Abstract
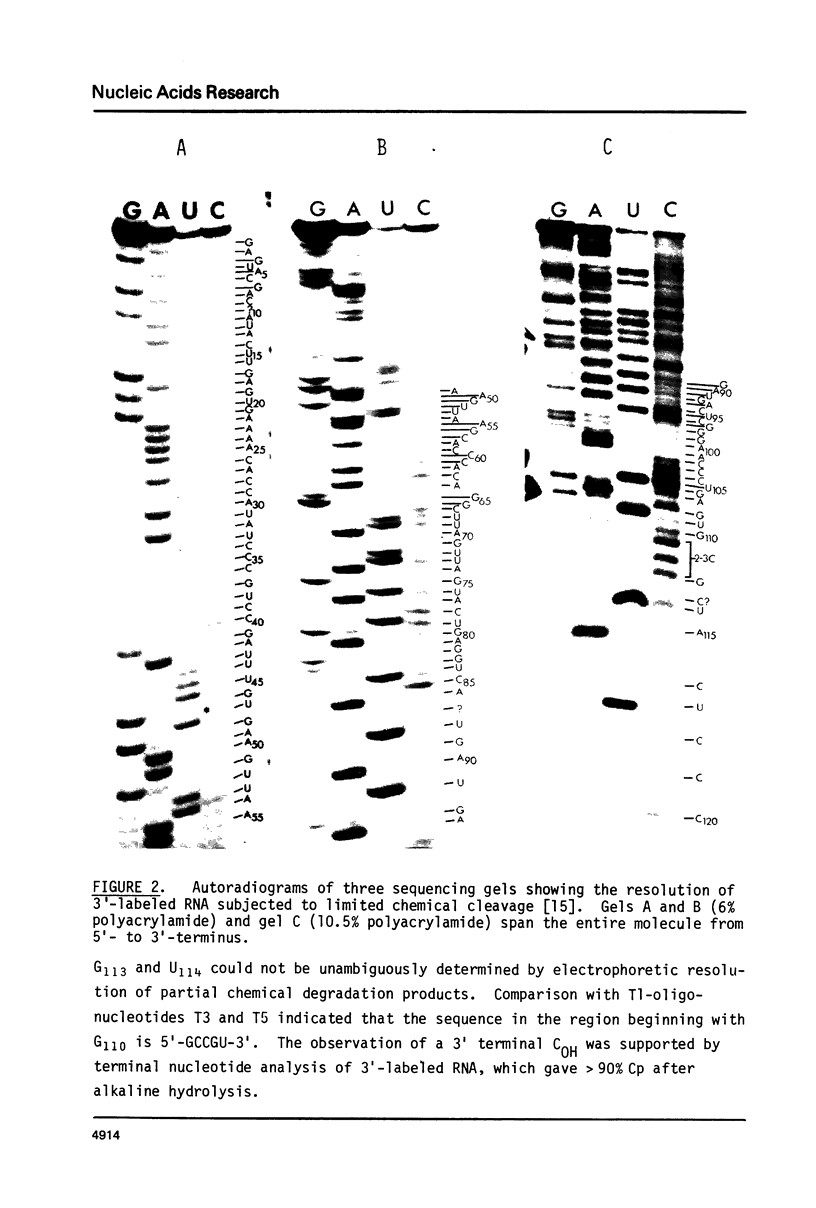
The complete nucleotide sequence of the cytosol 5S ribosomal ribonucleic acid of the trypanosomatid protozoan Crithidia fasciculata has been determined by a combination of T1-oligonucleotide catalog and gel sequencing techniques. The sequence is: GAGUACGACCAUACUUGAGUGAAAACACCAUAUCCCGUCCGAUUUGUGAAGUUAAGCACC CACAGGCUUAGUUAGUACUGAGGUCAGUGAUGACUCGGGAACCCUGAGUGCCGUACUCCCOH. This 5S ribosomal RNA is unique in having GAUU in place of the GAAC or GAUC found in all other prokaryotic and eukaryotic 5S RNAs, and thought to be involved in interactions with tRNAs. Comparisons to other eukaryotic cytosol 5S ribosomal RNA sequences indicate that the four major eukaryotic kingdoms (animals, plants, fungi, and protists) are about equally remote from each other, and that the latter kingdom may be the most internally diverse.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allington W. B., Cordry A. L., McCullough G. A., Mitchell D. E., Nelson J. W. Electrophoretic concentration of macromolecules. Anal Biochem. 1978 Mar;85(1):188–196. doi: 10.1016/0003-2697(78)90289-0. [DOI] [PubMed] [Google Scholar]
- Benhamou J., Jourdan R., Jordan B. R. Sequence of Drosophila 5S RNA synthesized by cultured cells and by the insect at different developmental stages. Homogeneity of the product and homologies with other 5S RNA's at the level of primary and secondary structure. J Mol Evol. 1977 May 13;9(3):279–298. doi: 10.1007/BF01796116. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann V. A. Collection of published 5S and 5.8S RNA sequences and their precursors. Nucleic Acids Res. 1979 Jan;6(1):r29–r44. doi: 10.1093/nar/6.1.419-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget B. G., Weissman S. M. Nucleotide sequence of KB cell 5S RNA. Science. 1967 Dec 29;158(3809):1695–1699. doi: 10.1126/science.158.3809.1695. [DOI] [PubMed] [Google Scholar]
- Fox G. E., Woese C. R. The architecture of 5S rRNA and its relation to function. J Mol Evol. 1975 Oct 3;6(1):61–76. doi: 10.1007/BF01732674. [DOI] [PubMed] [Google Scholar]
- Gray M. W. The ribosomal RNA of the trypanosomatid protozoan Crithidia fasciculata: physical characteristics and methylated sequences. Can J Biochem. 1979 Jun;57(6):914–926. doi: 10.1139/o79-111. [DOI] [PubMed] [Google Scholar]
- Hori H., Osawa S. Evolutionary change in 5S RNA secondary structure and a phylogenic tree of 54 5S RNA species. Proc Natl Acad Sci U S A. 1979 Jan;76(1):381–385. doi: 10.1073/pnas.76.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan B. R., Galling G., Jourdan R. Sequence and conformation of 5 S RNA from Chlorella cytoplasmic ribosomes: comparison with other 5 S RNA molecules. J Mol Biol. 1974 Aug 5;87(2):205–225. doi: 10.1016/0022-2836(74)90144-2. [DOI] [PubMed] [Google Scholar]
- LANE B. G., DIEMER J., BLASHKO C. A. END GROUP AND SEDIMENTATION DATA ON FRAGMENTED HIGH MOLECULAR WEIGHT RIBONUCLEATES. Can J Biochem Physiol. 1963 Sep;41:1927–1941. [PubMed] [Google Scholar]
- LANE B. G. The separation of adenosine, guanosine, cytidine and uridine by one-dimensional filter-paper chromatography. Biochim Biophys Acta. 1963 May 28;72:110–112. [PubMed] [Google Scholar]
- Lu A. L., Steege D. A., Stafford D. W. Nucleotide sequence of a 5S ribosomal RNA gene in the sea urchin Lytechinus variegatus. Nucleic Acids Res. 1980 Apr 25;8(8):1839–1853. doi: 10.1093/nar/8.8.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean F. I., Amiro E. R. The effect of solute concentration on the shape of the trypanosomatid flagellate Crithidia fasciculata. Can J Microbiol. 1973 Jul;19(7):878–880. doi: 10.1139/m73-140. [DOI] [PubMed] [Google Scholar]
- Nishikawa K., Takemura S. Nucleotide sequence of 5 S RNA from Torulopsis utilis. FEBS Lett. 1974 Mar 15;40(1):106–109. doi: 10.1016/0014-5793(74)80904-x. [DOI] [PubMed] [Google Scholar]
- Nishikawa K., Takemura S. Structure and function of 5S ribosomal ribonucleic acid from Torulopsis utilis. II. Partial digestion with ribonucleases and derivation of the complete sequence. J Biochem. 1974 Nov;76(5):935–947. [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Schwartz R. M., Dayhoff M. O. Origins of prokaryotes, eukaryotes, mitochondria, and chloroplasts. Science. 1978 Jan 27;199(4327):395–403. doi: 10.1126/science.202030. [DOI] [PubMed] [Google Scholar]
- Uchida T., Bonen L., Schaup H. W., Lewis B. J., Zablen L., Woese C. The use of ribonuclease U2 in RNA sequence determination. Some corrections in the catalog of oligomers produced by ribonuclease T1 digestion of Escherichia coli 16S ribosomal RNA. J Mol Evol. 1974 Feb 28;3(1):63–77. doi: 10.1007/BF01795977. [DOI] [PubMed] [Google Scholar]
- Walseth T. F., Johnson R. A. The enzymatic preparation of [alpha-(32)P]nucleoside triphosphates, cyclic [32P] AMP, and cyclic [32P] GMP. Biochim Biophys Acta. 1979 Mar 28;562(1):11–31. doi: 10.1016/0005-2787(79)90122-9. [DOI] [PubMed] [Google Scholar]
- Whittaker R. H. New concepts of kingdoms or organisms. Evolutionary relations are better represented by new classifications than by the traditional two kingdoms. Science. 1969 Jan 10;163(3863):150–160. doi: 10.1126/science.163.3863.150. [DOI] [PubMed] [Google Scholar]
- Woese C., Sogin M., Stahl D., Lewis B. J., Bonen L. A comparison of the 16S ribosomal RNAs from mesophilic and thermophilic bacilli: some modifications in the Sanger method for RNA sequencing. J Mol Evol. 1976 Apr 9;7(3):197–213. doi: 10.1007/BF01731489. [DOI] [PubMed] [Google Scholar]