Abstract
The brain capillary endothelial cell (BCEC) is a major functional component of the blood–brain barrier and is an underlying factor in the pathophysiology of various diseases, including brain ischemia, multiple sclerosis, and neurodegenerative disorders. We examined gene silencing in BCECs by using endogenous lipoprotein to introduce short-interfering RNA (siRNA) in vivo. A cholesterol-conjugated 21/23-mer siRNA targeting organic anion transporter 3 (OAT3) mRNA (Chol-siOAT3) was intravenously injected into mice after its incorporation into extracted endogenous lipoproteins. Chol-siOAT3 was not delivered to neurons or glia, but was successfully delivered into BCECs and resulted in a significant reduction of OAT3 mRNA levels when injected after its incorporation into high-density lipoprotein (HDL). Efficient delivery was not achieved, however, when Chol-siOAT3 was injected without any lipoproteins, or after its incorporation into low-density lipoprotein (LDL). Investigations in apolipoprotein E (ApoE)-deficient and LDL receptor (LDLR)-deficient mice revealed that the uptake of HDL-containing Chol-siOAT3 was mainly mediated by ApoE and LDLR in mice. These findings indicate that siRNA can be delivered into BCECs in vivo by using endogenous lipoprotein, which could make this strategy useful as a new gene silencing therapy for diseases involving BCECs.
Introduction
The blood–brain barrier (BBB) is composed of brain capillary endothelial cells (BCECs) with pericytes, astrocyte foot processes, and nerve endings terminating on the capillary surface.1 The BBB is a unique structure in the central nervous system that represents a physical barrier formed by endothelial tight junctions and a transport barrier resulting from selective membrane transporters and vesicular trafficking in the BCECs.2
BCECs are associated with the pathophysiology of various diseases, including brain ischemia, multiple sclerosis (MS), and neurodegenerative disorders.3 In vivo gene silencing in BCECs can be a potentially useful approach for treating these above diseases because BCECs express different molecules that are considered to be important for the pathology of each disease. Inflammatory cell adhesion molecules, such as the intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and selectins, are potential target molecules for the treatment of brain ischemia and MS. This is because the adhesion of activated leukocytes to BCECs induces secondary neuronal injury after reperfusion4,5 and immune-mediated demyelination in MS.6,7 In Alzheimer's disease (AD), inhibition of the receptor for advanced glycation end products (RAGE) can be expected to alleviate AD pathology, because RAGE expressed in BCECs mediates an influx transport of the neurotoxic amyloid-β peptide (Aβ) from the blood into the brain.8,9
RNA interference is a powerful tool to achieve post-transcriptional gene silencing. Efficient in vivo delivery of synthetic short-interfering RNA (siRNA) is the biggest challenge for the therapeutic application of this tool.10 We first reported the delivery of siRNA into BCECs with a hydrodynamic injection technique,11 and the same strategy was adopted in the subsequent reports.12,13 However, the hydrodynamic injection technique cannot be applied clinically because of the volume overload and extremely high hydrostatic pressure involved; therefore, there is a need to develop an alternate strategy that would be clinically feasible.
We hypothesized that the best in vivo carrier of siRNA into BCECs is the molecule that is taken up into BCECs but cannot pass through the BBB. Cholesterol meets these requirements: cholesterol is a major lipid of lipoproteins which can be endocytosed via lipoprotein receptors expressed in BCECs, but most cholesterol cannot enter the brain.14 Extracted endogenous lipoproteins have been reported to work as effective vectors for the delivery of siRNA to the liver by conjugation of cholesterol (Chol-siRNA).15 This report showed that although Chol-siRNA incorporated into high-density lipoprotein (HDL) or low-density lipoprotein (LDL) accumulated in the liver, kidney, adrenal gland, ovary, stomach, and intestine, it was not detected in the brain after intravenous injection.15 However, we suspect that it does not necessarily preclude the transport of Chol-siRNA into the BCECs, because the brain capillary endothelial volume is <0.1% of total brain1 and therefore the distribution to the BCECs might be below the detection limit. In the present study, we have used endogenous lipoproteins to develop an in vivo delivery system for Chol-siRNA to be taken up into the BCECs.
Results
Design of Chol-siRNA targeting OAT3 mRNA and its in vitro gene silencing effect
We designed a 21/23-mer siRNA to target mouse organic anion transporter 3 (OAT3) mRNA (NM_031194) so that there was an overhang of two nucleotides in the 3′-end of its antisense strand.16 OAT3 is exclusively expressed at the endothelial cells in the brain and plays an important role in the brain-to-blood efflux transport of uremic toxins and neurotransmitter metabolites.17 The siRNA sequence was taken from our previous report, which described the selection of a good sequence for the cleavage of OAT3 mRNA.11 Chemical modifications such as phosphorothioate linkages and 2′-O-methyl sugar modifications were introduced into the nucleotides at the 3′ side of the both strands of siRNA to enhance their resistance towards degradation by exo- and endonucleases.18 Cholesterol was covalently conjugated to the 3′-end of the sense strand by using a cholesteryl-triethyleneglycol phosphoramidite linker (Chol-siOAT3) (Figure 1a).
Figure 1.
Design of Chol-siRNA targeting organic anion transporter 3 (OAT3) mRNA and its in vitro gene silencing effect. (a) Chemical structure of cholesterol conjugated to the 3′ end of the sense strand. (b) In vitro gene silencing effect of cholesterol-conjugated short-interfering RNA (siRNA) targeting OAT3 mRNA (Chol-siOAT3). Luciferase activity was analyzed 24 hours after transfection of Neuro2a cells with the Renilla luciferase-fused OAT3 expression vector, firefly luciferase expression vector, and either unconjugated siRNA targeting OAT3 mRNA (siOAT3), Chol-siOAT3, or unrelated siRNA (unrelated siRNAs 1, 2, and 3 represent siRNAs targeting mouse claudin-5, apolipoprotein B, and superoxide dismutase-1, respectively) at the concentration of 5 nmol/l. The data shown are relative to the values of the control group (transfected without siRNA). Data are expressed as mean values ± SEM (n = 3). (c) In vitro gene silencing effect of Chol-siOAT3. Luciferase activity was analyzed 24 hours after transfection of Neuro2a cells with the Renilla luciferase-fused OAT3 expression vector, firefly luciferase expression vector, and Chol-siOAT3 at different concentrations. Data are expressed as mean values ± SEM (n = 5). siRNA, short-interfering RNA; OAT3, organic anion transporter 3.
Because the linker used to conjugate cholesterol was different from the pyrrolidine linker reported earlier,18 we examined the in vitro gene silencing effect of Chol-siOAT3 by cotransfection of cultured mouse neuroblastoma (Neuro2a) cells with a Renilla luciferase-fused OAT3 expression vector and a firefly luciferase expression vector. Chol-siOAT3 at the concentration of 5 nmol/l efficiently inhibited the expression of OAT3 by 86.6% relative to the control luciferase activity, an inhibition that was similar to that achieved by an unconjugated siRNA also targeting OAT3 mRNA (siOAT3) (Figure 1b). The expression of OAT3 was not inhibited by unrelated siRNAs that targeted the mRNA of mouse claudin-5 (NM_013805), mouse apolipoprotein-B (ApoB) (NM_009693), or mouse superoxide dismutase-1 (NM_011434). The examination of the silencing effect of Chol-siOAT3 at different concentrations showed that the half maximal inhibitory concentration of Chol-siOAT3 was 0.51 nmol/l (Figure 1c). Thus, Chol-siOAT3 was considered to be highly efficient and specific in its cleavage of OAT3 mRNA.
Ex vivo incorporation of Chol-siOAT3 into extracted endogenous lipoproteins
We investigated the character of endogenous lipoproteins used as vectors for Chol-siOAT3 and optimized the composition of ex vivo mixture of endogenous lipoproteins and Chol-siOAT3 for animal experiments. We first obtained endogenous lipoproteins from mouse sera by ultracentrifugation. The endogenous HDL fraction was isolated from the sera of wild-type (WT) mice, whereas the endogenous LDL fraction was collected from the sera of LDL receptor (LDLR)-deficient (LDLR-/-) mice19 because WT mice only had small amounts of LDL in their sera.
An analysis of lipid profiles using high-performance liquid chromatography20 showed that the HDL fraction contained only HDL-cholesterol, with no cholesterol derived from any other lipoproteins (Figure 2a), and that the LDL fraction contained only LDL-cholesterol (Figure 2b). Moreover, western blotting revealed a characteristic apolipoprotein profile for each lipoprotein fraction: the HDL fraction contained apolipoprotein A-I (ApoA-I) and no ApoB, whereas the LDL fraction contained ApoB (ApoB-100 and ApoB-48) but no apolipoprotein A-I (Figure 2c). These results indicate that endogenous HDL and LDL had been successfully isolated.
Figure 2.
Ex vivo incorporation of Chol-siOAT3 into extracted endogenous lipoproteins. (a) HPLC analysis showing cholesterol levels of the serum and HDL fraction obtained from wild-type mice. (b) HPLC analysis showing cholesterol levels of the serum and LDL fraction obtained from LDL receptor-deficient (LDLR−/−) mice. (c) Western blotting of ApoA-I and ApoB in the HDL and LDL fractions. (d) Gel-shift assay of siOAT3 and Chol-siOAT3. Electrophoretic mobility was examined using a 12% polyacrylamide gel after 100 pmol of each short-interfering RNA (siRNA) were incubated with different volumes of the HDL fraction. ApoA-I, apolipoprotein A-I; ApoB, apolipoprotein B; Chol-siOAT3, cholesterol-conjugated siRNA targeting organic anion transporter 3 mRNA; HDL, high-density lipoprotein; HPLC, high-performance liquid chromatography; LDL, low-density lipoprotein; OAT3, organic anion transporter 3; siOAT3, unconjugated siRNA targeting OAT3 mRNA.
Next, we incubated siOAT3 or Chol-siOAT3 with the HDL or LDL fraction ex vivo and evaluated the electrophoretic mobility of the resulting mixtures using polyacrylamide gels. Single bands were seen for siOAT3, both with and without the HDL fraction, at the position of ~21 nucleotides (Figure 2d). In contrast, mixtures of 100 pmol of Chol-siOAT3 and 1 µl and more of the HDL fraction did not show any bands corresponding to 21 nucleotides (Figure 2d). These mixtures were considered to be stable for at least 24 hours in vivo because the intensity and mobility of the mixtures did not change after the incubation with mouse sera at 37 °C for 24 hours (data not shown). Similar patterns of bands were seen when siOAT3 or Chol-siOAT3 was mixed with the LDL fraction (data not shown). Taken together, these results indicate that Chol-siOAT3 is incorporated into HDL and LDL in a saturable manner, whereas siOAT3 is not incorporated into these lipoproteins at all. We decided to mix 1 µl of the HDL or LDL fraction per 100 pmol of Chol-siOAT3 for animal experiments because Chol-siOAT3 could be fully incorporated into HDL or LDL at this ratio.
Delivery of Chol-siOAT3 into BCECs by intravenous injection
After siOAT3 and/or Chol-siOAT3 were injected into mice intravenously, we examined the extent of their delivery to the brain histologically. For this purpose, we utilized siRNAs labeled with Cy3 at the 5′-ends of the antisense strands. We injected 10 mg/kg of siOAT3 or Chol-siOAT3 alone [in phosphate-buffered saline (PBS)], or the same dosage of Chol-siOAT3 after its incorporation into HDL or LDL, into the tail vein. Brains of mice were taken 1 hour after the injection, and the frozen sections of the striatum were subjected to confocal laser imaging. We found no Cy3 signals in the brains after the injection of siOAT3 alone (Figure 3a). When mice were injected with Chol-siOAT3 without lipoproteins (Chol-siOAT3/PBS) or Chol-siOAT3 with the LDL fraction (Chol-siOAT3/LDL), there were faint Cy3 signals along the blood capillary vessels in the striatum (Figure 3a). In contrast, robust Cy3 signals were observed when mice were injected with Chol-siOAT3 with the HDL fraction (Chol-siOAT3/HDL) (Figure 3a). Similar findings were seen in all other areas of the brain, including the cerebral cortex, hippocampus, cerebellum, and the brainstem.
Figure 3.
Delivery of Chol-siOAT3 into BCECs by intravenous injection. (a) Confocal laser images of frozen striatal sections prepared after intravenous injection of Cy3-labeled siOAT3 or Chol-siOAT3 (10 mg/kg), with and without the lipoprotein fraction. Bar = 100 µm. (b) Confocal laser images of frozen striatal sections prepared after intravenous injection of Cy3-labeled Chol-siOAT3 (10 mg/kg) along with HDL fraction. Sections were stained with Hoechst 33342 and immunolabeled with antibodies against vWF. Bar = 20 µm. (c) Northern blotting for the detection of antisense sequences of Chol-siOAT3 and the mouse U6 microRNA sequence in the small vascular fraction of the brain after injection of Chol-siOAT3 (10 mg/kg), with or without lipoprotein fractions. (d) The bars represent 21-mer band densities under the given conditions relative to band density of internal control, U6 microRNA. “Control ” means uninjected; data are expressed as mean values ± SEM (n = 3). BCEC, brain capillary endothelial cell; Chol-siOAT3, cholesterol-conjugated short-interfering RNA targeting organic anion transporter 3 mRNA; HDL, high-density lipoprotein; LDL, low-density lipoprotein; OAT3, organic anion transporter 3; siOAT3, unconjugated siRNA targeting OAT3 mRNA; vWF, von Willebrand factor.
In mice injected with Chol-siOAT3/HDL, higher magnification of the brain sections revealed that Cy3 signals were located in the cytoplasm of BCECs that were marked immunologically by antibodies against von Willebrand factor (Figure 3b). There were no Cy3 signals in other cells of the brain, such as neurons or glia, indicating that Chol-siOAT3 could not pass through the BBB. Moreover, we found no Cy3 signals in the endothelial cells of the aorta and lung which were taken simultaneously at euthanization (data not shown).
For the detection of Chol-siOAT3 itself in the BCECs, we injected Chol-siOAT3 (10 mg/kg) with and without the lipoprotein fraction and performed northern blotting of small RNAs extracted from the small vascular fraction of the brain21 3 hours after injection with a probe corresponding to the antisense strand of Chol-siOAT3. The content of BCECs in the small vascular fraction of the brain was previously estimated to be ~50%11 and thus, the detection of the antisense strand of Chol-siOAT3 by northern blotting was easier in this concentrated fraction than in the entire brain. The 21-mer band other than the 23-mer band was clearly detected when mice were injected with Chol-siOAT3/HDL (Figure 3c), suggesting that the 23-mer antisense strands had been cleaved by Dicer in the cytoplasm of BCECs. The density of the 21-mer band was much reduced when mice were injected with Chol-siOAT3/PBS or Chol-siOAT3/LDL (Figure 3c,d), demonstrating that the delivery of Chol-siOAT3 was only a little in these conditions. Taken together, these results indicate that Chol-siOAT3 is selectively delivered to the BCECs in the brain after intravenous injection and that the delivery is markedly enhanced when Chol-siOAT3 is incorporated into HDL before its injection into mice.
Gene silencing by intravenous injection of Chol-siOAT3 along with the HDL fraction
We assessed the extent of gene silencing after the intravenous injection of Chol-siOAT3. Mice were injected with 10 mg/kg Chol-siOAT3 with or without a lipoprotein fraction three times at 12-hour intervals, and were euthanized 6 hours after the last injection. Because OAT3 is exclusively expressed at endothelial cells in the brain, we could evaluate OAT3 mRNA levels in BCECs by analyzing these levels in the entire brain. Quantitative reverse transcriptase-PCR (RT-PCR), which was performed using total RNA extracted from whole-brain homogenates, showed almost no reduction in OAT3 mRNA levels when mice were injected with Chol-siOAT3/PBS or Chol-siOAT3/LDL (Figure 4a). In contrast, when mice were injected with Chol-siOAT3/HDL, OAT3 mRNA levels in the brain were reduced by ~50% (P < 0.01; Figure 4a). We also evaluated OAT3 mRNA levels in the kidneys of these mice because OAT3 was also expressed at the epithelial cells of the renal proximal tubules,22 but there was no reduction (data not shown). The mRNA levels of interferon-β, interferon-γ, and tumor necrosis factor-α were not increased in the brains of these mice and there was no increase in the levels of interferon-α in the blood samples which were collected at euthanization from the mice injected with Chol-siOAT3/HDL, suggesting that there was no immune stimulatory effect contributing to the silencing activity (data not shown).
Figure 4.
Gene silencing by intravenous injection of Chol-siOAT3 along with the HDL fraction. (a) Quantitative RT-PCR showing organic anion transporter 3 (OAT3) mRNA levels in whole-brain homogenates after the injection of Chol-siOAT3 (10 mg/kg), with and without lipoprotein fractions, three times at 12-hour intervals. The data shown are relative to claudin-5 mRNA levels and are expressed as mean values ± SEM (n = 4, *P < 0.01). (b) Quantitative RT-PCR showing OAT3 mRNA levels in whole-brain homogenates after the injection of different doses (1.0, 3.3, and 10 mg/kg) of Chol-siOAT3 with the HDL fraction, unrelated Chol-siRNA (10 mg/kg) with the HDL fraction, and HDL fraction only, three times at 12-hour intervals (Total injected dose is shown). The data shown are relative to claudin-5 mRNA levels and are expressed as mean values ± SEM (n = 4, *P < 0.01). Chol-siOAT3, cholesterol-conjugated siRNA targeting OAT3 mRNA; HDL, high-density lipoprotein; LDL, low-density lipoprotein; OAT3, organic anion transporter 3; PBS, phosphate-buffered saline; RT-PCR, reverse transcriptase-PCR.
When mice were injected three times each with 1.0, 3.3, or 10 mg/kg of Chol-siOAT3 along with the HDL fraction (for total doses of 3, 10, and 30 mg/kg Chol-siOAT3), the 10 and 30 mg/kg total doses resulted in significant and dose-dependent reductions in OAT3 mRNA levels (Figure 4b). We found no effects in the brain when mice were treated with an unrelated Chol-siRNA targeting ApoB mRNA (total dose 30 mg/kg) along with the HDL fraction, while there was a reduction of ~40% in the ApoB mRNA levels in the liver which was taken simultaneously at euthanization (data not shown). These results indicate that Chol-siOAT3 efficiently cleaves OAT3 mRNA in BCECs when Chol-siOAT3 is incorporated into HDL before its injection into mice.
Receptor-mediated uptake of Chol-siOAT3/ HDL into BCECs
We investigated the mechanism of delivery of Chol-siOAT3/HDL into BCECs. We thought that apolipoprotein E (ApoE) in HDL would most probably work as a ligand because of its important role in lipid transport.23 First, we isolated endogenous HDL fractions from the sera of ApoE-deficient (ApoE−/− HDL)24 and WT mice (WT HDL). To be able to make appropriate comparisons, we adjusted the mixed volume of each HDL fraction to have the same molecular amount of HDL-cholesterol. We then injected Cy3-labeled Chol-siOAT3 (10 mg/kg) into WT mice along with WT or ApoE−/− HDL fraction and compared the histological findings 1 hour later. The brain sections revealed similar Cy3 signals along the blood capillary vessels under both conditions (Figure 5a), suggesting that exogenous ApoE−/− HDL must recruit ApoE rapidly from the endogenous lipoproteins in the blood circulation of WT mice. Next, we injected the same dose of Cy3-labeled Chol-siOAT3 into ApoE−/− mice along with WT or ApoE−/− HDL fraction. Robust Cy3 signals were observed when Cy3-labeled Chol-siOAT3 was injected along with WT HDL fraction (Figure 5b), indicating that the receptors for HDL were intact in the BCECs of ApoE−/− mice. In contrast, there were remarkably reduced Cy3 signals when Cy3-labeled Chol-siOAT3 was injected into ApoE−/− mice along with ApoE−/− HDL fraction (Figure 5b), demonstrating that the delivery of Chol-siOAT3/HDL was mainly ApoE-dependent.
Figure 5.
Receptor-mediated uptake of Chol-siOAT3/HDL into BCECs. (a) Confocal laser images of frozen striatal sections of WT mice after the injection of Cy3-labeled Chol-siOAT3 (10 mg/kg) with the HDL fraction extracted from the sera of WT or ApoE-deficient (ApoE–/–) mice. Bar = 100 µm. (b) Confocal laser images of frozen striatal sections of ApoE–/– mice after the injection of Cy3-labeled Chol-siOAT3 (10 mg/kg) with the HDL fraction extracted from the sera of WT or ApoE–/– mice. Bar = 100 µm. (c) Confocal laser images of frozen striatal sections of WT and LDLR–/– mice after the injection of Cy3-labeled Chol-siOAT3 (10 mg/kg) with the HDL fraction extracted from the sera of WT mice. Bar = 100 µm. (d) Quantitative RT-PCR showing organic anion transporter 3 (OAT3) mRNA levels in whole-brain homogenates of WT, ApoE–/–, and LDLR–/– mice after the injection of Chol-siOAT3 (10 mg/kg) with the HDL fraction, three times at 12-hour intervals. WT and LDLR–/– mice were injected with WT HDL, and ApoE–/– mice were injected with ApoE–/– HDL. The data shown are relative to claudin-5 mRNA levels and are expressed as mean values ± SEM (n = 4, *P < 0.01). ApoE, apolipoprotein E; BCEC, brain capillary endothelial cell; Chol-siOAT3, cholesterol-conjugated siRNA targeting OAT3 mRNA; HDL, high-density lipoprotein; LDLR, low-density lipoprotein receptor; OAT3, organic anion transporter 3; WT, wild-type.
We tried further to identify the putative receptor for Chol-siOAT3/HDL in BCECs. Among several lipoprotein receptors expressed in BCECs, we hypothesized that LDLR was most likely to be responsible for the uptake of Chol-siOAT3/HDL because of its notably high affinity for ApoE.23,25 The brain sections of LDLR−/− mice injected with Cy3-labeled Chol-siOAT3 along with WT HDL fraction revealed remarkably reduced Cy3 signals compared to the brain sections of WT mice injected with the same solution (Figure 5c), suggesting that Chol-siOAT3/HDL was taken up into BCECs mainly via LDLR.
Moreover, we assessed the gene silencing effect in the brains of ApoE−/− and LDLR−/− mice. ApoE−/− mice were injected with 10 mg/kg Chol-siOAT3 with ApoE−/− HDL and LDLR−/− mice were injected with 10 mg/kg Chol-siOAT3 with WT HDL three times at 12-hour intervals, and were euthanized 6 hours after the last injection. Quantitative RT-PCR showed no reduction in OAT3 mRNA levels in the brains of ApoE−/− and LDLR−/− mice (Figure 5d). Taken together, these results indicate that the uptake of Chol-siOAT3/HDL into BCECs was mainly mediated by ApoE and LDLR in mice.
In vitro examinations of receptor-mediated uptake
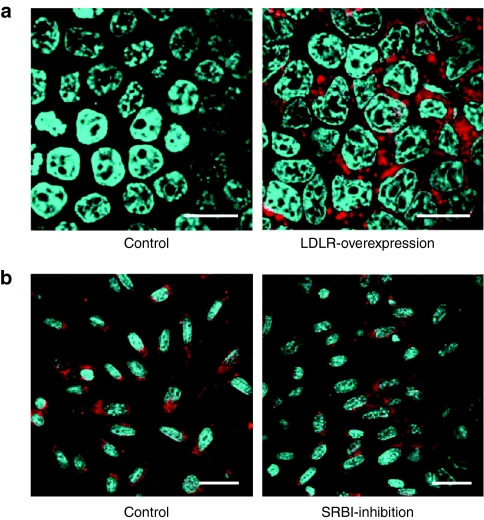
We assessed in vitro whether LDLR could actually mediate the uptake of endogenous HDL. For this purpose, we utilized HEK293T cells transfected with a LDLR-expressing plasmid or a mock plasmid. The HDL fraction extracted from the sera of WT mice was mixed with a fluorescent lipid probe of BODIPY and was incubated in the culture medium of the HEK293T cells for 3 hours. LDLR-overexpressing cells showed marked BODIPY signals in the cytoplasm, whereas the cells transfected with mock plasmid showed almost no signals (Figure 6a), suggesting that extracted endogenous HDL could be taken up via LDLR.
Figure 6.
In vitro examinations of receptor-mediated uptake. (a) Confocal laser images of HEK293T cells transfected with a mock plasmid (Control) or a LDLR-expressing plasmid (LDLR-overexpression) 3 hours after incubation in the culture medium containing BODIPY-labeled HDL (red). Cells were stained with Hoechst 33342. Bar = 20 µm. (b) Confocal laser images of TM-BBB cells incubated with 1 µmol/l of Cy3-labeled Chol-siOAT3/HDL for 30 minutes with and without a pretreatment by 1 µmol/l of SRBI inhibitor. “Control ” means no pretreatment by SRBI inhibitor. Cells were stained with Hoechst 33342. Bar = 20 µm. Chol-siOAT3, cholesterol-conjugated siRNA targeting OAT3 mRNA; HDL, high-density lipoprotein; LDLR, low-density lipoprotein receptor; OAT3, organic anion transporter 3; SRBI, scavenger receptor class B type I.
Moreover, we evaluated in vitro whether the uptake of Chol-siOAT3/HDL into BCECs was mediated by scavenger receptor class B type I (SRBI) because SRBI was known as a major HDL receptor in the liver and was reported to be also expressed at BCECs.26 For this purpose, we utilized the culture cells of conditionally immortalized brain capillary endothelium of mice origin, TM-BBB,27 in which we confirmed the expression of SRBI mRNA by RT-PCR (data not shown). The cells were treated with a SRBI inhibitor, BLT-1,28 and then incubated with 1 µmol/l of Cy3-labeled Chol-siOAT3/HDL for 30 minutes. Cy3 signals in the cytoplasm of TM-BBB cells were not decreased by BLT-1 (Figure 6b), suggesting that SR-BI was not a major receptor responsible for the uptake of Chol-siOAT3/HDL into BCECs.
Discussion
In this study, we achieved efficient delivery of siRNA into BCECs by utilizing endogenous lipoproteins. We showed that Chol-siOAT3 was efficiently delivered into BCECs when incorporated into HDL before its intravenous injection, and that the mechanism of delivery was mainly receptor-mediated uptake.
When Chol-siOAT3 was injected without any lipoproteins, the delivery into BCECs was quite limited and resulted in very little gene silencing. Two explanations may account for this result. First, Chol-siOAT3 not incorporated into lipoproteins is rapidly removed from blood circulation through filtration in the kidneys and by uptake into the reticuloendothelial system such as the spleen, lymph nodes, and bone marrow.10 Second, Chol-siOAT3 probably forms aggregates with other serum proteins, especially albumin,15 resulting in its decreased incorporation into endogenous, circulating HDL. Chol-siOAT3 may attach itself to the plasma membrane of BCECs by virtue of its lipophilicity, but it is unlikely to be taken up by a nonspecific fluid-phase mechanism because there is minimal pinocytosis in BCECs.1
BCECs express lipoprotein receptors such as the LDLR, SRBI, and LDLR-related proteins.2,26,29 The interaction of lipoproteins and BCECs has been investigated mostly from the perspective of lipoprotein transport to the brain across the BBB, rather than into the BCECs themselves. Plasma LDL can bind to LDLR in BCECs and pass through them via transcytosis to supply lipids to the brain.29,30 Moreover, it has been suggested that plasma HDL can transport α-tocopherol to the brain by binding to SRBI and move via transcytosis across the BCECs.26 Serum lipoproteins must also get transported into the BCECs themselves, especially for supplying cholesterol, because an earlier study using primary cultures of microvascular endothelial cells have shown that cholesterol synthesis in these cells decreases after addition of lipoproteins to the medium.31 However, the precise mechanism of this transport—which lipoproteins are taken up into the BCECs and which receptors are responsible for this in vivo uptake—has not been elucidated.
Our results indicate that HDL works as an efficient vector for transporting Chol-siOAT3 into the BCECs, being mainly mediated by ApoE as the ligand and LDLR as the receptor. LDL binds to LDLR with ApoB-100 as a ligand, whereas HDL is also bound tightly to LDLR with ApoE as a ligand.32,33 Investigations using human fibroblasts demonstrated that ApoE is necessary for HDL to not only bind to LDLR, but also to be internalized into the cells.33 In the examinations using LDLR-overexpressing cells, we have also demonstrated that LDLR can mediate the uptake of endogenous HDL. On the other hand, SRBI was not considered to be a major receptor responsible for the uptake of Chol-siOAT3/HDL into BCECs because SRBI-inhibitor did not prevent the uptake of Chol-siOAT3/HDL into TM-BBB cells. SRBI is essentially responsible for the reverse cholesterol transport into hepatocytes and is mainly associated with the collection of cholesterol from the vascular endothelial cells into HDL, not the supply of cholesterol from HDL to these cells.34 Therefore, we think it reasonable that the contribution of SRBI to the uptake of Chol-siOAT3/HDL into BCECs might be small. We suppose that in addition to LDLR, LDLR-related proteins can possibly mediate this uptake because LDLR-related proteins may be responsible for the supply of cholesterol into BCECs by virtue of its high affinity for ApoE.
Here we can ask why the delivery of Chol-siOAT3/LDL to BCECs via LDLR was not sufficiently achieved in WT mice, in spite of LDL uptake being the main function of LDLR. One possible explanation is the unique lipoprotein profile in rodents, which is different from those seen in other species. Because the rate of hepatic LDL clearance in rodents is 40 times greater than that in humans,35 Chol-siOAT3/LDL may be getting rapidly eliminated from the blood circulation into the liver, resulting in limited distribution to other tissues or organs in mice. This explanation is consistent with the findings of an earlier study showing that Chol-siRNA was delivered almost exclusively to the mouse liver when it was incorporated into LDL, whereas with HDL, it was also distributed to the kidney, adrenal gland, ovary, stomach, and intestine.15 Our negative result of OAT3 mRNA suppression in the kidneys by Chol-siOAT3/HDL is probably and simply because the epithelial cells of the renal proximal tubules, in which OAT3 is expressed, are lined by the basement membrane and therefore do not directly face the blood circulation. As another explanation of the insufficient delivery of Chol-siOAT3/LDL to BCECs, we cannot rule out the possibility that LDL in LDLR-/- mice may have some unknown property that may be causing low affinity for LDLR. As a therapeutic approach in humans, LDL as well as HDL has a potential to work as another effective vector of Chol-siRNA to deliver the siRNA to BCECs in humans.
Our strategy of siRNA delivery using an HDL vector accomplished a 50–60% reduction in the target mRNA levels in BCECs. Depending on the target molecules, even this partial gene silencing effect can be expected to achieve a sufficient therapeutic outcome. A promising candidate gene for the treatment of AD is RAGE, which facilitates the transport of Aβ across the BBB into the brain8 and therefore introduces the associated oxidative stress and neuroinflammation that characterize the disease.9 The levels of RAGE are reported to be ~2.5-fold higher in the brains of AD patients than in age-matched controls.8 Moreover, it is demonstrated that the inhibition of RAGE in BCECs could alleviate AD pathology in a transgenic AD mouse model in which the level of RAGE was approximately twice in the cerebral cortex of transgenic AD mice when compared with their nontransgenic littermate controls.9 Thus, even a 50–60% reduction in the levels of RAGE in BCECs could be beneficial as a therapeutic intervention for AD patients.
The practicality of our strategy using endogenous lipoprotein should be also discussed. The volume of the HDL and LDL fraction in our experiments was half of mouse serum, and from a single series of ultracentrifugations, we obtained these fractions for 25 injections when mixed with 10 mg/kg of Chol-siRNA. We needed just one mouse for obtaining the HDL fraction for three injections with 10 mg/kg of Chol-siRNA, which accomplished a 50–60% reduction of target mRNA levels in BCECs. The dosing volume of 10 ml/kg body weight in our experiments is generally employed in the actual intravenous administration of blood products to patients, suggesting the feasibility of our strategy in the clinical situations.
Because lipoprotein vectors have been reported to accumulate mainly in the liver,15 an enhancing their tropism toward BCECs would help to decrease the dose of Chol-siRNA and the lipoprotein vector. A recent study accomplished the delivery of increased amounts of a gene to BCECs by inserting polypeptides, selected from a phage library by in vivo panning, into the binding site of the adeno-associated virus vector to its receptor.36 Similar approaches of engineering with polypeptides for Chol-siRNA and lipoprotein vectors should facilitate their enhanced targeting to BCECs and result in greater efficiency in gene silencing.
As another possible improvement of our strategy, recombinant lipoproteins can be better siRNA vectors because of their purity and homogeneity. Concerning the ApoE-based delivery, synthetic carriers including micelles and liposomes have been actually utilized for the delivery to BCECs in vitro.37,38 However, it is not certain that recombinant lipoproteins can incorporate Chol-siRNA in the same manner as endogenous lipoprotein and that these artificial lipoproteins can be efficiently delivered to BCECs by the same receptor-mediated mechanism in vivo.
In summary, this is the first report to demonstrate the concept of an endogenous lipoprotein vector for the efficient delivery of siRNA into BCECs in mice via an intravenous injection. This concept of siRNA delivery can be advanced as a promising clinical strategy for gene silencing to treat various diseases involving BCECs.
Materials and Methods
siRNAs. All siRNAs were chemically synthesized at Hokkaido System Science (Sapporo, Japan). Cholesterol-conjugated siRNA targeting OAT3 mRNA (Chol-siOAT3) was constituted from the corresponding sense strand: 5′-CCA UUA UCU UGA AUG UGG AAU*-cholesterol-3′ and antisense strand: 5′-AUU CCA CAU UCA AGA UAA UGg* u*G-3′. The lower case letters represent 2′-O-methyl sugar modification and the asterisks represent phosphorothioate linkages. Unconjugated siRNA targeting OAT3 mRNA (siOAT3) was composed of the sense strand: 5′-CCA UUA UCU UGA AUG UGG AA*U-3′ and the same antisense strand as Chol-siOAT3. The unrelated siRNAs prepared for in vitro mRNA targeting were as follows: mouse claudin-5 (sense strand: 5′-CGU UGG AAA UUC UGG GUC UUU-3′, antisense strand: 5′-AGA CCC AGA AUU UCC AAC GUU-3′); mouse superoxide dismutase-1 (sense strand: 5′-GGU GGA AAU GAA GAA AGU ACA AAG ACU-3′, antisense strand: 5′-AGU CUU UGU ACU UUC UUC AUU UCC ACC UU-3′); and cholesterol-conjugated siRNA for in vitro and in vivo targeting of apolipoprotein-B mRNA (sense strand: 5′-GUC AUC ACA CUG AAU ACC AAU*-cholesterol-3′, antisense strand: 5′-AUU GGU AUU CAG UGU GAU GAc* a*C-3′).
Cholesterol was covalently conjugated via a cholesteryl-triethyleneglycol phosphoramidite linker (10-1975; Glen Research, Sterling, VA). For histological examinations, the 5′-ends of the antisense strands of siOAT3 and Chol-siOAT3 were labeled with Cy3. For the generation of siRNA duplexes, equimolar amounts of sense and antisense strands were heated in PBS at 95 °C for 5 minutes and slowly cooled to room temperature.
In vitro gene silencing. Mouse OAT3 cDNA was subcloned from pGEM-HEN/Roct (OAT3) into the Renilla luciferase expression vector, psiCHECK-1 (Promega, Fitchburg, WI). Neuro2a cells were transfected with 20 ng of a Renilla luciferase-fused OAT3 expression vector, 50 ng of a firefly luciferase expression vector, pGL3 (Promega), and siRNA at different concentrations in each well of 24-well plates. Changes in Renilla luciferase activity were normalized to firefly luciferase activity. The luciferase activities were analyzed 24 hours after transfection by using the Dual Luciferase System (Promega).
Animals. Female WT imprinting control region mice (Oriental Yeast, Tokyo, Japan) were used to evaluate the extent of delivery of Chol-siOAT3 with and without lipoprotein fractions. Investigations of the mechanism of delivery of Chol-siOAT3/HDL were conducted in female LDLR-/- and ApoE-/- mice on a C57BL/6J background (The Jackson Laboratory, Bar Harbor, ME) and also with female WT C57BL/6J mice (Oriental Yeast). Mice were 8–10 weeks of age at the time of the studies and were kept on a 12-hour light/dark cycle in a pathogen-free animal facility with free access to food and water. All animal experiments were performed in accordance with the Ethical and Safety Guidelines for Animal Experiments of the Tokyo Medical and Dental University.
Isolation of lipoproteins. Lipoproteins were isolated from the sera of WT, LDLR−/−, and ApoE−/− mice by ultracentrifugation, according to a modification of a method described earlier.39 First, a half volume of a solution of density 1.006 g/ml was layered onto one volume of mouse serum and centrifuged for 2.4 hours at 337,000g at 16 °C. Second, one volume of the lower solution was mixed with a half volume of a solution of density 1.182 g/ml and centrifuged for 3.6 hours at 337,000g at 16 °C. The half volume of the upper solution was set aside for use in experiments as the LDL fraction, and one volume of the lower solution was mixed with a half volume of a solution of density 1.478 g/ml and centrifuged for 7.5 hours at 266,000g at 16 °C. The half volume of the upper solution obtained after this third centrifugation contained HDL and was used in experiments as the HDL fraction.
Western blotting analysis. Two microliters of HDL or LDL fraction were collected in 18 µl of homogenate buffer [20 mmol/l Tris–HCl (pH 7.4), 0.1% SDS, 0.1% Triton X-100, 0.01% sodium deoxycholate, and 1× complete protease inhibitor cocktail [Roche Diagnostics, Mannheim, Germany)]. The total protein mixture was separated by electrophoresis in a 5–20% polyacrylamide gel (ATTO, Tokyo, Japan) and transferred onto polyvinylidene difluoride membranes. Blots were probed with a goat antibody against ApoA-I (1:500, sc-23605; Santa Cruz Biotechnology, Santa Cruz, CA) and ApoB (1:500, sc-11795; Santa Cruz Biotechnology), and then incubated with an anti-goat secondary antibody (1:2,000, sc-2020; Santa Cruz Biotechnology) tagged with horseradish peroxidase. Blots were visualized with the aid of SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific, Waltham, MA) and analyzed with a ChemiDoc System (Bio-Rad, Hercules, CA).
Gel-shift assay. One hundred pmol of siOAT3 and Chol-siOAT3 were added to 0–10 µl of the HDL or LDL fraction. The samples were resolved by electrophoresis in a 12% polyacrylamide gel for 1 hour at 100 V. Two micrograms of siRNA ladder marker (Takara Bio, Otsu, Japan) was used as a size standard for siRNA. The siRNAs were visualized under ultraviolet light after staining the gel with ethidium bromide in Tris-borate-EDTA buffer.
Injection of siRNAs. Each siRNA was injected slowly into the tail veins of mice at a dosing volume of 10 ml/kg body weight in a single dose of 10 mg/kg for histological examinations and northern blotting analyses, and was injected three times in doses of 1.0, 3.3, or 10 mg/kg body weight at 12-hour intervals for quantitative RT-PCR.
Histological examination. Brains of mice were fixed in 4% paraformaldehyde/PBS for 12 hours. The fixed specimens were snap-frozen in liquid nitrogen and brain sections of 16-µm thickness were prepared by using a LEICA CM3050 S cryostat (Leica Microsystems, Wetzlar, Germany). The sections were stained with Hoechst 33342 (Sigma-Aldrich, St Louis, MO) to visualize the nuclei and were immunolabeled with antibodies against von Willebrand factor (1:100, A0082; Dako, Glostrup, Denmark) to visualize vascular endothelial cells. This was followed by incubation with a fluorescein isothiocyanate-conjugated secondary antibody (1:50, AP106F; Millipore, Billerica, MA). All images were acquired with an LSM 510 confocal laser scanning microscope (Carl Zeiss MicroImaging, Göttingen, Germany).
Small vascular fractionation of the brain and northern blotting analysis. The small vascular fraction of the brain was prepared according to a modification of a method reported earlier.21 Briefly, brains of mice were homogenized in PBS and centrifuged for 5 minutes at 800g. The pellet was suspended in a 15% dextran solution and centrifuged for 10 minutes at 4,500g at 4 °C. The pellet was resuspended in 5 mmol/l PBS and, after incubation for 10 minutes, was centrifuged for 5 minutes at 800g. Small RNAs containing fewer than 200 nucleotides were extracted from the above final pellet of small vessels using MirVana (Ambion, Austin, TX). The RNA was condensed with Ethachinmate (Nippon Gene, Tokyo, Japan) and 1.2 µg of the RNA was separated by electrophoresis in a 14% polyacrylamide-urea gel and transferred to a Hybond-N+ membrane (Amersham Biosciences, Piscataway, NJ). The blot was hybridized with a probe corresponding to the siRNA antisense sequence or with the mouse U6 microRNA sequence (as an internal control) which had been labeled with fluorescein using a Gene Images 3′-Oligolabelling Kit (Amersham Biosciences). The signals were visualized by Gene Images CDP-star Detection Kit (Amersham Biosciences).
Quantitative RT-PCR assay. Total RNA was extracted from whole-brain homogenates with Isogen (Nippon Gene). DNase-treated RNA (2.5 µg) was reverse-transcribed with Super Script III and Random Hexamers (Life Technologies, Carlsbad, CA). The cDNAs were amplified by the quantitative TaqMan system by using the Light Cycler 480 Real-Time PCR Instrument (Roche Diagnostics). The primers and probes for mouse OAT3 (Mm00459534_m1), claudin-5 (Mm00727012_s1), interferon-β (Mm00439546_s1), interferon-γ (Mm00801778_m1), and tumor necrosis factor-α (Mm00443258_m1) were designed by Life Technologies. Relative OAT3 mRNA levels were calculated in comparison to claudin-5 mRNA levels, which were used as a BCEC-specific internal control.
Interferon-α analysis. The levels of interferon-α in the blood samples were analyzed by mouse interferon-α ELISA Kit (PBL Biomedical Laboratories, Piscataway, NJ) according to the manufacturers' protocol.
In vitro examinations of receptor-mediated uptake. Mouse serum HDL was labeled with BODIPY by a mixture of the HDL fraction and the solution of cholesteryl BODIPY 542/563 C11 (Life Technologies). HEK293T cells were grown in poly--lysine 4-well culture slides (BD, Franklin Lakes, NJ) and were transfected with 600 ng of mouse LDLR-expressing plasmid (Origene, Rockville, MD) or the same dose of mock plasmid by using Lipofectamine 2000 (Life Technologies). Twenty four hours after the transfection of the plasmids, the mixed solution containing 11 µl of the HDL fraction was added to 400 µl of the culture medium. After incubation for 3 hours, the cells were stained with Hoechst 33342 (Sigma-Aldrich) and were fixed in 4% paraformaldehyde/PBS.
TM-BBB cells (generously provided by Tetsuya Terasaki, PhD, Graduate School of Pharmaceutical Sciences, Tohoku University) were grown in collagen-I 4-well culture slides (BD) at 33 °C. The cells were treated with 1 µmol/l of SRBI inhibitor, BLT-1 (373210; Merck, Darmstadt, Germany) for 1 hour and then, were incubated with 1 µmol/l of Cy3-labeled Chol-siOAT3/HDL. After incubation for 30 minutes, the cells were stained with Hoechst 33342 (Sigma-Aldrich) and were fixed in 4% paraformaldehyde/PBS.
All images were acquired with an LSM 510 confocal laser scanning microscope (Carl Zeiss MicroImaging).
Statistical analysis. All data represent means ± SEM. Student's t-test was used to determine the significance of differences between two groups in quantitative RT-PCR assays.
Acknowledgments
The authors thank Sumio Ohtsuki, PhD, Graduate School of Pharmaceutical Sciences, Tohoku University, for his helpful advice, and Mizuko Osaka, PhD, Department of Life Science and Medical Ethics, Graduate School, Tokyo Medical and Dental University, for her technical support. This work was supported by grants from the Ministry of Health, Labour, and Welfare of Japan (#2212070 and #2212148) and a grant from the Ministry of Education, Science and Culture of Japan (#20659138). This work was done in Tokyo, Japan. The authors declared no conflict of interest.
REFERENCES
- Pardridge WM. Blood-brain barrier genomics. Stroke. 2007;38 2 Suppl:686–690. doi: 10.1161/01.STR.0000247887.61831.74. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR., and, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Frijns CJ., and, Kappelle LJ. Inflammatory cell adhesion molecules in ischemic cerebrovascular disease. Stroke. 2002;33:2115–2122. doi: 10.1161/01.str.0000021902.33129.69. [DOI] [PubMed] [Google Scholar]
- Danton GH., and, Dietrich WD. Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol. 2003;62:127–136. doi: 10.1093/jnen/62.2.127. [DOI] [PubMed] [Google Scholar]
- Correale J., and, Villa A. The blood-brain-barrier in multiple sclerosis: functional roles and therapeutic targeting. Autoimmunity. 2007;40:148–160. doi: 10.1080/08916930601183522. [DOI] [PubMed] [Google Scholar]
- Simka M. Blood brain barrier compromise with endothelial inflammation may lead to autoimmune loss of myelin during multiple sclerosis. Curr Neurovasc Res. 2009;6:132–139. doi: 10.2174/156720209788185605. [DOI] [PubMed] [Google Scholar]
- Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A.et al. (1996RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease Nature 382685–691. [DOI] [PubMed] [Google Scholar]
- Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E.et al. (2003RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain Nat Med 9907–913. [DOI] [PubMed] [Google Scholar]
- Whitehead KA, Langer R., and, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hino T, Yokota T, Ito S, Nishina K, Kang YS, Mori S.et al. (2006In vivo delivery of small interfering RNA targeting brain capillary endothelial cells Biochem Biophys Res Commun 340263–267. [DOI] [PubMed] [Google Scholar]
- Campbell M, Kiang AS, Kenna PF, Kerskens C, Blau C, O'Dwyer L.et al. (2008RNAi-mediated reversible opening of the blood-brain barrier J Gene Med 10930–947. [DOI] [PubMed] [Google Scholar]
- Fuest C, Bankstahl M, Winter P, Helm M, Pekcec A., and, Potschka H. In vivo down-regulation of mouse brain capillary P-glycoprotein: a preliminary investigation. Neurosci Lett. 2009;464:47–51. doi: 10.1016/j.neulet.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Pfrieger FW. Cholesterol homeostasis and function in neurons of the central nervous system. Cell Mol Life Sci. 2003;60:1158–1171. doi: 10.1007/s00018-003-3018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G, Pandey RK.et al. (2007Mechanisms and optimization of in vivo delivery of lipophilic siRNAs Nat Biotechnol 251149–1157. [DOI] [PubMed] [Google Scholar]
- Sano M, Sierant M, Miyagishi M, Nakanishi M, Takagi Y., and, Sutou S. Effect of asymmetric terminal structures of short RNA duplexes on the RNA interference activity and strand selection. Nucleic Acids Res. 2008;36:5812–5821. doi: 10.1093/nar/gkn584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuki S, Asaba H, Takanaga H, Deguchi T, Hosoya K, Otagiri M.et al. (2002Role of blood-brain barrier organic anion transporter 3 (OAT3) in the efflux of indoxyl sulfate, a uremic toxin: its involvement in neurotransmitter metabolite clearance from the brain J Neurochem 8357–66. [DOI] [PubMed] [Google Scholar]
- Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M.et al. (2004Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs Nature 432173–178. [DOI] [PubMed] [Google Scholar]
- Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE., and, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui S, Hara Y, Hosaki S., and, Okazaki M. A new on-line dual enzymatic method for simultaneous quantification of cholesterol and triglycerides in lipoproteins by HPLC. J Lipid Res. 2002;43:805–814. [PubMed] [Google Scholar]
- Kanda T, Yoshino H, Ariga T, Yamawaki M., and, Yu RK. Glycosphingolipid antigens in cultured bovine brain microvascular endothelial cells: sulfoglucuronosyl paragloboside as a target of monoclonal IgM in demyelinative neuropathy [corrected] J Cell Biol. 1994;126:235–246. doi: 10.1083/jcb.126.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizwan AN., and, Burckhardt G. Organic anion transporters of the SLC22 family: biopharmaceutical, physiological, and pathological roles. Pharm Res. 2007;24:450–470. doi: 10.1007/s11095-006-9181-4. [DOI] [PubMed] [Google Scholar]
- Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Zhang SH, Reddick RL, Piedrahita JA., and, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- Hatters DM, Peters-Libeu CA., and, Weisgraber KH. Apolipoprotein E structure: insights into function. Trends Biochem Sci. 2006;31:445–454. doi: 10.1016/j.tibs.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Balazs Z, Panzenboeck U, Hammer A, Sovic A, Quehenberger O, Malle E.et al. (2004Uptake and transport of high-density lipoprotein (HDL) and HDL-associated alpha-tocopherol by an in vitro blood-brain barrier model J Neurochem 89939–950. [DOI] [PubMed] [Google Scholar]
- Hosoya K, Tetsuka K, Nagase K, Tomi M, Saeki S, Ohtsuki S.et al. (2000Conditionally immortalized brain capillary endothelial cell lines established from a transgenic mouse harboring temperature-sensitive simian virus 40 large T-antigen gene AAPS PharmSci 2E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieland TJ, Shaw JT, Jaipuri FA, Duffner JL, Koehler AN, Banakos S.et al. (2008Identification of the molecular target of small molecule inhibitors of HDL receptor SR-BI activity Biochemistry 47460–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goti D, Balazs Z, Panzenboeck U, Hrzenjak A, Reicher H, Wagner E.et al. (2002Effects of lipoprotein lipase on uptake and transcytosis of low density lipoprotein (LDL) and LDL-associated alpha-tocopherol in a porcine in vitro blood-brain barrier model J Biol Chem 27728537–28544. [DOI] [PubMed] [Google Scholar]
- Dehouck B, Fenart L, Dehouck MP, Pierce A, Torpier G., and, Cecchelli R. A new function for the LDL receptor: transcytosis of LDL across the blood-brain barrier. J Cell Biol. 1997;138:877–889. doi: 10.1083/jcb.138.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux FS, Mokni R, Hughes CC, Clouet PM, Lefauconnier JM., and, Bourre JM. Lipid synthesis by rat brain microvessel endothelial cells in tissue culture. J Neuropathol Exp Neurol. 1989;48:437–447. doi: 10.1097/00005072-198907000-00005. [DOI] [PubMed] [Google Scholar]
- Jeon H., and, Blacklow SC. Structure and physiologic function of the low-density lipoprotein receptor. Annu Rev Biochem. 2005;74:535–562. doi: 10.1146/annurev.biochem.74.082803.133354. [DOI] [PubMed] [Google Scholar]
- Mahley RW., and, Innerarity TL. Interaction of canine and swine lipoproteins with the low density lipoprotein receptor of fibroblasts as correlated with heparin/manganese precipitability. J Biol Chem. 1977;252:3980–3986. [PubMed] [Google Scholar]
- Connelly MA., and, Williams DL. Scavenger receptor BI: a scavenger receptor with a mission to transport high density lipoprotein lipids. Curr Opin Lipidol. 2004;15:287–295. doi: 10.1097/00041433-200406000-00008. [DOI] [PubMed] [Google Scholar]
- Dietschy JM., and, Turley SD. Control of cholesterol turnover in the mouse. J Biol Chem. 2002;277:3801–3804. doi: 10.1074/jbc.R100057200. [DOI] [PubMed] [Google Scholar]
- Chen YH, Chang M., and, Davidson BL. Molecular signatures of disease brain endothelia provide new sites for CNS-directed enzyme therapy. Nat Med. 2009;15:1215–1218. doi: 10.1038/nm.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer I, Dunay IR, Weisgraber K, Bienert M., and, Dathe M. An apolipoprotein E-derived peptide mediates uptake of sterically stabilized liposomes into brain capillary endothelial cells. Biochemistry. 2005;44:2021–2029. doi: 10.1021/bi048080x. [DOI] [PubMed] [Google Scholar]
- Leupold E, Nikolenko H., and, Dathe M. Apolipoprotein E peptide-modified colloidal carriers: the design determines the mechanism of uptake in vascular endothelial cells. Biochim Biophys Acta. 2009;1788:442–449. doi: 10.1016/j.bbamem.2008.11.025. [DOI] [PubMed] [Google Scholar]
- Hatch FT. Practical methods for plasma lipoprotein analysis. Adv Lipid Res. 1968;6:1–68. [PubMed] [Google Scholar]