Abstract
Although immunotherapy with Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes (CTLs) can treat EBV-associated Hodgkin and non-Hodgkin lymphoma (HL/NHL), more than 50% of such tumors are EBV negative. We now describe an approach that allows us to consistently generate, in a single line, CTLs that recognize a wide spectrum of nonviral tumor-associated antigens (TAAs) expressed by human HL/NHL, including Survivin, MAGE-A4, Synovial sarcoma X (SSX2), preferentially expressed antigen in melanoma (PRAME) and NY-ESO-1. We could generate these CTLs from nine of nine healthy donors and five of eight lymphoma patients, irrespective of human leukocyte antigen (HLA) type. We reactivated TAA-directed T cells ex vivo, by stimulation with dendritic cells (DCs) pulsed with overlapping peptide libraries spanning the chosen antigens in the presence of an optimized Th1-polarizing, prosurvival/proliferative and Treg inhibitory cytokine combination. The resultant lines of CD4+ and CD8+, polycytokine-producing T cells are directed against a multiplicity of epitopes expressed on the selected TAAs, with cytolytic activity against autologous tumor cells. Infusion of such multispecific monocultures may extend the benefits of CTL therapy to treatment even of EBV negative HL and NHL.
Introduction
T-cell therapy has the potential to eradicate malignant disease.1,2,3,4 Our group has recently demonstrated that in vitro expanded cytotoxic T lymphocytes (CTLs) targeting the subdominant tumor-associated Epstein-Barr virus (EBV) antigen LMP2 can produce tumor responses in patients with relapsed/refractory EBV-associated Hodgkin (HL) and non-Hodgkin lymphoma (NHL), including in subjects with bulky disease.1 Though effective, this approach is only applicable to the ~40% of HL and NHL which are EBV-associated.5 Moreover, EBV antigen expression can be deleted under immune-mediated pressure as a tumor immune evasion mechanism.6
Nonviral tumor-associated antigens (TAAs) are also potential targets for T-cell therapy, but as self antigens, they are relatively weak stimulators of T-cell immunity, since self-reactive T cells are anergized or tolerized. Hence, efforts to extend T-cell therapy to nonviral tumors has been limited by an inability to consistently activate and expand endogenous tumor-reactive T cells directed against the antigens expressed by malignant cells. Although tumor-specific CTLs have been evaluated clinically, these cells were usually activated using single human leukocyte antigen (HLA) class I-restricted epitopes from single antigens, and were thus restricted by HLA genotype.7,8,9 Moreover the CD8+ T cells were short-lived and may have lacked essential growth and survival signals from CD4+ T-helper cells.7,8,9 Finally, tumor-specific T cells are susceptible to immune evasion strategies employed by the tumor, including downregulation or mutation of the expressed target epitopes/antigens, a common response when only a single epitope/antigen is targeted.6,9,10,11
We now describe a novel strategy to generate CTL lines capable of targeting multiple antigens expressed by lymphomas. We use dendritic cells (DCs) loaded with a mix of peptide libraries spanning the sequence of the TAAs SSX2, MAGEA4, Survivin, PRAME, and NY-ESO-1. By supplementing our cultures with a combination of Th1-polarizing, prosurvival and pro-proliferative cytokines, these single cultures contain tumor-cytotoxic T cells from both CD4+ and CD8+ populations with specificity for a multiplicity of epitopes on several TAAs. We could generate these CTLs from both healthy donors and cancer patients, irrespective of HLA type, supporting the use of such multispecific monocultures of CTLs for the treatment of subjects with EBV negative HL/NHL and related disorders.
Results
Generation of CTL lines with broad spectrum antigen specificity
With a goal of generating T-cell lines containing CD4+ and CD8+ T cells with specificity for multiple epitopes in multiple antigens, we stimulated peripheral blood mononuclear cells (PBMCs) with antigen-loaded DCs in the presence of exogenous cytokines. For initial experiments we loaded DCs with peptide mixtures (pepmixes) of overlapping 15mer peptides spanning three antigens, MAGEA4, SSX2 and Survivin, which are highly expressed by lymphoma cells.12,13,14,15 We supplemented our cultures with different combinations of Th1-promoting (interleukin-12 (IL-12), IL-27, IL-18),16,17,18 prosurvival (IL-15),19,20 proliferation enhancing (IL-7, IL-15, IL-2)21 and Treg inhibitory (IL-6) cytokines.22,23 The cytokines IL-7 (10 ng/ml), IL-12 (10 ng/ml) and IL-15 (5 ng/ml) (basic cytokine cocktail) were included in all conditions, as we have previously shown that this combination was necessary to reactivate tumor-directed peptide-specific T cells in vitro.24,25 In addition, the cultures received IL-6 (100 ng/ml) and/or IL-27 (10 ng/ml) and/or IL-18 (10 ng/ml). We determined the specificity of the resulting T-cell lines on day 23 of culture after two DC stimulations on days 9 and 16 using interferon-γ (IFN-γ) enzyme-linked immunospots (ELIspots) as a readout.
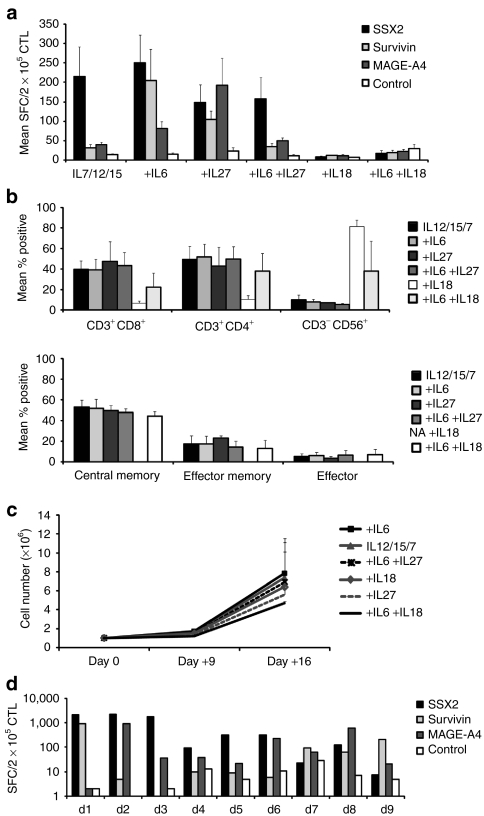
Cultures supplemented with IL-18 alone showed no specific activity for any of the stimulating antigens, while T cells cultured in the presence of the basic cytokine cocktail (IL-7, 12, 15) or with IL-7, 12, 15, 6 + 27 were specific solely for SSX2, with weak/undetectable reactivity against MAGE-A4 or Survivin (n = 5) (Figure 1). In contrast, T-cell lines cultured in the presence of IL-7, 12, 15 + 6 recognized two or all three of the stimulating antigens, with a mean of 249.8 spot-forming cells (SFC)/2 × 105 CTL ± 71.8 SEM, (SSX2), 81.1 SFC/2 × 105, CTL ± 17.7 SEM, (MAGE-A4) and 204.4 SFC/2 × 105 CTL ± 81 SEM (Survivin). Similarly, cells expanded in the presence of IL-7, 12, 15 + 27 also recognized two or three antigens, with specificity for SSX2 (mean 148.8 SFC/2 × 105 CTL ± 44.9 SEM), MAGE-A4 (mean 192.6 SFC/2 × 105 CTL ± 69.1 SEM) and Survivin (mean 105 SFC/2 × 105 CTL, ± 86.7 SEM) (Figure 1a). These two best culture conditions activated both CD3/CD4+ and CD3/CD8+ T cells (mean 48.4 ± 3.8% and 42.3 ± 4%, respectively, n = 5), and contained effector (defined as CD3+/CD45RA+/CD62L-; 4.9 ± 2.7%), central memory (CD3+/CD45RO+/CD62L+; 50.7 ± 6.9%), and effector memory cells (CD3+/CD45RO+/CD62L-; 20.1 ± 5.2%) and a minor population of natural killer cells (mean 7.4 ± 1.8% SEM). There was no evidence of regulatory T-cell outgrowth (as assessed by Foxp3/CD25 co-staining) using these conditions (data not shown). However, supplementation with IL-18 resulted in the outgrowth of natural killer cells (mean 81.5 ± 6.4% SEM) rather than T cells (mean 17.5 ± 7.8% SEM), which was partially reversed when IL-6 was added to the cocktail (Figure 1b). There was no significant difference in the proliferative potential of CTL lines generated in the presence of the different cytokine combinations assessed by cell counting using trypan blue exclusion (median 6.7-fold expansion, range 4.7–7.9) (Figure 1c). In subsequent experiments, we therefore used the optimal cytokine cocktails IL-7, 12, 15, 6 and IL-7, 12, 15, 27. Figure 1d shows that this strategy was robust, since we were successfully able to generate multi-TAA CTL from nine of nine healthy individuals with specificity for at least two of the three stimulating antigens as evaluated by IFN-γ ELIspot, performed after the 2nd or 3rd round of DC stimulation. The data shown for donors 1, 3, 4, 5, 7, 9 represent data from CTL lines initiated in the presence of IL-7, 12, 15, 6 and for donors 2, 6, 8 the lines were initiated using IL-7, 12, 15, 27.
Figure 1.
Generation of multi-TAA-specific CTL. Peripheral blood mononuclear cells were stimulated with autologous dendritic cells loaded with pepmixes spanning SSX2, Survivin, and MAGE-A4 in the presence of IL-7, IL-12, IL-15 + IL-6, + IL-27, + IL-6/ IL-27, + IL-18 or + IL-6/ IL-18. (a) The specificity of multi-TAA CTL lines generated from five donors as measured by IFN-γ release (IFN-γ ELIspot) in response to stimulation with the pepmix antigens SSX2 (black bars), Survivin (gray bars), and MAGE-A4 (dark gray bars) is shown. Results are expressed as spot-forming cells (SFC)/2 × 105, input cells ± SEM. Control was IFN-γ release in response to stimulation with irrelevant pepmix (white bars). (b) Phenotype of the multi-TAA-specific CTLs. (c) CTL proliferation was measured by cell counting, using trypan blue exclusion at days 0, 9 and 16 after stimulation (n = 4 donors). (d) TAA specificity was designated positive if SFC ≥2x of control activity. CTL, cytotoxic T lymphocyte; IFN-γ, interferon-γ IL, interleukin; TAA, tumor-associated antigen.
multi-TAA CTL are polyclonal
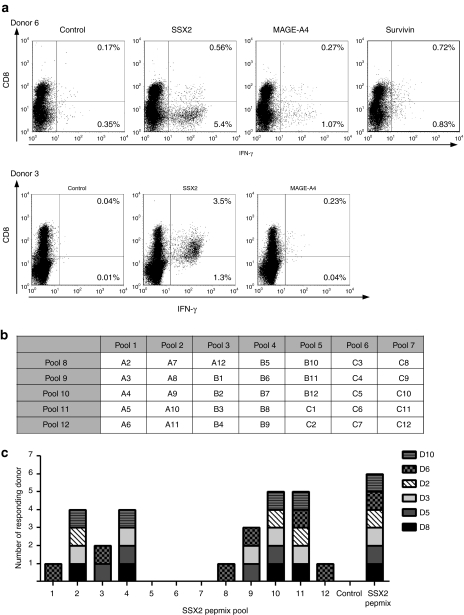
The pepmixes used for stimulation were designed to contain all potential CD8+ epitopes, but the size of each peptide (15 amino acids) and the overlap (11 amino acids) means they may not present some relevant CD4+ epitopes. A lack of CD4+ tumor-specific T cells in the infusion product may compromise its in vivo survival and activity. We therefore assessed the phenotype of responding cells in our cultures following stimulation with individual pepmixes spanning SSX2, MAGEA4, Survivin, or an irrelevant pepmix (control). Figure 2a (upper panel) shows IFN-γ production from both CD4+ (CD8-; bottom right quadrants) (1.07%-MAGE-A4, 5.4%-SSX2, 0.83%-Survivin, 0.35% negative control) and the CD8+ T-cell fractions (27%-MAGE-A4, 0.56%-SSX-2, Survivin-0.72%, 0.17%-negative control; top right quadrants) T cells from Donor 6. Donor 3 (lower panel) had a predominantly CD8-mediated TAA response with 3.5%-SSX2 and 0.23%-MAGE-A4 specific IFN-γ secreting T cells (0.04% negative control) detectable in the CD8+ T-cell fraction, 1.3%-SSX2 and 0.04%-MAGE-A4 specific IFN-γ–secreting T cells were detected in CD8- (CD4+) T cells (0.01%-negative control). Thus, pepmix stimulation can successfully reactivate both CD4+ and CD8+ T cells.
Figure 2.
multi-TAA-specific CTLs are polyclonal. (a) Cytokine production in CD3+CD8+ and CD3+CD8- (CD4+) T cells as detected by intracellular IFN-γ cytokine staining in two representative donors is shown. (b) The SSX2 peptide pool grid is shown. (c) CTL reactivity after stimulation with the single SSX2 peptide pools in six donor CTL lines is demonstrated. Specificity was evaluated by IFN-γ ELIspot and positive responses of each donor to each pool plotted in a bar diagram. Unstimulated CTL (no addition of peptide) was used as negative control, and the SSX2 pepmix was used as a positive control. CTL, cytotoxic T lymphocytes; IFN-γ, interferon-γ TAA, tumor-associated antigen.
To evaluate breadth of TAA reactivity and to detect novel T-cell epitopes, we mapped the SSX2-directed epitope specificities of six CTL lines generated from three HLA A2+ and three HLA A2- donors. For this screening process, CTL lines were stimulated with peptide pools (designated A2–A12, B1–B12, C1–C12) comprising thirty five 20-mer peptides overlapping by 15aa. We arranged these peptides in 12 pools so that each 20-mer peptide was represented in two pools (Figure 2b).26 Using the SSX2 pepmix as a stimulus, we confirmed antigen-specificity in all six CTL lines. The peptide pools recognized by each line are shown in Figure 2c. The most frequently recognized pools were 10 and 11, but all pools except 5, 6, and 7, were recognized by at least one donor. More than one epitope peptide was recognized by five out of six CTL lines, since we identified specificity directed against two or more distinct peptide regions mapping to overlapping 25mer peptides. The donor HLA types and peptide sequences recognized by the different lines are shown in Table 1. We confirmed the identity of several previously published CD4+ and CD8+ epitopes and their HLA restriction elements. For example, Figure 2a upper panel shows a major SSX2-directed response to be CD4-restricted in Donor 6 and to map to four distinct peptide regions, two of which contain previously published CD4+ T-cell epitope peptides (Table 1). In addition, we identified at least five novel responses. These include reactivity to the 25mer NDP peptide (aa86-110) recognized by both Donor 8 and Donor 10 (who share HLA-B44) and the 25mer peptide GND (aa81-105), recognized by Donors 5 and 3 (who share HLA DR1) (Table 1).
Table 1. Peptide specificity of CTL lines of HLA-A2 and non HLA-A2 donors.

multi-TAA CTL are polyfunctional
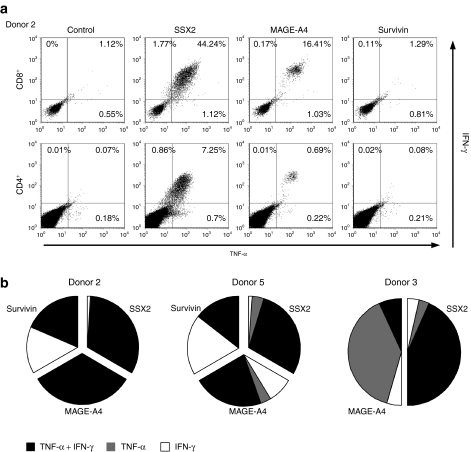
It has been suggested that production of multiple proinflammatory cytokines correlates with cytolytic function and is important for in vivo activity.27,28 Therefore, we assessed whether the reactivated CTL were able to produce more than one cytokine in response to antigenic stimulation. We found that the majority of IFN-γ producing cells also produced tumor necrosis factor-α (TNF-α) in both the CD8+ and CD4+ populations as demonstrated by intracellular staining in Figure 3a (donor 2). In this donor, of 47.13% of the CD3/CD8+ T cells that were SSX2-specific (upper left + upper right + lower right quadrants), 44.24% produced both IFN-γ and TNF-α. Similarly, of 8.81% of this donor's CD3/CD4+ T cells specific for the same antigen, 7.25% (upper right quadrant) produced both IFN-γ and TNF-α. The majority of the CD3+ CD8+ cells reactive to MAGEA4 also produced both cytokines. The results from three donors are summarized in Figure 3b. Each chart is subdivided to represent the different antigen specificities within the line, and each sub-section is further divided to show the proportion of T cells that are single cytokine producing, IFN-γ (shown in white) and TNF-α (shown in gray) or double cytokine producing (shown in black). Overall, most T cells reactive against SSX2 and MAGEA4 are polyfunctional, while reactivity against Survivin is mediated equally by single (IFN-γ secreting) and double cytokine producing T cells.
Figure 3.
multi-TAA CTLs produce multiple cytokines. (a) The production of the Th1 cytokines IFN-γ and TNF-α in CD3+ CD8+ or CD3+ and CD4+ (CD8-) cells in one representative donor (Donor 2) is shown. Cells were stimulated using the tumor antigen-specific pepmixes SSX-2, MAGE-A4, and Survivin. (b) Summary results from three donors showing the fractions of antigen-specific IFN-γ+, TNF α+ double cytokine (black) and IFN-γ+ (white) or TNF α+ (gray) single cytokine producing T cells within the cultures is shown. CTL, cytotoxic T lymphocytes; IFN-γ, interferon-γ TAA, tumor-associated antigen; TNF-α, tumor necrosis factor-α.
multi-TAA T-cell lines are cytolytic in vitro
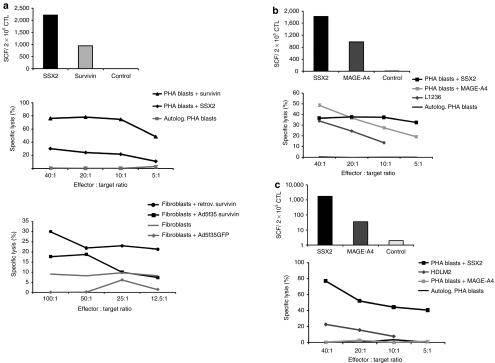
To determine whether the polyclonal, cytokine producing multi-TAA CTL were also cytolytic, we incubated donor 1 T cells with specificity for SSX2 (2211 SFC/2 × 105 CTL) and Survivin (945 SFC/2 × 105 CTL) as evaluated by IFN-γ ELIspot, with autologous phytohemagglutinin (PHA) blasts pulsed with an irrelevant pepmix (negative control) or with the SSX2 and Survivin pepmixes, and measured specific Chromium (Cr51) release after 4 hours of incubation. At an effector:target (E:T) ratio of 40:1, CTLs killed both SSX2- and Survivin-pulsed target cells (76% and 30% Cr51 release, respectively), with minimal recognition of control target cells (1% release). To determine whether these CTL could also kill target cells expressing naturally processed target peptides, we used autologous fibroblasts transduced either with a retroviral or an adenoviral vector (Ad5f35) encoding Survivin. At an E:T of 100:1 we observed specific lysis of Survivin-expressing fibroblasts (30% and 18%, retroviral- and adenoviral-transduced, respectively) with no recognition of control mock-transduced fibroblasts (Figure 4a). These results were confirmed in two additional CTL lines (Figure 4b,c). Figure 4b shows a CTL line with specificity for MAGE-A4 (982 SFC/2 × 105 CTL) and SSX2 (1821 SFC/2 × 105 CTL) that efficiently lysed autologous PHA blasts pulsed with pepmixes spanning the same antigens (49% MAGEA4, 36%-SSX2, E:T 40:1) as well as naturally processed peptides on the Hodgkin line L1236 cell line matched with the CTLs at HLA-A2 (34% specific lysis at an E:T ratio 40:1). Finally, Figure 4c shows that a CTL line with specificity for both SSX2 (1762 SFC/2 × 105 CTL) and MAGE-A4 (36 SFC/2 × 205 CTL) was able to kill autologous PHA blasts pulsed with SSX2 (77%, E:T ratio 40:1). This line failed to kill MAGEA4-pulsed PHA blasts, likely because the frequency of these cells in the culture was too low to produce specific lysis in the less sensitive Cr51 release assay. Nevertheless, this CTL line killed the HDLM2 HL cell line which expresses both SSX2 and MAGEA4 and is HLA-A1 and -B8 matched with the CTLs. Tumor antigen expression in the partially HLA-matched HL cell lines L1234 and HDLM2 was confirmed by immunohistochemistry (Supplementary Figure S1) as well as quantitative reverse transcriptase PCR (data not shown). multi-TAA CTL lines were devoid of alloreactivity assessed using HLA-mismatched PHA blasts as targets in a 4-hour chromium release assay (data not shown).
Figure 4.
multi-TAA CTLs are cytotoxic in vitro. (a) To determine whether the multi-TAA CTL could also lyse tumor antigen-expressing target cells we tested the ability of the line generated from donor1 (upper panel) to kill autologous pepmix-loaded PHA blasts in a standard 4-hour Cr51 release assay. PHA blasts pulsed with pepmixes spanning an irrelevant antigen served as a control. E:T ratios of 40:1, 20:1, 10:1 to 5:1 were tested (lower panel). The lower right panel shows the cytolytic activity of this line against autologous fibroblast targets, endogenously transgenically expressing Survivin following retroviral or adenoviral vector (Ad5f35) transduction. Fibroblasts alone, or mock-transduced fibroblasts were used as negative controls (E:T—100:1, 50:1, 25:1 and 12.5:1). (b,c) show the cytolytic ability of two additional multi-TAA CTL lines, whose specificity was first assessed by interferon-γ (IFN-γ) ELIspot (upper panels). These lines were able to kill pepmix-loaded autologous PHA blasts as well as partially HLA-matched TAA expressing lymphoma cell lines (L1236 donor 2, HDLM2 donor 3). Specific lysis was evaluated by standard Cr51 release assay—E:T ratio of 40:1, 20:1, 10:1 and 5:1. CTL, cytotoxic T lymphocyte; TAA, tumor-associated antigen.
Generation of multi-TAA-specific CTLs from lymphoma patients
Since the clinical use of TAA-specific T cells for the treatment of lymphoma relies on the generation of CTL from autologous T cells, we next determined whether our CTL generation protocol would be effective using material from patients with lymphoma who have been treated with lymphocyte-directed chemotherapy and irradiation. We collected blood from eight patients with relapsed HL or NHL and reactivated CTL against SSX2, MAGEA4, and Survivin (n = 5) or SSX2, MAGEA4, Survivin, PRAME, and NY-ESO1 (n = 3); we added two additional antigens for the latter group since both are expressed in the lymphoma cell lines HDLM2, L1236 (Supplementary Figure S1) and are also frequently detected in primary tumor cells (Supplementary Figure S2).15,29,30 Of the five patient-derived CTL lines stimulated with SSX2, Survivin and MAGEA4, three (LP069, LP092, and LP096) showed specificity against at least two of the stimulating antigens. Of the three patient lines generated using SSX2, MAGEA4, Survivin, PRAME, and NY-ESO-1 pepmixes as the stimulant, all T-cell lines showed specificity for three or more antigens (Figure 5a). Thus we observed reactivity against SSX2 in 4/8 lines (median 90 SFC/2 × 105, range 66–492), MAGEA4 in 6/8 lines (median 52 SFC/2 × 105, range 8–1048), Survivin in 2/8 lines (median, 119 SFC/2 × 105, range 14–223), PRAME in 3/3 lines (median 663 SFC/2 × 105, range 624–1668), and NY-ESO-1 in 3/3 lines (median 42 SFC/2 × 105, range 16–56).
Figure 5.
multi-TAA CTL can be generated from lymphoma patients. (a) Peripheral blood mononuclear cells of patients LP058, LP066, LP069, LP092, and LP096 were stimulated with the TAA SSX2, Survivin and MAGE-A4. For patients LP071, LP090 and LP178 the stimulating antigen panel was extended to include PRAME and NY-ESO-1. (b) Intracellular cytokine staining to detect IFN-γ and TNF-α in two representative patient lines is shown. Cells were gated on CD3+ CD8+ or CD3+ CD4+ (CD8-). The cytolytic activity of the CTL line generated from lymphoma patient LP071 was tested in a 4-hour Cr51 release assay using partially matched lymphoma cell lines (HDLM2, L1236) as targets at E:T ratios ranging from 80:1 to 10:1. (c) The HLA-mismatched lymphoma cell line Raji, was used as negative control at a E:T ratio of 40:1. (d) TAA specificity (IFN-γ ELIspot assay) (upper panel) and cytolytic function (lower panel), as measured by Cr51 release, against autologous PHA blasts pulsed with relevant (SSX2, MAGE-A4) and irrelevant pepmixes (negative control) at E:T ratios from 40:1 to 5:1 is demonstrated. CTL, cytotoxic T lymphocyte; IFN-γ, interferon-γ TAA, tumor-associated antigen; TNF-α, tumor necrosis factor-α.
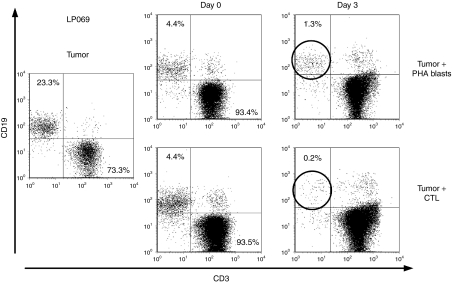
Lymphoma patients are often lymphopenic and have immunological defects, further it is likely that TAA-specific T cells from lymphoma patients are tolerized by their tumors. Therefore to confirm polyfunctionality of TAA-specific T cells from our patient-derived multi-TAA CTL we measured secretion of IFN-γ and TNF-α from the CD4+ and CD8+ T-cell compartments. Figure 5b, upper panel shows for patient LP178 that specificity for PRAME and Survivin was predominantly detected in CD4+ T cells, and, as for healthy donors, most T cells were either dual or TNF-α single cytokine producing. Similar results were observed for PRAME, SSX2 and NY-ESO-1 in donor LP071 (Figure 5b, lower panel). This CTL line was also capable of killing partially HLA-matched TAA-expressing cell lines HDLM2 and L1236 in a Cr51 release assay (33% and 29% specific lysis of HDLM2 and L1236 at an E:T of 80:1) with no nonspecific lysis of an HLA-mismatched lymphoma cell line (Raji) (Figure 5c). Similar cytokine secretion and cytolytic activity was observed in another patient-derived CTL line, LP069, which was specific for MAGE-A4 (1000 SFC/2 × 105 CTL) and SSX2 (909 SFC/2 × 105 CTL) (Figure 5d, upper panel), and lysed autologous PHA blasts pulsed with these antigens in a 4-hour Cr51 release assay (53%-MAGE-A4 and 10%-SSX2, E:T 40:1) with no recognition of control targets (PHA blasts alone). Finally, we cocultured multi-TAA CTLs (LP069) at a 1:1 ratio with autologous lymphoma cells, consisting of 23.3% CD19+ malignant lymphoblasts and 73.3% CD3+ T cells. In control cultures, we mixed tumor cells with activated autologous nonspecific T cells (PHA blasts) at the same ratio (Figure 6). The ratio of T cells (CD3+) to tumor cells (CD19+) was assessed on days 0 and 3 of culture by FACS analysis. By day 3, CD19+ tumor cells had been almost completely eliminated (0.2% remaining-lower right) by the multi-TAA CTL, but in the control condition (upper right) residual tumor cells persisted (1.3%) confirming that these in vitro generated multi-TAA CTL should be of sufficient avidity with adequate cytolytic function to kill malignant cells in vivo postadoptive transfer (Figure 6).
Figure 6.
multi-TAA kill autologous tumor cells. To assess the ability of patient-derived multi-TAA CTL to kill autologous tumor we cocultured the CTL with autologous CD19+ tumor cells at a CTL:tumor ratio of 1:1 for 3 days. Tumor cells cultured with autologous non-antigen specific PHA blasts were used as a negative control. The presence of CD19+ tumor cells was assessed by FACS analysis on day 0 and on day 3 of coculture. CTL, cytotoxic T lymphocyte; TAA, tumor-associated antigen.
Discussion
We have shown that we can generate polyclonal, Th1-polarized CD4+ and CD8+ CTLs with specificities toward a multiplicity of lymphoma-associated antigens by loading professional antigen presenting cells with a combination of peptide libraries. Our approach generates high-affinity CTLs that produce Th1 effector cytokines upon stimulation from both healthy donors and cancer patients, regardless of HLA genotype. These CTLs are cytotoxic to HLA-matched antigen-expressing cell lines and to autologous tumor cells.
The therapeutic efficacy of CTLs for lymphoma, as for other tumors, is dependent on the nature of the antigen(s) targeted. The ideal target antigen should be immunogenic to T cells, uniquely or highly expressed by tumor cells compared to normal tissues and directly involved in maintaining the oncogenic tumor phenotype, thereby limiting the emergence of tumor escape mutants. In the current study, we focused on preparing T cells specific for the cancer testis antigens SSX2, MAGEA4, PRAME, and NY-ESO-1, and for the TAA Survivin. Cheever et al. recently identified Survivin, NY-ESO-1 and SSX2 as high priority targets for cancer therapy based upon criteria including tumor specificity, oncogenicity, expression level, and number of identified epitopes.31 Survivin is a member of the inhibitor of apoptosis protein family that is expressed during fetal development but present only at low levels in normal adult tissues.32 It is, however, abundantly expressed in various tumor tissues and cell lines and as such is being widely investigated as a target in both T-cell and vaccine studies.32,33,34 The cancer testis antigens, SSX2, MAGEA4, PRAME, and NY-ESO-1 are heterogeneously expressed in multiple tumors but are absent in healthy organs, with the exception of MHC class I-deficient germ line tissue.35 The expression of our chosen antigens was heterogeneous on lymphoma, both at the intra- and inter-tumor levels13,14,15,29,30 and Supplementary Figure S2. However, almost all cells of all lymphomas express at least one of these target antigens so that an approach, that produces—in a single culture—T cells capable of targeting several different antigens, may have superior clinical efficacy than targeting a single antigen.
Some TAAs are expressed, albeit at low levels, in normal tissues as well, an observation that raises potential safety concerns. Indeed, previous studies by Warren and colleagues reported pulmonary toxicity when minor histocompatibility antigen-directed CD8+ T-cell clones were adoptively-transferred to patients with relapsed acute leukemia after myeloablative allogeneic stem cell transplant. This toxicity correlated with the level of expression of the mHAg-encoding genes in lung tissue. Fortunately these adverse events were cell dose dependent and rapidly and effectively controlled with steroids.7 Four of our five target antigens (NY-ESO-1, SSX2, PRAME, and MAGEA4) have not been detected on normal tissue, and CTLs directed to these antigens are therefore not expected to induce “off target” toxicities. For example, we have recently shown that PRAME peptide-reactive T cells significantly reduced progenitor colony formation from chronic myelogenous leukemia samples without affecting normal hematopoietic precursor cells, thus attesting to the safety of this approach.36 Survivin is, however, expressed by some normal cells, albeit at lower levels of expression than tumors.32 Fortunately, Survivin-directed CTLs—induced using peptides, peptide-loaded DCs, mRNA vaccines or oncolytic viruses—have revealed no major systemic toxicities.33 Of note, however, Leisegang et al.37 recently explored Survivin as a tumor target, using adoptively-transferred transgenic lymphocytes expressing high avidity, HLA-A2 restricted, Survivin-specific T-cell receptors (Tg-TCRs). These authors found that the CTLs expressing these survivin-specific TCR killed one another due to recognition of endogenous Survivin expressed in the activated lymphocytes. We did not replicate these results with our Survivin-peptide induced CTLs from healthy donors (D1, D6) and lymphoma patients (LP178) (Figures 1d, 4a, and 5b). Thus, we were able to expand these cultures for up to 7 weeks, without observing fratricide of Survivin or other antigen-specific T cells. These conflicting findings likely reflect the starting material; in our study we reactivated circulating, autologous Survivin-specific T cells while Leisegang et al. cloned the TCR from allogenic high-affinity clones.
The preparation of TAA-directed CTL with potent antitumor effects requires both the reactivation of T cells with broad and high avidity specificities for TAA and the expansion of these cells in vitro without loss of specificity or function. Meeting these requirements has been problematic since T cells directed against “self” TAAs are present in small numbers in peripheral blood, are often anergized/tolerized, and have poor proliferative capabilities.20 We have previously shown that effective induction of antitumor immunity directed against single epitope peptides requires not only optimal antigen presentation by professional antigen presenting cells such as DCs, but also the presence of immune modulating and growth promoting cytokines IL-12, IL-7, and IL-15 at the priming stage.24,25,36 These cytokines enhance the induction of TH1/Tc1 antitumor immunity and restore the function of T cells that have been anergized or tolerized.24,25,36 Unfortunately, this cytokine cocktail was insufficient for reactivation of CTL with multi-antigen specificity (Figure 1a) and instead additional supplementation with either IL-6 or IL-27 was required. The proinflammatory cytokine IL-6, which signals through Stat3, allows escape of T memory/effector cells from regulatory T cell-mediated suppression,22 while recent reports have emphasized the importance of combining IL-12 and IL-27 (an IL-12 cytokine family member) for suppression of inhibitory cytokines from polarized Th2 cells and induction of TAA-specific CD4+ Th1-polarized effector cells.38 Thus both cytokines likely help repolarize circulating tumor-reactive T cells and break tolerance. The cytokine combinations used for this purpose and the sequence of their introduction needs careful analysis, since individually effective cytokines (e.g., IL-6 + IL-27 or IL-6 + IL-18) may produce antagonistic or even paradoxical effects when combined (Figure 1a). Although we describe the generation of CTLs directed to lymphoma-associated TAAs, we also found that the cytokine combinations we described also enabled the successful reactivation of T cells targeting TAAs commonly expressed in leukemia (WT1, PRAME, PR3, Survivin) and hepatocellular carcinoma (MAGE1, MAGE3, AFP) (data not shown), demonstrating that our approach can be generalized.
To achieve our objective of generating multi-TAA CTL from any individual with any HLA phenotype we stimulated T cells with DCs loaded with a mix of pepmixes spanning our antigens of interest. The use of peptides derived from the full-length of the antigen is essential for this process. First, this allows natural selection of the highest affinity CTL available, rather than forced stimulation of T cells with a predetermined peptide.36,39 Second, since multiple epitopes are presented, T cells that recognize multiple target sequences can be stimulated, thus minimizing the likelihood of tumor immune escape due to emergence of epitope loss variants. Third, both HLA class I and class II binding epitopes can be presented, increasing the probability that both CD4+ and CD8+ T cells will be generated.39,40 This in turn should favor the subsequent persistence and survival of the transferred cells in vivo. In the current study, we used peptide libraries (15mer peptides overlapping by 11 amino acids) as a stimulus, and though these peptides contain all CD8+ but possibly not all CD4+ epitopes and require trimming by endogenous exo- and endopeptidases prior to loading on HLA class I molecules, we were nevertheless successfully able to reactivate a significant tumor-directed helper T-cell component as well as CD8+ CTLs in the majority of lines generated.
Although the TAA-specific T cells we described have direct cytotoxic activity against human lymphoma cells, simple infusion of these CTLs may be insufficient to produce optimal antitumor activity. Recently, however, immune-modulating drugs such as demethylating agents have been shown to upregulate expression of TAAs on lymphoma and other tumor cells, thereby increasing their susceptibility to killing by tumor-directed T cells.41 Moreover, engraftment of transferred T cells can be facilitated by prior immunodepletion with cyclophosphamide or total body irradiation, since this favors subsequent homeostatic T-cell expansion postinfusion. Hence, the combination of small molecule therapeutics with the TAA-specific CTLs we have described may be of considerable value for the treatment of HL and NHL.
Materials and Methods
Donors and cell lines. PBMCs and tumor material was obtained from healthy volunteers and lymphoma patients with informed consent on Baylor College of Medicine Institutional Review Board-approved protocols. PBMCs were used to generate DCs, CTL lines, and PHA blasts.
PHA blasts were generated from PBMC (2 × 106/ml) using PHA (5 µg/ml) for initial stimulation. PHA blasts where maintained in CTL Media [RPMI 1640 (Gibco-BRL, San Francisco, CA) supplemented with 45% Click's medium (Irvine Scientific, Santa Ana, CA), 2 mmol/l GlutaMAX TM-I (Invitrogen, Carlsbad, CA), and 5% Human AB Serum (Valley Biomedical, Winchester, VA)] and supplemented with IL-2 (100 U/ml) (R&D Systems, Minneapolis, MN), which was replenished every 3 days.
The following cell lines were used: Raji (CD19+ Burkitt lymphoma cell line), HDLM-2 (CD19-negative HL cell line) and L1236 (CD19-negative HL cell line). All cells were purchased from American Type Culture Collection and maintained in culture in RPMI-1640 supplemented and 10% fetal bovine serum (FBS) (Hyclone, Waltham, MA) and 2 mmol/l GlutaMAX TM-I. Cell line identity and HLA types were confirmed by SSP-PCR within 6 months of performing experiments.
Monocyte isolation and DC generation. DCs were generated as previously described.42 Briefly, monocytes were isolated from fresh or frozen PBMCs by CD14 selection using MACS Beads (Miltenyi, Bergisch Gladbach, Germany) and cultured in DC media (CellGenix supplemented with 2 mmol/l GlutaMAX TM-I) (CellGenix USA, Antioch, IL) with 800 U/ml GM-CSF (Sargramostim Leukine; Immunex, Seattle, WA) and 1,000 U/ml IL-4 (R&D Systems) for 5 days. IL-4 and GM-CSF were replenished on day 3. On day 5, DCs were matured in DC media using a cytokine cocktail containing 100 ng/ml IL-6, 10 ng/ml IL-1β, 10 ng/ml TNF-α (R&D Systems), 1 µg/ml PGE2 (Sigma, St Louis, MO), 800 U/ml GM-CSF and 1,000 U/ml IL-4 for 48 hours.
Loading DCs with pepmixes. Mature DCs were pelleted and pulsed with a cocktail of pepmixes spanning MAGE-A4, SSX-2 and Survivin, or MAGE-A4, SSX-2, Survivin, PRAME and NY-ESO for 60 minutes at 37 °C, 5% CO2 (100 ng/pepmix was used for DC loading). All pepmixes, which are overlapping peptide libraries (15mers overlapping by 11 amino acids) spanning the entire protein sequence of the antigens of interest were purchased from JPT Technologies (Berlin, Germany). After incubation DCs were resuspended at a concentration of 2 × 105/ml in CTL Media.
CTL generation. CD14 negative PBMCs were used as responder cells and stimulated with pepmix-pulsed DCs at a stimulator:responder (S:R) ratio of 1:10. Cells were cultured in CTL media supplemented with 10 ng/ml IL-7, 10 ng/ml IL-12, 5 ng/ml IL-15 and 100 ng/ml IL-6 and/or 10 ng/ml IL-27 and/or 10 ng/ml IL-18 (R&D Systems). On day 10 CTL were harvested, counted to assess viability using trypan blue exclusion, and restimulated with pepmix-pulsed DCs at a S:R ratio of 1:10. Cells were cultured in CTL media for a further 7 days with IL-7 (10 ng/ml) at the day of restimulation and consecutively fed with IL-2 (50 U/ml, Proleukin; Chiron, Emeryville, CA) or IL-15 (5 ng/ml) twice weekly from day 14. On day 17 after initial stimulation. cells were harvested, counted and their phenotype, specificity and functional capacity analyzed. To further expand the cells CTL were restimulated weekly at a S:R ratio of 1:10 with pepmix-pulsed DCs in CTL media, supplemented with IL-2 (50 U/ml) or IL-15 (5 ng/ml) twice weekly.
Flow cytometry
Immunophenotyping: CTLs were surface-stained with monoclonal antibodies to: CD3, CD4, CD8, CD14, CD16, CD56, CD19, CD45RO, and CD62L (Becton Dickinson BD, Franklin Lakes, NJ). Cells were washed once with phosphate-buffered saline (PBS) (Sigma) containing 2% FBS (HyClone; Thermo Fisher Scientific, Hudson, NH), pelleted, and antibodies added in saturating amounts (10 µl). After 15 minutes incubation at 4 °C in the dark, cells were washed twice and analyzed. Approximately 20,000 live cells from each population were analyzed. Samples were acquired on a FACSCalibur flow cytometer and the data analyzed using Cell Quest software (BD).
Intracellular cytokine staining: After 2–4 rounds of DCs stimulations CTLs were harvested, resuspended at a concentration of 5 × 106/ml in CTL media and plated at 200 µl/well in a 96-well plate. The cells were then stimulated with 100 ng of the equivalent tumor antigen-spanning pepmix or control irrelevant pepmix in the presence of Brefeldin A (1 µg/ml) (BD) for 5–7 hours. Afterwards, CTLs were washed once with PBS containing 2% FBS, pelleted, and surface stained with CD4, CD8, and/or CD3, (BD) (10 µl/antibody/tube) antibodies. After 15 minutes incubation at 4 °C in the dark, the cells were washed twice, pelleted, fixed and permeabilized with Cytofix/Cytoperm solution (BD) for 20 minutes at 4 °C in the dark. After washing twice with PBS/2%FBS containing 0.1% saponin (Calbiochem; EMD Chemicals, Gibbstown, NJ) cells were incubated with 20 µl IFN-γ and TNF-α antibodies (BD) for 30 minutes at 4 °C in the dark. Cells were then washed twice with cold PBS/2%FBS containing 0.1% saponin and at least 200,000 live cells from each population were analyzed with a FACSCalibur equipped with Cell Quest software (BD).
ELIspot assay. We used ELIspot analysis to quantify mono and multi-TAA-specific T cells. The populations were serially diluted from 2 × 105 to 2.5 × 104, cells/well, and tumor-specific activity measured after direct stimulation with pepmixes spanning MAGE-A4, SSX2, Survivin, PRAME, and NY-ESO. Each culture condition was run in triplicate. After 20 hours of incubation, plates were developed, dried overnight at room temperature in the dark, then sent to Zellnet Consulting, New York, NY for quantification. SFC and input cell numbers were plotted, and a linear regression calculated after excluding plateau data points. The frequency of T cells specific to each antigen was expressed as specific SFC per input cell numbers.
SSX2 overlapping peptide library. A peptide library consisting of thirty five 20-mer peptides with 15-aa overlap covering the complete sequence of SSX-2 was purchased from Alta Bioscience, Birmingham, UK. Lyophilized peptides were reconstituted at 5 mg/ml in DMSO. As described previously,26 these peptides were pooled into a total of 12 pools in such a manner that each 20-mer peptide was represented in 2 pools, according to the grid shown in Figure 2b.
Cytotoxicity assay. We measured the cytotoxic specificity of each T-cell population in a standard 4–6-hour Cr51 release assay, using E:T ratios of 0:1 to 5:1. Generated CTLs were used as effectors. The targets were lymphoma cell lines, fibroblasts or PHA blasts. Fibroblasts were either retrovirally transduced to express Survivin or transduced with an adenoviral vector expressing Survivin or green fluorescent protein as a control. PHA blasts were pulsed with pepmixes spanning MAGE-A4, SSX2 and/or survivin. PHA blasts alone or loaded with an irrelevant pepmix were used as controls. The target cells were labeled simultaneously for 1 hour with Cr51. The percentage of specific lysis was calculated as specific lysis [(experimental release - spontaneous release)/(maximum release - spontaneous release)] × 100.
Coculture experiments. Prior to coculture, tumor material was characterized phenotypically using FACS analysis (CD19/CD20) and by immunhistochemistry to assess tumor antigen expression. multi-TAA CTL were mixed with tumor cells at a tumor:T cell ratio of 1:1 in CTL medium supplemented with 5 ng IL-15 and as a control, PHA blasts from the same donor were incubated with tumor at the same ratio. After 3 days of coculture, cells were collected, counted and stained with monoclonal antibodies to detect both T lymphocytes (CD3) and tumor cells (CD19/CD20), and then analyzed by flow cytometry (FACScan; BD).
Immunohistochemistry. Cell lines and primary HL samples were resuspended in PBS, mounted onto glass slides (Shandon Cytospin 4 Cytocentrifuge; Thermo Scientific, Waltham, MA) and then placed in the steamer for 10 min in Target retrieval solution (DAKO). Slides were incubated in preblock/diluent for 30 min followed by incubation with mouse anti-human MAGE-A4, PRAME, Survivin or NY-ESO-1 antibody (all AbCam, Cambridge, MA) and SSX2 (ORIGENE, Rockville, MD), all diluted 1:50 in diluant for 1 h at room temperature. Anti-mouse horseradish peroxidase/biotin was used to detect positive cells, followed by enzymatic conversion using the chromogenic substrate 3,3 diaminobenzidine.
Viral vectors. Ad5f35-EGFP was purchased from the vector development laboratory of the Center for Cell and Gene Therapy at Baylor College of Medicine, Houston, TX. Ad5f35-Survivin was generated by subcloning the Survivin cDNA (InvivoGen, San Diego, CA) into pShuttle (Clontech, Moutain View, CA). From pShuttle, the Survivin expression cassette, containing the CMV promoter, Survivin and the BGH poly A, was cloned into the E1/E3 deleted adenoviral backbone vector pAd5F35 using pI-Sce I and I-Ceu I sites.43 The resultant plasmids were sequenced to confirm the sequence (SEQwright, Houston, TX). Recombinant adenoviruses were generated as described in the literature.43 Plaques positive for Survivin expression by western blot were expanded, purified, and titered by standard procedures. A retrovirus encoding Survivin was constructed by subcloning the Survivin cDNA (InvivoGen) into pMSCV-IRES-GFP (gift from Elio Vanin, Nothwestern University, Chicago, IL). RD114 pseudotyped retroviral particles were generated as previously described.44
SUPPLEMENTARY MATERIAL Figure S1. SSX2, Survivin, MAGE-A4, PRAME and NY-ESO-1 expression in lymphoma cell lines. Figure S2. SSX2, Survivin, MAGE-A4, PRAME and NY-ESO-1 expression in primary tumor cells.
Acknowledgments
We thank Tara Gray for sample collection. This work was supported in by an NIH-NCI P50 CA126752 SPORE in lymphoma. U.G. is supported by the Leukemia and Lymphoma Society. H.E.H. is supported by a Dan L Duncan chair and M.K.B. by a Fayez Sarofim chair. The authors declare no competing financial interests.
Supplementary Material
SSX2, Survivin, MAGE-A4, PRAME and NY-ESO-1 expression in lymphoma cell lines.
SSX2, Survivin, MAGE-A4, PRAME and NY-ESO-1 expression in primary tumor cells.
REFERENCES
- Bollard CM, Gottschalk S, Leen AM, Weiss H, Straathof KC, Carrum G.et al. (2007Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer Blood 1102838–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA.et al. (2010Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients Blood 115925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Spiess P., and, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233:1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- Straathof KC, Bollard CM, Popat U, Huls MH, Lopez T, Morriss MC.et al. (2005Treatment of nasopharyngeal carcinoma with Epstein-Barr virus–specific T lymphocytes Blood 1051898–1904. [DOI] [PubMed] [Google Scholar]
- Gulley ML, Eagan PA, Quintanilla-Martinez L, Picado AL, Smir BN, Childs C.et al. (1994Epstein-Barr virus DNA is abundant and monoclonal in the Reed-Sternberg cells of Hodgkin's disease: association with mixed cellularity subtype and Hispanic American ethnicity Blood 831595–1602. [PubMed] [Google Scholar]
- Gottschalk S, Ng CY, Perez M, Smith CA, Sample C, Brenner MK.et al. (2001An Epstein-Barr virus deletion mutant associated with fatal lymphoproliferative disease unresponsive to therapy with virus-specific CTLs Blood 97835–843. [DOI] [PubMed] [Google Scholar]
- Warren EH, Fujii N, Akatsuka Y, Chaney CN, Mito JK, Loeb KR.et al. (2010Therapy of relapsed leukemia after allogeneic hematopoietic cell transplantation with T cells specific for minor histocompatibility antigens Blood 1153869–3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley ME, Wunderlich J, Nishimura MI, Yu D, Yang JC, Topalian SL.et al. (2001Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma J Immunother 24363–373. [DOI] [PubMed] [Google Scholar]
- Lozupone F, Rivoltini L, Luciani F, Venditti M, Lugini L, Cova A.et al. (2003Adoptive transfer of an anti-MART-1(27-35)-specific CD8+ T cell clone leads to immunoselection of human melanoma antigen-loss variants in SCID mice Eur J Immunol 33556–566. [DOI] [PubMed] [Google Scholar]
- Khong HT, Wang QJ., and, Rosenberg SA. Identification of multiple antigens recognized by tumor-infiltrating lymphocytes from a single patient: tumor escape by antigen loss and loss of MHC expression. J Immunother. 2004;27:184–190. doi: 10.1097/00002371-200405000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamshchikov GV, Mullins DW, Chang CC, Ogino T, Thompson L, Presley J.et al. (2005Sequential immune escape and shifting of T cell responses in a long-term survivor of melanoma J Immunol 1746863–6871. [DOI] [PubMed] [Google Scholar]
- Adida C, Haioun C, Gaulard P, Lepage E, Morel P, Briere J.et al. (2000Prognostic significance of survivin expression in diffuse large B-cell lymphomas Blood 961921–1925. [PubMed] [Google Scholar]
- Chambost H, Van Baren N, Brasseur F, Godelaine D, Xerri L, Landi SJ.et al. (2000Expression of gene MAGE-A4 in Reed-Sternberg cells Blood 953530–3533. [PubMed] [Google Scholar]
- Colleoni GW, Capodieci P, Tickoo S, Cossman J, Filippa DA., and, Ladanyi M. Expression of SSX genes in the neoplastic cells of Hodgkin's lymphoma. Hum Pathol. 2002;33:496–502. doi: 10.1053/hupa.2002.124909. [DOI] [PubMed] [Google Scholar]
- Shafer JA, Cruz CR, Leen AM, Ku S, Lu A, Rousseau A.et al. (2010Antigen-specific cytotoxic T lymphocytes can target chemoresistant side-population tumor cells in Hodgkin lymphoma Leuk Lymphoma 51870–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owaki T, Asakawa M, Morishima N, Hata K, Fukai F, Matsui M.et al. (2005A role for IL-27 in early regulation of Th1 differentiation J Immunol 1752191–2200. [DOI] [PubMed] [Google Scholar]
- Giermasz AS, Urban JA, Nakamura Y, Watchmaker P, Cumberland RL, Gooding W.et al. (2009Type-1 polarized dendritic cells primed for high IL-12 production show enhanced activity as cancer vaccines Cancer Immunol Immunother 581329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Carr AL, Donald EJ, Skitzki JJ, Okuyama R, Stoolman LM.et al. (2005Synergistic effects of IL-12 and IL-18 in skewing tumor-reactive T-cell responses towards a type 1 pattern Cancer Res 651063–1070. [PubMed] [Google Scholar]
- Kaneko S, Mastaglio S, Bondanza A, Ponzoni M, Sanvito F, Aldrighetti L.et al. (2009IL-7 and IL-15 allow the generation of suicide gene-modified alloreactive self-renewing central memory human T lymphocytes Blood 1131006–1015. [DOI] [PubMed] [Google Scholar]
- Teague RM, Sather BD, Sacks JA, Huang MZ, Dossett ML, Morimoto J.et al. (2006Interleukin-15 rescues tolerant CD8+ T cells for use in adoptive immunotherapy of established tumors Nat Med 12335–341. [DOI] [PubMed] [Google Scholar]
- Liu S, Riley J, Rosenberg S., and, Parkhurst M. Comparison of common gamma-chain cytokines, interleukin-2, interleukin-7, and interleukin-15 for the in vitro generation of human tumor-reactive T lymphocytes for adoptive cell transfer therapy. J Immunother. 2006;29:284–293. doi: 10.1097/01.cji.0000190168.53793.6b. [DOI] [PubMed] [Google Scholar]
- Goodman WA, Levine AD, Massari JV, Sugiyama H, McCormick TS., and, Cooper KD. IL-6 signaling in psoriasis prevents immune suppression by regulatory T cells. J Immunol. 2009;183:3170–3176. doi: 10.4049/jimmunol.0803721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G.et al. (2008IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells Proc Natl Acad Sci USA 10518460–18465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintarelli C, Dotti G, De Angelis B, Hoyos V, Mims M, Luciano L.et al. (2008Cytotoxic T lymphocytes directed to the preferentially expressed antigen of melanoma (PRAME) target chronic myeloid leukemia Blood 1121876–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster AE, Leen AM, Lee T, Okamura T, Lu A, Vera J.et al. (2007Autologous designer antigen-presenting cells by gene modification of T lymphocyte blasts with IL-7 and IL-12 J Immunother 30506–516. [DOI] [PubMed] [Google Scholar]
- Kern F, Faulhaber N, Khatamzas E, Frömmel C, Ewert R, Prösch S.et al. (1999Measurement of anti-human cytomegalovirus T cell reactivity in transplant recipients and its potential clinical use: a mini-review Intervirology 42322–324. [DOI] [PubMed] [Google Scholar]
- Kannanganat S, Ibegbu C, Chennareddi L, Robinson HL., and, Amara RR. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. J Virol. 2007;81:8468–8476. doi: 10.1128/JVI.00228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badr G, Bédard N, Abdel-Hakeem MS, Trautmann L, Willems B, Villeneuve JP.et al. (2008Early interferon therapy for hepatitis C virus infection rescues polyfunctional, long-lived CD8+ memory T cells J Virol 8210017–10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proto-Siqueira R, Figueiredo-Pontes LL, Panepucci RA, Garcia AB, Rizzatti EG, Nascimento FM.et al. (2006PRAME is a membrane and cytoplasmic protein aberrantly expressed in chronic lymphocytic leukemia and mantle cell lymphoma Leuk Res 301333–1339. [DOI] [PubMed] [Google Scholar]
- Staege MS, Banning-Eichenseer U, Weissflog G, Volkmer I, Burdach S, Richter G.et al. (2008Gene expression profiles of Hodgkin's lymphoma cell lines with different sensitivity to cytotoxic drugs Exp Hematol 36886–896. [DOI] [PubMed] [Google Scholar]
- Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT.et al. (2009The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research Clin Cancer Res 155323–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen MH, Svane IM, Becker JC., and, Straten PT. The universal character of the tumor-associated antigen survivin. Clin Cancer Res. 2007;13:5991–5994. doi: 10.1158/1078-0432.CCR-07-0686. [DOI] [PubMed] [Google Scholar]
- Otto K, Andersen MH, Eggert A, Keikavoussi P, Pedersen Lø, Rath JC.et al. (2005Lack of toxicity of therapy-induced T cell responses against the universal tumour antigen survivin Vaccine 23884–889. [DOI] [PubMed] [Google Scholar]
- Wobser M, Keikavoussi P, Kunzmann V, Weininger M, Andersen MH., and, Becker JC. Complete remission of liver metastasis of pancreatic cancer under vaccination with a HLA-A2 restricted peptide derived from the universal tumor antigen survivin. Cancer Immunol Immunother. 2006;55:1294–1298. doi: 10.1007/s00262-005-0102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meklat F, Li Z, Wang Z, Zhang Y, Zhang J, Jewell A.et al. (2007Cancer-testis antigens in haematological malignancies Br J Haematol 136769–776. [DOI] [PubMed] [Google Scholar]
- Quintarelli C, Dotti G, Hasan ST, De Angelis B, Hoyos V, Errichiello S.et al. (2011High-avidity cytotoxic T lymphocytes specific for a new PRAME-derived peptide can target leukemic and leukemic-precursor cells Blood 1173353–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisegang M, Wilde S, Spranger S, Milosevic S, Frankenberger B, Uckert W.et al. (2010MHC-restricted fratricide of human lymphocytes expressing survivin-specific transgenic T cell receptors J Clin Invest 1203869–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassi E, Braga M, Longhi R, Gavazzi F, Parmiani G, Di Carlo V.et al. (2009Non-redundant role for IL-12 and IL-27 in modulating Th2 polarization of carcinoembryonic antigen specific CD4 T cells from pancreatic cancer patients PLoS ONE 4e7234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welters MJ, Kenter GG, Piersma SJ, Vloon AP, Löwik MJ, Berends-van der Meer DM.et al. (2008Induction of tumor-specific CD4+ and CD8+ T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine Clin Cancer Res 14178–187. [DOI] [PubMed] [Google Scholar]
- Melief CJ., and, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer. 2008;8:351–360. doi: 10.1038/nrc2373. [DOI] [PubMed] [Google Scholar]
- Goodyear O, Agathanggelou A, Novitzky-Basso I, Siddique S, McSkeane T, Ryan G.et al. (2010Induction of a CD8+ T-cell response to the MAGE cancer testis antigen by combined treatment with azacitidine and sodium valproate in patients with acute myeloid leukemia and myelodysplasia Blood 1161908–1918. [DOI] [PubMed] [Google Scholar]
- Gerdemann U, Christin AS, Vera JF, Ramos CA, Fujita Y, Liu H.et al. (2009Nucleofection of DCs to generate Multivirus-specific T cells for prevention or treatment of viral infections in the immunocompromised host Mol Ther 171616–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotnda P, Onishi H, Heslop HE, Shayakhmetov D, Lieber A, Brenner M.et al. (2001Efficient infection of primitive hematopoietic stem cells by modified adenovirus Gene Ther 8930–937. [DOI] [PubMed] [Google Scholar]
- Rossig C, Bollard CM, Nuchtern JG, Rooney CM., and, Brenner MK. Epstein-Barr virus-specific human T lymphocytes expressing antitumor chimeric T-cell receptors: potential for improved immunotherapy. Blood. 2002;99:2009–2016. doi: 10.1182/blood.v99.6.2009. [DOI] [PubMed] [Google Scholar]
- Ayyoub M, Merlo A, Hesdorffer CS, Speiser D, Rimoldi D, Cerottini JC.et al. (2005Distinct but overlapping T helper epitopes in the 37-58 region of SSX-2 Clin Immunol 11470–78. [DOI] [PubMed] [Google Scholar]
- Ayyoub M, Stevanovic S, Sahin U, Guillaume P, Servis C, Rimoldi D.et al. (2002Proteasome-assisted identification of a SSX-2-derived epitope recognized by tumor-reactive CTL infiltrating metastatic melanoma J Immunol 1681717–1722. [DOI] [PubMed] [Google Scholar]
- Ayyoub M, Hesdorffer CS, Montes M, Merlo A, Speiser D, Rimoldi D.et al. (2004An immunodominant SSX-2-derived epitope recognized by CD4+ T cells in association with HLA-DR J Clin Invest 1131225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyoub M, Hesdorffer CS, Metthez G, Stevanovic S, Ritter G, Chen YT.et al. (2004Identification of an SSX-2 epitope presented by dendritic cells to circulating autologous CD4+ T cells J Immunol 1727206–7211. [DOI] [PubMed] [Google Scholar]
- Neumann F, Wagner C, Stevanovic S, Kubuschok B, Schormann C, Mischo A.et al. (2004Identification of an HLA-DR-restricted peptide epitope with a promiscuous binding pattern derived from the cancer testis antigen HOM-MEL-40/SSX2 Int J Cancer 112661–668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SSX2, Survivin, MAGE-A4, PRAME and NY-ESO-1 expression in lymphoma cell lines.
SSX2, Survivin, MAGE-A4, PRAME and NY-ESO-1 expression in primary tumor cells.