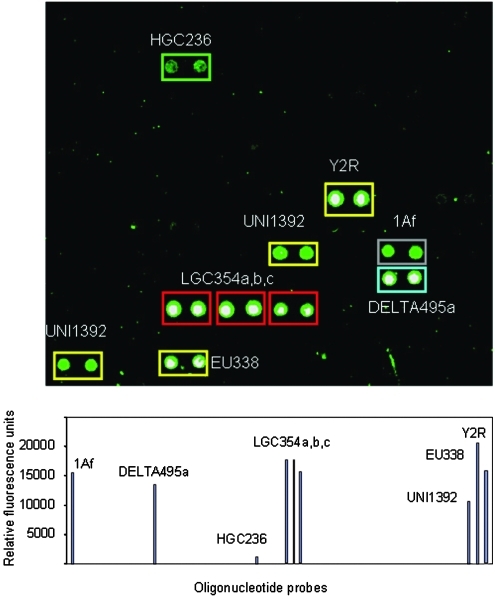
Detection of bacteria by DNA hybridization in sample 200. Microarray image showed positive fluorescent spots (top figure) corresponding to universal eubacterial probes (UNI1392, EU338, Y2R), high-GC-content Gram-positive bacteria (Actinomycetes group, probe HGC236), the low-GC-content Gram-positives Firmicutes including the Lactobacillus subgroup (LGC354a), the Bacillus subgroup (LGC354b), and the Streptococcus subgroup (LGC354c); Deltaproteobacteria (DELTA495a); and Archaea (1Af). This last probe corresponds to a highly degenerated oligonucleotide considered specific for Archaea. Because the fluorescent probe was amplified with primers for Eubacteria, we did not expect positive results with 1Af unless it came from similar sequences from unknown bacteria. (Bottom figure) Plot showing the quantification of fluorescent signals from nonsaturated images. Color images available online at www.liebertonline.com/ast
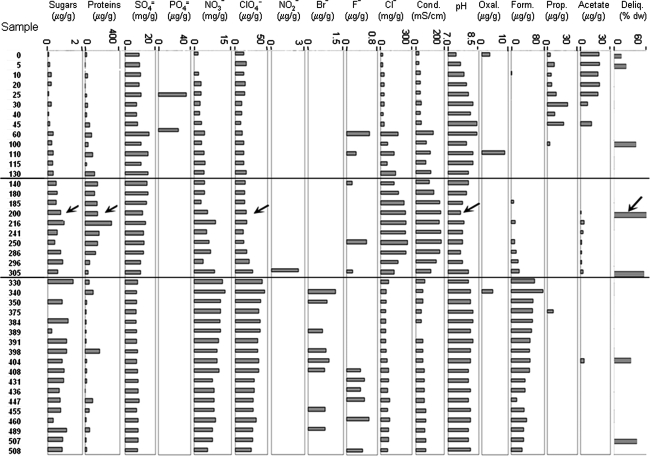
FIG. 6.
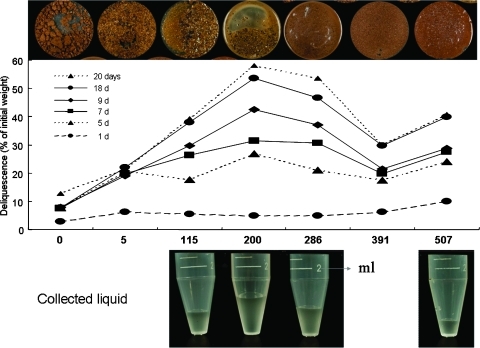
Biogeochemical analyses of the samples collected along the whole core. Sample names and depths, the assayed compound or parameter, the units, and the scale are indicated at the top of the figure. Arrows point to sample 200. The horizontal lines delimit the samples with the higher halite and protein concentration, the highest deliquescence capacity, and a small decrease in pH, defining a geological unit for a subsurface habitat. (Deliq) Deliquescence capacity of the samples after 20 days at 4°C and 75% relative humidity, expressed as the percentage (% dw) of adsorbed water with respect to the initial dry weight (2 g per sample), showing a maximum of 58% at sample 200 (See Figs. 9 and 10).
3.3. Geochemical analyses revealed a hypersaline habitat in the Atacama subsurface
The analysis of all powder- and chip-containing samples collected during drilling by ion chromatography showed a specific geochemical pattern at around 2 m deep (Fig. 6), which is in agreement with the results obtained in the field. A slight decrease in the pH of water solutions is coincident with a high increase of salt content (NaCl) and conductivity, both decreasing just when nitrates, perchlorates, and formate contents increase and remain steady down to the bottom of the borehole (5.2 m). In contrast, sulfate content is slightly higher in the first 2–3 m. A peak of total protein and sugar profiles is consistent with the salinity (halite), conductivity, the maximal deliquescence capacity, and LDChip300 results, while the pH of the water solution used for ion chromatography analysis (Section 2.18) showed a small acidification. This subsurface habitat is characterized by the presence of high amounts of nitrates, sulfates, and perchlorates, all of which are capable of working as electron acceptors for bacterial respiration. The presence of small organic acids is also highly remarkable, with propionate and acetate dominating in the upper part, while formate increases from 2 m deep (Fig. 6). All of them can be used as electron donors to support bacterial nitrate, sulfate, and perchlorate reduction. Additionally, amino acids were also detected by gas chromatography–mass spectrometry in polymeric material extracted with guanidine-hydrochloride buffer (see Section 2.14, Materials and Methods) from different depths (Table 4). These amino acids may come either from extracellular or intracellular fractions because we cannot rule out the cell lysis with the extraction method used.
Table 4.
Amino Acids Detected in Polymeric Material (>1200 Da) Extracted by Guanidine Chloride Buffer
| Sample | Amino acids |
|---|---|
| 0 | Gly, Ala, +3 unidentified amines |
| 5 | Glu, Ser, Gly, Ala, +3 unidentified amines |
| 60 | Asp (traces), Glu (traces), Ser, Gly, Ala, unidentified amines |
| 140 | Asp (traces), Glu (traces), Ser, Gly, Ala, 5 unidentified amines |
| 200 | Asp, Glu, Ser, Gly, Ala, unidentified amines |
| 286 | Asp (traces), Glu (traces), Ser, Ala, Pro, Val, 2 unidentified amines |
| 305 | Asp (traces), Glu (traces), Ser, Gly, Ala, 2 unidentified amines |
| 398 | Asp, Glu, Ser, Gly, Ala, 2 unidentified amines |
| 431 | Asp (traces), Glu (traces), Ser, Gly, Thr, Ala |
| 460 | ND |
| 507 | Asp, Glu, Ser, Gly, Ala, 3 unidentified amines |
ND, Not detected.
3.4. Microscopy and molecular phylogeny confirmed the presence of bacteria and archaea in the Atacama subsurface
Further analyses in the laboratory were performed to obtain new information and to validate the results obtained in the field. Core samples were assayed for microscopic evidence of cells either by SEM or optical microscopy. The scanning electron microscope images (Fig. 7) show the presence of cellular and biofilm morphologies together with mineralized cellular moulds excavated in the halite, which has a high signal for organic carbon. Fluorescent DNA-specific staining (DAPI) and microscopy analysis revealed the presence of cells with different morphologies: coccoid-, rod-, and comma-shaped, and vibrioid (Fig. 8A). After addition of water to the sample, some of these cells showed flagellar movement under the microscope, with and without a quick DAPI staining (see Section 2.13). These cells were metabolically active probably due to the water captured by the core during transport and storage in a cold room.
FIG. 7.
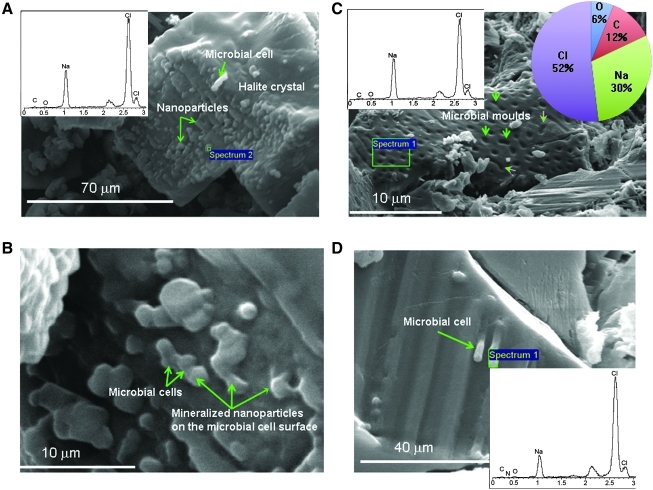
Microbial forms under the Atacama surface bound to halite crystals. Scanning electron microscope images and energy dispersive X-ray (EDX) spectra showed halite crystals with incipiently mineralized microbial cells. (A) Halite crystal with elongated morphology and rough surface showing nanoparticles and mineralized microbial cell-like structures (arrows) on its surface. EDX spectrum of the crystal (inset) indicates that it is composed of Na and Cl (halite). (B) Close-up of two microbial cells from (A) attached to the halite crystal surface (arrows). (C) Halite crystal showing empty voids which correspond to mineralized moulds of degraded microbial cells, most probably bacteria (arrows), some of them with clear vibriod form. Note that EDX spectrum displays Na, Cl, C, O, and N. The four latter elements come, most probably, from organic compounds of microbial cells. The pie chart shows the percentage of the main elements. (D) Close-up of two microbial cells attached to the halite crystal surface (arrow) and the EDX spectrum of one of them. The EDX spectra correspond to the green squares. Color images available online at www.liebertonline.com/ast
FIG. 8.
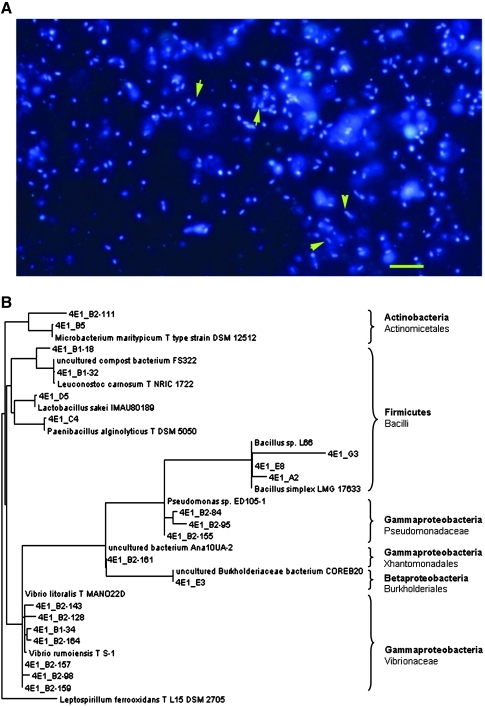
Microbial cells and phylogenetic analysis of bacterial DNA from 2 m deep. (A) Sample 200 was stained with DNA-specific stain (DAPI). Rod- and comma-shaped or vibrioid forms (arrows) can be detected. Bar, 5 μm. (B) Phylogenetic tree obtained with the 16S rRNA gene sequences from sample 200 (see text for explanations). Sequences obtained from sample 200 were named as 4E1.…Color images available online at www.liebertonline.com/ast
Phylogenetic analyses of the bacterial 16S rRNA gene (Fig. 8B) indicated that the most abundant clones found at 2 m deep were closely related to the Gammaproteobacteria class (most of them Vibrio spp. and some Pseudomonas spp.). Additionally, many clones corresponded to the phylum Firmicutes (most of them Bacillus spp.), and others from the phylum Actinobacteria (Microbacterium spp.). These results are again consistent with those obtained with LDChip300 (Table 3), where the antibodies show positive reactions that can be grouped into Gammaproteobacteria (Shewanella, Pseudomonas, strains or natural extracts enriched in these types of microorganisms like Acidithiobacillus, Halothiobacillus, and environmental extracts from Río Tinto), the Gram-positive Firmicutes, and Actinobacteria. Additionally, we obtained several sequences closely related to the archaeal genus Halorubrum. Again, this result is in agreement with LDChip and corroborated the first report about archaea in the Atacama subsoil.
4. Discussion
4.1. LDChip300 detected microbes and molecular biomarkers in the Atacama subsurface
We previously described the performance of former versions of LDChip (Parro et al., 2008b; 2011a, 2011b; Rivas et al., 2008). Herein, we demonstrate the unprecedented capacity of an immunosensor chip for the detection of microbes and molecular biomarkers in the subsurface of one of the best Mars analogues on Earth: the Atacama Desert (Fig. 3). Samples were immediately analyzed in the field after their collection from the core. Thirty samples can be assayed simultaneously with LDChip300 and the results obtained after 3 h, which allowed us to perform nearly real-time monitoring. It was highly relevant that several positive reactions obtained around 2 m deep corresponded to antibodies against Gram-positive bacterial antigens from the group Firmicutes (Table 3). This is the case for IVI19C1, raised against Desulfosporosinus meridiei (Clostridiales, Gram-positive sulfur-reducing bacteria); Lmo, for Listeria monocitogenes (Bacillales, Listeriaceae); G+Bact, Gram-positive lipoteichoic acids (cross reacts with Bacillales); IVH1C1, for Bacillus subtilis spores (Bacillaceae); or LTA, for LTA from the Gram-positive bacterium Listeria monocitogenes (Bacillales, Listeriaceae). The presence of bacteria from the Firmicutes group was demonstrated in the field by DNA extraction from the same sample, PCR amplification and fluorescent labeling of the bacterial 16S rRNA gene, and hybridization with a phylogenetic oligonucleotide microarray (Fig. 5).
Some of the antibodies raised against the Río Tinto acidic environment or acidophilic strains showed significant fluorescent signals, which indicates the presence of similar molecular biomarkers or microorganisms, or both. This is the case for peaks 1–7 in Fig. 3 (Table 3), which correspond to extracellular polymeric material or cellular fractions obtained from water, biofilms (filaments), sulfur-rich samples (dry crusts, sediments, jarosite and iron-sulfate precipitates), and peaks 8 and 9 from iron-sulfur bacteria of Acidithiobacillus genus. The presence of molecular biomarkers and bacteria characteristic of acidic environments might be related to the fact that Atacama soils may contain strong acids in accumulated dust particles, as reported by Quinn et al. (2005). They concluded that extremely low pH resulting from acid accumulation combined with limited water availability and high oxidation potential may result in acid-mediated reactions at the soil surface during low-moisture transient wetting events (i.e., thin films of water). These micro-niches might support the growth of sulfur-oxidizing bacteria like Acidithiobacillus spp. and Hallothiobacillus spp., iron-reducing bacteria like Acidocella spp. and Acidiphilium spp., which may tolerate up to pH 5 (Lu et al., 2010), or iron-oxidizing bacteria like Acidimicrobium spp.
The relatively high fluorescent signal obtained with antibodies IVI21S1, IVJ8C1, EPS_SP raised against Salinibacter ruber, Halorubrum spp., and exopolymeric substances from the Santa Pola solar saltern (Alicante, Spain), respectively, indicates the presence of archaea at 2 m deep. This fact was confirmed by molecular phylogenetic studies in the laboratory by amplification and sequencing the archaeal 16S rRNA gene. Archaea have been reported in superficial samples from the Atacama (Lizama et al., 2001; Demergasso et al., 2004), but until very recently they were not detected in the subsurface (Gramain et al., 2011). Our work further confirms and expands the presence of these prokaryotes in the Atacama soil subsurface.
Sugars and proteins followed a parallel pattern up to near 3 m, where proteins drop dramatically but sugars continue with relatively high levels. The peak in protein content could be associated with a more active microbial population, as a consequence of higher water availability due, in turn, to the presence of materials with higher deliquescence capacity. The microbial activity would produce acids that might explain the small drop in the pH of water solutions in these samples. By contrast, the permanent sugar level below 3 m might be due to the accumulation of extracellular polysaccharides (EPS) produced as a response to lower water activity, as it has been reported in some bacteria (e.g., Roberson and Firestone, 1992) or in soil environments (Foster, 1981). The strong correlation between protein content, halite, small acidification, and maximal deliquescence capacity (Fig. 6) with LDChip results, together with microscopic and molecular phylogenetic analyses, indicates the presence of an actual or recently colonized habitat, a true microbial oasis.
4.2. A hygroscopic mineral-driven subsurface hypersaline habitat: implications for Mars
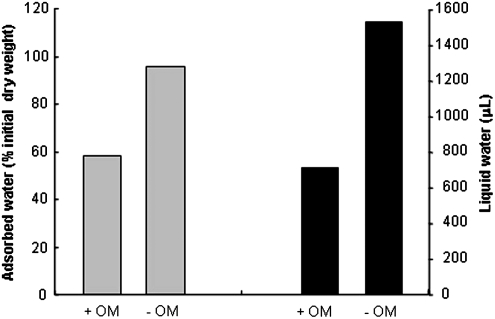
We described a new hypersaline subsurface habitat where the presence of hygroscopic minerals (halite, perchlorate, and anhydrite) may be responsible for water retention. Samples contained 10–41 ppm (mg Kg−1) of perchlorate and a peak of NaCl of 260 g Kg−1 at 2 m depth, high enough to promote deliquescence. In fact, these samples showed the higher deliquescence capacity (Figs. 6 and 9) by accumulating nearly 60% of the initial weight of liquid water in 20 days (e.g., sample 200). After 5 days, no significant differences were observed; but later, a clear increase in the capacity of water absorption and deliquescence was recorded for sample 200 and those in close proximity to it, coincident with the highest halite content. Moreover, deliquescence increases when organic matter (ca. 1.9% in sample 200) is removed (Fig. 10). These results are consistent with those of Davila et al. (2008), which demonstrated that water vapor condenses and deliquesces on the halite surface and pores at low relative humidity (75%). The ability of halite and perchlorate salts to absorb water and form a liquid solution (deliquesce) is highly relevant for the search for life on Mars. Zorzano et al. (2009) demonstrated that small amounts of sodium perchlorate spontaneously absorb moisture and melt into a liquid solution under martian atmospheric conditions. In addition, Davila et al. (2010) reported the deliquescence capacity of hygroscopic salts and their hypothetical capacity to support microbial growth or metabolic activity under simulated martian conditions.
FIG. 9.
Deliquescence capacity of the samples at different depths. (Top) Photographs showing the aspect of the samples after 20 days of deliquescence assay (see Section 2.17). The accumulation of liquid water is clearly visible. (Middle) The water retained by each of the samples was estimated as the percentage of the initial dry weight along the time course: 1, 5, 7, 9, 18, and 20 days. (Bottom) Liquid water recovered from some of the samples. Color images available online at www.liebertonline.com/ast
FIG. 10.
The presence of ca. 2% organic matter in the samples diminishes the deliquescence capacity to about 50%. Two aliquots of 2 g of sample 200 were dried, their organic matter kept (+OM) or baked at 550°C to destroy it (-OM), and finally subjected to the deliquescence capacity assay for 20 days (see Materials and Methods). The adsorbed water was measured as the percentage of the initial dry weight (gray) and as the total liquid water recovered (black). Total organic carbon in sample 200 was 1.98±0.15%.
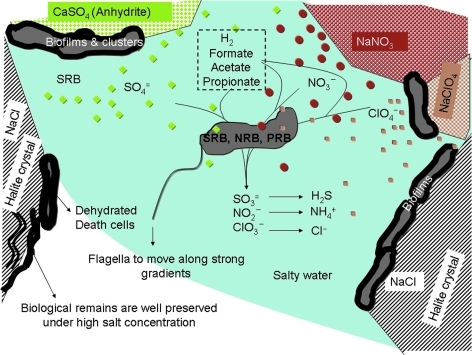
Our AtacaMars field campaign demonstrates that subsurface hygroscopic salt environments may be true oasis for microbial life and further supports the possibility of a subsurface martian habitat based on the deliquescence capacity of similar hygroscopic salts. We hypothesize an ecosystem fueled by reduced compounds from hydrothermal activity (H2, SH2, acetate, formate, etc.). Potential martian microorganisms could use perchlorates, nitrates, or sulfates as electron acceptors as shown in the Atacama subsurface (Fig. 11). Strong gradients of salts can be formed, and microorganisms would use flagella to move in search of nutrients. When water is scarce, microorganisms produce abundant exopolymeric material that helps them to avoid desiccation and attaches them to mineral surfaces, where they form small biofilms or cell clusters. After the death of the microorganisms, the polymeric remains (EPS, proteins, nucleic acids, etc.) or other metabolites are retained by electrostatic charges of mineral and salt crystals. These interactions with minerals, in addition to high salt concentrations, hinder enzymatic degradation. Similar niches could be present in the martian subsurface, where small organic acids can be supplied from the partial photodestruction of complex meteoritic organic matter. Halite and perchlorate salts have two main advantages to sustain a hypothetical subsurface martian ecosystem: they trap and form liquid water and simultaneously lower its freezing point. Additionally, high salt concentrations inhibit enzymes like DNAses, which allows better preservation of biological polymers.
FIG. 11.
A potential martian biota based on the Atacama's hypersaline subsurface habitat. Deliquescence on mineral crystals would dissolve salts and small organic compounds to provide energy sources and nutrients for microorganisms (see Section 4.2 for explanation). SRB, NRB, and PRB: sulfate-, nitrate-, and perchlorate-reducing bacteria, respectively. Color images available online at www.liebertonline.com/ast
4.3. LDChip for life detection on Mars
Our LDChip is suited to the search for microbial life in planetary exploration, particularly for Mars. We hypothesize that microorganisms living in similar habitats under similar physicochemical parameters share similar molecular mechanisms to deal with such conditions. Those mechanisms will produce similar biomolecules that constitute a good set of molecular biomarkers. We suggest two strategies for target selection: (i) a direct approach, in which well-known biomolecules or their diagenetic products from those listed by Parnell et al. (2007) and Parro et al. (2008a) are used as antigens to produce antibodies; and (ii) a shotgun strategy where whole or fractionated biochemical extracts from terrestrial Mars analogues are used as targets for antibody production (Parro et al., 2008a, 2011a; Rivas et al., 2008; Parro and Muñoz-Caro 2010). We have produced polyclonal antibodies against biochemical extracts from environments that are acidic and iron-sulfur-rich, hydrothermal, permanently frozen, hypersaline, and so on (Table 2). Samples were taken from water, sediments, mineral deposits (sulfate precipitates, jarosite, hematite, etc.), rocks, and subsurface cores during drilling campaigns. Simultaneously, we can produce antibodies against different kinds of universal macromolecules like EPS, anionic polymers, cell wall components, nucleic acids, nucleotides and derivatives, phylogenetically conserved proteins, and so on. There are commercial antibodies against single- or double-stranded DNA, all recognizing structures and features of terrestrial DNA. In the case of an eventual martian DNA made up of slightly different nucleotides, it probably would not be recognized with antibodies against terrestrial counterparts, except in those cases where the antibodies were recognizing common structures. It is possible to make antibodies against modified nucleotides, but it is unknown which modifications to target. Alternative nucleobases (e.g., xantine, aminated, or methylated derivatives of the four known nucleotides) can be used as targets. However, considering that some nucleobases (e.g., adenine) can be obtained abiotically (Yuasa et al., 1984), the detection of these types of compounds by itself is not a biomarker, unless they were forming part of a polymer like DNA or a secondary structure like a double or triple helix. Universal modified nucleotides that are widely used for life as signal-transducers, for example, cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) (Gomelsky, 2011), are good targets for antibody production. There are commercial antibodies against these compounds (Table 2), and we have also produced our own stock. In fact, LDChip300 showed strong signals for antibodies against these compounds (see peaks 46, 49, and 50, all against cGMP, in Fig. 3 and Table 3), which indicates that some microorganisms might be producing and even secreting cGMP or its derivatives. In fact, it has been recently reported that the Alphaproteobacterium Rhodospirillum centenum secretes relatively high amounts of cGMP to trigger the formation of metabolically dormant cells, called cysts, which are highly resistant to desiccation (Berleman and Bauer, 2004; Marden et al., 2011). High salt concentration inhibits degrading enzymes, among them the nucleases, which may explain why, in this hypersaline environment, DNA and nucleotide derivatives are well-preserved. Additionally, the binding to mineral surfaces may be an additional factor of stabilization and aggregation (Romanowski et al., 1993). Whether the positive signals obtained with anti-cGMP antibodies are indeed due to the presence of cGMP is yet to be determined. Positive signals from a SMI indicate that we are detecting at least a dimer, an oligomeric or polymeric compound, or other types of aggregate, because at least two antigen-binding sites are necessary; one to bind to the capturing antibody and another to bind to the fluorescent tracer.
LDChip300 contains antibodies against other universal compounds like protoporphyrin IX, lipidA, peptidoglycan, amino acids, vitamin K12, some antibiotics, and antibodies against proteins and peptides from relevant microbial metabolisms: nitrogen fixation; sulfate, nitrate, and perchlorate reduction; iron oxi-reduction; and so on. (Table 2). Theoretically, it is possible to produce antibodies against any type of terrestrial biomolecule or even molecules that could be considered an extraterrestrial substitute. But, given that we know only terrestrial life, we must focus on making antibodies for that.
5. Conclusion
We have discovered a new hypersaline subsurface habitat in the Atacama. The presence of highly hygroscopic salt deposits associated with the microorganisms indicates that this habitat constitutes a true microbial oasis. Hygroscopic salts promote the deliquescence of water on the mineral surfaces and form thin liquid films that can maintain microbial growth. Our LDChip300 detected microbes and molecular biomarkers in this hypersaline subsurface environment. The relatively high number of antibodies against a rational and structured panel of target molecular biomarkers makes LDChip a suitable tool for the search for life for planetary exploration. Halite- and perchlorate-rich subsurface environments on Mars should be considered targets for future life-detection missions. The known destructive effects on organic matter by thermal oxidation promoted by perchlorates (Navarro-González et al., 2010) are not a constraint for our LDChip-SOLID system (Parro et al., 2011a). The use of a SOLID-like Life Detector Chip and instrumentation as part of the payload of future missions could be an important contribution to the search for life on Mars.
Appendix
Table 2.
Antibodies Developed and Used in This Work
| Ab No | Ab name | Source/Strain | Sample/Culture conditions | Immunogen/Fraction | Host | Supp. References |
|---|---|---|---|---|---|---|
| 1 | IA1C1 | Río Tinto (3.2 water dam) | Water | Cellular fraction | Rb | Rivas et al.,2008 |
| 2 | IA1S1 | Río Tinto (3.2 water dam) | Water | Supernatant | Rb | Rivas et al.,2008 |
| 3 | IA1S2 | Río Tinto (3.2 water dam) | Water | Supernatant from EDTA wash | Rb | Rivas et al.,2008 |
| 4 | IA1C2 | Río Tinto (3.2 water dam) | Water | Insoluble cell pellet from S100 | Rb | Rivas et al.,2008 |
| 5 | IA1S100 | Río Tinto (3.2 water dam) | Water | Soluble cellular fraction S100 | Rb | Rivas et al.,2008 |
| 6 | IA1C3 | Río Tinto (3.2 water dam) | Water | EDTA washed cells (sonicated) | Rb | Rivas et al.,2008 |
| 7 | IA2C1 | Río Tinto (3.1 stream) | Green filaments | Cellular fraction | Rb | Rivas et al.,2008 |
| 8 | IA2S1 | Río Tinto (3.1 stream) | Green filaments | Supernatant | Rb | Rivas et al.,2008 |
| 9 | IA3C1 | Río Tinto (3.1 stream) | Black filaments | Cellular fraction | Rb | Rivas et al.,2008 |
| 10 | IA3S1 | Río Tinto (3.1 stream) | Black filaments | Supernatant | Rb | Rivas et al.,2008 |
| 11 | IC1C1 | Río Tinto (Playa 3.2) | Sediments | Cellular fraction | Rb | Rivas et al.,2008 |
| 12 | IC1S1 | Río Tinto (Playa 3.2) | Sediments | Supernatant | Rb | Rivas et al.,2008 |
| 13 | IC1S2 | Río Tinto (Playa 3.2) | Sediments | Supernatant from EDTA wash | Rb | Rivas et al.,2008 |
| 14 | IC2C1 | Río Tinto (3.1 water dam) | Dark red sediments | Cellular fraction | Rb | Rivas et al.,2008 |
| 15 | IC2S2 | Río Tinto (3.1 water dam) | Dark red sediments | Supernatant from EDTA wash | Rb | Rivas et al.,2008 |
| 16 | IC2C3 | Río Tinto (3.1 water dam) | Dark red sediments | EDTA washed cells (sonicated) | Rb | Rivas et al.,2008 |
| 17 | IC3C1 | Río Tinto (Main spring) | Brown filaments | Cellular fraction | Rb | Rivas et al.,2008 |
| 18 | IC3S2 | Río Tinto (Main spring) | Brown filaments | Supernatant from EDTA wash | Rb | Rivas et al.,2008 |
| 19 | IC3C3 | Río Tinto (Main spring) | Brown filaments | EDTA washed cells (sonicated) | Rb | Rivas et al.,2008 |
| 20 | IC4C1 | Río Tinto (3.2 water dam) | Brown filaments | Cellular fraction | Rb | Rivas et al.,2008 |
| 21 | IC4S2 | Río Tinto (3.2 water dam) | Brown filaments | Supernatant from EDTA wash | Rb | Rivas et al.,2008 |
| 22 | IC5C1 | Río Tinto (Playa 3.1) | Red crusts | Cellular fraction | Rb | Rivas et al.,2008 |
| 23 | IC5S1 | Río Tinto (Playa 3.1) | Red crusts | Supernatant | Rb | Rivas et al.,2008 |
| 24 | IC6C1 | Río Tinto (Playa 3.1) | Red sediment 1–2 cm under crust | Cellular fraction | Rb | Rivas et al.,2008 |
| 25 | IC6S1 | Río Tinto (Playa 3.1) | Red sediment 1–2 cm under crust | Supernatant | Rb | Rivas et al.,2008 |
| 26 | IC7C1 | Río Tinto (3.2 water dam) | Dried wall sediments | Cellular fraction | Rb | Rivas et al.,2008 |
| 27 | IC8C1 | Río Tinto (3.1 stream's banks) | Green-orange sediments | Cellular fraction | Rb | Rivas et al.,2008 |
| 28 | IC8S1 | Río Tinto (3.1 stream's banks) | Green-orange sediments | Supernatant | Rb | Rivas et al.,2008 |
| 29 | IC9C1 | Río Tinto (3.1 mine's ruins) | Red-grey sulfate-rich precipitates | Cellular fraction | Rb | Rivas et al.,2008 |
| 30 | A138 | Río Tinto (“Nacimiento”) | Yellow mats (central stream) | Whole Gu/HCl extraction | Rb | Rivas et al.,2008 |
| 31 | A140 | Río Tinto (3.0 stream) | Pink superficial layer | Cellular fraction | Rb | Rivas et al.,2008 |
| 32 | A141 | Río Tinto (3.0 stream) | Pink superficial layer | Supernatant | Rb | Rivas et al.,2008 |
| 33 | A143 | Río Tinto (3.2 water dam) | Wall sediments. Lithified overgrowth | Whole Gu/HCl extraction | Rb | Rivas et al.,2008 |
| 34 | A152 | Río Tinto (3.0 stream) | Pink superficial layer | Whole Gu/HCl extraction | Rb | Rivas et al.,2008 |
| 35 | ID1C1 | Río Tinto (Main spring) | Iron sulfate–rich precipitates | Cellular fraction | Rb | Rivas et al.,2008 |
| 36 | ID1S1 | Río Tinto (Main spring) | Iron sulfate–rich precipitates | Supernatant | Rb | Rivas et al.,2008 |
| 37 | ID1S2 | Río Tinto (Main spring) | Iron sulfate–rich precipitates | Supernatant from EDTA wash | Rb | Rivas et al.,2008 |
| 38 | ID1C3 | Río Tinto (Main spring) | Iron sulfate–rich precipitates | EDTA washed cells (sonicated) | Rb | Rivas et al.,2008 |
| 39 | ID2S2 | Peña de Hierro (148 m deep) | 4-59c sample (MARTE project) | Supernatant from EDTA wash | Rb | Rivas et al.,2008 |
| 40 | ID3S2 | Peña de Hierro (96 m deep) | 4-39c sample (MARTE project) | Supernatant from EDTA wash | Rb | Rivas et al.,2008 |
| 41 | ID4S2 | Peña de Hierro (154 m deep) | 4-61a sample (MARTE project) | Supernatant from EDTA wash | Rb | Rivas et al.,2008 |
| 42 | ID5S2 | Peña de Hierro (141 m deep) | 4-56c sample (MARTE project) | Supernatant from EDTA wash | Rb | Rivas et al.,2008 |
| 43 | ID7S2 | Peña de Hierro (84–97 m deep) | 8-42b+43c+45abc+46bc (MARTE project) | Supernatant from EDTA/guanidinium | Rb | Rivas et al.,2008 |
| 44 | ID10S2 | Peña de Hierro (119–127 m deep) | 8-54a+54c+56c (MARTE project) | Supernatant from EDTA/guanidinium | Rb | Rivas et al.,2008 |
| 45 | ID11S2 | Peña de Hierro (138 m deep) | 8-60b+60c (MARTE project) | Supernatant from EDTA/guanidinium | Rb | Rivas et al.,2008 |
| 46 | ID12S2 | Peña de Hierro (147–152 m deep) | 8-63b+64b+64c+65b (MARTE project) | Supernatant from EDTA/guanidinium | Rb | Rivas et al.,2008 |
| 47 | ID13S2 | Peña de Hierro (2–3 m deep) | 8-2a (MARTE project) | Supernatant from EDTA/guanidinium | Rb | Rivas et al.,2008 |
| 48 | ID18S2 | Peña de Hierro (93 m deep) | 8-45b (MARTE project) | Supernatant from EDTA/guanidinium | Rb | Rivas et al.,2008 |
| 49 | ID14S2 | Peña de Hierro (105 m deep) | 8-49b (MARTE project) | Supernatant from EDTA/guanidinium | Rb | Rivas et al.,2008 |
| 50 | ID15S2 | Peña de Hierro (119 m deep) | 8-54a (MARTE project) | Supernatant from EDTA/guanidinium | Rb | Rivas et al.,2008 |
| 51 | ID16S2 | Peña de Hierro (152 m deep) | 8-65b (MARTE project) | Supernatant from EDTA/guanidinium | Rb | Rivas et al.,2008 |
| 52 | ID17C1 | Río Tinto (3.2 water dam) | Yellow Fe-S–rich salt precipitates | Cellular fraction | Rb | Rivas et al.,2008 |
| 53 | ID17S1 | Río Tinto (3.2 water dam) | Yellow Fe-S–rich salt precipitates | Supernatant | Rb | Rivas et al.,2008 |
| 54 | Ahyd | Yellowstone National Park | Hydrogenobacter acidophilus (Bacteria, Aquificae) | Whole cells | Rb | Schweitzer et al.,2005 |
| 55 | Acyan | Yellowstone National Park | Cyanidium spp. (Eukaryote, Rhodophyta) | Whole cells | Rb | Schweitzer et al.,2005 |
| 56 | A185 | Acidiphilium spp. | Batch culture | Whole cells (intact+sonicated) | Rb | Parro et al.,2005 |
| 57 | A139 | Leptospirillum ferrooxidans | Batch (N2 fixing) | Sonicated cells | Rb | Parro et al.,2005 |
| 58 | A186 | L. ferrooxidans | Batch culture | Whole cells (intact+sonicated) | Rb | Parro et al.,2005 |
| 59 | IVE1C1 | L. pherrifilum (LPH2) | Fermentor | Whole cells | Rb | Rivas et al.,2008 |
| 60 | IVE1S1 | L. pherrifilum (LPH2) | Fermentor | Culture supernatant | Rb | Rivas et al.,2008 |
| 61 | IVE1C2 | L. pherrifilum (LPH2) | Fermentor | Insoluble cell pellet from S100 | Rb | Rivas et al.,2008 |
| 62 | IVE1S100 | L. pherrifilum (LPH2) | Fermentor | Soluble cellular fraction S100 | Rb | Rivas et al.,2008 |
| 63 | IVE1BF | L. pherrifilum (LPH2) | Fermentor | Biofilm | Rb | Rivas et al.,2008 |
| 64 | IVE2C1 | L. pherrifilum spp. | Batch+Fe2+ | Whole cells | Rb | Rivas et al.,2008 |
| 65 | IVE2S1 | L. pherrifilum spp. | Batch+Fe2+ | Culture supernatant | Rb | Rivas et al.,2008 |
| 66 | IVE2S100 | L. pherrifilum spp. | Batch+Fe2+ | Soluble cellular fraction S100 | Rb | Rivas et al.,2008 |
| 67 | A182 | Acidithiobacillus ferrooxidans | Batch+Fe2+ | Whole cells | Rb | Parro et al.,2005 |
| 68 | A183 | At. ferrooxidans | Batch+Fe2+ | Whole cells (sonicated) | Rb | Parro et al.,2005 |
| 69 | IVE3C1 | At. ferrooxidans | Batch+Fe2+ | Whole cells | Rb | Rivas et al.,2008 |
| 70 | IVE3S1 | At. ferrooxidans | Batch+Fe2+ | Culture supernatant | Rb | Rivas et al.,2008 |
| 71 | IVE3C2 | At. ferrooxidans | Batch+Fe2+ | Insoluble cell pellet from S100 | Rb | Rivas et al.,2008 |
| 72 | IVE3S100 | At. ferrooxidans | Batch+Fe2+ | Soluble cellular fraction S100 | Rb | Rivas et al.,2008 |
| 73 | A184 | At. thiooxidans | Batch+S | Whole cells (intact+sonicated) | Rb | Parro et al.,2005 |
| 74 | IVE4C1 | At. thiooxidans | Batch+S | Whole cells | Rb | Rivas et al.,2008 |
| 75 | IVE4C2 | At. thiooxidans | Batch+S | Insoluble cell pellet from S100 | Rb | Rivas et al.,2008 |
| 76 | IVE4S100 | At. thiooxidans | Batch+S | Soluble cellular fraction S100 | Rb | Rivas et al.,2008 |
| 77 | IVE5C1 | At. albertensis | Batch+S | Whole cells | Rb | Rivas et al.,2008 |
| 78 | IVE5C2 | At. albertensis | Batch+S | Insoluble cell pellet from S100 | Rb | Rivas et al.,2008 |
| 79 | IVE5S100 | At. albertensis | Batch+S | Soluble cellular fraction S100 | Rb | Rivas et al.,2008 |
| 80 | IVE6C1 | At. caldus | Batch+S | Whole cells | Rb | Rivas et al.,2008 |
| 81 | IVE6S2 | At. caldus | Batch+S | Supernatant from EDTA wash | Rb | Rivas et al.,2008 |
| 82 | IVE6C2 | At. caldus | Batch+S | Insoluble cell pellet from S100 | Rb | Rivas et al.,2008 |
| 83 | IVE6S100 | At. caldus | Batch+S | Soluble cellular fraction S100 | Rb | Rivas et al.,2008 |
| 84 | IVE7C1 | Halothiobacillus neapolitanus | Batch+S | Whole cells | Rb | Rivas et al.,2011 |
| 85 | IVE8C1 | Acidimicrobium ferrooxidans | Biomass from DSM N°10331 | Whole cells | Rb | This work |
| 86 | IVE8S2 | Acidimicrobium ferrooxidans | Biomass from DSM N°10331 | Supernatant from EDTA wash | Rb | This work |
| 87 | IVF1S1 | Shewanella gelidimarina | Batch (Marine broth 15°C) | Culture supernatant | Rb | Rivas et al.,2008 |
| 88 | IVF2C1 | S. gelidimarina | Batch (Marine broth 4°C) | Whole cells | Rb | Rivas et al.,2008 |
| 89 | IVF2S2a | S. gelidimarina | Batch (Marine broth 4°C) | Supernatant from EDTA wash | Rb | Rivas et al.,2008 |
| 90 | IVF2S2b | S. gelidimarina | Batch (Marine broth 4°C) | Supernatant from EDTA wash | Rb | Rivas et al.,2008 |
| 91 | IVF2C2 | S. gelidimarina | Batch (Marine broth 4°C) | Insoluble cell pellet from S100 | Rb | Rivas et al.,2008 |
| 92 | IVF2S100 | S. gelidimarina | Batch (Marine broth 4°C) | Soluble cellular fraction S100 | Rb | Rivas et al.,2008 |
| 93 | IVF3C2 | Psychroserpens burtonensis | Batch (Marine broth 15°C) | Insoluble cell pellet from S100 | Rb | Rivas et al.,2008 |
| 94 | IVF4C1 | P. burtonensis | Batch (Marine broth 4°C) | Whole cells | Rb | Rivas et al.,2008 |
| 95 | IVF4S1 | P. burtonensis | Batch (Marine broth 4°C) | Culture supernatant | Rb | Rivas et al.,2008 |
| 96 | IVF4S2a | P. burtonensis | Batch (Marine broth 4°C) | Supernatant from EDTA wash | Rb | Rivas et al.,2008 |
| 97 | IVF4S2b | P. burtonensis | Batch (Marine broth 4°C) | Supernatant from EDTA wash | Rb | Rivas et al.,2008 |
| 98 | IVF4S100 | P. burtonensis | Batch (Marine broth 4°C) | Soluble cellular fraction S100 | Rb | Rivas et al.,2008 |
| 99 | IVF5C1 | Psychrobacter frigidicola | Batch (Harpo's medium 15°C) | Whole cells | Rb | Rivas et al.,2008 |
| 100 | IVF5S1 | Ps. frigidicola | Batch (Harpo's medium 15°C) | Culture supernatant | Rb | Rivas et al.,2008 |
| 101 | IVF5C2 | Ps. frigidicola | Batch (Harpo's medium 15°C) | Insoluble cell pellet from S100 | Rb | Rivas et al.,2008 |
| 102 | IVF5S100 | Ps. frigidicola | Batch (Harpo's medium 15°C) | Soluble cellular fraction S100 | Rb | Rivas et al.,2008 |
| 103 | IVF6C1 | Cryobacterium psychrophilum | Batch (TSA) | Whole cells | Rb | Rivas et al.,2008 |
| 104 | IVF6S1 | C. psychrophilum | Batch (TSA) | Culture supernatant | Rb | Rivas et al.,2008 |
| 105 | IVF6S2 | C. psychrophilum | Batch (TSA) | Supernatant from EDTA wash | Rb | Rivas et al.,2008 |
| 106 | IVF6C2 | C. psychrophilum | Batch (TSA) | Insoluble cell pellet from S100 | Rb | Rivas et al.,2008 |
| 107 | IVF6S100 | C. psychrophilum | Batch (TSA) | Soluble cellular fraction S100 | Rb | Rivas et al.,2008 |
| 108 | IVF7C1 | Colwellia psychrerythraea | Bath culture (Marine broth) | Whole cells | Rb | Rivas et al.,2011 |
| 109 | IVF7S1 | C. psychrerythraea | Bath culture (Marine broth) | Culture supernatant | Rb | This work |
| 110 | IVF7S2 | C. psychrerythraea | Bath culture (Marine broth) | Supernatant from EDTA wash | Rb | This work |
| 111 | IVG1C1 | Acidocella aminolytica DSM 11237 | Batch (DSMZ medium N°269) | Whole cells | Rb | Rivas et al.,2011 |
| 112 | IVG2C_185 | Acidiphilium spp. | Batch (DSMZ medium N°269) | Whole cells | Rb | This work |
| 113 | IVG2C1 | Acidiphilium sp. | Batch (DSMZ medium N°269) | Whole cells | Rb | Rivas et al.,2011 |
| 114 | IVG3C1 | Acidobacterium capsulatum DSM 11244 | Batch (DSMZ medium N°269) | Whole cells | Rb | Rivas et al.,2011 |
| 115 | IVG4C1 | Thermus scotoductus | Batch (TYG) | Whole cells | Rb | Rivas et al.,2011 |
| 116 | IVG4C2 | T. scotoductus | Batch (TYG) | Insoluble cell pellet from S100 | Rb | Rivas et al.,2011 |
| 117 | IVG5C1 | Sulfobacillus acidophilus | Biomass from DSM No 10332 | Whole cells | Rb | Rivas et al.,2011 |
| 118 | IVG6C1 | T. termophilus | Batch (TYG) | Whole cells | Rb | This work |
| 119 | IVH1C1 | Bacillus subtilis (spores) | Batch (Schaeffer medium) | Whole spores | Rb | Fernández-Calvo et al.,2006 |
| 120 | IVI1C1 | Pseudomonas putida | Batch (LB) | Whole cells | Rb | Rivas et al.,2008 |
| 121 | IVI1C2 | P. putida | Batch (LB) | Insoluble cell pellet from S100 | Rb | Rivas et al.,2008 |
| 122 | IVI1S100 | P. putida | Batch (LB) | Soluble cellular fraction S100 | Rb | Rivas et al.,2008 |
| 123 | IVI1RB | P. putida | Batch (LB) | Ribosome fraction | Rb | Rivas et al.,2008 |
| 124 | IVI2C1 | Bacillus spp. (environ. isol.)* | Batch (LB) | Whole cells | Rb | Rivas et al.,2008 |
| 125 | IVI2S2 | Bacillus spp. (environ. isol.)* | Batch (LB) | Supernatant from EDTA wash | Rb | Rivas et al.,2008 |
| 126 | IVI2C2 | Bacillus spp. (environ. isol.)* | Batch (LB) | Insoluble cell pellet from S100 | Rb | Rivas et al.,2008 |
| 127 | IVI2S100 | Bacillus spp. (environ. isol.)* | Batch (LB) | Soluble cellular fraction S100 | Rb | Rivas et al.,2008 |
| 128 | IVI3C1 | Shewanella oneidensis | Batch (LB) | Whole cells | Rb | Rivas et al.,2008 |
| 129 | IVI3S2 | S. oneidensis | Batch (LB) | Supernatant from EDTA wash | Rb | Rivas et al.,2008 |
| 130 | IVI3C2 | S. oneidensis | Batch (LB) | Insoluble cell pellet from S100 | Rb | Rivas et al.,2008 |
| 131 | IVI3S100 | S. oneidensis | Batch (LB) | Soluble cellular fraction S100 | Rb | Rivas et al.,2008 |
| 132 | IVI4C1 | Burkholderia fungorum | Batch (LB) | Whole cells | Rb | Rivas et al.,2008 |
| 133 | IVI4S2 | B. fungorum | Batch (LB) | Supernatant from EDTA wash | Rb | Rivas et al.,2008 |
| 134 | IVI4C2 | B. fungorum | Batch (LB) | Insoluble cell pellet from S100 | Rb | Rivas et al.,2008 |
| 135 | IVI4S100 | B. fungorum | Batch (LB) | Soluble cellular fraction S100 | Rb | Rivas et al.,2008 |
| 136 | IVI5C1 | S. oneidensis | Anaerobic (fumarate) | Whole cells | Rb | Rivas et al.,2008 |
| 137 | IVI5S1 | S. oneidensis | Anaerobic (fumarate) | Culture supernatant | Rb | Rivas et al.,2008 |
| 138 | IVI5C2 | S. oneidensis | Anaerobic (fumarate) | Insoluble cell pellet from S100 | Rb | Rivas et al.,2008 |
| 139 | IVI5S100 | S. oneidensis | Anaerobic (fumarate) | Soluble cellular fraction S100 | Rb | Rivas et al.,2008 |
| 140 | IVI6C3 | Azotobacter vinelandii | Batch culture (LB) | EDTA washed cells (sonicated) | Rb | Rivas et al.,2008 |
| 141 | IVI7C1 | B. subtilis 168 | Batch culture (LB) vegetative cells | Whole cells | Rb | Rivas et al.,2008 |
| 142 | IVI8C1 | B. subtilis 3610 | Biofilm | Whole cells | Rb | Rivas et al.,2008 |
| 143 | IVI8S1 | B. subtilis 3610 | Biofilm | Culture supernatant | Rb | Rivas et al.,2008 |
| 144 | IVI9C1 | Deinococcus radiodurans | Biomass from DSMZ No 20539 | Whole cells | Rb | Rivas et al.,2008 |
| 145 | IVI10C1 | Desulfovibrio vulgaris(vulgaris) | Biomass from DSMZ No 644 | Whole cells | Rb | Rivas et al.,2008 |
| 146 | IVI11C1 | Geobacter sulfurreducens | Biomass from DSMZ No 12127 | Whole cells | Rb | Rivas et al.,2008 |
| 147 | IVI12C1 | Geobacter metallireducens | Biomass from DSMZ No 7210 | Whole cells | Rb | Rivas et al.,2008 |
| 148 | IVI13C1 | Thermotoga maritima | Biomass from DSMZ No 3109 | Whole cells | Rb | Rivas et al.,2008 |
| 149 | IVI14C1 | Verrucomicrobium spinosum | Biomass from DSMZ No 4136 | Whole cells | Rb | Rivas et al.,2008 |
| 150 | IVI15C1 | Methylomicrobium capsulatum | Biomass from DSMZ No 6130 | Whole cells | Rb | Rivas et al.,2008 |
| 151 | IVI16C1 | Planctomyces limnophilus | Biomass from DSMZ No 3776 | Whole cells | Rb | Rivas et al.,2008 |
| 152 | IVI17C1 | Hydrogenobacter thermophilus | Biomass from DSMZ No 6534 | Whole cells | Rb | Rivas et al.,2008 |
| 153 | IVI19C1 | Desulfosporosinus meridiei | Biomass from DSM No 13257 | Whole cells | Rb | Rivas et al.,2011 |
| 154 | IVI20C1 | Salinibacter ruber M8 | Batch (SW25% marine salt) | Whole cells | Rb | Rivas et al.,2011 |
| 155 | IVI21C1 | S. ruber PR1 | Batch (SW25% marine salt) | Whole cells | Rb | Rivas et al.,2011 |
| 156 | IVI21C2 | S. ruber PR1 | Batch (SW25% marine salt) | Insoluble cell pellet from S100 | Rb | Rivas et al.,2011 |
| 157 | IVI21S1 | S. ruber PR1 | Batch (SW25% marine salt) | Culture supernatant | Rb | This work |
| 158 | IVJ1C1 | Haloferax mediterranei | Batch (SW25% marine salt) | Whole cells | Rb | Rivas et al.,2008 |
| 159 | IVJ2C1 | Methanococcoides burtonii | Biomass from DSMZ No 6242 | Whole cells | Rb | Rivas et al.,2008 |
| 160 | IVJ3C1 | Thermoplasma acidophilum | Biomass from DSMZ No 1728 | Whole cells | Rb | Rivas et al.,2008 |
| 161 | IVJ4C1 | Methanobacterium formicicum | Biomass from DSMZ No 1535 | Whole cells | Rb | Rivas et al.,2008 |
| 162 | IVJ5C1 | Methanosarcina mazeii | Biomass from DSMZ No 3647 | Whole cells | Rb | Rivas et al.,2008 |
| 163 | IVJ6C1 | Pyrococcus furiosus | Biomass from DSM No 3638 | Whole cells | Rb | Rivas et al.,2011 |
| 164 | IVJ8C1 | Halorubrum sp. | Batch (SW25% marine salt) | Whole cells | Rb | Rivas et al.,2011 |
| 165 | IVJ9C1 | Halobacterium sp. | Batch (SW25% marine salt) | Whole cells | Rb | Rivas et al.,2011 |
| 166 | A-Ecoli1 | Escherichia coli | E. coli culture | All serotypes | Rb | US Biological (E3500-26) |
| 167 | A-Hpylori | Helicobacter pylori | Batch culture (ATCC 43504) | Whole cells | Rb | BIODESIGN (B65660R) |
| 168 | A-Lmono | L. monocytogenes | Batch culture (ATCC 43251) | Whole cells | Rb | BIODESIGN (B65420R) |
| 169 | A-Mycob | Mycobacterium genus | Batch culture M. tuberculosis | Genus-specific antigens extract | Rb | BIODESIGN (B47827R) |
| 170 | A-Paer | P. aeruginosa | Batch culture (P. aeruginosa) | Outer membrane protein extract | Gpig | BIODESIGN (B47578G) |
| 171 | A-Salm | Salmonella sp. (O+H Ags) | Batch culture | Mix S. enteritidis, typhym., heidelburg | Rb | BIODESIGN (B65701R) |
| 172 | A-G+Ag | Gram-positive antigen | Culture | Gram-positive antigen | M(Mab) | BIODESIGN (C11333M) |
| 173 | A-G+Bac | Gram-positive Bacteria | Culture | Lipoteichoic acid Gram-positives | M(Mab) | BIODESIGN (C65380M) |
| 174 | A-PG | Peptidoglycan | Culture (Streptococcus mutans) | Insoluble PG extract obtained by TCA | M(Mab) | CHEM. INTER. (MAB997) |
| 175 | A-lipidA | Lipid A (E. coli O157) | Culture | Whole cells prep of lipid A | Goat | BIODESIGN (B65692G) |
| 176 | A-LTA | Lipoteichoic acid | Culture | Native Streptococcus pyrogenes | Rb | BIODESIGN (B30103R) |
| 177 | A-NAG | N-Acetylglucosamine | NAG on proteins | NAG-KLH conjugated | M(Mab) | BIODESIGN (H67108M) |
| 178 | A-DNA1 | ss, dsDNA | Bovine ss, dsDNA | Calf thymus DNA | M(Mab) | US Biological (D3878-05) |
| 179 | A-DNA2 | DNA102 (human) | dsDNA | Autoimmune | Hu | Schweitzer et al.,2005 |
| 180 | A-dsDNA(hu) | dsDNA (human plasma) | dsDNA | Autoimmune | Hu | BIODESIGN (K07112H) |
| 181 | A-RNApII | RNA polymerase II | Eukaryotic RNApol | Wheat germ RNApol II (purified) | M(Mab) | BIODESIGN (M55701M) |
| 182 | A-GroEL | GroEL (E. coli) | Recombinant | GroEL (purified) | Rb | Sigma-Aldrich (G6532) |
| 183 | A-Hsp70 | HSP-70 | Batch culture (M. tuberculosis) | HSP-70 (purified protein) | M(Mab) | BIODESIGN (H86313M) |
| 184 | A-Lasnase | L-Asparaginase (E. coli) | Batch culture | L-Asparaginase (purified) | Rb | BIODESIGN (K59171R) |
| 185 | A-BGal | beta d Galactosidase (E. coli) | Batch culture | Beta d Galactosidase (purified) | Rb | BIODESIGN (K03450R) |
| 186 | A-GluOxid | Glucose oxidase | Batch culture (Aspergillus niger) | Glucose oxidase (purified) | Rb | BIODESIGN (K59137R) |
| 187 | A-GST | Glutathione-S-transferase | Recombinant (S. japonicum) | GST (purified) | Rb | Sigma-Aldrich (G7781) |
| 188 | A-Stv | Streptavidin | Culture (Streptomyces avidinii) | Streptavidin (purified) | Rb | Sigma-Aldrich (S6390) |
| 189 | A-Thio | Thioredoxin (E. coli) | Recombinant | Thioredoxin (purified) | Rb | Sigma-Aldrich (T0803) |
| 190 | A-Bglu | B. subtilis beta-glucanase | Recombinant (B. subtilis) | Beta-glucanase SDS-PAGE purified | Rb | Rivas et al.,2008 |
| 191 | A-Csn | B. subtilis chitosanase | Recombinant (B. subtilis) | Csn SDS-PAGE purified | Rb | Rivas et al.,2008 |
| 192 | A-Hfer | Human ferritin | From human liver | Ferritin (purified) | M(Mab) | BIODESIGN (H53715) |
| 193 | A1484 | NIFH3 peptide | seq. AKGILKYANSGGVRL | KLH conjugate | Rb | Fernández-Calvo et al.,2006 |
| 194 | A1485 | NIFD2 peptide | seq. YRSMNYISRHMEEKY | KLH conjugate | Rb | Fernández-Calvo et al.,2006 |
| 195 | A1487 | HSCA2 peptide | seq. TFTIDANGILDVRAL | KLH conjugate | Rb | Fernández-Calvo et al.,2006 |
| 196 | A1490 | FDX2 peptide | seq. HVIITEGFDNLSPME | KLH conjugate | Rb | Fernández-Calvo et al.,2006 |
| 197 | A1492 | GLNB2 peptide | seq. VETALRIRTGETGDA | KLH conjugate | Rb | Fernández-Calvo et al.,2006 |
| 198 | A1494 | MODA2 peptide | seq. MLAPLHKKIVYANTL | KLH conjugate | Rb | Fernández-Calvo et al.,2006 |
| 199 | A1496 | NIFS2 peptide | seq. LPVNKEGRVEIETLK | KLH conjugate | Rb | Fernández-Calvo et al.,2006 |
| 200 | LectinPo | Lectin potato protein (not Ab) | SDS-PAGE purified protein | Not applicable | — | CALBIOCHEM (431788) |
| 201 | A-cAMP1 | Cyclic AMP | Cyclic AMP | 3′-5′-cAMP-2′-BSA conjugated | Rb | Sigma-Genosys (A0670) |
| 202 | A-cAMP2 | Cyclic AMP | Human cAMP | Succinylated cAMP-BSA conjugated | Rb | US Biological (C8450-05) |
| 203 | A-cAMP3 | Cyclic AMP | Cyclic AMP | Succinylated cAMP-BSA conjugated | Sheep | BIODESIGN (Q55101S) |
| 204 | A-Asp | Aspartate | Purified Asp | Asp-KLH conjugated | Rb | Sigma-Aldrich (A9684) |
| 205 | A-Glu | Glutamate | Purified Glu | Glu-KLH conjugated | Rb | Sigma-Aldrich (G6642) |
| 206 | A-Trp | Tryptophan | Tryptophan | Trp-glutaraldehyde-Poly-lysine | Rb | US Biological (T9013-05) |
| 207 | A-polyHis | PolyHistidine | Poly-His | His-Tagged fusion protein | M(Mab) | Sigma-Aldrich (H1029) |
| 208 | A-Cortisol | Cortisol | Cortisol | Cortisol-21-hemisucc.-Thyroglobulin | Rb | Sigma-Aldrich (C8409) |
| 209 | A-Estrad1 | Estradiol | 17-beta-Estradiol | 17-beta-Estradiol-BSA | M(Mab) | BIODESIGN (E86022M) |
| 210 | A-Estrad2 | Estradiol | 17-beta-Estradiol | Estradiol-17-beta-6-BSA conjugated | M(Mab) | BIODESIGN (H31100M) |
| 211 | A-Pen | Penicillin | Penicllin and derivatives | Penicillin-BSA conjugated | M(Mab) | BIODESIGN (G13011M) |
| 212 | A-Tc | Tetracycline | Tetracycline | Tetracycline-BSA | Rb | US Biological (T2965-05) |
| 213 | A-VitB12 | Vitamin B12 | Cyanocobalamine | Vitamin B12-BSA conjugated | Rb | US Biological (C8400-10) |
| 214 | A-Atrazine | Atrazine | Atrazine | Atrazine-BgG | Rb | BIODESIGN (K82102R) |
| 215 | A-HBVAg | Hepatitis B virus surface Ag | Hepatitis B virus surface antigen | Highly purified Hepatitis B antigen | Goat | ABCAM (ab9216) |
| 216 | A-Surfactin_N | Surfactin | Sigma S3523 | Surfactin-Amine-BSA | Rb | This work |
| 217 | A-ASB_11362 | Archaeoglobus fulgidus | ATP synthase, subunit B (Archaea) | Purified recombinant polipeptide | Rb | This work |
| 218 | A-ASF1_11355 | Thermotoga maritima | ATP synthase F1, subunit Alpha (Bacteria) | Purified recombinant polipeptide | Rb | This work |
| 219 | A-BaFER | Bacterioferritin | Bacterioferritin | Purified recombinant polipeptide | Rb | Rivas et al.,2011** |
| 220 | A-CrReTs_977 | T. scotoductus | Chromate reductase (membrane) | Purified recombinant polipeptide | Rb | Rivas et al.,2011 |
| 221 | A-CspA-11357 | P. putida | Cold shock protein A | Purified recombinant polipeptide | Rb | This work |
| 222 | A-DsrA-11365 | DsrA (Archaeoglobus fulgidus) | Dissimilatory sulfite reductase | Purified recombinant polipeptide | Rb | This work |
| 223 | A-DsrB_11368 | DsrB (Archaeoglobus fulgidus) | Dissimilatory sulfite reductase | Purified recombinant polipeptide | Rb | This work |
| 224 | A-EFG_11359 | Thermotoga maritima | Elongation factor G | Purified recombinant polipeptide | Rb | This work |
| 225 | A-FdhF | FDHF (E. coli K12) | Selenopolypeptide sub. Formate DeHase H | Purified recombinant polipeptide | Rb | This work |
| 226 | A-FeReTs_983 | Iron reductase (T. scotoductus) | Iron reductase | Purified recombinant polipeptide | Rb | This work |
| 227 | A-HtpG | HtpG (Nostoc PCC73102) | HtpG homologous to HSP90 | Purified recombinant polipeptide | Rb | This work |
| 228 | A-HupL | HupL (At. ferrooxidans) | Ni-Fe membrane hydrogenase L chain | Purified recombinant polipeptide | Rb | This work |
| 229 | A-HupS | HupS (L. ferrooxidans) | Ni-Fe membrane hydrogenase S chain | Purified recombinant polipeptide | Rb | This work |
| 230 | A-HyfG | HyfG (E. coli) | Hydrogenase 4, component B | Purified recombinant polipeptide | Rb | This work |
| 231 | A-McrB_11378 | McrB (Methanococcoides burtonii) | Methyl CoM reductase I, B subunit | Purified recombinant polipeptide | Rb | This work |
| 232 | A-NADH_11363 | NuoF (P. putida) | NADH dehydrogenase I (quinone) | Purified recombinant polipeptide | Rb | This work |
| 233 | A-NifD_11466 | NifD (G. metallireducens) | NifD protein | Purified recombinant polipeptide | Rb | This work |
| 234 | A-NirS_11369 | NirS (P. aeruginosa) | Nitrite reductase | Purified recombinant polipeptide | Rb | This work |
| 235 | A-NOR1_11375 | NOR1 (N. hamburgensis) | Nitrite oxidoreductase Beta subunit | Purified recombinant polipeptide | Rb | This work |
| 236 | A-NRA-11912 | NRA (G. metallireducens) | Nitrate reductase subunit Alpha | Purified recombinant polipeptide | Rb | This work |
| 237 | A-OmpA | OmpA (E. coli) | OmpA membrane protein | Purified recombinant polipeptide | Rb | This work |
| 238 | A-OppA | OppA (Bacillus subtilis) | Oligopeptide binding protein sub A | Purified recombinant polipeptide | Rb | This work |
| 239 | A-PfuDPS | DPS (Pyrococcus furiosus) | Fe-binding and storage protein DpS | Purified recombinant polipeptide | Rb | Rivas et al.,2011** |
| 240 | A-PfuFER | Ferritin (P. furiosus) | Ferritin Fe-binding and storage protein | Purified recombinant polipeptide | Rb | Rivas et al.,2011** |
| 241 | A-RbcL_RB11374 | RbcL (At. ferrooxidans) | Rubisco large subunit | Purified recombinant polipeptide | Rb | This work |
| 242 | A-Rusticyanin | Rusticyanin (At. ferooxidans) | Rusticyanin | Purified recombinant polipeptide | Rb | This work |
| 243 | A-SsoDPS | DPS (Sulfolobus solfataricus) | Fe-binding and storage protein DpS | Purified recombinant polipeptide | Rb | Rivas et al.,2011** |
| 244 | A-ABCtrans | ABC transporter (T. scotoductus) | ABC-transporter protein | Purified recombinant polipeptide | Rb | Rivas et al.,2011 |
| 245 | A-ApsA-11754 | ApsA (Desulfovibrio desulfuricans) | HMMLREMREGRGPIYC | Conjugate | Rb | This work |
| 246 | A-BfR | BFR (D. desulfuricans) | CAENFAERIKELFFEP | Conjugate | Rb | This work |
| 247 | A-CcdA | CcdA (Geobacter sulfurreducens) | CLLGEKRLQVHRKPAGY | Conjugate | Rb | This work |
| 248 | A-cydA-11758 | CydA (S. oneidensis) | YILKKRDLPFARRSFAC | Conjugate | Rb | This work |
| 249 | A-DhnA1 | DhnA (Nostoc PCC73102) | LRNNAFKQDKDYHLAC | Conjugate | Rb | This work |
| 250 | A-DhnA2 | DhnA (Nostoc PCC73102) | SGRKAFQRPFEEGVKLC | Conjugate | Rb | This work |
| 251 | A-FtsZ | FtsZ Pseudomonas putida | CPFEGRKRMQIADEGIR | Conjugate | Rb | This work |
| 252 | A-GGDEF_11760 | Sensory box/GGDEF Domain | IDLDEFKRIN(DT)FGHKEGDKVC | Conjugate | Rb | This work |
| 253 | A-Groel | GroEL (G. metallireducens) | ETEMKEKKARVEDALC | Conjugate | Rb | This work |
| 254 | A-ICDH_11755 | ICDH-NAD (C. hydrogenoformans) | VL(ES)IKKNKVALKGPC | Conjugate | Rb | This work |
| 255 | A-IsiA1 | IsiA, PSII (Nostoc PCC73102) | EISRYKPEIPMGEQGC | Conjugate | Rb | This work |
| 256 | A-IsiA2 | IsiA, PSII (Nostoc PCC73102) | FHFEWNDPKLGLILC | Conjugate | Rb | This work |
| 257 | A-Ktrans_11750 | K+-transporter (G. metallireducens) | LAMSLGRKGEGGTIVC | Conjugate | Rb | This work |
| 258 | A-NifD1_12288 | NifD1 (Burkholderia xenovorans) | VFDKADPDKRPDFVSC | Conjugate | Rb | This work |
| 259 | A-NifD3_12289 | NifD3 (B. xenovorans) | VFGGDKKLDKIIDEIC | Conjugate | Rb | This work |
| 260 | A-NifH1_12291 | NifH1 (B. xenovorans) | CNERQTDKELELAEAL | Conjugate | Rb | This work |
| 261 | A-NifH2_12293 | NifH2 (B. xenovorans) | EFAPESKQAEEYRQLC | Conjugate | Rb | This work |
| 262 | A-OmcS | OmcS (G. sulfurreducens) | CHDPHGKYRRFVDGSI | Conjugate | Rb | This work |
| 263 | A-PhaC1 | PhaC (Pseudomonas putida) | CSLAPDSDDRRFNDPA | Conjugate | Rb | This work |
| 264 | A-PhaC2 | PhaC (P. putida) | CLGERAGALKKAPTRL | Conjugate | Rb | This work |
| 265 | A-PhcA1 | PhcA (Nostoc PCC73103) | NTELQSARGRYERAAC | Conjugate | Rb | This work |
| 266 | A-PhcA2 | PhCA (Nostoc PCC73104) | TPGPQFAADSRGKSKC | Conjugate | Rb | This work |
| 267 | A-PufM1 | PufM (Rhodospirillum rubrum) | SRLGGDREVEQITDRC | Conjugate | Rb | This work |
| 268 | A-PufM2 | PufM (Rhodospirillum rubrum) | SRLGGDREVEQITDRC | Conjugate | Rb | This work |
| 269 | A-RRO | Rubredoxin (D. desulfuricans G20) | CHTQDETMKALEIKKDV | Conjugate | Rb | This work |
| 270 | A-SodA | SodA (G. sulfurreducens) | CMLDYGLKRPDYIEAF | Conjugate | Rb | This work |
| 271 | A-SodF | SodF (G. sulfurreducens) | CARIDKDFGSFDKFKEE | Conjugate | Rb | This work |
| 272 | A-WshR | Water stress protein (P. putida F1) | CVRFTDEGKLDLPKGT | Conjugate | Rb | This work |
| 273 | A-AeKaC_S | AEKAC peptide | AEKAC-synthetic | Conjugate | Rb | This work |
| 274 | A-EPS_SP | Exopolysaccharides | Solar saltern (Santa Pola, Alicante, Spain) | EPS extract | Rb | This work |
| 275 | A-LPS_N | LPS (Pseudomonas sp.) | LPS-BSA | Rb | This work | |
| 276 | A-LipidA_43 | LipidA | Lipid A | Mab | Hycult biotech. (HM2046) | |
| 277 | A-AMP_N | AMP | AMP-OH-BSA | Rb | This work | |
| 278 | A-cAMP_N | cAMP | cAMP-OH-BSA | Rb | This work | |
| 279 | A-cAMP_sh | cAMP | Succinylated cAMP-BSA | Rb | Abcam (ab832) | |
| 280 | A-cGMP(bio) | cGMP | 2′O-succinyl guanosine-3′,5′ cGMP | Rb | MBL, Inc. (JM-3568-100) | |
| 281 | A-cGMP_N | cGMP | cGMP-OH-BSA | Rb | This work | |
| 282 | A-CoA_S | Coenzyme A | CoA-SH-BSA | Rb | This work | |
| 283 | A-Digoxin | Digoxin | Digoxin-KLH | Mab | SIGMA (D 8156) | |
| 285 | A-dinitroph. | Dinitrophenol | Dinitrophenol-BSA | Rb | SIGMA (D 9656) | |
| 286 | A-cGMP(ups) | cGMP | 2′-Monosuccinyl cyclic GMP-KLH | Rb | UPSTATE | |
| 287 | A-GMP_N | GMP | GMP-OH-BSA | Rb | This work | |
| 288 | A-Proto IX_N | Protoporphyrin IX | Protophorphyrin IX-OH-BSA | Rb | This work | |
| 289 | A-Thiamine | Thiamine | Thiamine-BSA | Rb | US Biological (T5004) | |
| 290 | A-Arg | Arginine | Arginine | Asymmetric NG-NG-dimethyl arginine | Rb | Santa Cruz Biotech.sc-57626 |
| 292 | A-Ala_N | Ala | Ala-NH2-BSA | Rb | This work | |
| 294 | A-aa_N | Amino acids | Cys-SH-BSA | Rb | This work | |
| 295 | A-Gly_N | Gly | Gly-NH2-BSA | Rb | This work | |
| 296 | A-His_N | His | His-NH2-BSA | Rb | This work | |
| 298 | A-Phe_N | Phe | Phe-NH2-BSA | Rb | This work | |
| 299 | A-Tyr_N | Tyr | Tyr-NH2-BSA | Rb | This work | |
| 300 | A-Val_N | Val | Val-NH2-BSA | Rb | This work | |
| 301 | P-139 | Pre-Immune serum | IgG fraction (protein A purified) | Pre-Immune serum | Rb | Rivas et al.,2008 |
| 302 | P-1496 | Pre-Immune serum | IgG fraction (protein A purified) | Pre-Immune serum | Rb | Rivas et al.,2008 |
| 303 | P-IA1S2 | Pre-Immune serum | IgG fraction (protein A purified) | Pre-Immune serum | Rb | Rivas et al.,2008 |
| 304 | P-IA1C1 | Pre-Immune serum | IgG fraction (protein A purified) | Pre-Immune serum | Rb | Rivas et al.,2008 |
| 305 | p-IA2S1 | Pre-Immune serum | IgG fraction (protein A purified) | Pre-Immune serum | Rb | This work |
| 306 | p-IA3C1 | Pre-Immune serum | IgG fraction (protein A purified) | Pre-Immune serum | Rb | This work |
| 307 | P-IC1S1 | Pre-Immune serum | IgG fraction (protein A purified) | Pre-Immune serum | Rb | Rivas et al.,2008 |
| 308 | P-IC1C1 | Pre-Immune serum | IgG fraction (protein A purified) | Pre-Immune serum | Rb | Rivas et al.,2008 |
| 309 | p-IC4C1 | Pre-Immune serum | IgG fraction (protein A purified) | Pre-Immune serum | Rb | This work |
| 310 | p-IC7C1 | Pre-Immune serum | IgG fraction (protein A purified) | Pre-Immune serum | Rb | This work |
| 311 | P-IC8C1 | Pre-Immune serum | IgG fraction (protein A purified) | Pre-Immune serum | Rb | Rivas et al.,2008 |
| 312 | p-IC9C1 | Pre-Immune serum | IgG fraction (protein A purified) | Pre-Immune serum | Rb | This work |
| 313 | P-ID4S2 | Pre-Immune serum | IgG fraction (protein A purified) | Pre-Immune serum | Rb | Rivas et al.,2008 |
| 314 | P-IVE1C1 | Pre-Immune serum | IgG fraction (protein A purified) | Pre-Immune serum | Rb | Rivas et al.,2008 |
| 315 | P-IVE3C1 | Pre-Immune serum | IgG fraction (protein A purified) | Pre-Immune serum | Rb | Rivas et al.,2008 |
| 316 | P-IVE4C1 | Pre-Immune serum | IgG fraction (protein A purified) | Pre-Immune serum | Rb | Rivas et al.,2008 |
| 317 | P-IVE5C1 | Pre-Immune serum | IgG fraction (protein A purified) | Pre-Immune serum | Rb | Rivas et al.,2008 |
| 318 | P-IVE6C1 | Pre-Immune serum | IgG fraction (protein A purified) | Pre-Immune serum | Rb | Rivas et al.,2008 |
| 319 | P-IVF2C1 | Pre-Immune serum | IgG fraction (protein A purified) | Pre-Immune serum | Rb | Rivas et al.,2008 |
| 320 | p-IVG2C1 | Pre-Immune serum | IgG fraction (protein A purified) | Pre-Immune serum | Rb | This work |
| 321 | p-IVG4C1 | Pre-Immune serum | IgG fraction (protein A purified) | Pre-Immune serum | Rb | This work |
| 322 | P-IVI2C1 | Pre-Immune serum | IgG fraction (protein A purified) | Pre-Immune serum | Rb | Rivas et al.,2008 |
| 323 | P-IVI8C1 | Pre-Immune serum | IgG fraction (protein A purified) | Pre-Immune serum | Rb | Rivas et al.,2008 |
| 324 | p-IVI10C1 | Pre-Immune serum | IgG fraction (protein A purified) | Pre-Immune serum | Rb | This work |
| 325 | p-IVI120C1 | Pre-Immune serum | IgG fraction (protein A purified) | Pre-Immune serum | Rb | This work |
| 326 | p-ABCtrans | Pre-Immune serum | IgG fraction (protein A purified) | Pre-Immune serum | Rb | This work |
| 327 | p-CrReTs-977 | Pre-Immune serum | IgG fraction (protein A purified) | Pre-Immune serum | Rb | This work |
| 328 | p-FeReTs-983 | Pre-Immune serum | IgG fraction (protein A purified) | Pre-Immune serum | Rb | This work |
| 329 | p-FeReTs-984 | Pre-Immune serum | IgG fraction (protein A purified) | Pre-Immune serum | Rb | This work |
| 330 | p-pool | Pre-Immune sera pool | IgG fraction (protein A purified) | Pre-Immune sera pool | Rb | Rivas et al.,2008 |
C1, cellular fraction; C2, insoluble cell pellet from S100; C3, EDTA washed cells; S1, culture supernatant; S2, supernatant from EDTA wash.
Rb, polyclonal antibodies from rabbit; M(Mab), monoclonal Abs from mouse; Mab, monoclonal antibody; Hu, human; Gpig, Guinea pig.
Environmental isolated from Río Tinto (kindly provided by Felipe Gómez-Gómez).
Antigens (purified proteins) were kindly provided by Dr. Trevor Douglas, Center for BioInspired Nanomaterials, Montana State University, Bozeman, MT.
Acknowledgments
This work has been funded by the Spanish Ministerio de Ciencia e Innovación (MICINN) grant No. AYA2008_04013. We thank Patricio Raúl Árias for his assistance in the field work, Compañía Minera Cordillera for their help during the field campaign, and Drs. Josefa Antón and Fernando Santos (Universidad de Alicante, Spain) for providing halophilic strains.
Abbreviations
BSA, bovine serum albumin; cAMP, cyclic adenosine monophosphate; EPS, exopolysaccharides; cGMP, cyclic guanosine monophosphate; LDChip, Life Detector Chip; MAAM, MultiArray Analysis Module; OTUs, operative taxonomic units; PAM, prokaryotic acidophile microarray; RDP, the Ribosomal Database Project; SAU, Sample Analysis Unit; SEM, scanning electron microscopy; SMI, sandwich microarray immunoassay; SPU, Sample Preparation Unit; SOLID, Signs Of LIfe Detector.
References
- Altschul S.F. Madden T.L. Schäffer A.A. Zhang J. Zhang Z. Miller W. Lipman D.J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berleman J. Bauer C.E. Characterization of the cyst cell formation in the purple photosynthetic bacterium Rhodospirillum centenum. Microbiology. 2004;150:383–390. doi: 10.1099/mic.0.26846-0. [DOI] [PubMed] [Google Scholar]
- Bobst A.L. Lowenstein T.K. Jordan T.E. Godfrey L.V. Ku T.L. Luo S. A 106 ka paleoclimate record from drill core of the Salar de Atacama, northern Chile. Palaeogeogr Palaeoclimatol Palaeoecol. 2001;173:21–42. [Google Scholar]
- Cabrol N.A. Chong-Diaz G.C. Wettergreen D. Stoker C.R. Dohm J.M. Reaten R. Schwher K. Gulick V.C. Landheim R. Lee P. Roush T.L. Zent A.P. Herrera-Lameli C. Jensen Iglesia A. Pereira Arredondo M. Dunfield G.C. Pedersen L. Bualat M.G. Christian D. Mina C. Sims M.H. Thomas H.J. Tucker D.J. Zbinden E. Science results of the Atacama Nomad rover field experiment, Chile: implications for planetary exploration. J Geophys Res. 2001;106:7664–7675. [Google Scholar]
- Cabrol N.A. Wettergreen D. Warren-Rhodes K. Grin E.A. Moersch J. Diaz G.C. Cockell C.S. Coppin P. Demergasso C. Dohm J.M. Ernst L. Fisher G. Glasgow J. Hardgrove C. Hock A.N. Jonak D. Marinangeli L. Minkley E. Ori G.G. Piatek J. Pudenz E. Smith T. Stubbs K. Thomas G. Thompson D. Waggoner A. Wagner M. Weinstein S. Wyatt M. Life in the Atacama: searching for life with rovers (science overview) J Geophys Res. 2007:112. doi: 10.1029/2006JG000298. [DOI] [Google Scholar]
- Cameron R. NASA Technical Repor 32-1378. National Aeronautics and Space Administration; Washington DC: 1969. Abundance of microflora in soils of desert regions. [Google Scholar]
- Chong-Díaz G. Mendoza M.N. García-Veigas J. Pueyo J.J. Turner P. Evolution and geochemical signatures in a Neogene forearc evaporitic basin: the Salar Grande (Central Andes of Chile) Palaeogeogr Palaeoclimatol Palaeoecol. 1999;151:39–54. [Google Scholar]
- Cole J.R. Wang Q. Cardenas E. Fish J. Chai B. Farris R.J. Kulam-Syed-Mohideen A.S. McGarrell D.M. Marsh T. Garrity G.M. Tiedje J.M. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37:D141–D145. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartnell L.R. Desorgher L. Ward J.M. Coates A.J. Modeling the surface and subsurface martian radiation environment: implications for astrobiology. Geophys Res Lett. 2007:34. doi: 10.1029/2006GL027494. [DOI] [Google Scholar]
- Davila A.F. Gómez-Silva B. de los Rios A. Ascaso C. Olivares H. McKay C.P. Wierzchos J. Facilitation of endolithic microbial survival in the hyperarid core of the Atacama Desert by mineral deliquescence. J Geophys Res. 2008:113. doi: 10.1029/2007JG000561. [DOI] [Google Scholar]
- Davila A.F. Duport L.G. Melchiorri R. Jänchen J. Valea S. de Los Rios A. Fiaren A.G. Möhlmann D. McKay C.P. Ascaso C. Wierzchos J. Hygroscopic salts and the potential for life on Mars. Astrobiology. 2010;10:617–628. doi: 10.1089/ast.2009.0421. [DOI] [PubMed] [Google Scholar]
- Delong E.F. Archaea in coastal marine environments. Proc Natl Acad Sci USA. 1992;89:5685–5689. doi: 10.1073/pnas.89.12.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demergasso C. Casamayor E.O. Chong G. Galleguillos P. Escudero L. Pedrós-Alió C. Distribution of prokaryotic genetic diversity in athalassohaline lakes of the Atacama Desert, Northern Chile. FEMS Microbiol Ecol. 2004;48:57–69. doi: 10.1016/j.femsec.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Dubois M. Gilles K.A. Hamilton J.K. Rebers P.A. Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- Ewing B. Hillier L. Wendl M. Green P. Basecalling of automated sequencer traces using phred I Accuracy assessment. Genome Res. 1998;8:175–185. doi: 10.1101/gr.8.3.175. [DOI] [PubMed] [Google Scholar]
- Fernández-Calvo P. Näke C. Rivas L.A. García-Villadangos M. Gómez-Elvira J. Parro V. A multi-array competitive immunoassay for the detection of broad range molecular size organic compounds relevant for astrobiology. Planet Space Sci. 2006;54:1612–1621. [Google Scholar]
- Fish S.A. Shepherd T.J. McGenity T.J. Grant W.D. Recovery of 16S ribosomal RNA gene fragments from ancient halite. Nature. 2002;417:432–436. doi: 10.1038/417432a. [DOI] [PubMed] [Google Scholar]
- Foster R.C. Polysaccharides in soil fabrics. Science. 1981;214:665–667. doi: 10.1126/science.214.4521.665. [DOI] [PubMed] [Google Scholar]
- Garrido P. González-Toril E. García-Moyano A. Moreno-Paz M. Amils R. Parro V. An oligonucleotide prokaryotic acidophile microarray (PAM): its validation and its use to monitor seasonal variations in extreme acidophilic environments with total environmental RNA. Environ Microbiol. 2008;10:836–850. doi: 10.1111/j.1462-2920.2008.01477.x. [DOI] [PubMed] [Google Scholar]
- Glavin D.P. Cleaves H.J. Schubert M. Aubrey A. Bada J.L. New method for estimating bacterial cell abundances in natural samples by use of sublimation. Appl Environ Microbiol. 2004;70:5923–5928. doi: 10.1128/AEM.70.10.5923-5928.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomelsky M. cAMP, c-di-GMP, c-di-AMP and now cGMP: bacteria use them all! Mol Microbiol. 2011;79:562–565. doi: 10.1111/j.1365-2958.2010.07514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D. Viewing and editing assembled sequences using Consed. In: Baxevanis A.D., editor; Davison D.B., editor. Current Protocols in Bioinformatics. John Wiley & Sons; New York: 2004. pp. 11.2.1–11.2.43. [DOI] [PubMed] [Google Scholar]
- Gramain A. Díaz G.C. Demergasso C. Lowenstein T.K. McGenity T.J. Archaeal diversity along a subterranean salt core from the Salar Grande (Chile) Environ Microbiol. 2011;13:2105–2121. doi: 10.1111/j.1462-2920.2011.02435.x. [DOI] [PubMed] [Google Scholar]
- Hecht M.H. Kounaves S.P. Quinn R.C. West S.J. Young S.M. Ming D.W. Catling D.C. Clark B.C. Boynton W.V. Hoffman J. Deflores L.P. Gospodinova K. Kapit J. Smith P.H. Detection of perchlorate and the soluble chemistry of martian soil at the Phoenix lander site. Science. 2009;325:64–67. doi: 10.1126/science.1172466. [DOI] [PubMed] [Google Scholar]
- Huber T. Faulkner G. Hugenholtz P. Bellerophon; a program to detect chimeric sequences in multiple sequence alignments. Bioinformatics. 2004;20:2317–2319. doi: 10.1093/bioinformatics/bth226. [DOI] [PubMed] [Google Scholar]
- Kminek G. Bada J.L. The effect of ionizing radiation on the preservation of amino acids on Mars. Earth Planet Sci Lett. 2006;245:1–15. [Google Scholar]
- Lizama C. Monteoliva-Sánchez M. Prado B. Ramos-Cormenzana A. Weckesser J. Campos V. Taxonomic study of extreme halophilic archaea isolated from the “Salar de Atacama”, Chile. Syst Appl Microbiol. 2001;24:464–474. doi: 10.1078/0723-2020-00053. [DOI] [PubMed] [Google Scholar]
- Lester E.D. Satomi M. Ponce A. Microflora of extreme arid Atacama Desert soils. Soil Biol Biochem. 2007;39:704–708. [Google Scholar]
- Lu S. Gischkat S. Reiche M. Akob D.M. Hallberg K.B. Küsel K. Ecophysiology of Fe-cycling bacteria in acidic sediments. Appl Environ Microbiol. 2010;76:8174–8183. doi: 10.1128/AEM.01931-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marden J.N. Dong Q. Roychowdhury S. Berleman J.E. Bauer C.E. Cyclic GMP controls Rhodospirillum centenum cyst development. Mol Microbiol. 2011;79:600–615. doi: 10.1111/j.1365-2958.2010.07513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan S.M. Bell J.F., III Calvin W.M. Christensen P.R. Clark B.C. de Souza P.A. Farmer J. Farrand W.H. Fike D.A. Gellert R. Ghosh A. Glotch T.D. Grotzinger J.P. Hahn B. Herkenhoff K.E. Hurowitz J.A. Johnson J.R. Johnson S.S. Jolliff B. Klingelhöfer G. Knoll A.H. Learner Z. Malin M.C. McSween H.Y. Pocock J. Ruff S.W. Soderblom L.A. Squyres S.W. Tosca N.J. Watters W.A. Wyatt M.B. Yen A. Provenance and diagenesis of the evaporite-bearing Burns Formation Meridiani Planum Mars. Earth Planet Sci Lett. 2005;240:95–121. [Google Scholar]
- Murchie S.L. Mustard J.F. Ehlmann B.L. Miliken R.E. Bishop J.L. McKeown N.K. Dobrea E.Z.N. Seelos F.P. Buczkowski D.L. Wiseman S.M. Arvidson R.E. Wray J.J. Swayze G. Clark R.N. Des Marais D.J. McEwen A.S. Bibring J.P. A synthesis of martian aqueous mineralogy after 1 Mars year of observations from the Mars Reconnaissance Orbiter. J Geophys Res. 2009:114. doi: 10.1029/2009JE003342. [DOI] [Google Scholar]
- Navarro-González R. Rainey F.A. Molina P. Bagaley D.R. Hollen B.J. de la Rosa J. Small A.M. Quinn R.C. Grunthaner F.J. Cáceres L. Gómez-Silva B. McKay C.P. Mars-like soils in the Atacama Desert, Chile, and the dry limit of microbial life. Science. 2003;302:1018–1021. doi: 10.1126/science.1089143. [DOI] [PubMed] [Google Scholar]
- Navarro-González R. Vargas E. de la Rosa J. Raga A.C. McKay C.P. Reanalysis of the Viking results suggests perchlorate and organics at mid-latitudes on Mars. J Geophys Res. 2010:115. doi: 10.1029/2010JE003599. [DOI] [Google Scholar]
- Osterloo M.M. Hamilton V.E. Bandfield J.L. Glotch T.D. Baldridge A.M. Christensen P.R. Tornabene L.L. Anderson F.S. Chloride-Bearing Materials in the Southern Highlands of Mars. Science. 2008;319:1651–1654. doi: 10.1126/science.1150690. [DOI] [PubMed] [Google Scholar]
- Parnell J. Cullen D. Sims M.R. Bowden S. Cockell C.S. Court R. Ehrenfreund P. Gaubert F. Grant W. Parro V. Rohmer M. Sephton M. Stan-Lotter H. Steele A. Toporski J. Vago J. Searching for life on Mars: selection of molecular targets for ESA's Aurora ExoMars mission. Astrobiology. 2007;7:578–604. doi: 10.1089/ast.2006.0110. [DOI] [PubMed] [Google Scholar]
- Parro V. Antibody microarrays for environmental monitoring. In: Timmis K.N., editor. Handbook of Hydrocarbon and Lipid Microbiology. Springer-Verlag Berlin; 2010. pp. 2699–2710. [Google Scholar]
- Parro V. Muñoz-Caro G. Solid carbonaceous and organic matter in space, macromolecular complexes, biomarkers, and microarray-based instrumentation for in situ detection. In: Basiuk V.A., editor. Astrobiology: Emergence, Search and Detection of Life. American Scientific Publishers; Los Angeles: 2010. pp. 376–398. [Google Scholar]
- Parro V. Rodríguez-Manfredi J.A. Briones C. Compostizo C. Herrero P.L. Vez E. Sebastián E. Moreno-Paz M. García-Villadangos M. Fernández-Calvo P. González-Toril E. Pérez-Mercader J. Fernández-Remolar D. Gómez-Elvira J. Instrument development to search for biomarkers on mars: terrestrial acidophile iron-powered chemolithoautotrophic communities as model systems. Planet Space Sci. 2005;53:729–737. [Google Scholar]
- Parro V. Rivas L.A. Gómez-Elvira J. Protein microarrays-based strategies for life detection in astrobiology. Space Sci Rev. 2008a;135:293–311. [Google Scholar]
- Parro V. Fernández-Calvo P. Rodríguez Manfredi J.A. Moreno-Paz M. Rivas L.A. García-Villadangos M. Bonaccorsi R. González-Pastor J.E. Prieto-Ballesteros O. Schuerger A.C. Davidson M. Gómez-Elvira J. Stoker C.R. SOLID2: an antibody array-based life-detector instrument in a Mars drilling simulation experiment (MARTE) Astrobiology. 2008b;8:987–999. doi: 10.1089/ast.2007.0126. [DOI] [PubMed] [Google Scholar]
- Parro V. de Diego-Castilla G. Rodríguez-Manfredi J.A. Rivas L.A. Blanco-López Y. Sebastián E. Romeral J. Compostizo C. Herrero P.L. García-Marín A. Moreno-Paz M. García-Villadangos M. Cruz-Gil P. Peinado V. Martín-Soler J. Pérez-Mercader J. Gómez-Elvira J. SOLID3: a multiplex antibody microarray-based optical sensor instrument for in situ life detection in planetary exploration. Astrobiology. 2011a;11:15–28. doi: 10.1089/ast.2010.0501. [DOI] [PubMed] [Google Scholar]
- Parro V. Fernández-Remolar D. Rodríguez-Manfredi J.A. Cruz-Gil P. Rivas L.A. Ruiz-Bermejo M. Moreno-Paz M. García-Villadangos M. Gómez-Ortiz D. Blanco-López Y. Menor-Salván C. Prieto-Ballesteros O. Gómez-Elvira J. Classification of modern and old Río Tinto sedimentary deposits through the biomolecular record using a life marker biochip: implications for detecting life on Mars. Astrobiology. 2011b;11:29–44. doi: 10.1089/ast.2010.0510. [DOI] [PubMed] [Google Scholar]
- Piatek J.L. Hardgrove C. Moersch J.E. Drake D.M. Wyatt M.B. Rampey M. Carlisle O. Warren-Rhodes K. Dohm J.M. Hock A.N. Carbol N.A. Wettergreen D.S. Grin E.A. Diaz G.C. Coppin P. Weinstein S. Cockell C.S. Marinangeli L. Ori G.G. Smith T. Jonak D. Wagner M. Stubbs K. Thomas G. Pudenz E. Glasgow J. Surface and subsurface composition of the life in the Atacama field sites from rover data and orbital image analysis. J Geophys Res. 2007:112. doi: 10.1029/2006JG000317. [DOI] [Google Scholar]
- Quinn R.C. Zent A.P. Grunthaner F.J. Ehrenfreund P. Taylor C.L. Garry J.R.C. Detection and characterization of oxidizing acids in the Atacama Desert using the Mars Oxidation Instrument. Planet Space Sci. 2005;53:1376–1388. [Google Scholar]
- Rivas L.A. García-Villadangos M. Moreno-Paz M. Cruz-Gil P. Gómez-Elvira J. Parro V. A 200-antibody microarray biochip for environmental monitoring: searching for universal microbial biomarkers through immunoprofiling. Anal Chem. 2008;80:7970–7979. doi: 10.1021/ac8008093. [DOI] [PubMed] [Google Scholar]
- Rivas L.A. Aguirre J. Blanco Y. González-Toril E. Parro V. Graph-based deconvolution analysis of multiplex sandwich microarray immunoassays: applications for environmental monitoring. Environ Microbiol. 2011;13:1421–1432. doi: 10.1111/j.1462-2920.2011.02442.x. [DOI] [PubMed] [Google Scholar]
- Roberson E.B. Firestone M.K. Relationship between desiccation and exopolysaccharide production in a soil Pseudomonas sp. Appl Environ Microbiol. 1992;58:1284–1291. doi: 10.1128/aem.58.4.1284-1291.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanowski G. Lorenz M.G. Wackernagel W. Plasmid DNA in a groundwater aquifer microcosm-adsorption, DNAase resistance and natural genetic transformation of Bacillus subtilis. Mol Ecol. 1993;2:171–181. doi: 10.1111/j.1365-294x.1993.tb00106.x. [DOI] [PubMed] [Google Scholar]
- Ruiz-Bermejo M. Menor-Salván C. Osuna-Esteban S. Veintemillas-Verdaguer S. Prebiotic microreactors: a synthesis of purines and dihydroxy compounds in aqueous aerosol. Orig Life Evol Biosph. 2007;37:123–142. doi: 10.1007/s11084-006-9026-5. [DOI] [PubMed] [Google Scholar]
- Schloss P.D. Westcott S.L. Ryabin T. Hall J.R. Hartmann M. Hollister E.B. Lesniewski R.A. Oakley B.B. Parks D.H. Robinson C.J. Sahl J.W. Stres B. Thallinger G.G. Van Horn D.J. Weber C.F. Introducing mothur: open-source platform-independent community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer M.H. Wittmeyer J. Avci R. Pincus S. Experimental support for an immunological approach to the search for life on other planets. Astrobiology. 2005;5:30–47. doi: 10.1089/ast.2005.5.30. [DOI] [PubMed] [Google Scholar]
- Shafaat H.S. Cable M.L. Ikeda M.K. Kirby J.P. Pelletier C.C. Ponce A. Towards an in situ endospore detection instrument. Proceedings of the IEEE Aerospace Conference; Institute of Electrical and Electronics Engineers (IEEE); New York. 2005. pp. 660–669. [Google Scholar]
- Shkrob I.A. Chemerisov S.D. Marin T.W. Photocatalytic decomposition of carboxylated molecules on light-exposed martian regolith and its relation to methane production on Mars. Astrobiology. 2010;10:425–436. doi: 10.1089/ast.2009.0433. [DOI] [PubMed] [Google Scholar]
- Skelley A.M. Scherer J.R. Aubrey A.D. Grover W.H. Ivester R.H.C. Ehrenfreund P. Grunthaner F.J. Bada J.L. Mathies R.A. Development and evaluation of a microdevice for amino acid biomarker detection and analysis on Mars. Proc Natl Acad Sci USA. 2005;102:1041–1046. doi: 10.1073/pnas.0406798102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P.K. Krohn R.I. Hermanson G.T. Mallia A.K. Gartner F.H. Provenzano M.D. Fujimoto E.K. Goeke N.M. Olson B.J. Klenk D.C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stahl D.A. Amann R. Development and application of nucleic acid probes. In: Stackebrandt E., editor; Goodfellow M., editor. Nucleic Acid Techniques in Bacterial Systematics. John Wiley & Sons; Chichester, UK: 1991. pp. 205–248. [Google Scholar]
- Stan-Lotter H. Leuko S. Legat A. Fendrihan S. The assessment of the viability of halophilic microorganisms in natural communities. In: Oren A., editor; Rainey F., editor. Methods in Microbiology. Extremophiles. Elsevier; Oxford: 2006. pp. 569–584. [Google Scholar]
- Tuross N. Stathoplos L. Ancient proteins in fossil bones. Methods Enzymol. 1993;224:121–129. doi: 10.1016/0076-6879(93)24010-r. [DOI] [PubMed] [Google Scholar]
- Weete J.D. Abril M. Blackwell M. Phylogenetic distribution of fungal sterols. PLoS One. 2010;5:e10899. doi: 10.1371/journal.pone.0010899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein S. Pane D. Ernst L.A. Warren-Rhodes K. Dohm J.M. Hock A.N. Piatek J.L. Emani S. Lanni F. Wagner M. Fisher G.W. Minkley E. Dansey L.E. Smith T. Grin E.A. Stubbs K. Thomas G. Cockell C.S. Marinangeli L. Ori G.G. Heys S. Teza J.P. Moersch J.E. Coppin P. Diaz G.C. Wettergreen D.S. Cabrol N.A. Waggoner A.S. Application of pulsed-excitation flourescence imager for daylight detection of sparse life in tests in the Atacama Desert. J Geophys Res. 2008:113. doi: 10.1029/2006JG000319. [DOI] [Google Scholar]
- Yuasa S. Flory D. Basile B. Oró J. Abiotic synthesis of purines and other heterocyclic compounds by the action of electrical discharges. J Mol Evol. 1984;21:76–80. doi: 10.1007/BF02100630. [DOI] [PubMed] [Google Scholar]
- Zorzano M.P. Mateo-Martí E. Prieto-Ballesteros O. Osuna S. Renno N. Stability of liquid saline water on present day Mars. Geophys Res Lett. 2009:36. doi: 10.1029/2009GL040315. [DOI] [Google Scholar]