Abstract
Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes (CTLs) can be modified to function as heterologous tumor directed effector cells that survive longer in vivo than tumor directed T cells without virus specificity, due to chronic stimulation by viral antigens expressed during persistent infection in seropositive individuals. We evaluated the nonviral piggyBac (PB) transposon system as a platform for modifying EBV-CTLs to express a functional human epidermal growth factor receptor 2-specific chimeric antigen receptor (HER2-CAR) thereby directing virus-specific, gene modified CTLs towards HER2-positive cancer cells. Peripheral blood mononuclear cells (PBMCs) were nucleofected with transposons encoding a HER2-CAR and a truncated CD19 molecule for selection followed by specific activation and expansion of EBV-CTLs. HER2-CAR was expressed in ~40% of T cells after CD19 selection with retention of immunophenotype, polyclonality, and function. HER2-CAR-modified EBV-CTLs (HER2-CTLs) killed HER2-positive brain tumor cell lines in vitro, exhibited transient and reversible increases in HER2-CAR expression following antigen-specific stimulation, and stably expressed HER2-CAR beyond 120 days. Adoptive transfer of PB-modified HER2-CTLs resulted in tumor regression in a murine xenograft model. Our results demonstrate that PB can be used to redirect virus-specific CTLs to tumor targets, which should prolong tumor-specific T cell survival in vivo producing more efficacious immunotherapy.
Introduction
Immunotherapy with tumor-antigen specific cytotoxic T lymphocytes (CTLs) is increasingly becoming an option for the treatment for intractable cancers that resist standard chemotherapies and radiation. T cells use highly specific mechanisms for tumor-cell recognition that depend on the expression by the tumor of antigens not expressed on healthy tissues. Many tumors inappropriately express fetal antigens such as survivin and carcinoembryonic antigen or testis antigens like MAGE or SSX that may serve as tumor antigens.1,2 However, in practice these antigens are difficult to target, first because high affinity T cells are tolerized, naïve or anergized and therefore difficult to reactivate and expand in vitro and second because tumors inhibit the processing and presentation of antigens by MHC proteins on the cell surface. Further, not all tumors express known unique antigens.
T cells of any specificity can be retargeted to known tumor antigens by transgenic expression of recombinant antigen receptors. One class of retargeting receptor that recognizes whole antigens on the cell surface are known as chimeric antigen receptors (CARs), because they combine the antigen-binding domains of antibodies with the ζ chain of the T cell receptor (TCR) to induce tumor cell killing.3 Since tumor cells rarely express costimulatory molecules and commonly inactivate professional antigen presenting cells that might otherwise present tumor antigens in an immunostimulatory manner, second generation CARs also contain intracellular signaling domains from costimulatory molecules, such as CD28, OX40, or 41BB to induce antigen-dependent proliferation and cytokine secretion.4,5 The cytolytic function of nonspecifically activated T cells (ATCs) can thus be targeted to poorly immunogenic tumors and this strategy is currently being evaluated in clinical trials for lymphoma using CD20 and CD19-CARs, neuroblastoma using CD171 and GD2-CARs and lung and brain tumors using human epidermal growth factor receptor 2-chimeric antigen receptor (HER2-CAR).6,7,8,9,10 So far retroviral and lentiviral vectors have been used for gene transfer in clinical trials.
HER2 is overexpressed on a range of tumors including ovarian cancer, gastric cancer, lung cancer, and breast cancer, and has been a successful target of trastuzumab (Herceptin) antibody therapy.11,12,13,14 However, many tumors express HER2 at levels ineffectively recognized by Herceptin.15 We have previously reported that CD3-ATCs redirected to HER2 by expression of a HER2-CAR from a retroviral vector could recognize even low levels of antigen on HER2-positive glioblastoma, osteosarcoma or medulloblastoma cells, efficiently kill the tumor cells in vitro, and reduce the tumor burden in xenogeneic murine models.16,17
Lack of T cell expansion and persistence is a major hurdle of T-cell therapy. Even when expressing costimulatory domains from CARs, T cells may be actively suppressed by tumors that commonly express inhibitory ligands, like TGFβ and PD-L1 and recruit inhibitory cell types.18 Expression of multiple costimulatory domains in CARs may provide additional benefits to T cells, but such modified T cells may become hypersensitive to stimulation with potentially toxic results.4,9 Therefore we have evaluated Epstein-Barr virus-specific cytotoxic T lymphocytes (EBV-CTLs) as hosts for CARs, since EBV-CTLs have proven safe in multiple clinical trials and EBV antigens expressed by naturally infected B cells at distant sites may produce a beneficial vaccine effect.8,19 Indeed EBV-CTLs expressing GD2-CARs expanded more and persisted longer than similarly transduced, CD3-ATCs in patients with relapsed neuroblastoma.8
Transposons are capable of integrating into the human genome and stably expressing transgenes,20,21,22,23,24 and are less expensive and easier to manufacture than viral vectors. However, effective transposon modification of antigen-specific T cells has not yet been reported and the piggyBac (PB) transposon has not yet been evaluated for immunotherapy in an in vivo model. We therefore evaluated the potential of EBV-CTLs expressing a HER2-CAR from the PB transposon to eliminate tumors in a mouse model. The PB transposon system has high gene-transfer efficiency and large coding capacity in mouse primary cells, human cell lines, and inducible pluripotent stem cells.21,23 We have recently demonstrated the high efficiency of PB gene transfer into resting human T cells,24,25 and shown that PB did not preferentially integrate into or near to proto-oncogenes using genome-wide mapping of PB integration sites, compared to retrovirus and lentivirus.22,24 We now show that EBV-CTLs can be modified to express a HER2-CAR using PB and demonstrate that transgenic CTLs can be selected using truncated-CD19 expressed as a second transgene. Finally, we show that HER2-CAR-modified EBV-CTLs (HER2-CTLs) can eliminate HER2-expressing tumor cells both in vitro and in a NOD-SCID xenograft model.
Results
Production, selection and expansion of EBV-CTLs expressing HER2-CAR
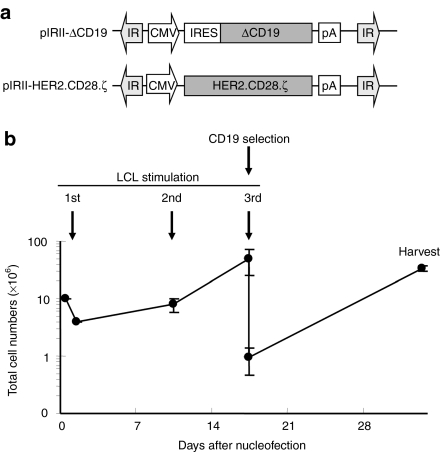
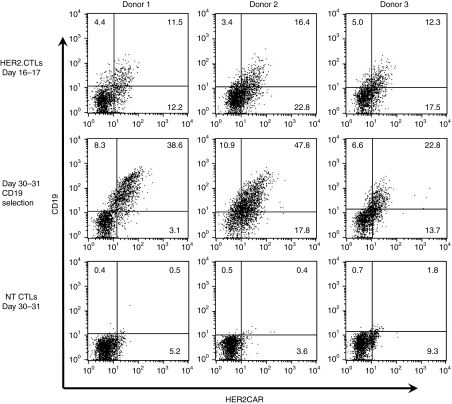
10 × 106 peripheral blood mononuclear cells (PBMCs) were cultured overnight in IL-7-containing T cell medium (TCM) and then nucleofected with two transposon vectors (pIRII-HER2.CD28.ζ and pIRII-ΔCD19) and with pCMV-PB. One day after nucleofection, PBMC numbers had declined to 3.9 ± 0.6 (range 3.9–4.0) × 106 mostly due to the cell damage induced by DNA nucleofection. Surviving cells were stimulated with the autologous EBV-transformed B lymphoblastoid cell lines (LCLs) at a 40:1 ratio of PBMCs to LCL in the presence of IL-4 and IL-7 for 9 days and then restimulated with LCLs at a 4:1 ratio in the presence of IL-15. This combination of cytokines improved the viability and expansion of the transfected T cells (not shown). Since the IL-7 receptor is progressively downregulated on ATCs, IL-15 was used as a growth and survival factor during the second and all subsequent stimulations. Cell numbers increased up to 49.6 ± 23.7 (range 22.4–65.3) × 106 after the second EBV-LCL stimulation (Figure 1b). Phenotypic analysis on day 9 or 10 showed that 24.2% ± 4.6% (range 20.7–22.5) of CTLs expressed both HER2-CAR and CD19. On day 16 or 17, the expanded CD19 transgene-expressing EBV-CTLs were selected using anti-CD19 microbeads, and immediately cocultured with EBV-LCLs for further 2 weeks. Prior to selection, 30.9% ± 7.8% (range 23.7–39.2) of cells expressed HER2-CAR, and 13.4% ± 2.6% (range 11.5–16.4) expressed both HER2-CAR and CD19. After CD19 selection this increased to 47.9 ± 15.5% (range 36.5–65.5) for HER2-CAR and 36.4 ± 12.6% (22.8–47.8) for both HER2-CAR and CD19. By day 30 of culture, the selected HER2-CTLs reached 33.3 ± 3.5 (range 30.0–36.9) × 106 (Figure 2). Thus PB-transposed HER2-CAR-expressing EBV-CTLs can be produced, expanded, and enriched using magnetic-beads.
Figure 1.
Generation of human epidermal growth factor receptor 2-chimeric antigen receptor (HER2-CAR) expressing Epstein-Barr virus-specific T cells (EBV-CTLs). (a) Schema of piggyBac-transposons used to nucleofect peripheral blood mononuclear cells (PBMCs). pIRII-HER2.28.ζ and pIRII-ΔCD19 are piggyBac-transposon plasmids. pCMV-PB was the piggyBac-transposase plasmid. IR, inverted terminal repeat; CMV, cytomegalovirus immediate early promoter; IRES, internal ribosomal entry site; pA, polyadenylation sequence; HER2.28.ζ, Her2/neu-specific chimeric antigen receptor, CD28 and T cell receptor ζ chain; ΔCD19, truncated CD19. (b) Production of HER2-CTLs. On day-1, 10 × 106 PBMCs were incubated in interleukin (IL)-7 containing T-cell medium [IL-7-T-cell medium (TCM)] for 20–24 hours. On day 0, IL-7-treated PBMCs were nucleofected with pIRII-HER2.28.ζ pIRII-ΔCD19 and pCMV-PB using the Nucleofector device and immediately transferred into medium containing IL-7-TCM for 20–24 hours. On day 1, to generate EBV-CTLs, nucleofected PBMCs were stimulated with 40 Gy γ-irradiated autologous EBV-transformed B lymphoblastoid cell lines (EBV-LCLs) at a responder: stimulator ratio of 40: 1 in IL-4/IL-7-TCM. On day 9 or 10, the cells were restimulated with LCLs at a 4:1 ratio in IL-15-TCM. On day 16-17, EBV-CTLs were selected for CD19 expression using anti-CD19 MACS beads, immediately transferred in 30 ml of IL-15-TCM in a gas-permeable cell culture device (GRex) and stimulated with 5 × 106 autologous LCLs. On day 30–31, EBV-CTLs were harvested, analyzed, and cryopreserved. The total cell numbers of HER2-CAR- and ΔCD19-nucleofected EBV-CTLs were determined using trypan blue exclusion on day 1, day 9–10, day 16–17, and day 30–31. Data shows the mean ± s.d. of experiments from three donors.
Figure 2.
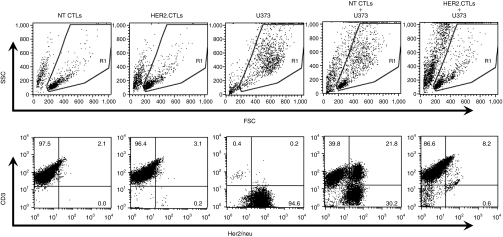
Human epidermal growth factor receptor 2-chimeric antigen receptor (HER2-CAR) expression on nucleofected EBV-CTLs. HER2-CAR- and ΔCD19-nucleofected EBV-CTLs (HER2-CTLs) were analyzed for transgene expression on day 16–17 (before selection) and on day 30–31 (after selection). Nontransfected EBV-CTLs (NT CTLs) were also analyzed as controls. Shown are results from three separate donors. CTLs, cytotoxic T lymphocytes; EBV, Epstein-Barr virus; NT, nontransfected.
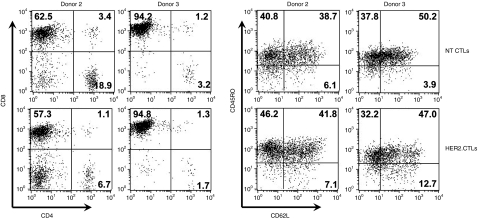
PB modification did not markedly affect the phenotype of EBV-CTLs: The CD4:CD8 subset ratio in HER2-CTLs did not differ greatly between stably transfected and nontransfected (NT) EBV-CTLs (Figure 3). About 40% of HER2-CTLs expressed an effector memory phenotype characterized by both CD45RO and CD62L and was similar to the frequency observed in NT EBV-CTLs (Figure 3).
Figure 3.
Immunophenotype of HER2-CTLs selected and expanded in vitro. Immunophenotyping using anti-CD4, anti-CD8, anti-CD45RO and anti-CD62L antibodies was performed after selection and expansion of HER2-CTLs in vitro. Shown are representative results from donor 2 and donor 3 of four donors. CTLs, cytotoxic T lymphocytes. CTLs, cytotoxic T lymphocytes; HER2, human epidermal growth factor receptor 2.
HER2-CTLs specifically target both HER2 antigen and EB virus antigens
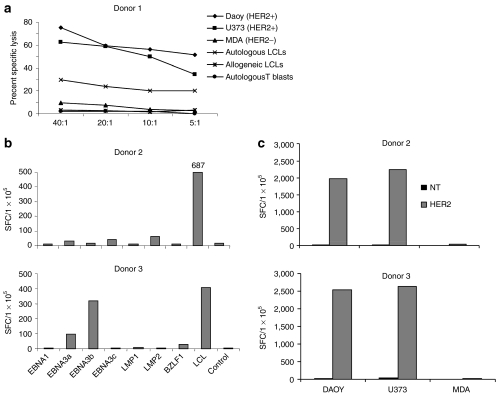
To confirm coexisting HLA-independent HER2-specfic killing and HLA-restricted EBV-specific killing we performed 4 hour cytotoxicity assays, using HER2 and EBV antigen-expressing tumor cells labeled with 51Cr. HER2-CTLs showed superior lysis of HER2-positive tumor cell lines, U373, and Daoy (Figure 4a) compared to NT EBV-CTLs (not shown) and equivalent killing of autologous EBV-LCLs. Neither HER2-CTLs nor NT EBV-CTLs killed the HER2-negative breast cancer cell line, MDA-MB-468, allogeneic LCLs, or autologous ATCs (Figure 4a).
Figure 4.
Antigen specificity of HER2-CTLs. After CD19-selection and 14 days-expansion in vitro, HER2-CTLs were tested for their ability to target HER2-positive tumor cell lines (Daoy and U373), a HER2-negative tumor cell line (MDA-MB-468), and autologous and allogeneic EBV-LCLs. (a) Cytotoxic specificity for Daoy, U373, MDA, autologous and allogeneic EBV-LCLs, and autologous activated T blasts using Cr51 release assay. NT EBV-CTLs were tested as controls. (b) Interferon (IFN)-γ production by HER2-CTLs in response to representative EBV antigens, EBNA1, EBNA3A, 3B, 3C, LMP1, LMP2 and BZLF1, and autologous LCLs was measured using ELISPOT assays. (c) IFN-γ production in response to HER2-positive tumor cell lines (Daoy and U373) and HER2-negative tumor cell line (MDA) using ELISPOT assay. NT EBV-CTLs were used as controls in all assays. Similar results were obtained in four donors. CTLs, cytotoxic T lymphocytes; EBV, Epstein-Barr virus; HER2, human epidermal growth factor receptor 2; LCLs, B lymphoblastoid cell lines; NT, nontransfected.
The ability of the T cells to produce interferon (IFN)-γ in response to specific antigens was also tested in ELIspot assays. HER2-CTLs produced IFN-γ in response to autologous EBV-LCLs and to specific EBV antigens presented as peptide mixes by ATCs (Figure 4b) and to HER2-positive tumor cell lines U373 and Daoy (Figure 4c). The NT EBV-CTLs demonstrated a similar pattern of reactivity against the EBV-associated targets but did not recognize the HER2-positive targets. Neither the HER2-CTLs nor NT EBV-CTLs produced IFNγ when cultured with the HER2-negative tumor cell line, MDA-MB-468. Data from two donors (a total of four experiments) is shown.
We also evaluated the ability of HER2-CTLs to kill tumor targets in coculture assays. HER2-CTLs were cultured with the HER2+ glioblastoma cell line, U373, at a ratio of 1: 1 or 2: 1 CTLs to tumor in the absence of cytokines for 5 days. Figure 5 shows HER2-CTLs, NT CTLs, and U373 tumor cells cultured alone and together. HER2-CTLs eliminated virtually all HER2+ tumor cells by day 5 both at 2:1 and at 1: 1 ratios, while NT EBV-CTLs did not inhibit tumor growth.
Figure 5.
Eradication of HER2-expressing tumor cells by HER2-CTLs. On day 7 after the 4th LCL-stimulation, 1 × 106 HER2-CTLs were cocultured with 1 × 106 HER2+ U373 cells. CTLs and U373 cells were plated alone in TCM without cytokines in each well of a 24-well plate as controls. Five days later, cultures were stained with anti-CD3-FITC antibody and anti-Her2/neu-PE antibody and immediately analyzed by flow cytometry. CTLs, cytotoxic T lymphocytes; HER2, human epidermal growth factor receptor 2; LCLs, B lymphoblastoid cell lines.
HER2.CAR expression is increased by stimulation through the CAR or the TCR
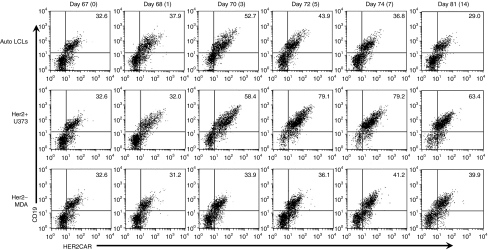
To evaluate the stability and antigen-inducibility of the transgenic CAR expression from PB, we serially analyzed CAR expression on HER2-CTLs that had received five weekly stimulations with LCLs and were then restimulated (on day 67) through their TCR with EBV.LCLs, through their CAR with HER2-positive tumor cells or with HER2-negative tumor cells and then cultured for 14 days. The expression of the HER2.CAR was then measured over the next 14 days. On day 67 of culture the HER2-CAR was expressed on ~32% of cells with a mean fluorescent intensity (MFI) of 33.3. After restimulation with LCLs, CAR-expression transiently increased (up to 52.7%) but returned to 36.8% by day 8 and 29% by day 14 after stimulation (day 81 of culture) (Figure 6 top panel). By contrast after stimulation through the CAR using U373 cells as stimulators, transgene expression increased to ~80% by day 5 post stimulation and remained high for up to 14 days (day 81 of culture) (Figure 6, middle panel). There was a slight increase in HER2-CAR expression after stimulation with HER2 negative MDA cells (Figure 6, lower panel). These results suggest that antigen stimulation via a native TCR or CAR can increase CAR expression from a PB vector, and that significant basal levels were maintained stably in long term culture.
Figure 6.
Transient and reversible increase of chimeric antigen receptor (CAR) expression on HER2-CTLs by antigen-specific stimulation. On day 67 of culture, 14 days after the 5th stimulation with autologous LCLs, HER2-CTLs were stimulated with autologous LCLs, HER2-positive U373, or HER2-negative MDA cell lines. HER2-CTLs were analyzed for HER2-CAR- and ΔCD19-expression before the stimulation on day 67 of culture (day 0 prestimulation), and on day 68 (day 1 after stimulation), 70 (3), 72 (5), 74 (7) and 81 of culture (day 14 after stimulation). CTLs, cytotoxic T lymphocytes; HER2, human epidermal growth factor receptor 2; LCLs, B lymphoblastoid cell lines.
HER2-CTLs survive long-term with stable CAR-expression
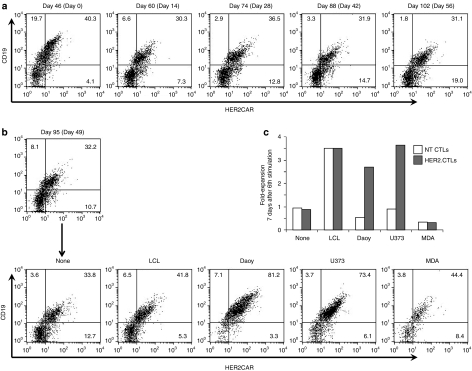
To determine how long HER2-CTLs can persist and stably express HER2-CAR in vitro, we assessed cell survival and CAR-expression using transfected EBV-CTLs that were maintained in culture without antigenic stimulation but in the presence of IL-15 for 2 months after the 5th stimulation (on day 49) with EBV-LCLs. Surprisingly, HER2-CTLs survived, albeit with minimal expansion for over 100 days after gene-transfer and 56 days after the final LCL-stimulation and continued to express the CAR stably (range 38.0–50.1%) (Figure 7a). Of note, the T cells did not survive in the absence of cytokines (data not shown). To determine if the long-lived HER2-CTLs maintained their antigen specificity and growth ability, we restimulated the HER2-CTLs with EBV-LCLs or HER2+/− tumor cell lines on day 95 after gene transfer (on day 49 after the 5th stimulation). Seven days later, the long-lived HER2-CTLs showed a dramatic increase of CAR frequency after stimulation with HER2+ tumors, U373 and Daoy, (Figure 7b), accompanied by three to fivefold expansion while as expected NT CTLs, which were maintained for equally long in culture, did not respond to HER2-positive tumor stimulation (Figure 7b,c). EBV stimulation induced cell expansion regardless of CAR engineering. These results suggest that HER2-CTLs could survive for several months in the absence of specific stimulation and for longer periods in the presence of EBV-infected cells or HER2+ tumor.
Figure 7.
Long term survival and stable chimeric antigen receptor (CAR)-expression of HER2-CTLs in vitro. (a) HER2-CTLs received 5th LCL-stimulation at an effector: target ratio of 4: 1 on day 46 post nucleofection, and thereafter they were maintained without any antigen-stimulation in IL-15-TCM. Cells were analyzed for human epidermal growth factor receptor 2-chimeric antigen receptor (HER2-CAR) and ΔCD19 expression every 2 weeks until day 102 post nucleofection. (b,c) On day 95 post nucleofection in a, 1 × 106 cells of 5th–stimulated HER2-CTLs were further stimulated at a 4:1 ratio with autologous LCLs, or HER2 + Daoy or U373 cells or with HER2-MDA cells, or were unstimulated. Seven days later, cells were analyzed for HER2-CAR and ΔCD19 expression by flow cytometry in b and counted using trypan blue exclusion for viable cells in c. As control, NT CTLs were also stimulated with LCLs, analyzed by flow cytometry and counted as same above. CTLs, cytotoxic T lymphocytes; HER2, human epidermal growth factor receptor 2; LCLs, B lymphoblastoid cell lines; NT, nontransfected.
Cultured HER2-CTLs are polyclonal
To determine if the transfection and culture procedure resulted in clonal expansion, we analyzed TCR gene rearrangement using the BIOMED-2 multiplex PCR assay in combination with GeneScan analysis, which is a highly sensitive and reliable method in routine testing of T-cell clonality26,27 and in which the detection rate of clonal rearrangement of regions of TCRβ and TCRγ ranged from 94 to 98% in patients with T-cell malignancies (see Supplementary Materials and Methods).28,29 The results of Biomed-2 analysis are shown in Supplementary Figure S1. As a control, we used PBMCs from donor 1 and Jurkat cells. GeneScan analyses of the PCR products revealed typical polyclonal Gaussian curves in PBMCs (B) and predominant monoclonal peaks in Jurkat cells (A) for TCRβ (tube a: 265 nucleotides (nt), tube c: 311 nt) and TCRγ (tube d: 212 nt; tube e: 116 nt). EBV-LCLs (C) from donor 1 displayed monoclonal peaks for TCRβ (tube c: 305 nt) in the absence of T cells (CD3+ cells <1%). HER2-CTLs (D) from donor 1 (including γ-irradiated EBV-LCLs from donor 1 as stimulator) after a 30-day-culture showed monoclonal peaks for TCRβ (tube c: 305 nt) in accordance with the regions of TCR gene rearrangement of EBV-LCLs used as antigen-presenting cells for HER2-CTL culture. HER2-CTLs did not show any other monoclonal peaks either for TCRβ or TCRγ, but instead showed polyclonal patterns.
Regression of xenografts after administration of HER2.CTL
To evaluate the in vivo antitumor activity of HER2-CTLs we used a xenograft model. To allow serial bioluminescence imaging in vivo, we transduced the human HER2-positive tumor cell line, HT-1080, with a retroviral vector encoding an eGFP-firefly luciferase fusion gene (eGFP.FFLuc). Cells were then sorted for GFP positivity and firefly luciferase functionality was confirmed in vitro using a luminometer (data not shown).
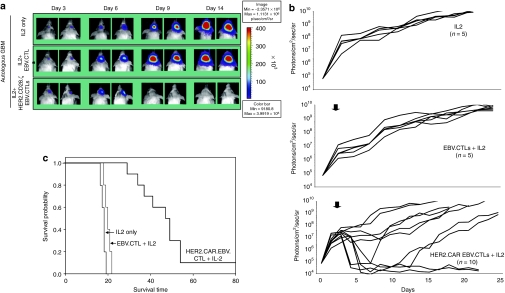
5 × 104 eGFP.FFLuc HT-1080 cells were injected stereotactically into the right frontal cortex of SCID mice. On day 6 after tumor-cell injection, mice received an intratumoral injection of 2 × 106 HER2-CTLs (n = 10) or NT CTLs from the same donor (n = 5). A subset of animals was sham injected (n = 5). All animals received 1500U IL-2 intraperitoneally three times weekly for 2 weeks. We quantified tumor growth by serial bioluminescence. In untreated animals, and in animals treated with NT CTLs, tumors grew exponentially over time (Figure 8a). In contrast, photon emission decreased significantly in all tested mice after HER2-CTL injection, indicating tumor regression (Figure 8b). This was confirmed by histological examination in a subset of animals. Kaplan–Meier survival studies 80 days after tumor injection showed that untreated mice and mice receiving NT CTLs had a median survival of 19 and 20 days respectively. In contrast, mice treated with HER2-CTLs had a median survival of 55 days (P < 0.007; Figure 8c).
Figure 8.
Adoptively transferred HER2-CTLs induce regression of HER2-positive xenografts in vivo. 5 × 104 eGFP.FFLuc-expressing HT-1080 cells were injected stereotactically into the caudate nucleus of 9–12 week old SCID mice followed by intratumoral injection of 2 × 106 HER2-CTLs or NT EBV-CTLs on day 6 after tumor inoculation. (a) Tumors grew progressively in untreated mice as shown for two representative animals (upper row) and in mice receiving NT EBV-CTLs (middle row), while tumors regressed over a period of 2–5 days in response to a single injection of HER2-CTLs generated from the same donor (lower row). (b) Quantitative bioluminescence imaging: HER2-CTLs induced tumor regression when compared to NT EBV-CTLs (two-tailed P value = 0.006, Mann–Whitney U test). Solid arrows: time of T-cell injection; open arrows: background luminescence (mean~105 photon/sec/cm2/sr); n, number of animals tested in each group. (c) Kaplan–Meier survival curve: Survival analysis performed 80 days after tumor establishment. Mice treated with HER2-CTLs had a significantly longer survival probability (P < 0.007) in comparison to untreated mice and mice that received NT EBV-CTLs. CTLs, cytotoxic T lymphocytes; HER2, human epidermal growth factor receptor 2; EPV, Epstein-Barr virus; NT, nontransfected.
Discussion
We and others have demonstrated that resting human T cells can be transfected stably with transposons.24,30 However ATCs are intolerant to transfection and it has been difficult to establish stable transfection of activated antigen-specific T cells. We have now developed a strategy for the successful expression of transgenes in activated, antigen-specific human T cells using the PB transposon system. We used PB to introduce both a HER2-CAR and a selectable marker into PBMCs from which stably transfected, EBV-specific T cells could subsequently be selected and expanded to clinically relevant numbers. We further demonstrated that these CTL could be maintained in culture for over 100 days while retaining stable transgene expression even in the absence of antigenic stimulation. Transgene expression increased transiently in response to stimulation through both the endogenous TCR and the CAR and then returned to baseline. Both EBV antigen-expressing and HER2-expressing tumor cells could be eliminated in in vitro assays, while regression of HER2-expressing sarcoma cells could be induced in a NOD-SCID xenograft model.
Manuri et al. showed that T cells modified with PB to carry a CD19-CAR could lyse CD19-positive tumor cells in vitro.30 These T cells expanded on CD19-expressing artificial antigen presenting cells but the specificities of the native TCRs were unknown. Such CAR-expressing T cells rely on tumor cells and normal B cells to provide stimulatory signals and both of these cell types can be tolerogenic.31 To ensure T cell activation upon encounter with tumor a number of groups have evaluated the addition of intracellular signaling domains from costimulatory molecules like CD28, OX40, and 41BB.4,5 These molecules may not capitulate the spatio-temporal requirements for T cell activation and may produce T cells that are excessively reactive, with resultant clinical toxicity when multiple costimulatory domains are combined in a single receptor.10,32 Further, costimulatory domains may not provide resistance to multiple inhibitory ligands produced by tumors and their accessory cells. An alternative strategy to promote the in vivo survival/activity of CAR-expressing T cells could be to use T cells specific for vaccine antigens as carriers for CARs, allowing vaccination after infusion to support T cell maintenance.33 We have previously investigated the use of EBV-specific T cells as carriers for CARs since these T cells may be stimulated and maintained in vivo by “normal” EBV-infected B cells through their endogenous TCRs.8,34 Hence when patients with relapsed neuroblastoma were treated with both CD3-activated ATCs and EBV-specific CTLs expressing a GD2-CAR from a retrovirus vector, EBV-specific T cells preferentially expanded and persisted longer than CD3-ATCs after infusion, resulting in three complete remissions among 11 patients treated.8
We evaluated the stability of transgene expression after transfection of antigen-specific T cells with PB vectors, but transfection resulted in excessive death of ATCs and we were unable to achieve outgrowth of transgene-expressing CTLs. However when combined with a selectable marker, antigen-specific T cells could be reactivated and then selectively expanded from transfected PBMCs. Methods of transfection other than nucleofection provided no significant advantages (not shown). Surprisingly, if PBMCs were cultured in IL-7 prior to transfection and were then activated with antigen in the presence of both IL-4 and IL-7, the viability of transgene-expressing antigen-specific T cell precursors could be maintained and we could expand transgenic, antigen-specific T cell lines. Although IL-4 is generally considered a TH2 cytokine, EBV-specific T cells grown in IL-4 and IL-7, maintained their cytolytic activity and their ability to secrete TH1 cytokines (IFNγ and IL-2) following stimulation through both endogenous and transgenic receptors over long term culture. Like many cytokines, the activity of IL-4 is likely to be contextual35 and it appears to have no repolarizing effect on established memory T cells specific for strong viral antigens. The transduced T cells were clearly polyclonal as evidenced by their reactivity with multiple EBV antigens and their polyclonal TCR rearrangements. The single monoclonal TCR Vβ peak seen in the LCL was unexpected, but TCR gene rearrangement is found in B-cell-derived clonal cells and Biomed-2 protocols have detected TCR gene rearrangement in 35% of precursor B-cell acute lymphoblastic leukemia and 27% of mature B-cell malignancies coincident with immunoglobulin gene rearrangement (although not so far reported in EBV-LCLs).36,37
Retrovirus vectors present several problems for clinical gene therapies. They have a propensity to integrate into or near proto-oncogenes and their prohibitive expense for phase I/II clinical trials, requiring the generation of master producer cell banks and master virus banks. Transposons, by contrast, can be introduced into cells as plasmids at less than 1/10 of the cost. Further, the PB transposon has a large cargo capacity, enabling the introduction of multiple transgenes in a single or multiple transposons,21,24,38,39 potentially allowing the expression of additional genes that could protect T cells from tumor-mediated inhibition. The use of any integrating gene delivery system poses the potential risk of genotoxicity when used for human application. We have previously mapped PB integration sites in primary human T cells and found a lack of preference for integrating into or near known proto-oncogenes.24,25 Further, we found that the outgrowing T cells had a polyclonal pattern of TCR Vβ and Vγ gene usage, with no indication of mono- or oligoclonality (Supplementary Figure S1). Nonetheless, codelivery of a suicide gene can be used for improved safety. We have previously demonstrated PB-mediated delivery of an inducible caspase suicide gene into primary human T cells with chemical dimerizer induced complete cell ablation.24,40,41 Our use of a truncated CD19 molecule could also be used for cell ablation via using anti-CD19 antibodies to target transfected cell removal if needed.42
The aim of this study was to develop a nonviral gene-transfer method for EBV-CTLs and we successfully generated EBV-CTLs engineered to express a HER2-CAR using the nonviral PB-transposon system. Up to ~50% of HER2-CTLs expressed an effector memory phenotype which was similar to that of retrovirally transduced EBV-specific CTLs that persisted for up to nine years after infusion into stem cell transplant recipients.19 Moreover, after infusion into NOD/SCID mice bearing HER2-positive tumors, HER2-CTLs induced tumor regression in vivo and resulted in significant extension of the life span of mice harboring tumors. The long term stability of transgene expression is likely to be advantageous for long term tumor control and transient increase in CAR expression after antigen-specific stimulation via native or chimeric TCRs should increase their potency after antigenic challenge.
Using this protocol to generate HER2-CTLs, we obtained ~30 × 106 dually specific T cells from ~10 ml of peripheral blood within about 30 days. In clinical trial for pediatric patients with neuroblastoma using retrovirally GD2-CAR-engineered EBV-CTLs, we infused 2 × 107 to 2 × 108/m2 of CTLs into the patients, which resulted in clinical efficacy and tumor regression. If we collect 30–40 ml peripheral blood from pediatric patients with HER2+ medulloblastoma, we could obtain ~108 of HER2-CTLs within 30 days using this protocol. Therefore, engineering of EBV-CTLs to express tumor-specific CARs by PB-transposon system presents an alternative approach to viral vectors for the gene modification of human T cells for therapeutic purposes
Materials and Methods
Plasmid construction. The PB-transposase plasmid, pCMV-PB, and PB-transposon plasmid, pIRII-ΔCD19, have been described previously.24 pIRII-IRES-ΔCD19 is a transposon encoding the echomyocarditis virus internal ribosome entry site (IRES) followed by a CD19 gene from which the intracellular signaling domain has been deleted.16 Both vectors are transcriptionally regulated by the cytomegalovirus (CMV) immediate early gene enhancer/promoter sequence (Figure 1a). We cloned the HER2-CAR with a CD28.ζ signaling domain from a retroviral vector (SFG.HER2.CD28.ζ) (HER2-CAR)43 into pIRII-IRES-ΔCD19 by replacing the IRES-ΔCD19 element with the HER2-CAR (Figure 1a). All plasmid constructs were confirmed by restriction digestion and DNA sequencing.
Blood donors and cell lines. PBMCs from EBV-seropositive healthy volunteers were obtained with informed consent from the Baylor College of Medicine institutional review board. EBV-transformed B lymphoblastoid cell lines (EBV-LCLs) were produced as previously described,43 and maintained in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum and 2 mmol/l L-glutamine (GlutaMAX-I; Invitrogen, Carlsbad, CA). The glioblastoma cell line, U373, the medulloblastoma cell line, Daoy, the sarcoma line HT-1080, and the breast tumor cell line, MDA-MB-468 (MDA) were purchased from the American Type Culture Collection and grown in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum and 2 mmol/l -glutamine for use as target cells in various assays.
Stable transfection of EBV-CTLs with HER2-CAR. We have previously reported efficient gene-transfer of the PB-transposon system into primary human T cells.24 To generate PB gene-modified EBV-CTLs, bulk PBMCs (>10 × 106) were cultured overnight in complete T-cell medium (TCM) [Advanced RPMI (Gibco, Gaithersburg, MD)] supplemented with 5% fetal bovine serum and 2 mmol/l -glutamine] containing recombinant human interleukin (IL)-7 (10 ng/ml). The next day 10 × 106 IL-7-treated PBMCs were nucleofected with pIRII-HER2.CD28.ζ transposon (5 µg), pIRII-ΔCD19 transposon (5 µg), and pCMV-PB transposase (5 µg) using nucleofection program U-014 and the Human T-cell Nucleofector Kit (Lonza, Basel, Switzerland). Nucleofected PBMCs were immediately transferred to TCM with IL-4 (1,000 IU/ml) and IL-7 (10 ng/ml) (R&D Systems, Minneapolis, MN), plated into 2 cm2 wells of a 24-well plate at 2 × 106 per well for a further 20–24 hours, and then stimulated with 5 × 104 autologous EBV-LCLs that had been irradiated with 40 Gy at a responder: stimulator (R:S) ratio of 40:1 as previously described.43 On day 9 or 10 after nucleofection, EBV-CTLs were counted and restimulated with irradiated autologous LCLs at an R:S ratio of 4:1 in TCM with IL-15 (5 ng/ml) (Proleukin; Chiron, Emeryville, CA). To enrich HER2-CAR-expressing cells, on day 16 or 17, EBV-CTLs were incubated with CD19-microbeads (Miltenyi Biotec, Bisley, UK) for 15 minutes at 4 °C, then positively selected using Miltenyi Mini-MACS column according to the manufacturer's instructions. The selected cells were immediately cocultured with 5 × 106 irradiated autologous LCLs and IL-15 (5 ng/ml) for a further 2 weeks in 30 ml of TCM in a cell culture device with a gas-permeable surface membrane area of 10 cm2 (GRex10; Wilson-Wolf Manufacturing, New Brighton, MN).44 IL-15 (5 ng/ml) was added to the medium twice weekly. On day 30 or 31, EBV-CTLs were analyzed for HER2-CAR expression and then cryopreserved for further experiments.
Flow cytometry. We analyzed PB transfected EBV-CTLs for transgene expression and phenotype using a FACSCalibur with Cell Quest software (Becton Dickinson, Franklin Lakes, NJ). Cell-surface expression of HER2-CAR was detected with a recombinant human HER2-Fc chimera (R&D Systems). Briefly, stably transfected (HER2-CTLs) and NT EBV-CTLs were incubated with HER2-Fc for 30 minutes at 4 °C, and detected with a goat anti-human Fc fluorescein isothiocyanate (FITC)-conjugated secondary antibody (Chemicon, Temecula, CA), peridinin chlorophyll protein (PerCP)-conjugated CD3 monoclonal antibody (MAb) (Becton Dickinson) and allophycocyanin (APC)-conjugated CD19 MAb (Becton Dickinson).
To determine subpopulation and memory phenotype of the generated HER2-CTLs, we stained the cells with phycoerythrin (PE)-conjugated CD4 (CD4-PE) MAb, CD8-APC MAb and CD3-PerCP MAb, CD62L-PE MAb, CD45-RO-APC MAb and CD3-PerCP MAb (all from Becton Dickinson), respectively, and analyzed them by flow cytometry. NT EBV-CTLs and appropriate isotype-matched antibodies were used as controls in each experiment.
Cytotoxicity assay. To determine if HER2-CTLs were able to lyse target cells, we performed cytotoxicity assays using a standard 51Cr release assay.43 As effectors, we used HER2-CTLs or NT EBV-CTLs 7 days after the 4th stimulation, and as targets we used HER2-positive tumor cell lines, U373 and Daoy, a HER2-negative tumor cell line, MDA-MB468, autologous and HLA-mismatched EBV-LCLs and autologous T cells activated with a CD3 antibody (100 ng/ml) (Ortho Biotech, Bridgewater, NJ) (ATCs). ATCs were used as target cells 7–14 days after stimulation in IL-15-containing TCM. Target cells were labeled for 1 hour with 51Cr sodium chromate (Na2CrO4), washed and then incubated with effector cells at an E:T ratio of 40:1, 20:1, 10:1, or 5:1 for 4 hours at 37 °C in 5% CO2 incubator. The percent specific lysis was calculated as (experimental release − spontaneous release)/(maximum release − spontaneous release) × 100.
Enzyme-linked immunospot assay. We measured interferon (IFN)-γ production by HER2-CTLs in response to stimulation with representative EBV antigens and tumor cell lines using enzyme-linked immunospot (ELISpot) assay. Briefly, transfected and NT EBV-CTLs were serially diluted from 2 × 105 to 5 × 104 cells/well, and we measured the virus-specific activity of responder cells after direct stimulation with autologous EBV-LCL (1 × 105 cells/well) and pepmixes (15 mers overlapping by 11 amino acids from JPT Technologies, Berlin, Germany) spanning the EBV latent and lytic antigens EBNA1, EBNA3a, EBNA3b, EBNA3c, LMP1, LMP2, and BZLF1. All pepmixes were used at a concentration of 100 ng/peptide/well. Tumor-specific activity was measured by incubating responder cells with the HER2-positive tumor cell lines, U373 and Daoy, and a HER2-negative tumor cell line, MDA-MB-468, all plated at 1 × 105 cells/well. Each culture condition was run in duplicate. After 20 hours of incubation, plates were developed as previously described, dried overnight at room temperature in the dark, then sent to Zellet Consulting, New York, NY for quantification.45 Spot-forming cells and input cell numbers were plotted, and a linear regression calculated after excluding plateau data points. The frequency of T cells specific to each antigen was expressed as specific spot-forming cells per input cell numbers.
Coculture experiments. To evaluate the ability of HER2-CTLs to eliminate HER2-positive(+) tumors, we cocultured 1 × 106 HER2-CTLs or NT EBV-CTLs (on day 7 after their 4th stimulation with LCLs) with non-irradiated HER2+ tumor cell, U373, at effector to target cell ratios of 2:1 or 1:1 in TCM in the absence of cytokines in 24-well plates. As controls, 1 × 106 of NT or CAR-engineered EBV-CTLs, and 0.5 × 106 U373 were also cocultured. Phenotypic analyses were performed on days 1, 3, and 5. T cells and tumor cells were detected using anti-CD3-FITC-conjugated CD3 MAb and anti-Her2/neu-PE-conjugated MAb by flow cytometry.
NOD-SCID xenograft model. All animal experiments were conducted on a protocol approved by the Baylor College of Medicine Institutional Animal Care and Use Committee. Recipient NOD-SCID mice were purchased from Taconic (C.B-Igh-1b/IcrTac-Prkdcscid; FOX CHASE CB-17 SCID ICR; Taconic, Hudson, NY). Male 9- to 12-week-old mice were anesthetized with rapid sequence inhalation isofluorane (Abbot Laboratories, Abbot Park, IL) followed by an intraperitoneal injection of 225–240 mg/kg Avertin solution and then maintained on isofluorane by inhalation throughout the procedure. The head was shaved, mice were immobilized in a Cunningham Mouse/Neonatal Rat Adaptor (Stoelting, Wood Dale, IL) stereotactic apparatus fitted into an E15600 Lab Standard Stereotaxic Instrument (Stoelting), and then scrubbed with 1% povidone-iodine. A 10 mm skin incision was made along the midline. The tip of a 31G ½ inch needle mounted on a Hamilton syringe (Hamilton, Reno, NV) served as the reference point. A 1 mm burr-hole was drilled into the skull, 1 mm anterior to and 2 mm to the right of the bregma. Firefly-luciferase expressing HER2-positive HT-1080 cells (5 × 104 in 2.5 µl) were injected 3 mm deep to the bregma, corresponding to the center of the right caudate nucleus over 5 minutes. The needle was left in place for 3 minutes, to avoid tumor cell extrusion, and then withdrawn over 5 minutes. Five days after tumor cell injection, animals were treated with 2 × 106 effector T cells in 5 µl to the same tumor coordinates. The incision was closed with 2–3 interrupted 7.0 Ethicon sutures (Ethicon, Somerville, NJ). Animals received 1500U IL-2 intraperitoneally three times weekly for 2 weeks. A subcutaneous injection of 0.03–0.1 mg/kg buprenorphine (Buprenex, Hull, England) was given for pain control.
Bioluminescence imaging. Isofluorane anesthetized animals were imaged using the IVIS system (IVIS; Xenogen, Alameda, CA) 10 minutes after 150 mg/kg -luciferin (Xenogen) was injected intraperitoneally. The photons emitted from luciferase-expressing cells within the animal body and transmitted through the tissue were quantified using “Living Image”, a software program provided by the same manufacturer. A pseudo-color image representing light intensity (blue least intense and red most intense) was generated and superimposed over the grayscale reference image. Animals were imaged every other day for 1 week after injections, then twice weekly for 2 weeks then weekly thereafter. They were regularly examined for any neurological deficits, weight loss, or signs of stress and euthanized according to preset criteria, in accordance the Baylor College of Medicine's Center for Comparative Medicine guidelines.
Statistical analysis. The data are presented as mean ± 1 SD. The Student's t-test was used to determine the statistical significance of differences between samples, and P values less than 0.05 were accepted as indicating a significant difference.
SUPPLEMENTARY MATERIAL Figure S1. Evaluation of T-cell clonality of HER2-CTLs using BIOMED-2 multiplex PCR and GeneScan analysis. Materials and Methods.
Acknowledgments
This work was supported in parts by a Specialized Centers for Cell-based Therapy (SCCT) grant from NIH-NHLBI 1 U54 HL1081007 and an NIH-NCI lymphoma SPORE P50 CA126752. M.H.W. is supported by a career development award from the Department of Veterans Affairs and the generous support of Dr. and Mrs. Harold M. Selzman. Y.N. was supported by JHIF scholarship awards in Herpesvirus Infections Research. The authors thank Malcolm Brenner for critical review of the manuscript.
Supplementary Material
Evaluation of T-cell clonality of HER2-CTLs using BIOMED-2 multiplex PCR and GeneScan analysis.
REFERENCES
- Schmidt SM, Schag K, Müller MR, Weck MM, Appel S, Kanz L.et al. (2003Survivin is a shared tumor-associated antigen expressed in a broad variety of malignancies and recognized by specific cytotoxic T cells Blood 102571–576. [DOI] [PubMed] [Google Scholar]
- Zendman AJ, Ruiter DJ., and, Van Muijen GN. Cancer/testis-associated genes: identification, expression profile, and putative function. J Cell Physiol. 2003;194:272–288. doi: 10.1002/jcp.10215. [DOI] [PubMed] [Google Scholar]
- Eshhar Z, Waks T, Gross G., and, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci USA. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulè MA, Straathof KC, Dotti G, Heslop HE, Rooney CM., and, Brenner MK. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005;12:933–941. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Finney HM, Akbar AN., and, Lawson AD. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J Immunol. 2004;172:104–113. doi: 10.4049/jimmunol.172.1.104. [DOI] [PubMed] [Google Scholar]
- Park JR, Digiusto DL, Slovak M, Wright C, Naranjo A, Wagner J.et al. (2007Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma Mol Ther 15825–833. [DOI] [PubMed] [Google Scholar]
- Till BG, Jensen MC, Wang J, Chen EY, Wood BL, Greisman HA.et al. (2008Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells Blood 1122261–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G.et al. (2008Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma Nat Med 141264–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM., and, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Dudley ME., and, Rosenberg SA. Adoptive cell therapy: genetic modification to redirect effector cell specificity. Cancer J. 2010;16:336–341. doi: 10.1097/PPO.0b013e3181eb3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A.et al. (2001Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2 N Engl J Med 344783–792. [DOI] [PubMed] [Google Scholar]
- Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L.et al. (2002Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer J Clin Oncol 20719–726. [DOI] [PubMed] [Google Scholar]
- Mass RD, Press MF, Anderson S, Cobleigh MA, Vogel CL, Dybdal N.et al. (2005Evaluation of clinical outcomes according to HER2 detection by fluorescence in situ hybridization in women with metastatic breast cancer treated with trastuzumab Clin Breast Cancer 6240–246. [DOI] [PubMed] [Google Scholar]
- Hudis CA. Trastuzumab–mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- Serrano-Olvera A, Dueñas-González A, Gallardo-Rincón D, Candelaria M., and, De la Garza-Salazar J. Prognostic, predictive and therapeutic implications of HER2 in invasive epithelial ovarian cancer. Cancer Treat Rev. 2006;32:180–190. doi: 10.1016/j.ctrv.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Ahmed N, Ratnayake M, Savoldo B, Perlaky L, Dotti G, Wels WS.et al. (2007Regression of experimental medulloblastoma following transfer of HER2-specific T cells Cancer Res 675957–5964. [DOI] [PubMed] [Google Scholar]
- Ahmed N, Salsman VS, Kew Y, Shaffer D, Powell S, Zhang YJ.et al. (2010HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors Clin Cancer Res 16474–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada SA, Peggs KS, Simpson TR., and, Allison JP. Shifting the equilibrium in cancer immunoediting: from tumor tolerance to eradication. Immunol Rev. 2011;241:104–118. doi: 10.1111/j.1600-065X.2011.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA.et al. (2010Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients Blood 115925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Wu X, Li G, Han M, Zhuang Y., and, Xu T. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell. 2005;122:473–483. doi: 10.1016/j.cell.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Wu SC, Meir YJ, Coates CJ, Handler AM, Pelczar P, Moisyadi S.et al. (2006piggyBac is a flexible and highly active transposon as compared to sleeping beauty, Tol2, and Mos1 in mammalian cells Proc Natl Acad Sci USA 10315008–15013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MH, Coates CJ., and, George AL., Jr PiggyBac transposon-mediated gene transfer in human cells. Mol Ther. 2007;15:139–145. doi: 10.1038/sj.mt.6300028. [DOI] [PubMed] [Google Scholar]
- Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, Hämäläinen R.et al. (2009piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells Nature 458766–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa Y, Huye LE, Dotti G, Foster AE, Vera JF, Manuri PR.et al. (2009Optimization of the PiggyBac transposon system for the sustained genetic modification of human T lymphocytes J Immunother 32826–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan DL, Nakazawa Y, Kaja A, Kettlun C, Cooper LJ, Rooney CM.et al. (2009Genome-wide mapping of PiggyBac transposon integrations in primary human T cells J Immunother 32837–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg Y, van Gastel-Mol EJ, Verhaaf B, Lam KH, van Dongen JJ., and, Langerak AW. BIOMED-2 multiplex immunoglobulin/T-cell receptor polymerase chain reaction protocols can reliably replace Southern blot analysis in routine clonality diagnostics. J Mol Diagn. 2005;7:495–503. doi: 10.1016/S1525-1578(10)60580-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen JJ, Langerak AW, Brüggemann M, Evans PA, Hummel M, Lavender FL.et al. (2003Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936 Leukemia 172257–2317. [DOI] [PubMed] [Google Scholar]
- Brüggemann M, White H, Gaulard P, Garcia-Sanz R, Gameiro P, Oeschger S.et al. (2007Powerful strategy for polymerase chain reaction-based clonality assessment in T-cell malignancies Report of the BIOMED-2 Concerted Action BHM4 CT98-3936 Leukemia 21215–221. [DOI] [PubMed] [Google Scholar]
- Liu H, Bench AJ, Bacon CM, Payne K, Huang Y, Scott MA.et al. (2007A practical strategy for the routine use of BIOMED-2 PCR assays for detection of B- and T-cell clonality in diagnostic haematopathology Br J Haematol 13831–43. [DOI] [PubMed] [Google Scholar]
- Manuri PV, Wilson MH, Maiti SN, Mi T, Singh H, Olivares S.et al. (2010piggyBac transposon/transposase system to generate CD19-specific T cells for the treatment of B-lineage malignancies Hum Gene Ther 21427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo ME, El-Amine M, Tonnetti L, Fleischman L., and, Scott DW. Gene therapeutic approaches to induction and maintenance of tolerance. Int Rev Immunol. 2001;20:627–645. doi: 10.3109/08830180109045582. [DOI] [PubMed] [Google Scholar]
- Sadelain M, Brentjens R., and, Rivière I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21:215–223. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmeier S, Altvater B, Pscherer S, Eing BR, Kuehn J, Rooney CM.et al. (2007Gene-engineered varicella-zoster virus reactive CD4+ cytotoxic T cells exert tumor-specific effector function Cancer Res 678335–8343. [DOI] [PubMed] [Google Scholar]
- Rossig C, Bollard CM, Nuchtern JG, Rooney CM., and, Brenner MK. Epstein-Barr virus-specific human T lymphocytes expressing antitumor chimeric T-cell receptors: potential for improved immunotherapy. Blood. 2002;99:2009–2016. doi: 10.1182/blood.v99.6.2009. [DOI] [PubMed] [Google Scholar]
- Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T.et al. (2007IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways Nat Immunol 8967–974. [DOI] [PubMed] [Google Scholar]
- van der Velden VH, Brüggemann M, Hoogeveen PG, de Bie M, Hart PG, Raff T.et al. (2004TCRB gene rearrangements in childhood and adult precursor-B-ALL: frequency, applicability as MRD-PCR target, and stability between diagnosis and relapse Leukemia 181971–1980. [DOI] [PubMed] [Google Scholar]
- Evans PA, Pott Ch, Groenen PJ, Salles G, Davi F, Berger F.et al. (2007Significantly improved PCR-based clonality testing in B-cell malignancies by use of multiple immunoglobulin gene targets. Report of the BIOMED-2 Concerted Action BHM4-CT98-3936 Leukemia 21207–214. [DOI] [PubMed] [Google Scholar]
- Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P., and, Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–775. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlig KM, Saridey SK, Kaja A, Daniels MA, George AL., Jr, and, Wilson MH. Multiplexed transposon-mediated stable gene transfer in human cells. Proc Natl Acad Sci USA. 2010;107:1343–1348. doi: 10.1073/pnas.0910383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Freeman KW, Khan T, Pham E., and, Spencer DM. Improved artificial death switches based on caspases and FADD. Hum Gene Ther. 1999;10:2273–2285. doi: 10.1089/10430349950016924. [DOI] [PubMed] [Google Scholar]
- Straathof KC, Pulè MA, Yotnda P, Dotti G, Vanin EF, Brenner MK.et al. (2005An inducible caspase 9 safety switch for T-cell therapy Blood 1054247–4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tey SK, Dotti G, Rooney CM, Heslop HE., and, Brenner MK. Inducible caspase 9 suicide gene to improve the safety of allodepleted T cells after haploidentical stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:913–924. doi: 10.1016/j.bbmt.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, Ng CY, Heslop HE, Holladay MS, Richardson S, Turner EV.et al. (1995Production of genetically modified Epstein-Barr virus-specific cytotoxic T cells for adoptive transfer to patients at high risk of EBV-associated lymphoproliferative disease J Hematother 473–79. [DOI] [PubMed] [Google Scholar]
- Vera JF, Brenner LJ, Gerdemann U, Ngo MC, Sili U, Liu H.et al. (2010Accelerated production of antigen-specific T cells for preclinical and clinical applications using gas-permeable rapid expansion cultureware (G-Rex) J Immunother 33305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leen AM, Christin A, Khalil M, Weiss H, Gee AP, Brenner MK.et al. (2008Identification of hexon-specific CD4 and CD8 T-cell epitopes for vaccine and immunotherapy J Virol 82546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Evaluation of T-cell clonality of HER2-CTLs using BIOMED-2 multiplex PCR and GeneScan analysis.