Abstract
An ideal anticancer strategy should target only the malignant cells but spare the normal ones. In this regard, we established a platform, consisting of an antigen-delivering vehicle and a protein vaccine, for developing an immunotherapeutic approach with the potential for eliminating various cancer types. Mesenchymal stem cells (MSCs) have been demonstrated capable of targeting tumors and integrating into the stroma. Moreover, we have developed a protein vaccine PE(ΔIII)-E7-KDEL3 which specifically recognized E7 antigen and elicited immunity against cervical cancer. Taking advantage of tumor-homing property of MSCs and PE(ΔIII)-E7-KDEL3, we used E6/E7-immortalized human MSCs (KP-hMSCs) as an E7 antigen-delivering vehicle to test if this protein vaccine could effectively eliminate non-E7-expressing tumor cells. Animals which received combined treatment of KP-hMSCs and PE(ΔIII)-E7-KDEL3 demonstrated a significant inhibition of tumor growth and lung-metastasis when compared to PE(ΔIII)-E7-KDEL3 only and KP-hMSCs only groups. The efficiency of tumor suppression correlated positively to the specific immune response induced by PE(ΔIII)-E7-KDEL3. In addition, this combined treatment inhibited tumor growth via inducing apoptosis. Our findings indicated that KP-hMSCs could be used as a tumor-targeting device and mediate antitumor effect of PE(ΔIII)-E7-KDEL3. We believe this strategy could serve as a platform for developing a universal vaccine for different cancer types.
Introduction
Despite the advances in both clinical and basic research, dedicated to reducing mortality rates and improving survival, cancer remains the leading cause of death among patients younger than age 85 years in the United States.1 Ninety percent of cancer deaths do not result from the primary tumor but rather from subsequent organ metastases.2 Thus, an ideal cancer therapy should be able to systemically eradicate both the primary and metastatic tumors in the body. Currently, systemic therapy has been limited to chemotherapy and biologic response modifiers. While new therapeutic agents like docetaxel, pemetrexed, and erlotinib have been demonstrated efficacy in treating patients with advanced lung cancer, clinical responses to treatment and improved survival have been modest.3 The limited successes of systemic chemotherapy thus underscore the need for developing new therapeutic strategies. Although not without risks, one such novel therapy approach is vaccine therapy.4,5 Antigen-specific peptide or protein-based immunotherapy appears to be an attractive approach for cancer treatment because of its potential in eradicating systemic tumors at multiple sites and specificity in discriminating between normal and neoplastic cells. For instance, human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine has been reported highly effective against cervical infection and precancer caused by oncogenic HPV types.6 Although this vaccine offers protections against HPV-16 or HPV-18 infections and associated precancerous lesions, it has little effect against patients who have been burdened by fully developed cervical cancer. This limitation could be a result of immunoediting by cancer cells and intrinsically low immunogenicity of certain antigens such as E7.7 To overcome this problem, a fusion protein vaccine, PE(ΔIII)-E7-KDEL3, was previously developed by our collaborators.8 The improved vaccine potency and E7 antigen-presenting ability were attributed to the addition of retrograde-delivery and KDEL domains from exotoxin A of Pseudomonas aeruginosa. This engineered protein vaccine was shown to significantly enhance major histocompatibility complex (MHC) class I and II presentation of the linked E7 antigen, leading to increased immunological responses and antitumor effects in vivo.8
Another emerging cancer therapeutic tool is mesenchymal stem cell (MSC)-based therapy. Others and our laboratory have demonstrated that MSCs targeted to and infiltrated into the tumor and its stroma thus could be utilized in anticancer gene therapy.9,10,11,12,13 Taking advantage of the aforementioned MSCs' tumor-homing and infiltrating abilities, we established an immortalized human MSC cell line, termed KP-hMSCs using modified HPV-16 E6/E7 genes,14 which was then be utilized to target, infiltrate and tag primary and metastatic tumors.
In this study, we aimed to create an alternative cancer immunotherapeutic platform by combining our previously established protein vaccine, PE(ΔIII)-E7-KDEL3 and the E7 antigen carrying KP-hMSCs. We hypothesized that tumors could be targeted, infiltrated, and tagged by the E7-expressing KP-hMSCs so that E7-specific protein vaccine, PE(ΔIII)-E7-KDEL3, induced and mounted immunological attack to suppress or eliminate tumor cells. The first component of our system, a specially designed protein vaccine, PE(ΔIII)-E7-KDEL3 has been shown to greatly enhance antigen-specific immunologic responses against HPV-16 E7; it exhibited improved potency and efficacy when compared to its predecessors both in vitro and in vivo.8 Although effective, this protein vaccine is limited to targeting E7-expressing tumors such as cervical and ovarian cancers. In order for this fusion protein vaccine to be applied in a broader spectrum of cancer treatments, we introduced a second component to our system, an E7-expressing human MSC cell line KP-hMSCs. Previously, we demonstrated that KP-hMSCs were able to target microscopic tumors, proliferate, and integrate into the stroma. Importantly, these integrated MSCs did not become tumorigenic and presented a great therapeutic advantage.9 These intrinsic properties of MSCs make them an ideal tracer and delivery system in devising cell-based anticancer therapy. We demonstrated that KP-hMSCs as a tool in tumor targeting and infiltration in a subcutaneous and lung-metastasis mouse model.15,16 In this study, we combined the E7-expressing MSCs and PE(ΔIII)-E7-KDEL3 protein vaccine and examined their antitumor effects. This combined treatment significantly suppressed tumorigenesis and metastasis in the mouse model. More importantly, our system offered a platform for the future development of a universal antitumor treatment.
Results
Increased number of E7-specific CD4+ T-cell precursors and serum level of anti-E7 antibodies elicited by PE(ΔIII)-E7-KDEL3 protein vaccine
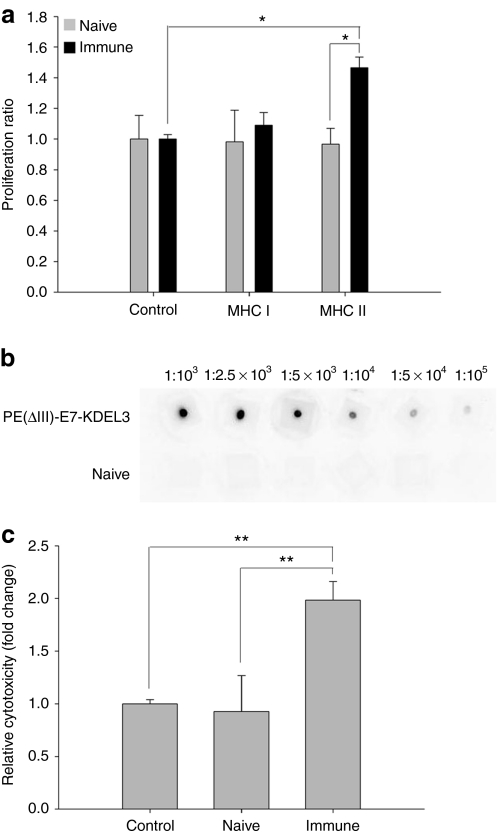
To investigate the immunological response stimulated by PE(ΔIII)-E7-KDEL3 protein vaccine, splenocytes isolated from vaccinated and naive mice were incubated with E7 peptide-containing MHC class I epitope (amino acid 49–57) or MHC class II epitope (amino acid 30–67). After a 5-day incubation period, splenocytes cultured with E7 peptide-containing MHC class II epitope exhibited a significantly increased proliferation rate (Figure 1a), suggesting that PE(ΔIII)-E7-KDEL3 protein vaccine stimulated significantly more E7-specific CD4+ T-cell precursors than CD8+. In vitro dot blot analysis was used to demonstrate the relative E7-specific antibody titer stimulated by the protein vaccine (Figure 1b). Then, we cocultured NG4TL4-TK and KP-hMSCs cells with serum from vaccine immunized mice to determine antibody-mediated tumor cytotoxicity. Our results demonstrated that anti-E7 serum increased the incidence of cell lysis in NG4TL4-TK/KP-hMSCs coculture (Figure 1c).
Figure 1.
In vitro immunological analyses of mice vaccinated with protein vaccine. (a) Splenocyte proliferation assay in response to E7 peptide in vitro. Splenocytes from mice vaccinated with PE(ΔIII)-E7-KDEL3 showed increased proliferation in response to E7 peptides containing a major histocompatibility complex (MHC) class II epitope. (b) Dot blot analysis of E7-specific antibodies purified from mice vaccinated with PE(ΔIII)-E7-KDEL3. (c) E7-specific antibodies mediated complement-dependent cytotoxicity. After incubation with the coculture of NG4TL4-TK and KP-hMSC cells, the sera from mice vaccinated with PE(ΔIII)-E7-KDEL3 induced more cell lysis than that from naive mice. Error bars represent SD among three independent experiments. *P < 0.05, **P < 0.01. hMSCs, human mesenchymal stem cells.
Tumor growth inhibition by PE(ΔIII)-E7-KDEL3 protein vaccine/MSC combined treatment monitored by noninvasive molecular imaging
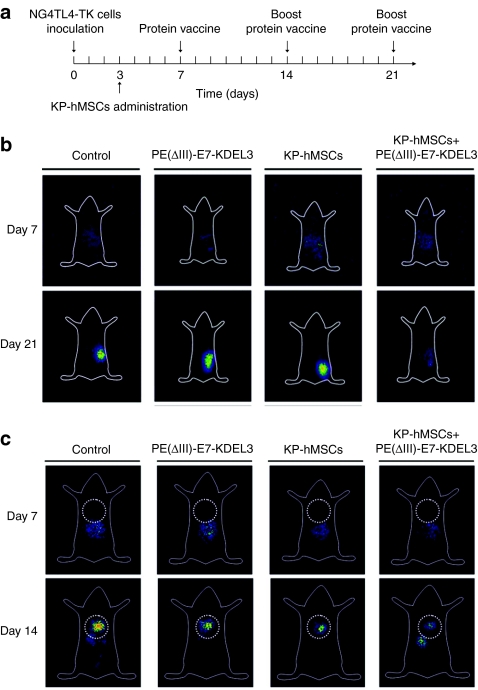
We utilized planer γ-imaging to evaluate the therapeutic effect of combined protein vaccine/MSC treatment in a mouse model. The time course of experiment was illustrated in Figure 2a. Briefly, mice were inoculated with NG4TL4-TK cells at day 0 followed by intravenous injection of KP-hMSCs cells on day 3. Mice were first immunized with PE(ΔIII)-E7-KDEL3 protein vaccine 7 days after tumor inoculation and received boost shots 1 and 2 weeks later. The experiment was divided into four groups, the control group (no treatment), vaccine only group (PE(ΔIII)-E7-KDEL3), MSCs only group (KP-hMSCs) and finally combined-treatment group receiving both the vaccine and MSCs (KP-hMSCs+PE(ΔIII)-E7-KDEL3). In subcutaneous tumor model, planer γ-imaging was obtained on day 7 and 21 post-tumor inoculation. The signal intensity reflected the extent and relative growth of tumor. Animals that received the combined treatment demonstrated a gradual decrease in signal intensity, reflecting reduced tumor burden over time as observed on day 21 whereas reversal was observed in the control animals (Figure 2b). In lung-metastasis model, mice received intravenous inoculation of NG4TL4-TK cells, were subjected to planar γ-imaging 7 and 14 days postinoculation. No signals were detected in the lungs (dot circle) on day 7 in all groups. On day 14, signals from NG4TL4-TK cells were detected in the lungs of all groups indicating lung metastasis had occurred. Among four experimental groups, mice that received the combined treatment exhibited the weakest tumor signal intensity (Figure 2c). Data from both subcutaneous and lung-metastasis models suggested the combined treatment posed an inhibitory effect on NG4TL4-TK tumor growth and lung metastasis.
Figure 2.
In vivo imaging of mice-bearing tumor with protein vaccine/mesenchymal stem cells (MSCs) combined treatment. (a) Illustration of experimental design. (b) Representative planar γ-camera images from the subcutaneous tumor model. Images were taken at 7 and 21 after subcutaneous (s.c.) injection of 5 × 104 NG4TL4-TK cells in the right flanks. (c) Representative planar γ-camera images of lung-metastasis model. Images were collected at 7 and 14 days after intravenous injection of 1 × 105 NG4TL4-TK cells. There are four groups in both models. Control group, tumor bearing with no treatment (PE(ΔIII)-E7-KDEL3) group, tumor bearing with protein vaccine treatment only; KP-hMSCs, tumor bearing with MSCs treatment only; KP-hMSCs+PE(ΔIII)-E7-KDEL3 group, tumor bearing with combined treatment. In the subcutaneous tumor model, mice with combined treatment showed a significantly lower level of signal intensity from the tumor than other groups at day 21. Similar to the subcutaneous model, signal intensity from the tumor in the lungs was significantly weaker in the combined treatment group. Dotted circle represents the position of the lungs.
Inhibition of primary and pulmonary metastatic tumor in mice using protein vaccine/MSC combined treatment
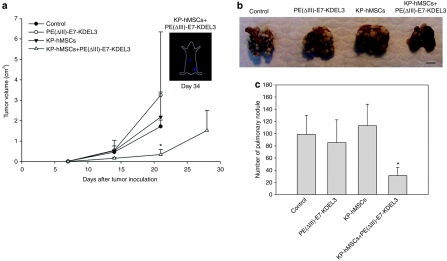
To validate data obtained from the in vivo imaging experiments, we calculated the volumes of subcutaneous tumors. The results showed that the tumors in combined-treatment group were significantly smaller than that in other groups on day 21. Except the mice in combined-treatment group, other mice were all sacrificed due to oversized or ulcerated tumors after day 21. The tumor volumes only slightly increased and the signal intensity of planar γ-imaging remained weak in combined-treatment group when examined on day 34 (Figure 3a). To evaluate pulmonary metastases, lung tissues of mice receiving tumor grafts were collected 21 days after tumor inoculation. The macromorphological analysis demonstrated that significantly fewer pulmonary nodules were observed in the combined-treatment group when compared to those in other groups (Figure 3b). After quantification, the mean numbers of pulmonary tumor nodules in mice that received the combined treatment were significantly lower than the animals treated with other approaches (Figure 3c). We also performed survival assay on lung-metastasis model. The result showed that the survival rate of combined-treatment group was ~25–35% higher than other groups (Supplementary Figure S1).
Figure 3.
Assessments of mice-bearing tumor with protein vaccine/mesenchymal stem cell (MSC) combined treatment. (a) The volumes of subcutaneous tumors from different treatment groups. The tumors in combined-treatment group were significantly smaller than that in other groups at day 21, and slightly increased at day 28. Even after 34 days, the signal intensity of planar γ-camera imaging remains weak in combined-treatment group (n = 6). (b) Representative macro-morphological images of pulmonary nodules from different treatment groups. The least number of tumor nodules were observed in the combined-treatment group. (c) Quantification of pulmonary nodules from control and treatment groups. A significant decrease was observed in the number of pulmonary nodules in the combined-treatment group when compared with other groups (n = 5). Error bars indicate SD. *P < 0.05, compared with control group.
Homing of MSCs to pulmonary metastatic tumor
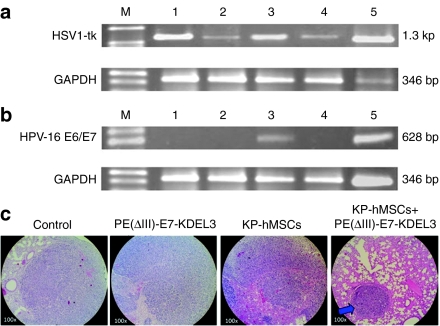
Next, we confirmed that the pulmonary tumors were originated from NG4TL4-TK cells by the presence of HSV1-tk gene using semiquantitative reverse transcription (RT)-PCR. The presence of HSV1-tk PCR product was detected in all treatment groups (Figure 4a). The mRNA level of HSV1-tk appeared to be lower in both vaccine only (Figure 4a, lane 2) and combined-treatment samples (Figure 4a, lane 4). In a parallel experiment, HPV-16 E6/E7 gene was utilized to identify MSCs. As expected, HPV-16 E6/E7 mRNA was detected in the lung tissue of mice that received MSC only treatment (Figure 4b, lane 3), verifying MSCs could target and infiltrate tumors. However, HPV-16 E6/E7 mRNA was not detected in the combined-treatment group (Figure 4b, lane 4). We reasoned that HPV-16 E6/E7 was undetectable in the combined-treatment group was due to the immunological attack launched by the vaccine against cells with E7 antigen including the injected MSCs. The effectiveness of protein vaccine/MSC combined treatment was also validated by histological analysis. In hematoxylin and eosin sections, the lung tissue of combined-treatment group demonstrated a higher degree of structural integrity as evident by the presence of intact pulmonary alveolus (arrow in Figure 4c).
Figure 4.
Ex vivo validation of mesenchymal stem cells (MSCs) targeting and vaccine/MSCs combined treatment in pulmonary metastatic tumor. (a) The presence of HSV1-tk gene in the lung tissues from different treatment groups was detected using reverse transcription (RT)-PCR. There were five different groups namely, without any treatment (lane 1), vaccine only treatment (lane 2), MSCs only treatment (lane 3), the combined treatment (lane 4), and positive control NG4TL4-TK cells (lane 5). HSV1-tk gene was used as an indicator for that the pulmonary tumors were developed by NG4TL4-TK cells, and could be detected in all groups. (b) RT-PCR detection of HPV-16 E6/E7 expression in lung tissues from the mice received different treatments. The group arrangement is the same as in a except lane 5, which represents a positive control, MSCs only. HPV-16 E6/E7 gene in the MSC was detected in MSCs only treatment group (lane 3) and MSCs control (lane 5). GAPDH was used as an internal standard. M represents DNA ladder. (c) Hematoxylin and eosin (H&E) stained parafilm sections of the lung tissues from different treatment groups. Pulmonary alveoli were appeared more intact in the combined-treatment group than in other groups. Blue arrow indicated tumor nodule formation.
Tumor regression by protein vaccine/MSC combined-treatment
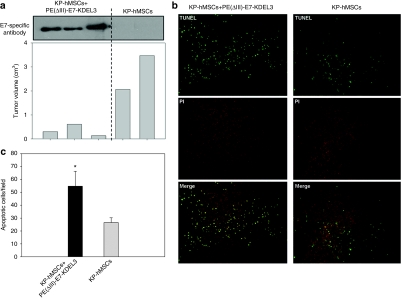
PE(ΔIII)-E7-KDEL3 protein vaccine-induced immunity was predominantly mediated by antibody-dependent mechanisms as shown in Figure 1. To validate this notion, we analyzed the relationship between serum concentration of anti-E7 antibodies and tumor volume. In the subcutaneous tumor model, a negative correlation between the titer of anti-E7 antibody and tumor volume was observed. In the combined-treatment group (KP-hMSCs+PE(ΔIII)-E7-KDEL3), three animals (six mice in total) showed a substantially smaller tumor burden than the animals that received stem cell treatment only (KP-hMSCs, Figure 5a) and this tumor suppression was correlated to the significantly higher serum concentration of anti-E7 antibody. We further examined whether the protein vaccine/MSC combined treatment could induce tumor apoptosis. TUNEL assay revealed that the number of apoptotic cells (green) in the tumor of mice treated with protein vaccine/MSC were higher than mice received only stem cells (KP-hMSCs) (Figure 5b). Quantitatively, an approximately twofold increase in apoptotic cells was observed in the tumor sections from protein vaccine/MSC group when compared to sections from the stem cells-treated group (Figure 5c).
Figure 5.
Evaluation of tumor inhibition by the protein vaccine/mesenchymal stem cells (MSCs) combined treatment. (a) Western blots probed with anti-E7-antibody from sera of the mice received the combined treatment. A negative correlation between the level of anti-E7 antibody and the tumor volume was observed. (b) TUNEL analysis of tumor sections obtained from the combined treatment and MSCs only treatment groups. TUNEL staining was shown in green and propidium iodide (PI) in red. The sample from the combined treatment contained more TUNEL-positive cells than the MSCs only group. (c) Quantification of TUNEL analysis (n = 6). Error bars represent SD among three independent experiments. *P < 0.05, compared with KP-hMSCs group.
Discussion
It is well documented that the immune system can control neoplastic development and growth in a process termed immunosurveillance by recognizing tumor antigens. Some of these antigens, so-called tumor-specific antigen, are expressed exclusively by tumors. Tumor-specific antigens usually arise from viral infection, genetic mutations, and/or translocations of normal cellular genes. Another group of antigens expressed on tumors more commonly are called tumor-associated antigen. Tumor-associated antigens are found on both normal cells and malignant cells but in a significantly higher number in the latter; generally a solid tumor is composed of heterogeneous cell population and their cellular heterogeneity often contributes to tumor's ability to escape host immunosurveillance. For instance, tumor-associated antigens are often self-antigens so responses are rendered by self-tolerance.17 Tumor cells downregulate the expression of MHC I/II and costimulatory molecules leading to anergic T or B cells and failure to respond to their cognate antigens under stimulation. Defects in antigen presentation by antigen-presenting cells, in particularly, the dendritic cells also contributes to tumor escape.18 The tumor stroma has also been shown to assist tumor immunoresistance by serving as a physical barrier between the tumor and immune cells.19 Therefore, there are two major obstacles encountered in cancer immunosurveillance: the lack of tumor-specific antigens and antigen-presenting ability. Hence, we purposed to overcome these two aforementioned problems with the combined usage of protein vaccine/MSC system and examine the feasibility of this approach in this study.
One of the two key components of our system was the HPV-16 E6/E7-immortalized human MSC cell line, KP-hMSCs. The insertion of HPV-16 E6/E7 genes served two functions. First, the human MSCs were immortalized so that they could be used indefinitely and variations between different batches of primary stem cells could also be avoided. The immortalized KP-hMSCs have been thoroughly characterized and shown to be nontumorigenic.14 Second, upon infiltration, KP-hMSCs delivered the E7 antigen into non-E7 expressing tumor cells thereby providing a tumor-specific antigen and expanding the therapeutic spectrum of the E7 antigen-based protein vaccine (PE(ΔIII)-E7-KDEL3). This claim was supported by several lines of experimental evidence in our study. First, E7 mRNA transcript was detected in the tumor biopsies. Second, tumor-bearing mice received E7-expressing KP-hMSCs and PE(ΔIII)-E7-KDEL3 protein vaccine exhibited the highest degree of tumor and metastasis suppression as shown by the planer γ-imaging. Third, lung tissues collected from lone treatment of KP-hMSCs or PE(ΔIII)-E7-KDEL3 protein vaccine contained similar numbers of nodules when compared to nontreatment control whereas the combined treatment contained significantly fewer nodules. Collectively, these findings indicated that KP-hMSCs successfully infiltrated into the tumor and elicited tumoricidal immunological responses.
The detection of E7 antigen in the tumor also provided evidence that human MSCs successfully survived in immunological competent mice. This attribute played an important role in our study and provided additional support that human MSCs could be engrafted into different species.20,21,22 Recent reports have suggested that mesenchymal cells are capable of suppressing immunological reactions by secreting various chemokines and nitric oxide.23,24 This immunosuppressing ability could play a major role in the initial survival of KP-hMSCs followed by the subsequent immunoediting ability of NG4TK4-tk tumor cells once KP-hMSCs were incorporated into the tumor microenvironment. This claim was supported by the detection of E7 antigen in the lung tissues obtained from tumor-bearing mice that received KP-hMSCs only as the treatment. However, E7 antigen was not detectable in the lung tissues collected from tumor-bearing mice receiving KP-hMSCs and PE(ΔIII)-E7-KDEL3 combined treatment. It is possible that E7-expressing KP-hMSCs within the tumor might be recognized and eliminated by the vaccine-mediated immunological attacks as evident by the disappearance of E7 antigen in the residual tumor tissue from the mice which received the combined treatment. In addition, E7-expressing KP-hMSCs was likely the primary target for vaccine-induced anti-E7 immunity as the result of adaptive immunity developed from previous inoculations. Based on this reason and limitation, booster shots containing KP-hMSCs could not be used in these vaccinated animals for eliminating residual tumors or secondary metastasis in present study. In the future, this limitation could be circumvented by an inducible promoter system where E7 gene expression can be regulated in the MSCs, allowing stealth homing of E7-expressing MSCs to the tumor without being recognized by the E7 protein vaccine-induced immunity. Two inducible promoter systems, including radiation-inducible25,26 and tetracycline-inducible promoters,27,28 are being investigated for the improvement of this current platform.
Tumor antigens have been shown primarily present in association with MHC class I molecules and recognized by tumor-specific CD8+ T cells,29,30 however they also have been found to be associated with MHC class II molecules and recognized by CD4+ T cells.31,32,33,34 In previous study, PE(ΔIII)-E7-KDEL3 protein vaccine elicited its antitumor effect via all venues of immunological responses including both CD4+ T, CD8+ T, and natural killer cells.8 However, CD4+ T cells and MHC II molecules appeared to be the predominant route utilized to deliver tumor suppressing effects in this study. The addition of KP-hMSCs might contribute to the different immunological responses triggered by PE(ΔIII)-E7-KDEL3 protein vaccine when compared to the results from the earlier study. As stated previously, MSCs are capable of suppressing immune system, thus it is possible that immunological reactions mediated by CD8+ T could be somehow downregulated by the incorporation of KP-hMSCs. In addition, the extent or amount of E7 antigen tagged to NG4TL4-TK tumors via the incorporation of E7-expressing KP-hMSCs could be much less when compared to the endogenous E7 antigen presented by TC-1 tumor cells mentioned previously.
The underlying molecular mechanisms responsible for the anticancer effects mediated by KP-hMSCs and PE(ΔIII)-E7-KDEL3 combined treatment could be unique due to the involvement of MSCs. Based on the observation that KP-hMSCs-infiltrated tumor cells were targeted and eliminated by anti-E7 antibody generated from PE(ΔIII)-E7-KDEL3 inoculation, we proposed two possible scenarios (Figure 6). One scenario involves the potential possibility that the infiltrated KP-hMSCs was integrated into tumor-associated stroma. Others and our previous studies have demonstrated that systemic delivery of MSCs could target to the tumor lesion and then contribute to the formation of tumor-associated stroma by differentiating in to vascular endothelial cells and stomal fibroblasts.9,35,36,37 Thus, it is possible that the tumor-associated stroma was tagged with E7 antigen as the result of KP-hMSCs incorporation. The circulating anti-E7 antibody generated from the protein vaccine recognized the antigen and inhibited the tumor via destroying the tumor-associated stroma and antiangiogenic effect. This possibility is currently being investigated in our laboratory.
Figure 6.
Proposed mechanism underlying the antitumor effect mediated by the vaccine/mesenchymal stem cell (MSC) combined treatment. Diagrammatic representation of antitumor mechanisms mediated by KP-hMSCs+PE(ΔIII)-E7-KDEL3 combined treatment. Antibody-mediated tumor immunity have been shown could be achieved by complement dependent cytotoxicity and antibody dependent cell-mediated cytotoxicity (NK cells). Hence, we proposed two possible scenarios that KP-hMSCs facilitated antitumor effect of PE(ΔIII)-E7-KDEL3. First, injected KP-hMSCs upon targeting and interacting with tumor cells, integrated into tumor-associated stroma by differentiating into vascular endothelial cells and stroma fibroblasts. The E7-antibody recongnized antigen and inhibited the tumor via destroying tumor-associated and antiangiogenic effect. Second, KP-hMSCs and the tumor cells underwent cellular fusion so that the hybrid progenies also expressed E7 antigen. Thus, antitumor effect was then achieved by anti-E7 antibodies from the immunization leading to the reduced tumor burden and metastasis.
Another possible scenario is that cell fusion may occur between tumor and MSCs. MSCs have been shown to be fusogenic in nature. For instance, inflammation has been suggested to trigger the fusion of myelo-lymphoid cells with nonhematopoietic cells including cardiomyocytes, skeletal muscle, hepatocytes, and Purkinjie neurons.38 More importantly, the formation of heterokaryon contains genetic information from both parental cells as demonstrated in the rescue of fumarylacetoacetate hydrolase-deficient hepatocytes by heterotypic cell fusion of transplanted bone marrow-derived stem cells.39 In addition, transplantation of rat bone marrow into mice led to the activation of dormant rat Purkinjie-specific genes in BMDC nuclei after fusion with mouse Purkinjie neurons accompanied with nuclear reprogramming.40 Based on these findings and to demonstrate that the fusogenic ability of E7-expressing MSCs was not limited to KP-hMSCs cell line, we conducted in vitro coculture experiments using MSCs isolated from cord blood (CB-MSCs) and NG4TL4-TK tumor cells. CB-MSCs was tranduced with nonfunctional (nontumorigenic) E7 antigen and puromycin resistant gene while NG4TL4-TK with neomycin resistant gene. We found that coculturing these two cells gave rise to fusion hybrid cells with resistance to both puromycin and neomycin. Among six single colonies after dual antibiotic selection, one colony appeared to contain both E7 and TK genes, indicating the successful cell fusion and the incorporation of parental genetic information into the progeny (Supplementary Figure S2). This finding suggested that spontaneous fusion and genetic reprogramming between MSCs and tumor cells might be more frequent than previously considered. Therefore, cell fusion phenomenon could be one of the potential mechanisms for KP-hMSCs and PE(ΔIII)-E7-KDEL3 combined treatment-induced tumoricidal effect.
In summary, the present study provided evidence for an alternative approach consisting of E7-expressing MSCs and its specific protein vaccine, PE(ΔIII)-E7-KDEL3. This combined treatment appeared to effectively suppress tumorigenesis and metastasis in the mouse model. These experimental findings served as a platform for the future development of a universal treatment for different cancer types.
Materials and Methods
Animals. Four- to six-week-old female FVB/N mice were purchased from the National Taiwan University (Taipei, Taiwan). The animal experiment protocol was approved by the Institutional Animal Care and Use Committee of Taipei Medical University.
Cell. Murine fibrosarcoma cell lines containing herpes simplex virus type I thymidine kinase (HSV1-tk) gene (NG4TL4-TK), lung-colonizing metastatic sarcoma cells, were cultured in minimum essential medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 10 µg/ml streptomycin, and 2 mmol/l -glutamine in a humidified atmosphere with 5% CO2 at 37 °C.41 Human MSC cell line KP-hMSCs was originally cultured from the bone marrow of a 61-year-old female donor, immortalized by retroviral-mediated transduction of human papilloma virus E6/E7 genes, and maintained in Dulbecco's modified Eagle's medium containing 1 mg/ml glucose and 10% fetal bovine serum, 100 U/ml penicillin, 10 µg/ml streptomycin, and 2 mmol/l -glutamine in a humidified atmosphere with 5% CO2 at 37 °C. This immortalized hMSC line is >99% positive for CD29, CD44, CD90, CD105, SH2, and SH3 MSC characteristic markers.14
Preparation and vaccination of protein vaccines. The PE(ΔIII)-E7-KDEL3 protein vaccine was kindly provided by Dr C.W. Liao, Animal Technology Institute Taiwan, Miaoli, Taiwan.8 The stock of PE(ΔIII)-E7-KDEL3 was diluted with phosphate-buffered saline and incubated for 2 hours in 37 °C before vaccination. Mice were immunized with 0.1 mg/mouse PE(ΔIII)-E7-KDEL3 mixed with 10% ISA206 adjuvant by subcutaneously injected into the backs of the mice. These animals were then boosted subcutaneously 1 and 2 weeks later using the same regimen.
Mixed leukocyte reaction and 5-bromo-2-deoxyuridine ELISA. Mice were immunized with 0.1 mg/mouse PE(ΔIII)-E7-KDEL3 and boosted with the same regimen 1 and 2 weeks later. Splenocytes were harvested 1 week after the last vaccination. Proliferation of splenocytes was determined in vitro using a 5-bromo-2-deoxyuridine cell proliferation assay kit (CHEMICON, Billerica, MA) according to the manufacturer's instructions. Before cell proliferation assay, 1 × 105 pooled splenocytes from vaccinated or naive mice were incubated for 6 days with either 1 µg/ml of E7 peptide (amino acid 49–57) containing an MHC class I epitope for detecting E7-specific CD8+ T cell precursors42 or 1 µg/ml of E7 peptide (amino acid 30–67) containing an MHC class II epitope for detecting E7-specific CD4+ T cell precursors.43
Dot blot assay. For dot blot analysis, E7 protein was blotted onto nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA). The membranes were blocked with blocking buffer (5% skim milk in Tris-buffered saline Tween-20) for 1 hour and then blocking solution was discarded. The nitrocellulose membrane was incubated with serial diluted serum at 4 °C overnight. Antibody binding was detected by incubation with goat anti-mouse immunoglobulin G-horseradish peroxidase and followed by Western Lighting Chemiluminescence Reagent Plus assay (PerkinElmer, Waltham, MA) according to the manufacturer's protocol.
Cytotoxicity assay. For this assay, NG4TL4-TK and KP-hMSCs cells/well (1:1, 1 × 104 in total) were seeded in 96-well plate overnight and followed by the addition of serum from vaccinated mice for 3 days. Cytotoxicity assay was performed by quantitative measurements of lactate dehydrogenase using CytoTox-One Homogeneous Membrane Integrity Assay (Promega, Madison, WI) according to vendor's protocol.
Tumor xenografts and protein vaccine inoculation. Tumor xenografts were established by subcutaneous injection of 5 × 104 or intravenous injection of 1 × 105 NG4TL4-TK cells at day 0 followed by intravenous injection of KP-hMSCs on day 3. Mice were first immunized with PE(ΔIII)-E7-KDEL3 protein vaccine 7 days after tumor inoculation and received boost shots 1 and 2 weeks later. The experiment was divided into four groups, the control group (no treatment), vaccine only group (PE(ΔIII)-E7-KDEL3), MSCs only group (KP-hMSCs) and finally combined-treatment group receiving both the vaccine and MSCs (KP-hMSCs+PE(ΔIII)-E7-KDEL3). In subcutaneous model, tumor volumes were calculated as previously described44 and planer γ-imaging was obtained at day 7 and 21 after the inoculation of NG4TL4-TK cells. After day 21, the animals in control, vaccine only and MSCs only group were sacrificed due to oversized or ulcerated tumors. In lung-metastasis model, planar γ-imaging was obtained from 7 and 14 days postinoculation. Planar γ-imaging was performed as described.16 Briefly, 3.7 MBq of 131I-FIAU were injected into the tail vein 24 hours before planar imaging. Images were collected with a digital γ-camera (Elscint SP-6), equipped with a high-energy pinhole collimator, a 364 keV ± 10% 131I photopeak energy window, and a 256 × 256 × 16 bit image matrix. In lung-metastasis model, mice were sacrificed and lung tissues were collected 21 days after tumor inoculation. Lungs were fixed in 4% paraformaldehyde and pulmonary tumor nodules in each mouse were evaluated and enumerated by experimenters blinded to sample identity. For histological analysis, the fixed lungs were embedded in paraffin and followed by cutting in 10 µm sections and stained with hematoxylin and eosin.
E7 and TK gene validation by PCR. Total RNA harvested from cells and tumor tissues was extracted using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA) and underwent RT followed by PCR amplification. RT was performed with Super-Script III (Invitrogen Life Technologies) and an Oligo (dT)12–18 primer. Four micrograms of RNA were added into a final volume of 21-µl solution containing 10 mmol/l deoxynucleotide triphosphate mix, 10× RT buffer, 25 mmol/l MgCl2, 0.1 mol/l dithiothreitol, RNase inhibitor, and RNase H. Six micrograms of RT product were used for the PCR amplification in a final volume of 50 µl containing 2.5 mmol/l deoxynucleotide triphosphate, 25 mmol/l MgCl2, primers, and Taq DNA polymerase (Invitrogen Life Technologies). PCR amplification of reverse-transcribed complementary DNA was performed with the following primers and conditions. The primers used for HSV1-tk are 5′ TGCAGCGACCCGCTTAACAGCGT 3′ (forward) and 5′ CATAGATCTGGATCCTTCCGGTATTGTCT 3′ (reverse). Tm: 55 °C, 35 cycles. The primers for HPV-16 E6/E7 are 5′ ATGCATAGTATATAGAGATGGGAAT 3′ (forward) and 5′ CTGCAGCATCAGCCATGGTAGA 3′ (reverse). Tm: 56 °C, 35 cycles. The primers for GAPDH are 5′ GCTCTCCAGAACATCATCCCTGCC 3′ (forward) and 5′ CGTTGTCATACCAGGAAATGAGCTT 3′ (reverse). Tm: 55 °C, 35 cycles. PCR products were then run on 1% agarose gels (AMRESCO, Solon, OH) and visualized with ethidium bromide staining. Images were analyzed using FloGel-I (Fluorescent Gel Image System; TOP BIO, Taipei, Taiwan). GAPDH was used as an internal control.
Western blot analysis. Recombinant E7 protein was loaded into a sodium dodecyl sulfate-polyacrylamide gel and separated by electrophoresis. For western blotting, proteins from gels were transferred to a polyvinylidene fluoride membrane (pore size, 0.45 µm; Millipore, Billerica, MA) using a Mini Trans-Blot Cell apparatus (Bio-Rad Laboratories). The polyvinylidene fluoride membrane was probed with a 166,000 dilution serum from mice with combined-treatment group or stem cells only group. The E7-specific antibody was detected with incubation with goat anti-mouse immunoglobulin G-horseradish peroxidase and followed by Western Lighting Chemiluminescence Reagent Plus assay (PerkinElmer) according to the manufacturer's protocol.
TUNEL assay. TUNEL assay was performed using DeadEnd colorimetric apoptosis detection system (Promega) according to the manufacturer's instructions. Briefly, subcutaneous tumor sections from mice of combined-treatment group or stem cells only group were made permeable with 20 µg/ml proteinase K for 10 minutes at room temperature and the fragmented DNA was labeled using the TdT (terminal deoxynucleotidyl transferase) reaction mixture containing fluorescein-12-dUTP for 1 hour at 37 °C according to supplier recommendations. Nuclei were stained with 1 µg/ml propidium iodide. The result of TUNEL assay was expressed quantitatively by the ratio of apoptotic cells/field of view. The group received KP-hMSCs+PE(ΔIII)-E7-KDEL3 treatment demonstrated a significantly higher number of apoptotic cells when compared to the control group which received KP-hMSCs only.
Statistical analysis. All data are expressed as the means ± SD for the number of experiments. Statistical significance (*P < 0.05; **P < 0.01) between experimental and control groups was calculated by paired t-test.
SUPPLEMENTARY MATERIAL Figure S1. Survival analysis of mice-bearing pulmonary metastatic tumor with protein vaccine/MSCs combined treatment. Figure S2. In vitro investigation of the interactions between mesenchymal stem cells and cancer cells.
Acknowledgments
This work was supported by National Science Council (NSC 97-2314-B-038-033-MY3 to W.-P.D.; NSC 99-2628-B-038-010-MY3 to A.T.H.W.); Core Facility grant (NSC 99-3112-B-010-015) and the Department of Health (DOH) to Taipei Medical University—Center of Excellence for Cancer Research (TMU-CECR, DOH100-TD-C-111-008). HealthBanks Biotech Co., Ltd., Purzer Pharmaceutical Co., Ltd., and Kooper Biotech Co., Ltd.
Supplementary Material
Survival analysis of mice-bearing pulmonary metastatic tumor with protein vaccine/MSCs combined treatment.
In vitro investigation of the interactions between mesenchymal stem cells and cancer cells.
REFERENCES
- Jemal A, Siegel R, Ward E, Hao Y, Xu J., and, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- Mehlen P., and, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6:449–458. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- Giovannetti E, Lemos C, Tekle C, Smid K, Nannizzi S, Rodriguez JA.et al. (2008Molecular mechanisms underlying the synergistic interaction of erlotinib, an epidermal growth factor receptor tyrosine kinase inhibitor, with the multitargeted antifolate pemetrexed in non-small-cell lung cancer cells Mol Pharmacol 731290–1300. [DOI] [PubMed] [Google Scholar]
- Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD.et al. (2006Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412 N Engl J Med 3551018–1028. [DOI] [PubMed] [Google Scholar]
- Offit PA. The Cutter incident, 50 years later. N Engl J Med. 2005;352:1411–1412. doi: 10.1056/NEJMp048180. [DOI] [PubMed] [Google Scholar]
- Paavonen J, Naud P, Salmerón J, Wheeler CM, Chow SN, Apter D, HPV PATRICIA Study Group et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- Cheng WF, Hung CF, Pai SI, Hsu KF, He L, Ling M.et al. (2002Repeated DNA vaccinations elicited qualitatively different cytotoxic T lymphocytes and improved protective antitumor effects J Biomed Sci 96 Pt 2675–687. [DOI] [PubMed] [Google Scholar]
- Liao CW, Chen CA, Lee CN, Su YN, Chang MC, Syu MH.et al. (2005Fusion protein vaccine by domains of bacterial exotoxin linked with a tumor antigen generates potent immunologic responses and antitumor effects Cancer Res 659089–9098. [DOI] [PubMed] [Google Scholar]
- Hung SC, Deng WP, Yang WK, Liu RS, Lee CC, Su TC.et al. (2005Mesenchymal stem cell targeting of microscopic tumors and tumor stroma development monitored by noninvasive in vivo positron emission tomography imaging Clin Cancer Res 117749–7756. [DOI] [PubMed] [Google Scholar]
- Kucerova L, Matuskova M, Pastorakova A, Tyciakova S, Jakubikova J, Bohovic R.et al. (2008Cytosine deaminase expressing human mesenchymal stem cells mediated tumour regression in melanoma bearing mice J Gene Med 101071–1082. [DOI] [PubMed] [Google Scholar]
- Hamada H, Kobune M, Nakamura K, Kawano Y, Kato K, Honmou O.et al. (2005Mesenchymal stem cells (MSC) as therapeutic cytoreagents for gene therapy Cancer Sci 96149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Ishii K, Kobune M.et al. (2005Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model Mol Ther 1196–104. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Ito Y, Kawano Y, Kurozumi K, Kobune M, Tsuda H.et al. (2004Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model Gene Ther 111155–1164. [DOI] [PubMed] [Google Scholar]
- Hung SC, Yang DM, Chang CF, Lin RJ, Wang JS, Low-Tone Ho L.et al. (2004Immortalization without neoplastic transformation of human mesenchymal stem cells by transduction with HPV16 E6/E7 genes Int J Cancer 110313–319. [DOI] [PubMed] [Google Scholar]
- Wang HE, Yu HM, Liu RS, Lin M, Gelovani JG, Hwang JJ.et al. (2006Molecular imaging with 123I-FIAU, 18F-FUdR, 18F-FET, and 18F-FDG for monitoring herpes simplex virus type 1 thymidine kinase and ganciclovir prodrug activation gene therapy of cancer J Nucl Med 471161–1171. [PubMed] [Google Scholar]
- Deng WP, Wu CC, Lee CC, Yang WK, Wang HE, Liu RS.et al. (2006Serial in vivo imaging of the lung metastases model and gene therapy using HSV1-tk and ganciclovir J Nucl Med 47877–884. [PubMed] [Google Scholar]
- Igney FH., and, Krammer PH. Immune escape of tumors: apoptosis resistance and tumor counterattack. J Leukoc Biol. 2002;71:907–920. [PubMed] [Google Scholar]
- Stewart TJ., and, Abrams SI. How tumours escape mass destruction. Oncogene. 2008;27:5894–5903. doi: 10.1038/onc.2008.268. [DOI] [PubMed] [Google Scholar]
- Singh S, Ross SR, Acena M, Rowley DA., and, Schreiber H. Stroma is critical for preventing or permitting immunological destruction of antigenic cancer cells. J Exp Med. 1992;175:139–146. doi: 10.1084/jem.175.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min JJ, Ahn Y, Moon S, Kim YS, Park JE, Kim SM.et al. (2006In vivo bioluminescence imaging of cord blood derived mesenchymal stem cell transplantation into rat myocardium Ann Nucl Med 20165–170. [DOI] [PubMed] [Google Scholar]
- Hou M, Yang KM, Zhang H, Zhu WQ, Duan FJ, Wang H.et al. (2007Transplantation of mesenchymal stem cells from human bone marrow improves damaged heart function in rats Int J Cardiol 115220–228. [DOI] [PubMed] [Google Scholar]
- Grinnemo KH, Månsson A, Dellgren G, Klingberg D, Wardell E, Drvota V.et al. (2004Xenoreactivity and engraftment of human mesenchymal stem cells transplanted into infarcted rat myocardium J Thorac Cardiovasc Surg 1271293–1300. [DOI] [PubMed] [Google Scholar]
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI.et al. (2008Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide Cell Stem Cell 2141–150. [DOI] [PubMed] [Google Scholar]
- Xu G, Zhang L, Ren G, Yuan Z, Zhang Y, Zhao RC.et al. (2007Immunosuppressive properties of cloned bone marrow mesenchymal stem cells Cell Res 17240–248. [DOI] [PubMed] [Google Scholar]
- Hallahan DE, Mauceri HJ, Seung LP, Dunphy EJ, Wayne JD, Hanna NN.et al. (1995Spatial and temporal control of gene therapy using ionizing radiation Nat Med 1786–791. [DOI] [PubMed] [Google Scholar]
- Senzer N, Mani S, Rosemurgy A, Nemunaitis J, Cunningham C, Guha C.et al. (2004TNFerade biologic, an adenovector with a radiation-inducible promoter, carrying the human tumor necrosis factor alpha gene: a phase I study in patients with solid tumors J Clin Oncol 22592–601. [DOI] [PubMed] [Google Scholar]
- Howe JR, Skryabin BV, Belcher SM, Zerillo CA., and, Schmauss C. The responsiveness of a tetracycline-sensitive expression system differs in different cell lines. J Biol Chem. 1995;270:14168–14174. doi: 10.1074/jbc.270.23.14168. [DOI] [PubMed] [Google Scholar]
- Barde I, Zanta-Boussif MA, Paisant S, Leboeuf M, Rameau P, Delenda C.et al. (2006Efficient control of gene expression in the hematopoietic system using a single Tet-on inducible lentiviral vector Mol Ther 13382–390. [DOI] [PubMed] [Google Scholar]
- Boël P, Wildmann C, Sensi ML, Brasseur R, Renauld JC, Coulie P.et al. (1995BAGE: a new gene encoding an antigen recognized on human melanomas by cytolytic T lymphocytes Immunity 2167–175. [DOI] [PubMed] [Google Scholar]
- van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B.et al. (1991A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma Science 2541643–1647. [DOI] [PubMed] [Google Scholar]
- Topalian SL, Gonzales MI, Parkhurst M, Li YF, Southwood S, Sette A.et al. (1996Melanoma-specific CD4+ T cells recognize nonmutated HLA-DR-restricted tyrosinase epitopes J Exp Med 1831965–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manici S, Sturniolo T, Imro MA, Hammer J, Sinigaglia F, Noppen C.et al. (1999Melanoma cells present a MAGE-3 epitope to CD4(+) cytotoxic T cells in association with histocompatibility leukocyte antigen DR11 J Exp Med 189871–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper R, Christian RE, Gonzales MI, Nishimura MI, Gupta G, Settlage RE.et al. (1999Biochemical identification of a mutated human melanoma antigen recognized by CD4(+) T cells J Exp Med 189757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RF, Wang X., and, Rosenberg SA. Identification of a novel major histocompatibility complex class II-restricted tumor antigen resulting from a chromosomal rearrangement recognized by CD4(+) T cells. J Exp Med. 1999;189:1659–1668. doi: 10.1084/jem.189.10.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW.et al. (2007Mesenchymal stem cells within tumour stroma promote breast cancer metastasis Nature 449557–563. [DOI] [PubMed] [Google Scholar]
- Hall B, Andreeff M., and, Marini F. The participation of mesenchymal stem cells in tumor stroma formation and their application as targeted-gene delivery vehicles. Handb Exp Pharmacol: 2007. pp. 263–283. [DOI] [PubMed]
- Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B.et al. (2009Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression PLoS ONE 4e4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K.et al. (2003Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes Nature 425968–973. [DOI] [PubMed] [Google Scholar]
- Vassilopoulos G, Wang PR., and, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422:901–904. doi: 10.1038/nature01539. [DOI] [PubMed] [Google Scholar]
- Johansson CB, Youssef S, Koleckar K, Holbrook C, Doyonnas R, Corbel SY.et al. (2008Extensive fusion of haematopoietic cells with Purkinje neurons in response to chronic inflammation Nat Cell Biol 10575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei SJ, Chao Y, Shih YL, Yang DM, Hung YM., and, Yang WK. Involvement of Fas (CD95/APO-1) and Fas ligand in apoptosis induced by ganciclovir treatment of tumor cells transduced with herpes simplex virus thymidine kinase. Gene Ther. 1999;6:420–431. doi: 10.1038/sj.gt.3300817. [DOI] [PubMed] [Google Scholar]
- Feltkamp MC, Smits HL, Vierboom MP, Minnaar RP, de Jongh BM, Drijfhout JW.et al. (1993Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells Eur J Immunol 232242–2249. [DOI] [PubMed] [Google Scholar]
- Cheng WF, Hung CF, Hsu KF, Chai CY, He L, Ling M.et al. (2001Enhancement of sindbis virus self-replicating RNA vaccine potency by targeting antigen to endosomal/lysosomal compartments Hum Gene Ther 12235–252. [DOI] [PubMed] [Google Scholar]
- Deng WP, Chao MW, Lai WF, Sun C, Chung CY, Wu CC.et al. (2006Correction of malignant behavior of tumor cells by traditional Chinese herb medicine through a restoration of p53 Cancer Lett 233315–327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Survival analysis of mice-bearing pulmonary metastatic tumor with protein vaccine/MSCs combined treatment.
In vitro investigation of the interactions between mesenchymal stem cells and cancer cells.