Abstract
The efficacy of B cell depletion therapy in rheumatoid arthritis (RA) has driven interest in understanding the mechanism. Because the decrease in autoantibododies in RA does not necessarily correlate with clinical outcome other mechanisms may be operative. We previously reported in proteoglycan-induced arthritis (PGIA), B cell depletion inhibits autoreactive T cell responses. Recent studies in B cell depletion therapy also indicate a role for B cells in suppressing regulatory mechanisms. Here we demonstrate that B cells inhibited both the expansion and the function of T regulatory (Treg) cells in PGIA. Utilizing an anti-CD20 mAb, we depleted B cells from mice with PGIA and assessed the Treg cell population. Compared to control antibody-treated mice, Treg cell percentages were elevated in B cell-depleted mice, with a higher proportion of CD4+ T cells expressing Foxp3 and CD25. On a per cell basis, CD4+CD25+ cells from B cell depleted mice expressed increased amounts of Foxp3 and were significantly more suppressive than those from control Ab-treated mice. The depletion of Treg cells with an anti-CD25 mAb concurrent with B cell depletion therapy restored the severity of PGIA to levels equal to untreated mice. Although titers of autoantibodies did not recover to untreated levels, CD4+ T cell recall responses to the immunizing antigen returned as measured by T cell proliferation and cytokine production. Thus, B cells have the capacity to regulate inflammatory responses by enhancing T effector cells along with suppressing Treg cells.
Keywords: B cells, T, Autoimmunity, Rheumatoid Arthritis
Introduction
Rheumatoid arthritis (RA) is a debilitating inflammatory disease of the synovial joints mediated by chronic activation of several different cell populations including T cells, B cells, neutrophils, and macrophages; although the precise contribution of each of these cell populations is unclear (1). There is a renewed interest in the involvement of B cells in RA based on the clinical efficacy of B cell depletion therapy with anti-CD20 antibody (Rituximab) (2, 3). B cell depletion reduces rheumatoid factor and anti-CCP antibodies, however, variable decreases in these antibodies irrespective of improvement in clinical disease activity suggests additional mechanisms for efficacy (4, 5). Removal of autoreactive B cells participating in antigen presentation, co-stimulation, and cytokine production likely play a role, but these have not been completely elucidated in RA (6). In other autoimmune diseases and in animal model of autoimmune disease the T cell compartment is altered after B cell depletion resulting in reduced T cell activation and cytokine production (7-12).
Our murine model of RA, proteoglycan-induced arthritis (PGIA), is similar to human disease by several criterion including clinical assessment, radiographic analysis, scintigraphic bone scans, laboratory tests and histological assessment of diarthrodial joints (13-18). In this model, PG-specific B cells are required as antibody secreting cells as well as antigen-specific antigen-presenting cells (APCs) (19). Secondary signals delivered by B cells through CD80/CD86 are essential for autoreactive T cell activation and the development of arthritis (20). B cells are required to maintain chronic inflammation as anti-CD20 mAb treatment inhibits arthritis, T cell proliferation and cytokine production (12).
Autoreactive T cells that escape central tolerance are regulated in the periphery by Treg cells (21). Treg cells are divided into natural Treg cells that are induced in the thymus and inducible Treg cells that are activated in the periphery. Treg cells are characterized by high expression of CD25 and the transcription factor Foxp3 which is essential for Treg cell activity (22-24). Treg cells quell inflammation using several suppressive mechanisms which are mediated through both soluble and membrane bound factors (25). In a number of autoimmune diseases there are documented defects in Treg cell numbers and function which could potentially allow T effector (Teff) cell escape from suppression by the production of proinflammatory cytokines by either Teff cells or APCs (26-30). IFN-γ produced by Th1 cells and IL-6, IL-21, TGF-β produced by APC that promote Th17 cells inhibit Treg cell differentiation (31-35). Thus, an inappropriate balance between Teff cells and Treg cells permits autoreactive responses.
A direct impact of B cells on Treg cell activity is not clearly defined, although some recent work suggests that there may be a link. In a mouse model of Crohn's disease, B cells exacerbate ileitis through suppression of Treg cell function (36). Suppression of disease in B cell depleted nonobese diabetes and thyroiditis is accompanied by an increase in CD25+ Foxp3+ Treg cells(37) (38, 39). In clinical studies, Foxp3 mRNA transcripts and Treg cell numbers are elevated in lupus patients following B cell depletion therapy, while in another study, Treg cells of patients with idiopathic thrombocytopenic purpura are defective prior to treatment, but had restored capacity to suppress after B cell depletion therapy (40, 41). Treg cells were also increased in rituximab-responsive cryoglobulinaemia vasculitis patients(11). Based on these data, we were interested in determining if the Treg cells are involved in the suppression of arthritis after B cells depletion.
Here we report that the inflammatory environment in autoimmune arthritis determines Treg cell activity. In B cell depleted mice, there was a significant decrease in Teff cell activity which corresponded to an increase in the percentage of CD4+CD25+Foxp3+ T cells and in the level of Foxp3 protein expression. CD4+CD25+ Treg cells from B cell-depleted mice were also significantly more effective at suppressing PG-specific CD4+ T cell proliferation than Treg cells from control mice. Treg cells contributed to the inhibition of PGIA as Treg cell suppression in B cell depleted mice was completely abolished by the simultaneous depletion of Treg cells with anti-CD25 mAb. Recovery of arthritis severity in mice depleted of both B cells and Treg cells was accompanied by the restoration of PG-specific CD4+ T cell responses. These data demonstrate that the activity of B cells in autoimmune arthritis tips the balance toward inflammatory Teff cells and away from Treg cell control of inflammation.
Materials and Methods
Mice, antigen, and assessment of arthritis
BALB/c wild type (WT) mice were obtained from National Cancer Institute (Bethesda, MD). BALB/c IFN-γ-deficient mice were obtained from Jackson Laboratory (Bar Harbor, ME). BALB/c B cell deficient mice (JHD) were provided by Dr. Mark Shlomchik (Yale University, New Haven, CT). Female mice (>3 months) were immunized intraperitoneally (i.p.) with 150 μg of human proteoglycan (PG) in 2 mg of dimethyldioctadecyl-ammonium bromide (DDA) adjuvant (Sigma-Aldrich, St. Louis, MO) in 200 μl phosphate buffered saline (PBS), pH 7.2 and boosted on day 21 with 100 μg PG in DDA as previously described (42). Human cartilage PG was purified from joint replacement surgeries by procedures approved by the Institutional Review Board of Rush University Medical Center (Chicago, IL), as previously described (43). All animal experiments were approved by the Animal Care and Use Committee at Rush University Medical Center (Chicago, IL). Arthritic scores were assessed by blinded observers 3 times per week and were evaluated by the extent of erythema and swelling of each paw on a scale of 0-4 providing a maximum score for each mouse of 16. Scoring of each paw was as follows: 0, normal; 1, mild erythema and swelling of several digits; 2, moderate erythema and swelling; 3 more diffuse erythema and swelling; and 4, severe erythema and swelling of complete paw with ankylosis. Arthritis score represents the mean ± SEM of the data.
Abs, treatments and B- and Treg cell-depletion
B cell depletion was performed by a single i.v. injection of 250 μg of anti-mouse CD20 (anti-mCD20) mAb (18B12, IgG2a) (Biogen Idec, San Diego, CA) or the control anti-human CD20 mAb (2B8) (Biogen Idec), which has no cross-reactivity to the mouse CD20 molecule as described previously (12). Depletion of Treg cells was achieved by weekly i.p. injections of 500 μg of anti-mouse CD25 mAb (PC 61.5.3, Rat IgG1) (BioXcell, West Lebanon, NH) or control anti-HRPN mAb (Rat IgG1) (BioXcell). All antibody treatments of PG-immunized mice began 4 days after the second PG-DDA immunization.
Treg flow cytometry
Spleen and lymph nodes of anti-mCD20 mAb and anti-hCD20 mAb-treated mice were harvested after PG-DDA immunization. Single-cell suspensions of each tissue were immunostained using anti-CD3 FITC, anti-CD4 PerCp-Cy5.5 and anti-CD25 APC (BD Biosciences, San Jose, CA) with anti-Foxp3 PE (eBiosciences, San Diego, CA). Using a FACS Canto II (BD BioSciences) stained cells were acquired and analyzed with FACSDiva software (BD Biosciences). Data represents the mean ± SEM.
In vitro Treg cell suppression assays
Spleens were harvested from untreated WT or WT treated with anti-mCD20 mAb or anti-hCD20 mAb mice after PG-DDA immunization. Splenic effector CD4+ T cells (Teff) from untreated arthritic WT mice were isolated using AutoMACS separation with negative selection microbeads (Miltenyi-Biotec, Bergisch Gladbach, Germany). Splenic Treg cells from arthritic mice treated with anti-mCD20 mAb or anti-hCD20 mAb were CD25 positive selected from negatively isolated CD4+ T cells (Miltenyi-Biotec). Varying concentrations of Treg cells (0-1.25 × 105) were co-cultured with Teff cells (1.25 × 105) and mitomycin-C treated WT naïve splenic APCs (2.5 × 105) with or without PG (10 μg/ml) in RPMI-1640 media containing 5% FCS, 100 μg/ml penicillin, 100 μg/ml streptomycin and 2 mM L-glutamine (complete media) in quadruplicate in 96-well Falcon plates (Fisher Scientific, Fair Lawn, NJ). WT naïve spleen cells were incubation at 1 × 107 cells per ml with 25 μg/ml mitomycin-C (Sigma, St. Louis, MO) for 30 min to inactivate proliferation. Proliferation was measured by 3H-thymidine incorporation overnight of a 5 day culture. Data represents the mean ± SEM.
Detection of anti-PG antibodies by ELISA
Mice were bled from the orbital plexus for serum at 4, 8 and 11 weeks after the first PG-DDA immunization. Anti-mouse PG and anti-human PG antibodies in serum samples were determined by ELISA. Individual mouse serum samples and internal standard samples (pooled sera from arthritic mice) were serially diluted (PBS/0.5% Tween-20) in EIA tissue culture 96 “half area” well plates (Costar, Corning, NY) that were coated overnight with 0.5 μg human PG, or 0.75 μg native mouse PG in carbonate buffer. Known concentrations of plate-bound unlabeled murine IgG1 and IgG2a antibody (Southern Biotechnology, Birmingham, Alabama, USA) without plate-bound PGs were utilized as standard curves. Unlabeled plate-bound antibodies in standard curve wells or serum antibodies bound to PG-coated wells were detected using HRP labeled anti-mouse IgG1 or IgG2a (Zymed, San Francisco, CA) secondary antibodies with o-phenylenediamine (OPDA). Spectrophotometer readings at 490nm determined colorimetric changes and concentrations of anti-PG serum antibodies. Data represent the mean ± SEM.
T cell proliferation and assessment of Th1 and Th17 cytokines
Spleens were isolated from individual untreated mice or mice treated with: anti-mCD20 mAb; anti-hCD20 mAb; anti-mCD20 mAb with anti-CD25 mAb; anti-mCD20 mAb with rat IgG1 anti-HRPN mAb; anti-CD25 mAb; or rat IgG1 anti-HRPN mAb after PG-DDA immunizations. Isolated CD4+ T cells (2.5 × 105) as described above were cultured with mitomycin-C treated naïve total spleen APCs (2.5 × 105) with or without PG (10μg/ml) for 5 days. Proliferation was measured by 3H-thymidine incorporation as described above. Data represents the mean ± SEM. Supernatants were removed from similarly established cultures at day 4 and cytokines analyzed for IL-17 or IFN-γ concentrations, respectively, with ELISA kits (IFN-γ (BD Biosciences) or IL-17 (R&D Systems, Minneapolis, MN) according to manufacturer instructions. Data represents the mean ± SEM.
Statistical analysis
The Mann-Whitney U test was used to compare nonparametric data for statistical significance. Significance is based on a value of p < 0.05
Results
Depletion of Treg cells prior to immunization but not after accelerates PGIA
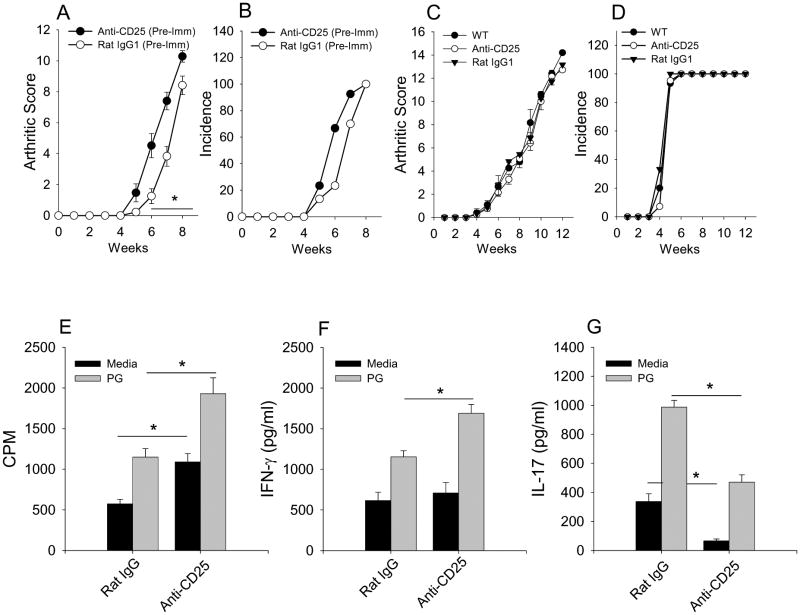
Previous studies have demonstrated that inflammatory conditions can limit Treg cell activity. To determine if the inflammatory environment in arthritis affects the function of Treg cells, we assessed Treg cell activity in PGIA. Treg cells were depleted prior to PG-DDA immunization or 4 days after the 2nd PG-DDA immunization when anti-PG-specific B and T cell responses have developed (12). BALB/c mice were depleted of Treg cells by treatment with anti-CD25 mAb i.p. every 7 days. CD25+Foxp3+ Treg cells were depleted in the blood, spleen and lymph nodes as measured by Foxp3+ and CD25+ staining of CD4+ cells (data not shown). Depletion of Treg cells 3 days prior to PG-DDA immunization resulted in the early onset of PGIA with enhanced arthritis severity (Fig. 1A & B). However, depletion of Treg cells 4 days after the second PG/DDA immunization at a time point when arthritis was initiated had no affect on PGIA (Fig. 1C & D). Although disease severity was not exacerbated, splenic CD4+ T cells from Treg cell depleted mice displayed a significant increase in PG-specific proliferation and IFN-γ production than CD4+ T cells from control Ab treated mice (Fig. 1E & E). However, the IL-17 secretion from CD4+ T cells was suppressed (Fig. 1G) likely do to the ability of IFN-γ to suppress IL-17 production. These data indicate that Treg cells are able to inhibit already primed Teff cells. However, the inability of Treg cell depletion to exacerbate arthritis after immunization suggests that the number or potency of Treg cells may be reduced in the inflammatory environment. Alternatively arthritis is at maximum severity and can not be further increased in absence of Treg cells.
Figure 1. Depletion of Treg cells prior to immunization but not after accelerates PGIA.
BALB/c mice were immunizations with PG in DDA twice with a 3 week interval. Mice depleted of Treg cells with anti-CD25 mAb (n=7) or treated with rat IgG1 (n=7) were injected intraperitoneally every 7 days beginning either 3 days prior to the initial PG-DDA immunization (A, B) or 4 days after the second PG-DDA immunization (C, D). Arthritis score (A, C) is the sum of paw inflammation scores divided by the number of arthritis mice. Incidence (B, D) is the percentage of mice with PGIA. At week 13 after the initial PG-DDA immunization, spleens were harvested. CD4+ T cells were purified from mouse spleens and co-cultured with mitomycin-C treated naïve total spleen cells with or without PG (10μg/ml) for 4 days. Proliferation (E) of CD4+ T cells was measured by 3H-thymidine incorporation. Supernatants were harvested and assayed by ELISA for IFN-γ (F) and IL-17 (G). Values are mean ± SEM and are representative of two independent experiments. Asterisk (*) denote significant differences (P ≤ 0.05).
CD4+ CD25+ Treg cell number and function increase in B cell depleted mice
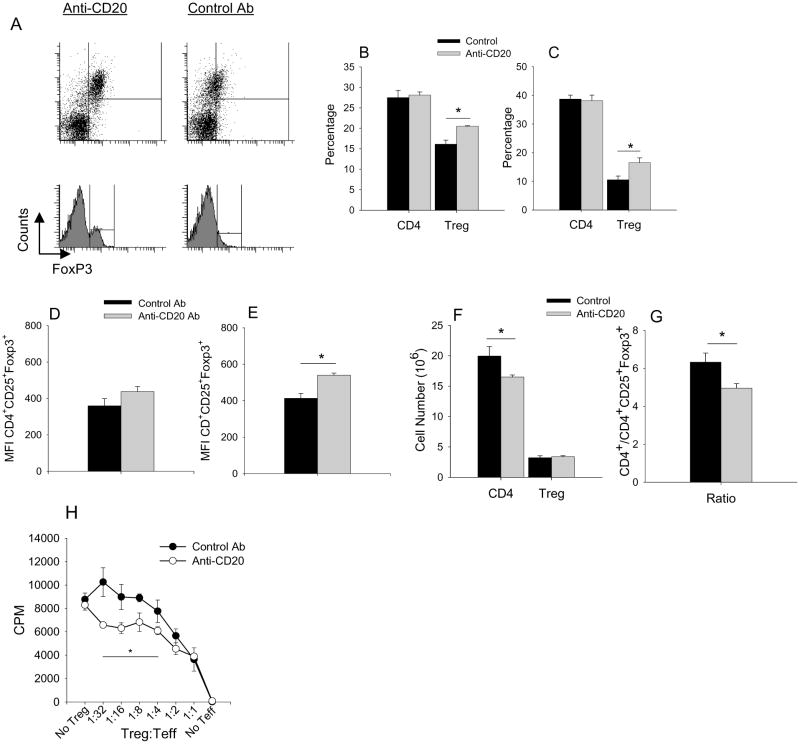
B cell depletion is well documented to suppress autoimmune disease. Reduction in autoantibodies does not always correlate with inhibition of disease suggesting other mechanisms may be involved. As described previously, depletion of B cells with anti-CD20 mAb treatment in early PGIA suppressed arthritis severity and inhibited PG-specific CD4+ T cell proliferation and IFN-γ and IL-17 cytokine production(12). Thus, the reduction in Teff cell responses may shift the balance away from Teff cells and toward Treg cells. To determine if the reduction in Teff cell activity in B cell depleted mice was in part due to an increase in Treg cells, we analyzed spleens and lymph nodes of mice after treatment with either anti-CD20 mAb or control mAb at a time point when B cell numbers had recovered. The percentages of CD4+ T cells were similar in anti-CD20 mAb and control mAb treated mice. However, there was a significant increase in the percentage of CD4+ Foxp3+CD25+ cells (Fig. 2A-C) in the spleen and lymph node and a significant increase in the expression of Foxp3 (reflected in the MFI) in Treg cells of the lymph nodes of B cell-depleted mice (Fig. 2D & E). This increase in the percentage of Treg cells was due to a reduction in CD4+ T cell numbers not an increase in Treg cell numbers (Fig. 2F) resulting in a reduction in the ratio of CD4+ T cells to CD4+CD25+Foxp3+ Treg cells in B cell depleted mice (Fig. 2G).
Figure 2. CD4+ CD25+ Treg cell number and function increase in B cell depleted mice.
A, Representative flow cytometry of Treg cells isolated from lymph nodes (B, D) and spleens (C, E) harvested after anti-CD20 mAb (n=5-6) or control Ab (n=5-6) treatment and analyzed by flow cytometry using anti-CD4, anti-Foxp3 and anti-CD25 fluorochrome labeled antibodies. Treg cells were measured as percentage of CD25+ Foxp3+ of CD4+ cells (B, C). Relative level of Foxp3 protein expression was determined by mean fluorescent intensities (MFI) of CD4+ CD25+ Foxp3+ cells (D, E). Cell number spleen (F) of CD4+ and CD4+CD25+ Foxp3+ from control and anti-CD20 treated mice. (G) Ratio of the cell number of CD4+ to CD4+CD25+Foxp3+. (H) CD4+CD25+ cells were isolated and pooled from spleens of 2 arthritic B cell-depleted (n=3 groups) or control Ab (n=3 groups) treated mice at 21 days or at peak of inflammation after Ab treatment. Purified CD4+ T cells (Teff) from untreated arthritic mice were co-cultured for 5 days with mitomycin-C treated naïve splenocytes, PG (10μg/ml) and titrated number of Treg cells. Proliferation of Teff cells was measured by 3H-thymidine incorporation. Values are mean ± SEM and are representative of 2-3 independent experiments. Asterisk (*) denote significant differences (P ≤ 0.05).
Since an increase in Foxp3 protein expression in Treg cells correlates with their suppressor capacity (44), we examined the suppression potency of Treg cells from B cell depleted mice in a suppressor assays. CD4+ CD25+ Treg cells were isolated from spleens of PG-immunized mice treated with either anti-CD20 mAb or control Ab. Titrated numbers of CD4+ CD25+ Treg cells were co-cultured with CD4+CD25- Teff cells from arthritic mice in the presence of PG and mitomycin-C treated naïve splenocytes as APCs. Parallel to the increased levels of Foxp3 expression, CD4+ CD25+ cells from B cell-depleted arthritic mice suppressed PG-specific CD4+ T cell proliferation more efficiently at lower concentrations of Treg cells than those from control Ab treated mice (Fig. 2H). At higher concentrations of Treg cells, a saturation effect on Teffs by CD4+ CD25+ cells was observed, as proliferation of Teff cells was similar for cultures containing CD4+ CD25+ cells from B cell-depleted or control Ab treated mice. These data demonstrate that B cell depletion results in an increase in the percentage and potency of Treg cells.
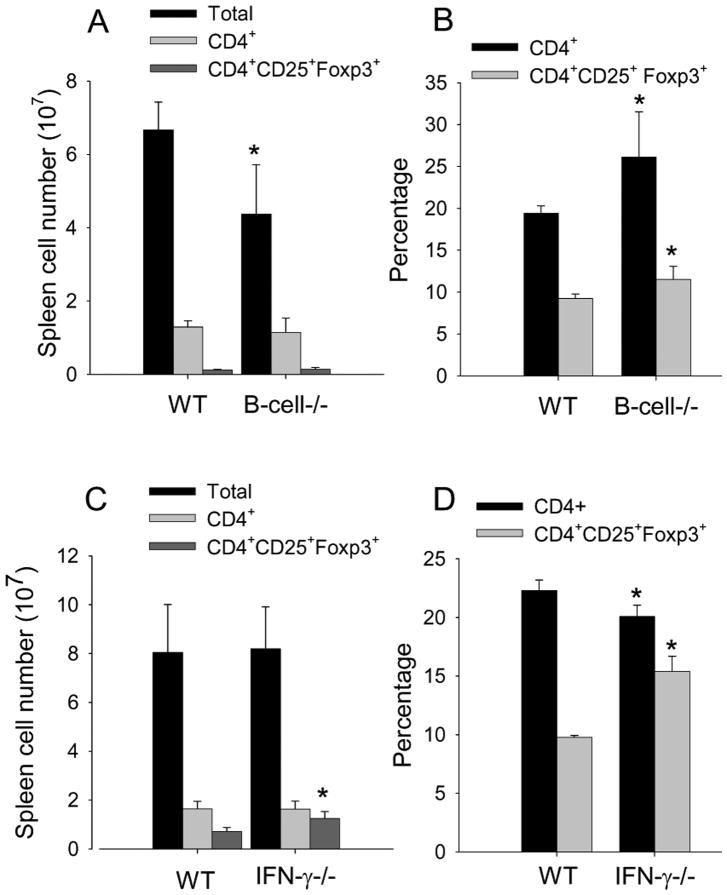
We next asked whether the change in Treg cells in B cell depleted mice was a consequence of immune activation. In naïve mice genetically deficient in B-cell there was the expected increase in the percentage of CD4+ with a concomitant increase in percentage of CD4+CD25+ Foxp3+ cells, however, the total cell number in B-cell deficient mice was reduced and the number of CD4+ and CD4+CD25+ Foxp3+ was similar to WT (Fig. 3A & B). Thus, these data suggest that a change in Treg cells in the B cell depleted mice is a consequence of inflammation. To further confirm in another system that reduction in inflammation results in an increase in Treg cells, we assessed Treg cells in PG-immunized IFN-γ-/- mice. We have previously reported that PGIA is suppressed in IFN-γ-/- mice (18, 43). In comparing Treg cells in WT and IFN-γ-/- mice we found the number and percentage of CD4+CD25+Foxp3+ was increased in IFN-γ-/- mice (Fig 3C & D). These data further support that inflammation suppresses Treg cells.
Figure 3. Inflammation suppresses the number and percentage Tre cells.
(A,B) Spleen cells from naïve WT or B-cell-deficient mice (n=4) were assessed for number and percentage CD4+ T cells and CD4+CD25+Foxp3+ by flow cytometry. (C,D). Spleen cells from PG-immunized WT and IFN-γ-/- mice (n=4) were analyzed for number and percentage of CD4+ T cells and CD4+CD25+Foxp3+ by flow cytometry. Values are mean ± SD and are representative of 2 independent experiments. Asterisk (*) denote significant differences (P ≤ 0.05).
Simultaneous Treg cell and B cell depletion restores PGIA and the autoreactive CD4+ T cell reactivity
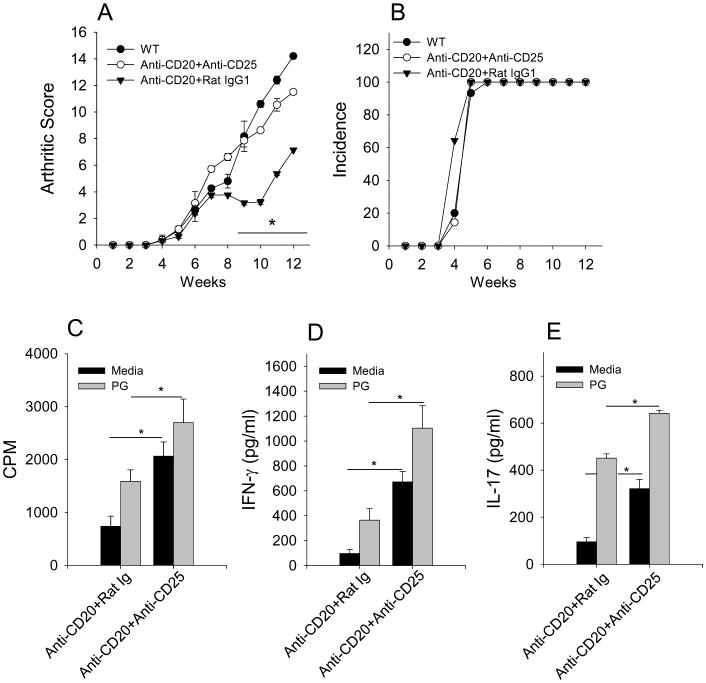
The increased suppressor phenotype of Treg cells suggests that the effectiveness of B cell depletion in PGIA may be in part due to enhanced Treg cell activity. To test this in vivo, we depleted Treg cells using anti-CD25 mAb treatment simultaneous with B cell depletion. The efficacy achieved with B cell depletion was completely reversed with the concomitant depletion of Tregs as mice treated with both anti-CD20 and anti-CD25 mAbs experienced a similar disease course as untreated WT mice (Fig. 4A & B). The absence of a resurgent autoantibody response in B cell-depleted mice with Treg cell depletion implicated the T cell compartment in the recovery of disease (data not shown). CD4+ T cells isolated from mice treated with anti-CD20 mAb and anti-CD25 mAb recovered their proliferative response to PG (Fig. 4C) as well as expression of IFN-γ and IL-17 (Fig. 4D & E) compared to mice treated with anti-CD20 mAb and rat IgG1 control Ab. These data demonstrate that Treg cells are a major contributor to the suppression of arthritis in B cell depleted mice by reducing Teff cell responses.
Figure 4. Simultaneous Treg and B cell depletion restores PGIA and autoreactive CD4+ T cell reactivity.
B cell-depleted mice were treated weekly with anti-CD25 mAb (n=7) or rat IgG1 (n=7) beginning on the same day as B cell depletion. Arthritis score (A) and incidence (B) of arthritis. Spleens were harvested 13 weeks after the initial PG-DDA immunization. CD4+ T cells were purified from indicated mouse spleens and cocultured with mitomycin-C treated naïve total spleen cells with or without PG (10μg/ml). Proliferation (C) of CD4+ T cells was measured by 3H-thymidine incorporation during the final 24 hours of 5 day cultures. IFN-γ (D) and IL-17 (E) was measured by ELISA of supernatants of 4 day cultures. Values are mean ± SEM and representative of two independent experiments. * p ≤ 0.05.
Discussion
We and others have suggested that B cell depletion therapy in autoimmune diseases is related to the suppression of autoreactive Teff cells due to the loss of sufficient antigen presentation by B cells (12, 19, 45). The continual activation of Teff cells in autoimmune disease indicates a failure to properly control the immune response to self-antigens. Treg cells are the main mechanism by which Teff cells are controlled (46). Accordingly, reduced total numbers or decreased suppressor capacity of Tregs has been reported in several autoimmune diseases (47)-46). We found that in PGIA, depletion of Treg cells led to exacerbated arthritis when depleted before PG-immunization but not after immunization. There are several possible explanations for these results; Treg cell potency or number may be reduced despite a clear increase in the Teff responses (Fig. 1). It is also possible that any exacerbation in arthritis due to Treg depletion could not be detected as arthritis was at maximum severity. This study was designed to determine if the mechanism for suppression of arthritis in B cell depleted mice involves Treg cells.
The ability of T-helper subsets with established effector phenotypes to convert to anti-inflammatory subsets or Treg cells to convert to pro-inflammatory subsets is an area of intense research and debate. A recent article by Rubtsov et al demonstrates convincingly that expression of Foxp3 in committed Treg cells is stable under physiological and many inflammatory conditions (48). However, the authors do mention several situations where Treg cells may lose Foxp3 expression such as cells under certain stress conditions or those that newly or transiently expressed Foxp3. In fact, epigentic studies demonstrated that many T-helper subsets differentiated in vitro maintain activating histone modifications of genes for master regulatory transcription factors responsible for other T-helper subset phenotypes (49). Adoptive transfer of Foxp3 expressing CD4+ T cells into T-cell deficient hosts demonstrated that Tregs are capable of losing Foxp3 expression and repopulating the T-follicular helper (TFH) cell compartment of Peyer's patches in vivo (50). The conversion of Treg cells to this proinflammatory phenotype in this model of homeostatic repopulation required the presence of B cells and their expression of CD40 (50). We reasoned that the depletion of B cells and the reduction in Teff cell responses might shift the balance toward the differentiation of Treg cells. Examination of the Treg cells in B cell depleted mice showed that the percentage and function of Treg cells increased (Fig 2). Similar increases in Treg cells were reported in B cell depleted mouse models of non-obese diabetes (NOD) and thyroiditis (39). Therefore, it appears that B cells have a profound impact on Treg cell presence and effectiveness during inflammatory conditions. However, this observation is not universal. In EAE, despite the reduction in Teff responses in B cell depleted mice after MOG immunization, Tregs are not increased and in some cases Treg cells are reduced (51, 52). There are numerous factors that are involved in the interplay between B cells, Teff cells and Treg cells in different models i.e. induced versus spontaneous disease models, strain of mouse, route of antigen exposure, timing of the B depletion, and timing of Treg analysis.
Although naïve B cell deficient mice do not have increased numbers of Treg cells (Fig. 3) (29), the loss of B cells at the time of inflammation may result in reduced polarization of naïve CD4+ T cells toward effector phenotypes and more towards regulatory populations. B cell depletion during PGIA results in significantly reduced PG-specific Th1 and Th17 activation (12). This reduction in the inflammatory cytokines IFN-γ and IL-17 during B cell depletion likely contributes to the increase in percentage of Treg cells in the spleens and lymph nodes of arthritic mice depleted of B cells (Fig. 2B & C). Studies show that Th1 IL-12, and IFN-γ, and Th2 IL-4 can inhibit TGF-β-induced Treg cell differentiation of naïve Th cells (53). Similarly a deficiency in IFN-γ in PGIA results in reduction in arthritis and an increase in Treg cells. Another example in vivo is the inflammation elicited by Toxoplasma gondii infection, which induces a substantial Th1 response which limits Treg cell conversion from naïve Th cells and the maintenance of their regulatory phenotype in vivo (54). The reduction in Th17 cells in B-depleted mice with PGIA may also affect Treg cells since in EAE the absence of IL-6 resulted in an overwhelming adaptive Treg cell response that suppressed the antigen-specific Th17 response and disease manifestation (55).
Along with the increase in the percentage of Treg cells, Foxp3 levels were elevated in CD4+ CD25+ T cells from mice depleted of B cells (Fig. 2). The suppressive capabilities of Treg cells has been directly related to the level of Foxp3 transcripts expressed by these cells (44). Thus, the increased in Foxp3 expression in B cell-depleted mice indicated a concurrent amplification of suppressor function. In vitro proliferation assays confirmed this supposition as CD4+ CD25+ T cells from B cell-depleted mice were significantly more effective at suppressing proliferation of CD4+ T cells from arthritic mice in response to re-stimulation with PG (Fig. 2H). In the clinic, similar findings have been observed with B cell depletion therapy. Elevated levels of Foxp3 mRNA are detected in peripheral blood mononuclear cells from lupus patients treated with anti-CD20 mAb (14). Also, defective Treg cell suppression is restored in idiopathic thrombocytopenic purpura patients after B cell depletion therapy (15). The findings that B cell depletion augments Treg cell Foxp3 expression and suppressor function support a Treg cell dependent mechanism of B cell depletion efficacy.
In vivo studies utilizing dual depletion of B cells and Treg cells resulted in a significant elevation in CD4+ T cell responsiveness to PG as measured by proliferation along with IFN-γ and IL-17 production (Fig. 4C-E). The return of PGIA in mice depleted of both B cells and Treg cells cannot be attributed to be a general response to Treg cell depletion as mice treated with anti-CD25 alone did not experience exacerbated arthritis (Fig. 1C & D). Treg cell influence on PGIA has previously been described to be insufficient under normal circumstances and is believed to be the result of an uncontrolled PG-specific CD4+ T cell response (56). In support of this, Treg cell depletion prior to PG-DDA immunization and the activation of PG-specific CD4+ T cells PGIA was exacerbated with an earlier onset (Fig. 1A & B). The reduced autoreactivity of the CD4+ T cells along with the strengthening of Treg cell populations in B cell depleted mice allow Treg cells to control pathogenic inflammation of PGIA. Our demonstration that Treg cells function to support the efficacy of B cell-depletion in autoimmune arthritis suggests that RA patients receiving B cell depletion therapies may benefit from a concomitant therapy that promotes further Treg expansion such as IL-2/anti-IL-2 complexes (57, 58).
Here we demonstrate a role for B cells in limiting Treg cell suppressor activity in the promotion of inflammation in a mouse model of autoimmune arthritis, PGIA. In this model, B cells contribute to an inflammatory environment that inhibits Treg cell expansion and function and/or promotes the differentiation of naïve CD4+ T cells preferentially to proinflammatory effector phenotypes rather than Treg cells. Alternatively, the efficiency of B cells to act as APCs may expand the pool of autoreactive Teff cells beyond the control of Treg cells. Further elucidation of the mechanisms utilized by B cells to suppress Treg cells will have a major impact on the development of new therapies for the treatment of chronic inflammatory diseases.
Acknowledgments
The authors thank Dr. Jeffrey Oswald and all the staff of the Comparative Research Center for their expert technical assistance.
1This research was supported by a grant from the National Institutes of Health (NIH) AR47657 to A. Finnegan.
3Abbreviations used in this paper
- RA
rheumatoid arthritis
- PG
proteoglycan
- PGIA
proteoglycan-induced arthritis
References
- 1.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 2.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 3.Emery P, Fleischmann R, Filipowicz-Sosnowska A, Schechtman J, Szczepanski L, Kavanaugh A, Racewicz AJ, van Vollenhoven RF, Li NF, Agarwal S, Hessey EW, Shaw TM. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum. 2006;54:1390–1400. doi: 10.1002/art.21778. [DOI] [PubMed] [Google Scholar]
- 4.Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, Keystone EC, Loveless JE, Burmester GR, Cravets MW, Hessey EW, Shaw T, Totoritis MC. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793–2806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 5.Cambridge G, Stohl W, Leandro MJ, Migone TS, Hilbert DM, Edwards JC. Circulating levels of B lymphocyte stimulator in patients with rheumatoid arthritis following rituximab treatment: relationships with B cell depletion, circulating antibodies, and clinical relapse. Arthritis Rheum. 2006;54:723–732. doi: 10.1002/art.21650. [DOI] [PubMed] [Google Scholar]
- 6.Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4(+) T cell immunity. Nat Rev Immunol. 2010;10:236–247. doi: 10.1038/nri2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sfikakis PP, Boletis JN, Tsokos GC. Rituximab anti-B-cell therapy in systemic lupus erythematosus: pointing to the future. Curr Opin Rheumatol. 2005;17:550–557. doi: 10.1097/01.bor.0000172798.26249.fc. [DOI] [PubMed] [Google Scholar]
- 8.Eming R, Nagel A, Wolff-Franke S, Podstawa E, Debus D, Hertl M. Rituximab exerts a dual effect in pemphigus vulgaris. J Invest Dermatol. 2008;128:2850–2858. doi: 10.1038/jid.2008.172. [DOI] [PubMed] [Google Scholar]
- 9.Stasi R, Del Poeta G, Stipa E, Evangelista ML, Trawinska MM, Cooper N, Amadori S. Response to B-cell depleting therapy with rituximab reverts the abnormalities of T-cell subsets in patients with idiopathic thrombocytopenic purpura. Blood. 2007;110:2924–2930. doi: 10.1182/blood-2007-02-068999. [DOI] [PubMed] [Google Scholar]
- 10.Yanaba K, Hamaguchi Y, Venturi GM, Steeber DA, St Clair EW, Tedder TF. B cell depletion delays collagen-induced arthritis in mice: arthritis induction requires synergy between humoral and cell-mediated immunity. J Immunol. 2007;179:1369–1380. doi: 10.4049/jimmunol.179.2.1369. [DOI] [PubMed] [Google Scholar]
- 11.Saadoun D, Rosenzwajg M, Landau D, Piette JC, Klatzmann D, Cacoub P. Restoration of peripheral immune homeostasis after rituximab in mixed cryoglobulinemia vasculitis. Blood. 2008;111:5334–5341. doi: 10.1182/blood-2007-11-122713. [DOI] [PubMed] [Google Scholar]
- 12.Hamel K, Doodes P, Cao Y, Wang Y, Martinson J, Dunn R, Kehry MR, Farkas B, Finnegan A. Suppression of proteoglycan-induced arthritis by anti-CD20 B Cell depletion therapy is mediated by reduction in autoantibodies and CD4+ T cell reactivity. J Immunol. 2008;180:4994–5003. doi: 10.4049/jimmunol.180.7.4994. [DOI] [PubMed] [Google Scholar]
- 13.Glant TT, Mikecz K, Arzoumanian A, Poole AR. Proteoglycan-induced arthritis in BALB/c mice. Clinical features and histopathology. Arthritis Rheum. 1987;30:201–212. doi: 10.1002/art.1780300211. [DOI] [PubMed] [Google Scholar]
- 14.Glant TT, Cs-Szabo G, Nagase H, Jacobs JJ, Mikecz K. Progressive polyarthritis induced in BALB/c mice by aggrecan from normal and osteoarthritic human cartilage. Arthritis Rheum. 1998;41:1007–1018. doi: 10.1002/1529-0131(199806)41:6<1007::AID-ART7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 15.Glant TT, Finnegan A, Mikecz K. Proteoglycan-induced arthritis: immune regulation, cellular mechanisms, and genetics. Crit Rev Immunol. 2003;23:199–250. doi: 10.1615/critrevimmunol.v23.i3.20. [DOI] [PubMed] [Google Scholar]
- 16.Finnegan A, Mikecz K, Tao P, Glant TT. Proteoglycan (aggrecan)-induced arthritis in BALB/c mice is a Th1-type disease regulated by Th2 cytokines. J Immunol. 1999;163:5383–5390. [PubMed] [Google Scholar]
- 17.Tunyogi-Csapo M, Kis-Toth K, Radacs M, Farkas B, Jacobs JJ, Finnegan A, Mikecz K, Glant TT. Cytokine-controlled RANKL and osteoprotegerin expression by human and mouse synovial fibroblasts: fibroblast-mediated pathologic bone resorption. Arthritis Rheum. 2008;58:2397–2408. doi: 10.1002/art.23653. [DOI] [PubMed] [Google Scholar]
- 18.Doodes PD, Cao Y, Hamel KM, Wang Y, Rodeghero RL, Mikecz K, Glant TT, Iwakura Y, Finnegan A. IFN-gamma regulates the requirement for IL-17 in proteoglycan-induced arthritis. J Immunol. 2010;184:1552–1559. doi: 10.4049/jimmunol.0902907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Neill SK, Shlomchik MJ, Glant TT, Cao Y, Doodes PD, Finnegan A. Antigen-specific B cells are required as APCs and autoantibody-producing cells for induction of severe autoimmune arthritis. J Immunol. 2005;174:3781–3788. doi: 10.4049/jimmunol.174.6.3781. [DOI] [PubMed] [Google Scholar]
- 20.O'Neill SK, Cao Y, Hamel KM, Doodes PD, Hutas G, Finnegan A. Expression of CD80/86 on B cells is essential for autoreactive T cell activation and the development of arthritis. J Immunol. 2007;179:5109–5116. doi: 10.4049/jimmunol.179.8.5109. [DOI] [PubMed] [Google Scholar]
- 21.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 23.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 24.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 25.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawson CA, Brown AK, Bejarano V, Douglas SH, Burgoyne CH, Greenstein AS, Boylston AW, Emery P, Ponchel F, Isaacs JD. Early rheumatoid arthritis is associated with a deficit in the CD4+CD25high regulatory T cell population in peripheral blood. Rheumatology (Oxford) 2006;45:1210–1217. doi: 10.1093/rheumatology/kel089. [DOI] [PubMed] [Google Scholar]
- 29.Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes. 2005;54:92–99. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 30.Sugiyama H, Gyulai R, Toichi E, Garaczi E, Shimada S, Stevens SR, McCormick TS, Cooper KD. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J Immunol. 2005;174:164–173. doi: 10.4049/jimmunol.174.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei J, Duramad O, Perng OA, Reiner SL, Liu YJ, Qin FX. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2007;104:18169–18174. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang JH, Kim YJ, Han SH, Kang CY. IFN-gamma-STAT1 signal regulates the differentiation of inducible Treg: potential role for ROS-mediated apoptosis. Eur J Immunol. 2009;39:1241–1251. doi: 10.1002/eji.200838913. [DOI] [PubMed] [Google Scholar]
- 33.Caretto D, Katzman SD, Villarino AV, Gallo E, Abbas AK. Cutting edge: the Th1 response inhibits the generation of peripheral regulatory T cells. J Immunol. 2010;184:30–34. doi: 10.4049/jimmunol.0903412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, Feng XH, Jetten AM, Dong C. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olson TS, Bamias G, Naganuma M, Rivera-Nieves J, Burcin TL, Ross W, Morris MA, Pizarro TT, Ernst PB, Cominelli F, Ley K. Expanded B cell population blocks regulatory T cells and exacerbates ileitis in a murine model of Crohn disease. J Clin Invest. 2004;114:389–398. doi: 10.1172/JCI20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marino E, Villanueva J, Walters S, Liuwantara D, Mackay F, Grey ST. CD4(+)CD25(+) T-cells control autoimmunity in the absence of B-cells. Diabetes. 2009;58:1568–1577. doi: 10.2337/db08-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu CY, Rodriguez-Pinto D, Du W, Ahuja A, Henegariu O, Wong FS, Shlomchik MJ, Wen L. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest. 2007;117:3857–3867. doi: 10.1172/JCI32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu S, Maiti PK, Dyson M, Jain R, Braley-Mullen H. B cell-deficient NOD.H-2h4 mice have CD4+CD25+ T regulatory cells that inhibit the development of spontaneous autoimmune thyroiditis. J Exp Med. 2006;203:349–358. doi: 10.1084/jem.20051438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sfikakis PP, Souliotis VL, Fragiadaki KG, Moutsopoulos HM, Boletis JN, Theofilopoulos AN. Increased expression of the FoxP3 functional marker of regulatory T cells following B cell depletion with rituximab in patients with lupus nephritis. Clin Immunol. 2007;123:66–73. doi: 10.1016/j.clim.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Stasi R, Cooper N, Del Poeta G, Stipa E, Laura Evangelista M, Abruzzese E, Amadori S. Analysis of regulatory T-cell changes in patients with idiopathic thrombocytopenic purpura receiving B cell-depleting therapy with rituximab. Blood. 2008;112:1147–1150. doi: 10.1182/blood-2007-12-129262. [DOI] [PubMed] [Google Scholar]
- 42.Hanyecz A, Berlo SE, Szanto S, Broeren CP, Mikecz K, Glant TT. Achievement of a synergistic adjuvant effect on arthritis induction by activation of innate immunity and forcing the immune response toward the Th1 phenotype. Arthritis Rheum. 2004;50:1665–1676. doi: 10.1002/art.20180. [DOI] [PubMed] [Google Scholar]
- 43.Finnegan A, Grusby MJ, Kaplan CD, O'Neill SK, Eibel H, Koreny T, Czipri M, Mikecz K, Zhang J. IL-4 and IL-12 regulate proteoglycan-induced arthritis through Stat-dependent mechanisms. J Immunol. 2002;169:3345–3352. doi: 10.4049/jimmunol.169.6.3345. [DOI] [PubMed] [Google Scholar]
- 44.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 45.Martin F, Chan AC. B cell immunobiology in disease: evolving concepts from the clinic. Annu Rev Immunol. 2006;24:467–496. doi: 10.1146/annurev.immunol.24.021605.090517. [DOI] [PubMed] [Google Scholar]
- 46.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10:849–859. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, Benoist C, Rudensky AY. Stability of the regulatory T cell lineage in vivo. Science. 329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, Cui K, Kanno Y, Roh TY, Watford WT, Schones DE, Peng W, Sun HW, Paul WE, O'Shea JJ, Zhao K. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, Hori S, Fagarasan S. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 51.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weber MS, Prod'homme T, Patarroyo JC, Molnarfi N, Karnezis T, Lehmann-Horn K, Danilenko DM, Eastham-Anderson J, Slavin AJ, Linington C, Bernard CC, Martin F, Zamvil SS. B-cell activation influences T-cell polarization and outcome of anti-CD20 B-cell depletion in central nervous system autoimmunity. Ann Neurol. 2010;68:369–383. doi: 10.1002/ana.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wei L, Laurence A, Elias KM, O'Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007;282:34605–34610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oldenhove G, Bouladoux N, Wohlfert EA, Hall JA, Chou D, Dos Santos L, O'Brien S, Blank R, Lamb E, Natarajan S, Kastenmayer R, Hunter C, Grigg ME, Belkaid Y. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31:772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G, Vollmar P, Stritesky GL, Kaplan MH, Waisman A, Kuchroo VK, Oukka M. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2008;105:18460–18465. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bardos T, Czipri M, Vermes C, Finnegan A, Mikecz K, Zhang J. CD4+CD25+ immunoregulatory T cells may not be involved in controlling autoimmune arthritis. Arthritis Res Ther. 2003;5:R106–113. doi: 10.1186/ar624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daniel C, Wennhold K, Kim HJ, von Boehmer H. Enhancement of antigen-specific Treg vaccination in vivo. Proc Natl Acad Sci U S A. 2010;107:16246–16251. doi: 10.1073/pnas.1007422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wesley JD, Sather BD, Perdue NR, Ziegler SF, Campbell DJ. Cellular requirements for diabetes induction in DO11.10xRIPmOVA mice. J Immunol. 2010;185:4760–4768. doi: 10.4049/jimmunol.1000820. [DOI] [PMC free article] [PubMed] [Google Scholar]