Abstract
The International Classification of Sleep Disorders, Second Edition (ICSD-2) distinguishes 5 subtypes of central sleep apnea syndromes (CSAS) in adults. Review of the literature suggests that there are two basic mechanisms that trigger central respiratory events: (1) post-hyperventilation central apnea, which may be triggered by a variety of clinical conditions, and (2) central apnea secondary to hypoventilation, which has been described with opioid use. The preponderance of evidence on the treatment of CSAS supports the use of continuous positive airway pressure (CPAP). Much of the evidence comes from investigations on CSAS related to congestive heart failure (CHF), but other subtypes of CSAS appear to respond to CPAP as well. Limited evidence is available to support alternative therapies in CSAS subtypes. The recommendations for treatment of CSAS are summarized as follows:
CPAP therapy targeted to normalize the apnea-hypopnea index (AHI) is indicated for the initial treatment of CSAS related to CHF. (STANDARD)
Nocturnal oxygen therapy is indicated for the treatment of CSAS related to CHF. (STANDARD)
Adaptive Servo-Ventilation (ASV) targeted to normalize the apnea-hypopnea index (AHI) is indicated for the treatment of CSAS related to CHF. (STANDARD)
BPAP therapy in a spontaneous timed (ST) mode targeted to normalize the apnea-hypopnea index (AHI) may be considered for the treatment of CSAS related to CHF only if there is no response to adequate trials of CPAP, ASV, and oxygen therapies. (OPTION)
The following therapies have limited supporting evidence but may be considered for the treatment of CSAS related to CHF after optimization of standard medical therapy, if PAP therapy is not tolerated, and if accompanied by close clinical follow-up: acetazolamide and theophylline. (OPTION)
Positive airway pressure therapy may be considered for the treatment of primary CSAS. (OPTION)
Acetazolamide has limited supporting evidence but may be considered for the treatment of primary CSAS. (OPTION)
The use of zolpidem and triazolam may be considered for the treatment of primary CSAS only if the patient does not have underlying risk factors for respiratory depression. (OPTION)
The following possible treatment options for CSAS related to end-stage renal disease may be considered: CPAP, supplemental oxygen, bicarbonate buffer use during dialysis, and nocturnal dialysis. (OPTION)
Citation:
Aurora RN; Chowdhuri S; Ramar K; Bista SR; Casey KR; Lamm CI; Kristo DA; Mallea JM; Rowley JA; Zak RS; Tracy SL. The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses. SLEEP 2012;35(1):17-40.
Keywords: Central sleep apnea, clinical guidelines, PAP, oxygen therapy, ASV
1.0 INTRODUCTION
The central sleep apnea syndromes (CSAS) are characterized by sleep disordered breathing associated with diminished or absent respiratory effort, coupled with the presence of symptoms including excessive daytime sleepiness, frequent nocturnal awakenings, or both. However, no recent evidence-based guidelines have been published. The purpose of this practice parameter is to review the available data for the treatment and management of CSAS in adults. When possible, a relative determination was made as to the most effective treatment option.
2.0 BACKGROUND
The International Classification of Sleep Disorders (ICSD)–22 identifies 6 different forms of CSAS: (1) Primary Central Sleep Apnea, (2) Central Sleep Apnea Due to Cheyne Stokes Breathing Pattern, (3) Central Sleep Apnea Due to Medical Condition Not Cheyne Stokes, (4) Central Sleep Apnea Due to High-Altitude Periodic Breathing, (5) Central Sleep Apnea Due to Drug or Substance, and (6) Primary Sleep Apnea of Infancy. The final category will not be reviewed in this document, as these guidelines pertain to CSAS treatment in adults.
While the ICSD-2 classification system for CSAS will be used to systemize these practice parameters, it is important to recognize that the underlying pathophysiology of central sleep apnea is due to 1 of 2 mechanisms: hyperventilation or hypoventilation. Post-hypocapnia hyperventilation is the underlying pathophysiological mechanism for central apnea associated with congestive heart failure, high altitude sickness, and primary CSAS. These patients chronically hyperventilate in association with hypocapnia during wake and sleep and demonstrate increased chemoresponsiveness and sleep state instability. CSAS in the absence of an identifiable etiology is referred to as “primary CSAS.” The presence and prevalence of this entity is uncertain.
Central sleep apnea due to hypoventilation results from the removal of the wakefulness stimulus to breathe in patients with compromised neuromuscular ventilatory control. Chronic ventilatory failure due to neuromuscular disease or chest wall disease may manifest with central apneas or hypopneas, at sleep onset or during phasic REM sleep. This is typically noted in patients with central nervous system disease (e.g., encephalitis), neuromuscular disease, or severe abnormalities in pulmonary mechanics (e.g., kyphoscoliosis3). The ventilatory motor output is markedly reduced and insufficient to preserve alveolar ventilation resulting in hypopneas. Thus, this type of central apnea may not necessarily meet the strict “central apnea” definition.
CSAS due to Cheyne Stokes breathing pattern (CSBP) or Cheyne-Stokes respiration (CSR) is characterized by an absence of air flow and respiratory effort followed by hyperventilation in a crescendo-decrescendo pattern. CSR most often occurs in patients with congestive heart failure (CHF). The prevalence is estimated to be approximately 30%4 to 40%5 in patients with CHF. However, this respiratory pattern can also be seen in patients with stroke or renal failure.
There is mounting evidence that CSAS/CSR may be an indicator of higher morbidity and mortality in CHF patients. Consequently, effective treatment of CSAS/CSR might improve the outcome of CHF patients with CSAS/CSR.
CSAS can occur in individuals with cardiac, renal, and neurological disorders but without a CSR pattern. This category is referred to CSAS Due to Medical Condition Not Cheyne Stokes.
CSAS associated with high altitude can be seen during the acclimatization period, during or after rapid ascent to high altitudes, typically 4000 meters or greater. Hyperventilation secondary to altitude-associated hypoxia is thought to be the trigger for high-altitude periodic breathing. Hence, individuals with a heightened or brisk response to hypoxia are more likely to develop CSAS Due to High-Altitude Periodic Breathing.2
The use of chronic opioid treatment for the management of chronic pain has increased over the last 10 years.6 Central Sleep Apnea Due to Drug or Substance is primarily a disorder related to opioid use. Patients who are on long-acting opioids for at least 2 months appear to be at increased risk for developing CSAS. In fact, CSAS has been reported to be present in as many as 30% of patients in methadone maintenance therapy.7 The exact mechanism of opioid-related CSAS is not well elucidated.
The following PICO (Patient Intervention Comparison Outcome) questions were addressed in the systematic review as shown in Box 1.
Box 1—PICO questions
What therapies improve mortality and apnea hypopnea index (AHI) in patients with primary CSAS?
Does positive airway pressure (PAP, including CPAP, BPAP, adaptive servo-ventilation [ASV]) improve clinical (transplant-free survival) or surrogate (left ventricular ejection fraction [LVEF] or AHI) outcomes in patients with CSAS and CHF?
Does oxygen improve clinical (transplant-free survival) or surrogate (LVEF or AHI) outcomes in patients with CSAS and CHF?
What other therapies exist for and do they improve transplant-free survival, LVEF, or AHI in patients with CSAS and CHF?
What therapies exist for and do they improve AHI in patients with high altitude periodic breathing?
What therapies exist for and do they improve AHI in ESRD patients with CSAS?
What therapies exist for and do they improve AHI in patients with CSAS due to drug or substance?
Optimally, standards of practice should be supported by scientific evidence based on controlled clinical trials. However, the number of studies on the clinical treatment outcomes for CSAS that meet this criterion is limited. The preponderance of the literature has focused on management of CSAS due to CSBP; therefore, caution is mandated when extrapolated to other forms of central apnea.
Many of the recommendations are based on studies that used the apnea-hypopnea index (AHI) as the primary outcome measure. Additional intermediate outcomes that were assessed included left ventricular ejection fraction (LVEF) and transplant-free survival in patients with CHF. Evidence from large population-based studies has shown that AHI correlates with survival and may be an appropriate severity metric.8–10 Nevertheless, most of the studies focused on obstructive sleep apnea and did not include a therapeutic intervention. Therefore, AHI is an acceptable surrogate outcome measure until long-term outcome data are available. Even more germane to the current topic is a fairly recent study11 that investigated patients with systolic heart failure and CSAS. The investigators found an AHI greater than 5 to be predictive of mortality even after accounting for confounders such as LVEF, NYHA functional class, heart rate, as well as multiple other patient factors. Hence, although the use of a surrogate marker such as the AHI has limitations, there are some advantages conferred by its use as an outcome measure. The AHI allows for relatively easy quantification of disease severity, and it has been shown to correlate with other outcomes of interest.
3.0 METHODS
3.1 Literature Search
The Standards of Practice Committee of the AASM commissioned this review in 2009. A search for articles on the medical treatment of CSAS was conducted using the PubMed database from 1966 through June 2010. The key words for searches were: (central sleep apnea treatment), (Cheyne-Stokes treatment), [(central sleep apnea) and (heart failure) and treatment], [(sleep apnea) and narcotics and treatment], and [(sleep-related breathing disorders) and narcotics and treatment]. The limits on these searches were humans, English, adults (+19 years), clinical trials, meta-analyses, and randomized controlled trials. A second set of more specific searches was done with the limits of humans, English, and adults (+19 years) with (central sleep apnea) and the following terms: 1) high altitude, 2) opioid, 3) traumatic brain injury, 4) [(end stage renal disease) or (renal disease) or ESRD], and pharmacotherapy. A total of 252 articles were identified using this process. The search was updated in June 2010 to include the latest research publications. Abstracts from these articles were reviewed to determine if they met inclusion criteria, which were a minimum of 5 patients plus clinical outcomes measures of mortality/transplant-free survival, left ventricular ejection fraction (LVEF), or apnea-hypopnea index (AHI). Complex sleep apnea was not included, as it is not currently listed as a disorder in the ICSD-2. Additionally, sleep disordered breathing had to be clearly differentiated between CSAS and OSA. CSAS was defined as greater than 50% central events including periodic breathing if subjects presented with both CSAS and OSA. Additional articles were identified by pearling (i.e., checking the reference sections of search results for articles otherwise missed). A total of 77 articles were reviewed, graded, and extracted.
3.2 Quality of Evidence
The assessment of evidence quality was performed according to the GRADE process. The GRADE system differs from other grading systems as each study is not only evaluated for study design and risk of bias, but, additionally, an estimate of effect (see footnote A following article) is generated for each outcome. Multiple aspects of quality are assessed including study limitations, imprecision, inconsistency of results, indirectness of evidence, and likeliness of publication bias. The quality of effects from observational studies can be adjusted by the presence of large magnitudes of effect, evidence of dose-response associations, and the presence of confounders.13 Quality refers to the confidence that the estimates of the effects are correct, and the quality rating is applied to a body of evidence and not to individual studies.1
Briefly, risk of bias includes aspects of study design (randomized control trials [RCTs] versus non-randomized controlled trials or before-after trials)14 and conduct such as blinding, allocation concealment, large loss to follow up, or selective outcome reporting.12 Imprecision refers to wide confidence intervals around the estimate of effect when there are relatively few patients and few events. Indirectness occurs when the question being addressed is different than the available evidence regarding population, intervention, comparator, or outcome. There is inconsistency when there is unexplained heterogeneity of the results. Reporting bias can occur if there is selective reporting of studies or outcomes, which may occur if the published evidence is limited to a small number of trials funded by a for-profit organization.12
As a first step, all individual studies were assessed by 2 task force members for study design and limitations to validity (bias) for each outcome of interest.15,16 Randomized control trials (RCTs) were considered a higher level of evidence than observational, nonrandomized, or before-after interventional studies (Table 1). Blinding for objective outcomes (mortality, AHI, if scoring was blinded) was not considered a threat to internal validity. Subsequently, the body of evidence for each outcome was assessed and graded, taking into account the results of the meta-analysis (if applicable) and other factors as described above. The final assessment, as defined in Box 2, was determined for each treatment and outcome measure.
Table 1.
A summary of GRADE's approach to rating quality of evidence1
| Study design | Initial quality of a body of evidence | Lower if | Higher if | Quality of a body of evidence |
|---|---|---|---|---|
| Radomized trials | High → | Risk of bias | Large effect | High (four plus:⊕⊕⊕⊕) |
| −1 Serious | +1 Large | |||
| −2 Very serious | +2 Very large | |||
| Inconsistency | Dose response | Moderate (three plus:⊕⊕⊕○) | ||
| −1 Serious | +1 Evidence of a gradient | |||
| −2 Very serious | ||||
| Observational studies | Low → | Indirectness | All plausible residual confounding | Low (two plus:⊕⊕○○) |
| −1 Serious | +1 Would reduce a demonstrated effect | |||
| −2 Very serious | ||||
| Imprecision | Very Low (one plus:⊕○○○) | |||
| −1 Serious | +1 Would suggest a spurious effect if no effect was observed | |||
| −2 Very serious | ||||
| Publication bias | ||||
| −1 Likely | ||||
| −2 Very likely |
Box 2—Final Assessments of Level of Bodies of Evidence1
High: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect.
Very low: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
The results are reported in summary tables in each section that include the number of studies, study design, limitations, inconsistency, indirectness, imprecision, and other considerations that went into the quality of evidence for each outcome of interest. Also reported are the number of patients that were studied, the overall effect that was calculated in the meta-analysis (reported as the mean difference [MD]), and a qualitative assessment of the relative importance of the outcome.
3.3 Meta-Analysis
All meta-analyses were performed using MIX software.17,18 The analyses were performed on the apnea-hypopnea index (AHI) and the LVEF when available. All analyses are presented using the random effects model.
The result of each meta-analysis is shown in a figure with several components. Each study of the meta-analysis is identified along the left-hand column, and adjacent to it is the year of the study, treatment (exposed, “e”) results, and control (“c”) results. The results are expressed as “n/M/SD” corresponding to “number/mean/standard deviation.” A graphical representation of the data is shown in the center of the figure. The vertical red line indicates the average response of all studies. The zero line represents no effect. The width of the red diamond at the bottom of the plot represents the standard deviation of the meta-analysis. If the red diamond does not touch the zero line, the meta-analysis results indicate that the treatment is different from zero (i.e., it has an effect). The magnitude of the effect across all studies is given by the value of the association measure along with the 95% confidence intervals.
Tables of the data used in the meta-analyses are presented at the end of the manuscript in the Appendix.
3.4 Recommendations
The Standards of Practice Committee (SPC) of the AASM developed and the Board of Directors of the AASM approved these practice parameters. All members of the AASM SPC and Board of Directors completed detailed conflict-of-interest statements and were found to have no conflicts of interest with regard to this subject. The recommendations were also critically reviewed by 2 outside experts, and the concerns that were raised were addressed by the SPC prior to approval by the Board.
These practice parameters define principles of practice that should meet the needs of most patients in most situations. These guidelines should not, however, be considered inclusive of all proper methods of care or exclusive of other methods of care reasonably directed to obtaining the same results. The ultimate judgment regarding propriety of any specific care must be made by the physician, in light of the individual circumstances presented by the patient, available diagnostic tools, accessible treatment options, and resources.
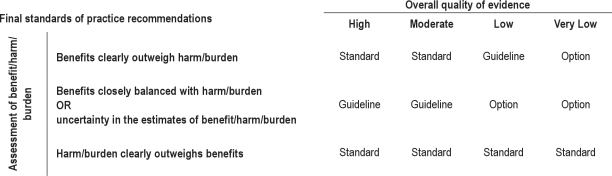
The AASM expects these guidelines to have an impact on professional behavior, patient outcomes, and, possibly, health care costs. These practice parameters reflect the state of knowledge at the time of publication and will be reviewed, updated, and revised as new information becomes available. Definitions of levels of recommendations used by the AASM appear in Table 2. Particularly noteworthy on this table is that when harm/burden clearly outweighs benefit, a STANDARD level of recommendation against the proposed therapy is given regardless of the overall quality of evidence. Sections titled “Values and Trade-offs” appear under each individual practice parameter. The Values and Trade-offs discussion elucidates the rationale leading to each recommendation. These sections are an integral part of the GRADE system and offer transparency to the process.19
Table 2.
AASM levels of recommendation
4.0 TREATMENT OF CENTRAL SLEEP APNEA (CSAS)
As previously stated, the ICSD-22 identifies 5 central sleep apnea syndromes that can affect the adult population. These are: (1) Primary CSAS; (2) Cheyne-Stokes breathing pattern; (3) High-altitude periodic breathing; (4) CSAS due to medical condition not Cheyne Stokes; and (5) CSAS due to drug or substance. The diagnosis of Complex Sleep Apnea Syndrome, also known as CPAP-emergent Central Sleep Apnea, is not firmly established and is not a part of the ICSD-2 nosology. Complex Sleep Apnea Syndrome is characterized by the emergence or persistence of central respiratory events during CPAP or BPAP titration for treatment of OSA. Four studies were found that provide limited evidence for efficacy of treatments. Two studies20,21 suggest that Complex Sleep Apnea Syndrome may resolve with continued CPAP therapy for some individuals, whereas 2 other studies22,23 suggest that ASV treatment may lower the AHI. The available evidence was considered insufficient to warrant a treatment recommendation.
4.1 Primary CSAS
Due to the infrequent occurrence of primary CSAS, there is limited evidence specifically addressing therapeutic interventions for primary CSAS. In fact, only 5 studies with a total of 51 participants with primary CSAS who met inclusion criteria were identified. These studies reported on 4 different treatments including supplemental carbon dioxide, acetazolamide, zolpidem, and triazolam. No studies were found that met inclusion criteria on the treatment of primary CSAS with CPAP, bilevel positive airway pressure in a spontaneous-timed mode (BPAP-ST), or ASV.
In 1 non-randomized treatment study of 6 patients, Xie et al.24 reported that carbon dioxide, either administered as a gas or by the addition of dead space, significantly decreased the AHI compared to room air (from 43.8 ± 17.0 to 5.9 ± 6.0). However, carbon dioxide is not readily available as a commercial gas and can be difficult to titrate in an open circuit design. Therefore, carbon dioxide is not currently recommended as a treatment option for primary CSAS.
Two non-randomized treatment studies reported on the use of acetazolamide for primary CSAS. One (DeBacker et al.25) looked at low-dose (250 mg/day) acetazolamide use, while the other (White et al.26) employed high-dose acetazolamide (1000 mg/day) therapy. Low dose acetazolamide was found to significantly decrease the AHI (from 37.2 ± 23.2 to 12.8 ± 10.8)25 in 14 patients at 1-month follow-up. The central apnea index significantly decreased (from 54 ± 29 to 12 ± 20) in 6 patients after 1 week of therapy with high-dose use.26 Additionally, an improvement in daytime sleepiness was reported with low-dose therapy.25
In another non-randomized treatment trial, Quadri et al.27 reported that zolpidem decreased AHI from 30.0 ± 18.1 to 13.5 ± 13.3 (P = 0.0001) over an average of 9 weeks of treatment in 20 patients. Zolpidem also decreased the central apnea hypopnea index (CAHI) and arousals, improved sleep quality and subjective excessive daytime sleepiness, but had mixed results in terms of its effect on obstructive events. In a randomized crossover trial with limitations, Bonnet et al.28 reported that triazolam decreased AHI (with borderline statistical significance, P = 0.05) and significantly decreased the central apnea index in 5 patients.
4.1.a: Positive airway pressure therapy may be considered for the treatment of primary CSAS. (OPTION)
Values and Trade-offs: The literature on the use of PAP therapy (CPAP, BPAP-ST, ASV) for the treatment of primary CSAS is very limited. However, PAP therapy offers the following benefits: (1) it has the potential to ameliorate central respiratory events; (2) it typically does not confer significant risks; and (3) it is readily available in most centers. Therefore, PAP therapy can be considered for the treatment of primary CSAS. The overall very low level of quality of evidence rendered an OPTION level recommendation.
4.1.b: Acetazolamide has limited supporting evidence but may be considered for the treatment of primary CSAS. (OPTION)
Values and Trade-offs: Given the low overall quality of evidence and the potential for side effects including paresthesias, tinnitus, gastrointestinal symptoms, metabolic acidosis, electrolyte imbalance, and drowsiness, the use of acetazolamide for the treatment of primary CSAS received an OPTION level recommendation.
4.1.c: The use of zolpidem and triazolam may be considered for the treatment of primary CSAS only if the patient does not have underlying risk factors for respiratory depression. (OPTION)
Values and Trade-offs: Due to the limited available evidence and the significant potential for adverse side effects especially respiratory depression, the use of zolpidem and triazolam in the setting of primary CSAS is not a preferable option and remains the last therapeutic option, to be considered only if the other therapeutic options listed above fail. Very close clinical follow-up must be provided to consider the use of these hypnotic agents.
4.2 CSAS Due to Congestive Heart Failure (CHF) Including Cheyne Stokes Breathing Pattern (CSBP) and Not Cheyne Stokes Breathing
Several treatment modalities, including assisted breathing devices and pharmacological therapies, have been studied to address CSAS and CSBP in CHF patients. Continuous positive airway pressure (CPAP), other fixed pressure devices (e.g., BPAP), adaptive servo-ventilation (ASV), oxygen, and acetazolamide have been most extensively investigated. Three outcomes measures were compiled and reported when there was sufficient data: transplant-free survival, LVEF, and AHI.
It is important to note that optimizing therapy for heart failure is central to treating CSAS. While appropriate pharmacological treatment is an essential element of therapy, implementing non-pharmacological therapies such as cardiac resynchronization therapy (CRT), atrial overdrive pacing (AOP), and cardiac transplant are also part of the armamentarium for CHF therapy. Although CSAS is not in and of itself an indication for CRT, AOP, or heart transplant, improvements in CSAS can be seen with the implementation of these interventions. As a point of interest, available data examining the effect of these procedures on CSAS has been included at the end of this section.
4.2.1 Continuous Positive Airway Pressure (CPAP)
Sixteen studies were found that addressed the effect of CPAP on CSAS associated with CHF.29–44 The studies included both non-randomized treatment and randomized controlled trials. Treatment lengths ranged from 1 night to 2 years.
A key limitation observed in many of the studies was that CPAP therapy was not titrated; therefore, its effectiveness was unclear. The overall grade for the body of evidence for transplant-free survival, LVEF, and the AHI RCTs was moderate as shown in Table 3. The low quality before-after AHI grade summary is also presented in the bottom row of the table.
Table 3.
Summary of quality and findings for CPAP
| Quality assessment |
Summary of findings |
Importance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Limitations | Inconsistency | Indirectness | Imprecision | Other considerations | No of patients |
Effect | Quality | ||
| CPAP | Control | Absolute (95% CI) | |||||||||
| Transplant-free survival (follow-up mean 2 years; event rates) | |||||||||||
| 329,30,33* | randomized trials | no serious limitations | no serious inconsistency | no serious indirectness | Serious | Not titrated | 71 | 125 | 9-33% event rate for suppressed CPAP vs. 24-56% event rate for controls | ⊕⊕⊕○ MODERATE |
CRITICAL |
| LVEF (follow-up 1-3 months; measured with: %; range of scores: 0-100; Better indicated by higher values) | |||||||||||
| 6;30,32–34,38,39 143 | randomized trials; non-randomized trial | no serious limitations | no serious inconsistency | no serious indirectness | Serious | Not titrated | 191 | 186 | MD 6.4 higher (2.4 to 10.5 higher) | ⊕⊕⊕○ MODERATE |
IMPORTANT |
| AHI (follow-up 1-3 months; measured with: No./hr; Better indicated by lower values) | |||||||||||
| 4;30,32,38,39 143 | randomized trials; non-randomized trial | no serious limitations | no serious inconsistency | no serious indirectness | no serious imprecision | Not titrated | 139 | 143 | MD 21 lower (25 to 17 lower) | ⊕⊕⊕○ MODERATE |
IMPORTANT |
| AHI (follow-up 1-84 days; measured with: No./hr; Better indicated by lower values) | |||||||||||
| 830–32,37–39,42,43 | Before-after trial data | very serious | no serious inconsistency | no serious indirectness | no serious imprecision | Not titrated | 19 | 0 | MD 30 lower (23 to 37 lower) | ⊕⊕○○ LOW |
IMPORTANT |
2 analyses were performed on the same study data (Bradley and Arzt).
4.2.1.1 Transplant-Free Survival
Evidence assessing the outcome of CPAP therapy on transplant free survival is limited. The CANPAP trial represents the foremost study addressing the impact of CPAP on transplant-free survival.30 While this study offered significant insight regarding CPAP therapy for CSAS/CSR, some limitations precluded concurrence with the investigators' conclusion that CPAP is ineffective in this patient population. What can be surmised from this trial is that CPAP therapy had no direct effect on cardiac function or survival if sleep disordered breathing was not adequately controlled.
Subsequently, Arzt et al.29 conducted a post hoc analysis of the data from the above study to determine the effect of CPAP on transplant-free survival when subjects were stratified based on residual disease with CPAP therapy and found that when CSAS was adequately treated, a positive effect on both LVEF and transplant-free survival (event rate 9% for CSAS-suppressed group versus 24% in control and 30% in non-suppressed groups) were noted.
Sin et al.33 also studied transplant-free survival and LVEF in patients with CHF with and without CSR-CSAS. Through stratification, significant trends towards lower mortality and cardiac transplantation rates were noted among the CPAP group versus the control group (33% event rate in the CPAP group versus 56% in the control group; relative risk reduction, 67%; 95% CI −4% to 89%; P = 0.059).
In summary, the available data suggests that PAP may improve survival if titrated to achieve a therapeutic reduction of AHI. Conversely, PAP therapy has no effect on survival if not adequately treated.
4.2.1.2 LVEF
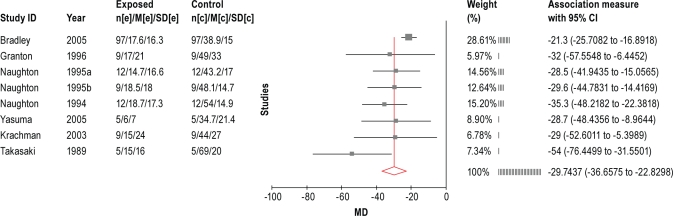
Eleven studies investigated the effects of CPAP on LVEF. A meta-analysis (Figure 1) was performed using the data from 7 trials that had control data available, including 6 RCT trials30,32–34,38,39 and 1 non-randomized trial.43 The random-effects meta-analysis showed that CPAP increased LVEF by 6% [95% CI 2.4 to 10.5%] on average when compared with the control group.
Figure 1.
Meta-analysis of LVEF from controlled CPAP treatment trials
4.2.1.3 AHI
Two meta-analyses were performed. The first (Figure 2) used the data from 5 trials that had control data available, including 4 RCT trials30,32,38,39 and 1 non-randomized trial.43 The random-effects meta-analysis showed that CPAP decreased AHI by 21/h [95% CI: 17 to 25] over controls. The second meta-analysis was conducted using before-after data from an additional 3 trials.31,37,42 The results are shown in Figure 3 and demonstrate a decrease of 30/h [95% CI: 23 to 37] with treatment compared to baseline. Notably, residual disease, with a mean AHI of 15 ± 4, remained in all the studies despite CPAP treatment.
Figure 2.
Meta-analysis of AHI from controlled CPAP treatment trials
Figure 3.
Meta-analysis of AHI from before-after CPAP treatment trials
4.2.1.4 AHI: Other analyses
A post hoc analysis (Arzt et al.29) performed on the Bradley et al.30 data found that only some of the participants had their CSAS suppressed by CPAP. Table 4 shows the results. These data indicate that either (1) a subgroup of patients respond to CPAP while others do not; or (2) adequate pressure was not given, as participants were treated with a pressure of 10 cm H2O or the maximum pressure tolerated.
Table 4.
AHI data: suppressed vs. unsuppressed
| Author, Year | Duration of CPAP | Stratification | AHI baseline (± SD) | AHI after CPAP (± SD) | n | Titrated |
|---|---|---|---|---|---|---|
| Arzt, 2007 / Ruttanaumpawan, 2009 | 3 mo. | Suppressed | 33.8 ± 12.7 | 6.2 ± 3.9 | 58 | No |
| Unsuppressed | 46.9 ± 14.9 | 34.6 ± 12.4 | 39 |
Two more studies further expanded on the former point that some patients respond to CPAP while others do not. The data in Table 5 summarize the results from Dohi et al.36 and Javaheri.44 Importantly, these patients were titrated to an endpoint of elimination of apneas and hypopneas.
Table 5.
AHI data: CPAP responders vs. nonresponders
| Author, Year | Duration of CPAP | Stratification | AHI baseline (± SD) | AHI after CPAP (± SD) | n | Titrated |
|---|---|---|---|---|---|---|
| Dohi, 2008 | 1 night | Responders | 50.6 ± 8.3 | 7.4 ± 3.5 | 11 | Yes |
| 1 night | Nonresponders | 54.4 ± 7.8 | 30.3 ± 11.7 | 9 | ||
| Javaheri, 2000 | 1 night | Responders | 62 ± 29 | 4 ± 2 | 9 | Yes |
| 1 night | Nonresponders | 62 ± 22 | 36 ± 15 | 12 |
4.2.1.a CPAP therapy targeted to normalize the apnea hypopnea index (AHI) is indicated for the initial treatment of CSAS related to CHF. (STANDARD)
Values and Trade-offs: The overall quality of evidence for the use of CPAP in the setting of CSAS related to CHF is moderate, but with a large effect size and consistent findings for reduction of AHI and improvement in LVEF. Post hoc analysis of the CANPAP data indicates that CPAP treatment targeted to an AHI < 15 has a positive effect on transplant-free survival in patients with CSAS and CHF. Given the relative ease of availability of this therapeutic intervention and overall familiarity with its use, a STANDARD level of recommendation was given. An alternate treatment option should be considered in the absence of adequate control of CSAS related to CHF with CPAP.
4.2.2 Bilevel positive airway pressure (BPAP)
BPAP may be used in patients who require high PAP level or as a pressure-support ventilatory method to augment alveolar ventilation. In fact, BPAP, in the spontaneous mode, may precipitate periodic breathing and central apnea and has been used experimentally for this purpose in sleep research laboratories. BPAP effects may be specific to the mode (spontaneous [S] or spontaneous-timed [ST] mode) or to the level of pressure support. Thus, it is difficult to isolate the independent effect of BPAP on central apnea.
4.2.2.1 BPAP-S
There was 1 study that met inclusion criteria for BPAP-S.45 This was a small RCT of 10 patients on BPAP-S with standard medical therapy vs. 11 patients on standard medical therapy alone. The change in LVEF from baseline at 3 months was reported to be +20.3% ± 8.2% with BPAP-S versus +3.2% ± 10.1% with standard medical therapy alone. The 1 night change in AHI was 28.3 ± 12.3/h at baseline to 5.2 ± 3.8 after BPAP-S. The patients were followed for a mean of 31.0 ± 2.3 months, and BPAP-S appeared to improve survival (10/10 patients using BPAP-S versus 7/11 controls survived); however, survival was not a stipulated outcome. BPAP in the spontaneous mode may aggravate central apnea caused by hyperventilation. Further investigations are necessary to more definitively characterize the association between BPAP-S and the outcomes of interest.
4.2.2.2 BPAP-ST
Four studies36,46–48 investigating the effect of BPAP-ST on CSAS related to CHF were found. The studies included non-randomized treatment trials as well as randomized controlled trials. Out of the 4 studies, 1 study evaluated the effect of BPAP-ST on CPAP non-responders, as defined by the persistence of central sleep apnea on CPAP (AHI ≥ 15).36 One study48 was not included in the analysis because it used a volume preset ventilator as opposed to BPAP-ST. The compiled grades for LVEF and AHI are very low as detailed in Table 6.
Table 6.
Summary of quality and findings for BPAP-ST
| Quality assessment |
Summary of findings |
Importance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Limitations | Inconsistency | Indirectness | Imprecision | Other considerations | No of patients |
Effect | Quality | ||
| BPAP-ST | control | Absolute | |||||||||
| LVEF (follow-up mean 3-6 months; Better indicated by higher values) | |||||||||||
| 236,46 | Non-randomized | serious | no serious inconsistency | no serious indirectness | serious | small sample size | 14 | 14* | See text | ⊕○○○ VERY LOW |
IMPORTANT |
| AHI (follow-up 1 night; Better indicated by lower values) | |||||||||||
| 336,46,47 | Non-randomized | serious | no serious inconsistency | no serious indirectness | serious | short-term | 25 | 25** | MD 44 lower (40 to 49 lower) | ⊕○○○ VERY LOW |
IMPORTANT |
Patients served as their own controls in 1 of the studies.
Control data are baseline values.
4.2.2.2.1 LVEF
Two studies36,46 reported the effects of BPAP-ST on LVEF. Dohi et al.36 reported the change in LVEF versus baseline on 7 patients after 6 months of treatment as +12.7% ± 10.0%. Kasai et al.46 reported in a non-randomized trial that the LVEF of the group of 7 patients receiving BPAP-ST improved 9.9% ± 8.6% over baseline versus the control group, in which the LVEF decreased by 1.4% ± 8.5%.
4.2.2.2.2 AHI
There were 3 studies that directly studied the effect of BPAP-ST on AHI. None of the trials had a control arm. The meta-analysis indicated an average decrease in the AHI by 44 [95% CI −40 to −49] with treatment versus baseline as shown in Figure 4. All studies showed that the fixed pressure devices decreased the average AHI to less than or equal to 10.
Figure 4.
Meta-analysis of AHI from before-after 1-night BPAP-ST treatment trials
4.2.2.3 BPAP-ST vs. CPAP
BPAP-ST was directly compared to CPAP in a 14-day randomized crossover trial by Köohnlein et al.49 Sixteen patients had CHF with a NYHA Class of 2.8 ± 0.4 and LVEF of 24 ± 7. Patients were not titrated. After PAP therapy was initiated, the NYHA Class improved to 2.0 ± 0.4 for CPAP users and to 1.9 ± 0.5 for BPAP users. Overall, CPAP and BPAP-ST were equally effective in lowering the AHI (from a baseline of 26.7 ± 10.7 to 7.7 ± 5.6 with CPAP and 6.5 ± 6.6 with BPAP-ST) and NYHA class.
4.2.2.a BPAP therapy in a spontaneous timed (ST) mode targeted to normalize the apnea hypopnea index (AHI) may be considered for the treatment of CSAS related to CHF only if there is no response to adequate trials of CPAP, ASV, and oxygen therapies. (OPTION)
Values and Trade-offs: There were a limited number of studies that examined the effectiveness of BPAP in the treatment of CSAS/CSR. ST mode was used more frequently compared with spontaneous mode in the available studies. The level of evidence for BPAP with spontaneous mode is comprised only of 1 trial that met inclusion criteria. Therefore, no recommendation can be made for this mode of BPAP until further evidence is available. BPAP-ST therapy offers many of the same advantages as CPAP therapy, such as low risk and easy availability. BPAP-ST may be considered only in those who fail CPAP, ASV, and oxygen therapy, as these latter options have substantially more evidence supporting their use. BPAP-ST is a form of noninvasive ventilation that requires specialized expertise. The cost is approximately $1900 compared with $400-$1000 for CPAP.50 The paucity of data allows only an OPTION level of recommendation at this time.
4.2.3 Adaptive Servo-Ventilation (ASV)
Adaptive servo-ventilation (ASV) is a form of closed-loop mechanical ventilation, pressure preset, and volume or flow cycled. It can be delivered at default settings or with variable inspiratory and expiratory pressure (to ensure upper airway patency). ASV alleviates central sleep apnea due to CSBP by providing dynamic (breath-by-breath) adjustment of inspiratory pressure support with a back-up rate to normalize breathing patterns relative to a predetermined target. Specifically, ASV mitigates hyperventilation and associated hypocapnia by delivering preset minute ventilation.
The ResMed ASV (AutoSet CS, AutoSet CS2, VPAP Adapt, or VPAP AdaptSV) provides EEP that can be adjusted to stabilize the upper airway obstruction. These devices target 90% of the calculated ventilatory assistance over a 3-minute moving window, to minimize hypo- and hyperventilation. ResMed ASV initially provides pressure support that varies between 3 and 15 cm H2O, and if not sufficient to maintain 90% of the calculated ventilatory assistance, it then increases the back-up respiratory rate.
The Respironics ASV (BiPAP autoSV or HEART PAP) targets the average peak flow, which is calculated over a 4-minute moving window. Similar to ResMed ASV, the EPAP in BiPAP autoSV serves to stabilize upper airway obstruction, while the IPAP max increases when the flow signal is below the target peak flow. If the flow target is reached, the device does not offer any additional pressure support or a minimum level of support if the IPAP min is set slightly above EPAP. Similar to Resmed ASV, the Respironics ASV has a back-up rate that can be set in auto mode or manually adjusted.
One study51 compared therapeutic to subtherapeutic ASV, 2 non-randomized trials52,53 compared ASV treatment to baseline, 3 studies45–47 compared ASV to CPAP, 2 studies54,55 compared it to BPAP-ST, 1 study56 compared ASV to either CPAP or BPAP (these 2 treatment results were combined), and 1 study compared ASV to oxygen.57
In summary, the data for LVEF and AHI are moderate as shown in Table 7. Meta-analyses indicate that ASV improves LVEF by 6% [95% CI 4%-8%] higher and decreases the AHI by 31 [95% CI −25 to −36] over baseline, and by 12-23/h compared with CPAP. Furthermore, it is worth noting 2 more recent studies58,59 (which were not included in this analysis as they fell outside the defined search dates) both confirmed significant improvements in AHI and LVEF with ASV treatment that were consistent with the analyzed data. Kasai et al.60 notes that although CPAP can suppress CSAS (e.g., 1-night data), compliance can be an issue limiting its effectiveness over time. They report that compliance was significantly better with ASV than with CPAP (5.2 ± 0.9 with ASV vs. 4.4 ± 1.1 h/night with CPAP). There are no long-term outcome data.
Table 7.
Summary of quality and findings for ASV
| Quality assessment |
Summary of findings |
Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Limitations | Inconsistency | Indirectness | Imprecision | Other considerations | No of patients | Effect | Quality | |
| Absolute | ||||||||||
| LVEF (follow-up 0.5-6 months; measured with: %; Better indicated by higher values) | ||||||||||
| 6 | 4 randomized51,54,60,61 and 2 non-randomized52,57 trials | 2 RCTs-no limitations; 2 RCTs – limitations; 2 NRTs – no other limitations | no serious inconsistency | no serious indirectness | no serious imprecision | Generally funded by manufacturers | 951 | MD 6.1 higher (3.9 to 8.4 higher) | ⊕⊕⊕○ MODERATE |
IMPORTANT |
| AHI (follow-up 0.005 - 6 months; measured with: No./hr sleep; Better indicated by lower values) | ||||||||||
| 9 | 6 randomized51,53–55,60,61 and 3 non-randomized52,56,57 trials | 2 RCTs – no limitations; 4 RCTs – limitations / 1 night of study / small n; 2 NRTs no other limitations; 1 NRT – only 1 night | no serious inconsistency | no serious indirectness | no serious imprecision | Generally funded by manufacturers | 1271 | MD 30.8 lower (36.4 to 25.3 lower) | ⊕⊕⊕○ MODERATE |
IMPORTANT |
Results vs. baseline, patients served as their own controls.
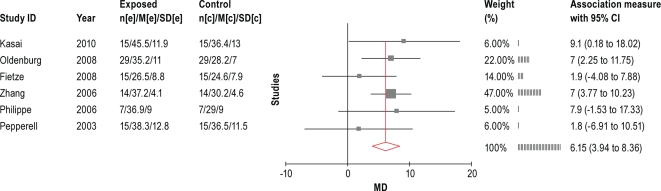
4.2.3.1 LVEF
Six studies51,52,54,57,60,61 reported on the effects of ASV on LVEF. The meta-analysis of the change in LVEF with treatment versus baseline is shown in Figure 5. The data show that ASV improves LVEF by 6.2% (95% CI 3.9% to 8.4%). Two longer-term (3-6 months) studies by Philippe et al.61 and Kasai et al.60 showed a statistically significant increase in LVEF with ASV, whereas CPAP did not. Though the study by Fietze et al. showed no effect of ASV on LVEF, BPAP-ST statistically significantly increased LVEF.54
Figure 5.
Meta-analysis of LVEF from before-after ASV treatment trials
4.2.3.2 AHI
Nine studies51–57,60,61 reported data on the effects of ASV on AHI and were consistent in showing that ASV improves AHI over baseline. Meta-analyses indicate that ASV decreases AHI by 31/h [95% CI −25 to −36] over baseline as shown in Figure 6. Furthermore, 6 of the studies51,52,55,56,60,61 showed a normalization of AHI to 5 or less.
Figure 6.
Meta-analysis of AHI from before-after ASV treatment trials
Four studies report that ASV decreases AHI by 12-23 compared to CPAP treatment.55,56,60,61 Two studies showed equivalence between ASV and BPAP-ST.54,55 One study57 compared ASV to oxygen and found that ASV decreased the AHI by 21 events/h compared to oxygen (a decrease of 81% vs. 19%, respectively).
4.2.3a Adaptive Servo-Ventilation (ASV) targeted to normalize the apnea-hypopnea index (AHI) is indicated for the treatment of CSAS related to CHF. (STANDARD)
Values and Tradeoff: The overall quality of evidence for ASV is moderate. While there is no survival or long-term data available for ASV at this time, there is a sufficient amount of data consistently demonstrating improvement in both the AHI and LVEF. Additionally, there was a study suggesting overall better compliance with ASV compared with CPAP. It is worth noting that most of the available studies are industry sponsored, and different manufacturers utilize different algorithms to detect respiratory events and determine characteristics of pressure delivery. Therefore, generalizability is not possible or appropriate. There is also some uncertainty as to what are the optimum settings, reflecting an overall lack of experience with using these devices.62 It should be mentioned that the cost of these devices50 is several-fold greater than the cost of CPAP, and availability is not universal. Nonetheless, the data for ASV is consistent and is at least comparable if not better than the data supporting CPAP use.
4.2.4 Oxygen
Oxygen has been used as a therapeutic intervention in patients with central sleep apnea secondary to heart failure. The mechanisms underlying the effect of oxygen on ventilatory control during sleep remain elusive. Potential mechanisms include reduced CO2 chemoreflex sensitivity or increased cerebral PCO2 level.63 Specifically, hyperoxia exposure can result in reduced controller gain due to inhibition of peripheral chemosensitivity.64 Several studies have reported on the effects of oxygen supplementation on the AHI and cardiac LVEF. In these studies, patients had systolic heart failure with NYHA class II to IV functional status52–60 and were receiving65–69 “optimal” pharmacotherapy for CHF. The duration of therapy with oxygen varied from a single night to 12 months. The summary of the grades and treatment effects are shown in Table 8.
Table 8.
Summary of quality and findings for oxygen
| Quality assessment |
Summary of findings |
Importance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Limitations | Inconsistency | Indirectness | Imprecision | Other considerations | No of patients |
Effect | Quality | ||
| oxygen | control | Absolute | |||||||||
| LVEF (follow-up 3-12 months; measured with: %; Better indicated by lower values) | |||||||||||
| 3 | 2 randomized69,70 and 1 non-randomized71 trials | 2 RCTs – no limitations; 1 NRT – no other limitations | no serious inconsistency | no serious indirectness | no serious imprecision | underpowered | 491 | 49 | MD 5.0 higher (0.3 to 9.8 higher) | ⊕⊕⊕○ MODERATE |
IMPORTANT |
| AHI (follow-up 1-12 months; measured with: No./hr; Better indicated by lower values) | |||||||||||
| 3 | randomized trials67,69,70 | no serious limitations | no serious inconsistency | no serious indirectness | no serious imprecision | Some issues with power | 42 | 42 | MD 15 lower (7 to 23 lower) | ⊕⊕⊕○ MODERATE |
IMPORTANT |
| AHI (follow-up 1-120 nights; measured with: No/hr; Better indicated by lower values) | |||||||||||
| 7 | 5 before/after57,71–74 and 2 randomized crossover66,68 without baseline data | 3 NRTs - no other limitations; 2 NRTs -underpowered; 2 NRTs- short term (1 night to 1 week) | no serious inconsistency | no serious indirectness | no serious imprecision | none | 129 | 02 | MD 18 lower (10 to 26 lower) | ⊕⊕⊕○ MODERATE |
IMPORTANT |
Another 24 patients were tested in the shorter-term trials (patients were their own controls).
Patients served as their own controls; another 9 patients were tested in Brostrom that were not included because the data were not normally distributed.
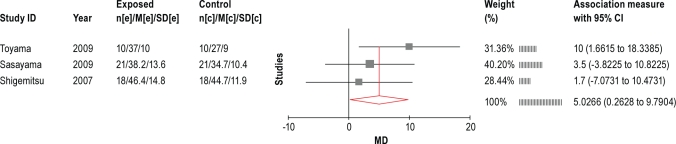
4.2.4.1 LVEF
Although none of the studies reported on mortality/transplant-free survival, 1 study70 reported no difference in the cumulative incidence rate of cardiac events between the oxygen therapy and control groups (hazard ratio for cardiac events 0.78; 95% CI, 0.30-2.05; P = 0.619 [log-rank test]). Six studies57,65,69–71,74 reported changes in LVEF following treatment with oxygen. The data consistently showed an improvement in LVEF with oxygen treatment. The 3 longest-term trials (3-12 months in duration, 2 RCTs and 1 non-randomized trial) were used in the meta-analysis. Figure 7 shows the results of the meta-analysis, indicating an average improvement in LVEF of 5% (95% CI 0.3 to 9.8). The analysis included only the before and after data since the trials comprised 2 trial designs. The control data from the 2 RCTs showed approximately a 1% improvement in LVEF.
Figure 7.
Meta-analysis of LVEF from controlled oxygen treatment trials
4.2.4.2 AHI
The studies reporting on the effect of oxygen on AHI fell into 2 groups: those that were randomized with control groups (RCT)65,67,69,70 and those that were either non-randomized before-after trials,57,71–74 or randomized for treatment but without baseline measurements.66,68 All except 1 study75 reported a statistically significant decrease in AHI with oxygen supplementation. Javaheri et al.73 further reported that there were responders (n = 14, AHI < 15/h) and partial responders (n = 22) to oxygen therapy. The responders had a lower initial AHI and higher PaCO2.
Meta-analyses on these 2 groups were performed separately. One study was excluded from analysis because the data were non-normally distributed.75 The meta-analyses provided similar results as shown in Figures 8 and 9. The RCT analysis showed an average AHI decrease of 15 [95% CI: −7 to −23] per hour with oxygen treatment over the control group. The meta-analysis of the before-after studies and studies with no baseline measurements showed an average AHI decrease of 18 [95% CI: −10 to −26] per hour from baseline after oxygen treatment.
Figure 8.
Meta-analysis of AHI from RCT oxygen treatment trials
Figure 9.
Meta-analysis of AHI from before-after oxygen treatment trials
Other germane findings from these studies include: a reduction in sympathetic nerve activity69 but with no demonstrable effect on cognitive function, patient symptoms (as measured by Epworth Sleepiness Scale or Visual Analogue Scale), or statistically significant improvements in sleep (decreased stage 1 sleep and arousals with increased stage 2 and slow wave sleep)66,67,72; however, daytime symptoms did not improve significantly.68 In a separate study,73 sleep parameters did not differ with the use of oxygen therapy for the group in total, but they did for “responders” who exhibited increased sleep efficiency and a decreased number of arousals. Other reported results include improvement65,70 or no change in QOL,75 improvement (decrease) in serum BNP levels,71 and a significant improvement in functional exercise capacity,67,75 although without significant improvement in LVEF in all cases.75 It is noteworthy that no adverse events were reported with prolonged oxygen supplementation in these studies.
In addition to the studies above, 2 studies directly compared oxygen to CPAP. In-laboratory titration was not performed in either study. Arzt et al.76 reported that both supplemental oxygen and CPAP reduced AHI, but only CPAP improved the apnea index to a statistically significant degree. It was not clear whether the apnea index was composed of central, obstructive, or both types of respiratory events. Furthermore, CPAP, not oxygen, significantly improved LVEF and ventilatory efficiency (V·E/V·CO2-slope) during exercise and exercise capacity (peakV·O2). In a second study of patients with severe CHF, Krachman et al.77 reported that both treatments significantly reduced AHI, with no difference between 2 treatment groups. Oxygen and CPAP were equally effective in improving mean oxygen saturation and decreasing mean percent time with oxygen saturation < 90%, but total sleep time and sleep efficiency decreased with CPAP therapy, while the arousal index was unchanged with both supplemental oxygen therapy and CPAP therapy. The data are presented in the Appendix.
4.2.4. Nocturnal oxygen therapy is indicated for the treatment of CSAS related to CHF. (STANDARD)
Values and Trade-offs: Based on above data, the benefits of oxygen supplementation for the treatment of CSAS are abundant and outweigh any potential disadvantages. While the variable duration of treatment in each study limits recommendations in regard to duration of oxygen therapy, the overall positive direction of results with respect to reducing AHI and improving LVEF confirms our recommendation. Although 1 paper reported that the cumulative incidence rate of cardiac events was no different between oxygen therapy and control groups, its effect on transplant free survival has not been assessed. The universal availability of oxygen therapy coupled with the overall quality of evidence discussed above influenced the level of recommendation. It should be noted that while oxygen therapy does not confer outcome advantages over CPAP therapy in the available evidence, supplemental oxygen can be easily administered and can be given for those individuals with CSAS related to CHF who are unable to comply with CPAP therapy. Consideration should be given to a repeat sleep study with oxygen to ensure adequate resolution of central sleep apnea events. In the US, the current cost of supplemental oxygen therapy is approximately $200 per month.
4.2.5 Direct comparison treatment studies with more than 2 treatment modalities
Three studies directly compared more than 2 treatment modalities: Hu et al.78 evaluated the effectiveness of CPAP, BPAP, oxygen, and high-frequency jet ventilation; Teschler et al.79 evaluated the effectiveness of CPAP, BPAP-ST, oxygen, and ASV; and in a retrospective review, Allam et al.23 reported on the effects of ASV, CPAP (although all the patients were selected to have suboptimal response to CPAP), BPAP-ST, and ASV in patients with CSAS and CSAS/CSR. Allam's study also included patients without CHF (68% of CSAS patients and 40% of CSAS/CSR patients) and those on opioids (23% of those with CSAS). In Hu's78 study, all treatment modalities improved AHI to a statistically significant extent; however, residual disease persisted, as shown in the table. Comparing all treatment modalities, only BPAP was statistically significantly better than the other treatment modalities in reducing the AHI. Teschler et al.79 reported that all treatment devices (oxygen, CPAP, BPAP, and ASV) significantly reduced AHI compared to baseline. Furthermore, ASV was significantly superior to all other treatment devices. Additional analysis showed that BPAP also was significantly better than CPAP (P = 0.027). Allam et al.23 reported that ASV performed almost equivalently to BPAP-ST for patients with CSAS and equivalent to CPAP (and better than BPAP-ST) for patients with CSAS/CSR. The data are presented in Table 9.
Table 9.
AHI data for head-to-head studies comparing more than 2 treatment modalities
| Author, Year | Duration of Trial | AHI baseline (± SD) | AHI after Test* Treatment (± SD) | AHI CPAP (± SD) | AHI BPAP (± SD) | AHI oxygen (± SD) |
|---|---|---|---|---|---|---|
| Hu, 2006 | 1 night | 30.9 ± 8.3 | 20.1 ± 4.1 | 18.5 ± 5.0 | 14.3 ± 3.9 | 23.6 ± 6.6 |
| Teschler, 2001 | 1 night | 44.5 ± 12.7 | 6.3 ± 3.4 | 26.8 ± 17.2 | 14.8 ± 8.6 | 28.2 ± 12.7 |
| Allam, 2006**: CSAS | 1 night | 60 (40.5–72.5) | 7 (4–11) | 68.5 (34.3–77.8) | 11 (5.5–61) | N/A |
| Allam, 2006**: CSAS/CSR | 1 night | 50 (38–69) | 4 (0–14) | 12 (4.5–35.3) | 26 (19–49) | N/A |
Test treatment for Hu was high-frequency jet ventilation, and for Teschler and Allam it was ASV.
Data reported as median with interquartile range
4.2.6 Alternate therapies for CSAS related to CHF
Several alternate therapies have been examined for the treatment of CSAS/CSR associated with CHF. These include pharmacological agents such as acetazolamide,80 theophylline,81,82 carvedilol,83,84 and captopril.85 Additionally, one study evaluated the use of erythropoietin and intravenous iron in patients with CHF and anemia.86 Lastly, the use of carbon dioxide has also been examined.87,88 The detailed results are presented in the Appendix.
In a randomized crossover study, Javaheri80 showed statistically significant improvement in both objective (AHI) and subjective measures (patient-reported sleep quality and daytime fatigue) with acetazolamide. However, no statistically significant improvement in LVEF was observed in this study.
There were 2 studies looking at the use of theophylline for the treatment of CHF related CSAS: 1 randomized crossover (Javaheri et al.81) and 1 non-randomized treatment trial (Hu et al.82). Both studies demonstrated statistically significant declines in the AHI, and 1 study82 showed a statistically significant decrease in EEG arousals with theophylline use. However, no statistically significant changes in sleep architecture,82 sleep efficiency,82 or LVEF81 were observed.
In 2 well-conducted but preliminary studies,83,84 Tamura et al. reported statistically significant improvements in both LVEF (32% ± 7.4% to 45% ± 9.8%, P < 0.001) and AHI (34 ± 13 to 14 ± 13, P = 0.003) with 10-20 mg/d of the β-blocker carvedilol. However, the mechanisms through which the improvement in the CAI is effected are not clearly delineated. While there is some evidence that β-blockers decrease central chemosensitivity, it is likely that improvement in LVEF plays a key role in the concomitant decline seen in central respiratory events.
In a non-randomized treatment trial with limitations, Walsh et al.85 showed a statistically significant decrease in AHI with captopril in participants with mild to moderate CHF. An increase in slow wave sleep and REM sleep times, subjective sleep quality, and daytime energy levels were noted. End-tidal CO2 concentrations and daytime minute ventilation were reduced. No significant changes in cardiac output and oxygen uptake were observed. However, it is well established that ACE inhibitors help enhance cardiac function in the setting of CHF, and this may contribute to the reduction in CSAS seen in this particular study.
Overall, the use of β-blockers and ACE inhibitors has become part of the standard regimen for the treatment of CHF. So, while the 3 studies discussed above provided data showing improvement in central respiratory events with the use of these medications, it remains difficult to confidently state that these agents independently treat CSAS that is associated with CHF. This further highlights that optimization of CHF therapy in this setting is essential.
In a non-randomized treatment trial consisting of participants with CHF and anemia, Zilberman et al.86 reported that the AHI statistically significantly improved when anemia was corrected with erythropoietin and intravenous iron. The decrease in AHI correlated with an increase in hemoglobin levels. In all 38 participants (of whom one had no sleep disordered breathing and 2 had OSA), statistically significant improvements were seen in the ESS, NYHA class (2.9 ± 9.4 to 1.7 ± 0.7), and daytime sleepiness as measured by a visual analog scale. However, given the low overall level of evidence coupled with the potential side effects, no recommendation could be made regarding the use of erythropoietin and intravenous iron.
In addition to the pharmacological therapies, 2 studies reported on the effect of carbon dioxide on AHI and sleep. In a randomized crossover study, Andreas et al.87 reported that 1 night of supplemental oxygen at 2 liters per minute (LPM) admixed with carbon dioxide at 0.2–1 LPM significantly decreased AHI (from 36.7 ± 21.9 in air to 5.4 ± 3.6 with oxygen and CO2) and duration of CSR compared to room air in 9 patients. CSR with apneas were noted in only 2 patients receiving oxygen plus carbon dioxide compared to 8 patients receiving room air. However, sleep architecture did not improve, arousals were not reduced, and there was evidence of increased sympathetic activation with the admixture of oxygen and carbon dioxide. This study did not measure the effect of oxygen alone. Steens et al.88 reported that 3% carbon dioxide, compared to room air, eliminated CSR/CSAS in patients with severe CHF, reducing AHI from 41.1 ± 28.9 to 1.0 ± 1.7 in the 6 patients. The central apnea index was not provided in either of these studies. In total, only 15 patients were studied for 1 night each. As previously mentioned, carbon dioxide is not universally available and is difficult to administer. Carbon dioxide is not a recommended treatment option.
4.2.6.a The following therapies have limited supporting evidence but may be considered for the treatment of CSAS related to CHF, after optimization of standard medical therapy, if PAP therapy is not tolerated, and if accompanied by close clinical follow-up: acetazolamide and theophylline. (OPTION)
Values and Trade-offs: There is only 1 study for acetazolamide and 2 studies for theophylline. Therefore the data for each agent are very low. Furthermore, the benefits vs. harms are unclear. Side effects of acetazolamide have been previously outlined. Theophylline is also associated with a number of potential adverse effects such as cardiac arrhythmias, CNS excitability, and gastrointestinal symptoms. Additionally, it has a narrow therapeutic index, and therefore close monitoring of levels is important. These pharmacological therapies require further research to generate more confidence in their effectiveness and to justify more than an OPTION level of recommendation.
4.2.7 Cardiac Interventions and CSAS
Cardiac resynchronization therapy (CRT) involves simultaneous pacing of one or both ventricles in patients with bundle branch blocks. Ventricular dyssynchrony in patients with CHF can further impair cardiac pump function of an already failing ventricle. CRT may improve pump performance and reverse the deleterious process of ventricular remodeling. The available data examining the effect of CRT on CSAS had a methodological limitation as the studies were not randomized, resulting in a moderate level of evidence. Table 10 shows the overall results. All studies showed statistically significant improvement in LVEF with CRT.89–94 The meta-analysis indicated an increase in LVEF by 8% [95% CI: 5% to 12%] as shown in Figure 10. Five of the 6 studies showed statistically significant improvement in AHI.68–71,73,74 One study did not show a statistically significant improvement in AHI but did show a statistically significant decrease in CSR events.93 The meta-analysis of all 6 studies showed an average decrease in AHI by 12 with CRT [95% CI: −9 to −14] as shown in Figure 11.
Table 10.
Summary of quality and findings for CRT
| Quality assessment |
Summary of findings |
Importance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Limitations | Inconsistency | Indirectness | Imprecision | Other considerations | No of patients | Effect | Quality | ||
| CRT | control | Absolute | |||||||||
| LVEF (follow-up 3-6 months; measured with: %; Better indicated by higher values) | |||||||||||
| 6 | Non-randomized treatment trials | serious | no serious inconsistency | no serious indirectness | no serious imprecision | None | 111 | 1111 | MD 8.2 higher (4.9 to 11.5 higher) | ⊕⊕⊕○ MODERATE |
IMPORTANT |
| AHI (follow-up 0.005-6 months; measured with: No./hr sleep; Better indicated by lower values) | |||||||||||
| 7 | Non-randomized treatment trials | serious | no serious inconsistency | no serious indirectness | no serious imprecision | none | 123 | 1231 | MD 11.8 lower (9.1 to 14.4 lower) | ⊕⊕⊕○ MODERATE |
IMPORTANT |
Patients served as their own controls (post-CRT or pre-CRT).
Figure 10.
Meta-analysis of LVEF from CRT treatment trials
Figure 11.
Meta-analysis of AHI from CRT treatment trials
Atrial overdrive pacing (AOP) paces the atria at a higher rate, usually 15-20 beats above the baseline heart rate. AOP helps CSAS probably by increasing cardiac output, decreasing pulmonary venous congestion, and shortening the circulation time. In the second part of Lüuthje's90 study, 30 patients were studied in a randomized crossover manner for 1 night. There was no statistically significant improvement with the addition of AOP to CRT (AHI 25.7 ± 17.5 vs. 23.7 ± 17.9, P = 0.07).
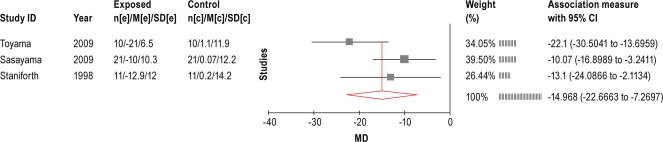
Cardiac transplant is most commonly used for patients with cardiomyopathy. In a prospective non-randomized controlled study,95 the association between heart transplant and CSAS was investigated in a post hoc analysis. In this randomized controlled trial, 13 participants with CHF + CSAS and 9 subjects with CHF but without CSAS underwent cardiac transplant. AHI, LVEF, and urinary norepinephrine excretion (UNE) were assessed at the time of enrollment and at an average of 13 months post operatively. In participants with CSAS, the AHI dropped from 28 ± 15 to 7 ± 6. Six patients were effectively cured (AHI < 5), CSAS persisted (AHI = 12.3 ± 0.9) in 3 patients, and 4 subjects developed OSA. No one in the control group (those without CSAS) developed OSA after heart transplant. Thus, even after normalization of cardiac function post-transplant, CSAS may persist, and OSA may develop. Both groups had significant improvements in LVEF (CSAS group, 19.2% ± 9.3% to 53.7% ± 6.1%, P < 0.001) and significant reductions in UNE (CSAS group, 48.1 ± 30.9 to 6.5 ± 4.8, P < 0.01).
The optimization of pharmacological therapy and the use of non-pharmacological methods to treat CHF or cardiomyopathy can lead to an improvement in CSAS. Interventions including CRT, AOP, and cardiac transplant are procedures that require specialized skills, have significant morbidity, are costly, and are not readily available. Despite the improvement noted in CSAS with these therapies, indications for these interventions are limited to those settings delineated by the cardiology literature.
4.3 CSAS Due to Medical Condition Not Cheyne Stokes: ESRD
CSAS can also occur with diseases and conditions other than congestive heart failure such as end-stage renal disease (ESRD). Since there are only 4 studies, all using different treatments, and all having significant limitations, the quality of evidence is very low. This precluded conducting a meta-analysis. The treatments fell into 2 groups: ventilatory (oxygen or CPAP) and dialysis (types of buffers or nocturnal).
In a non-randomized study, Kumagai et al.96 reported on the effect of oxygen in 11 peritoneal dialysis patients with sleep apnea syndrome. The nocturnal average oxygen saturation and minimum nocturnal oxygen saturation improved significantly. The AHI decreased from 31.1 ± 8.8 to 12.7 ± 8.5/h, and the central apnea index decreased from 4.0 ± 4.0 to 0.8 ± 1.2/h with oxygen. The authors note that the greatest effect of oxygen was on central apneas and hypopneas with little effect on obstructive apneas. In a non-randomized treatment study without blinded scoring, Pressman et al.97 reported the effect of 1 night of CPAP on 6 renal failure patients with CSAS and mixed apneas. CPAP was effective reducing the overall AHI (from 60.8 ± 43.4 to 6.0 ± 3.8) and improving oxygen saturation.
In a study limited by imprecision, Jean et al.98 reported the effect of bicarbonate versus acetate buffer during hemodialysis on 10 patients. While there were no significant differences in blood gas with either agent, there as an expected significant increase in serum bicarbonate with both buffers. Fewer central apneas were observed with the bicarbonate buffer compared with acetate buffer (3 [range, 0-15] on bicarbonate and 33 [range, 0-180] on acetate). There were no differences in oxygen saturation. The central apnea index decreased from 5.5 to 0.6/h on bicarbonate. Hypopneas were also significantly reduced with bicarbonate (19 vs. 13 per night).
In a non-randomized crossover study, Hanly and Pierratos99 compared nocturnal hemodialysis to conventional hemodialysis in 7 patients with chronic renal failure sleep disordered breathing events that were an equal distribution of central, mixed, and obstructive apneas. After a treatment period of 6-15 months, the central apnea hypopnea index compared to baseline values was lower with nocturnal dialysis (4 ± 2) vs. conventional hemodialysis (24 ± 27).
4.3. The following possible treatment options for CSAS related to end stage renal disease may be considered: CPAP, supplemental oxygen, bicarbonate buffer use during dialysis, and nocturnal dialysis. (OPTION)
Values and Trade-offs: At this time, the level of evidence is very low and the estimate of benefits vs. harms is unclear regarding any specific mode of therapy in ESRD patients with CSAS; therefore, an OPTION level of recommendation has been accorded. However, despite the very low level of evidence, it is clear that bicarbonate buffer is preferable during hemodialysis in these patients. Further studies are needed to elucidate the role of oxygen, CPAP, bicarbonate buffer use during dialysis, and nocturnal hemodialysis in patients with ESRD.
4.4 CSAS Due to High-Altitude Periodic Breathing
Periodic breathing during sleep results during the acclimatization period after rapid ascent to high altitudes. Three studies evaluated the effect of pharmacologic drugs on periodic breathing at high altitude. The pharmacologic treatments included acetazolamide,100 theophylline,100 temazepam,101 zolpidem,102 and zaleplon.102 A meta-analysis could not be performed as there were not a sufficient number of studies investigating any one particular therapy. The reported outcome measures were different for the different studies. Two100,102 reported AHI, while 1 reported percent time spent in periodic breathing.101
In 1 study with 33 participants, theophylline was found to be equally effective compared with acetazolamide in normalizing high-altitude periodic breathing (median AHI on the first night at altitude for placebo was 16.2 [range 3-92], acetazolamide was 2.5 [0-11], and theophylline was 4.2 [0-19]).100 Additionally, only acetazolamide significantly improved basal oxyhemoglobin saturation during sleep (86.2% ± 1.7% versus 81.0% ± 3.0%). While no major side effects were noted, 60% of the subjects on acetazolamide developed paresthesias in their hands and feet and impaired taste of fizzy drinks. Of the subjects on theophylline, 70% reported heart palpitations. Nearly all subjects (including placebo) reported increased diuresis, especially during the night.
Nickol et al.101 reported that temazepam significantly decreased the proportion of time in periodic breathing (16% to 9.4%, median) without any adverse effect on next-day reaction time, maintenance of wakefulness, or cognition. However, temazepam use was associated with a small decrease in oxygen saturation, 78% (65% to 84%) to 76% (64% to 83%) (P = 0.013).
Beaumont et al.102 also studied the effect of zolpidem and zaleplon vs. placebo, and found no change in ventilatory parameters in simulated high altitude; however, slow wave sleep improved. The apnea index and AHI significantly increased at altitude, and all apnea episodes observed were central apneas. In comparison to placebo, the overall apnea index and the mean or lowest SaO2 were not significantly changed; however, there was a trend towards a decrease (of 50%) in the apnea index with zolpidem in the first 3 h of the night.
At this time, the level of evidence is very low regarding use of a particular pharmacological agent to prevent CSAS related to high altitude and precludes the formation of a recommendation at this time. These medications should be used only for a short period of time due to the nature of the disorder. Further information on standard treatments for acute mountain sickness can be found in Imray et al.103
4.5 CSAS due to Drug or Substance
The literature assessing treatment of CSAS due to drug or substances is markedly limited, with a total of only 4 studies found on the topic of CSAS associated with chronic opioid use. No other data on the effectiveness of CPAP on CSAS due to drug or substance were found that met inclusion criteria. A small non-randomized study6 with limitations addressed the treatment of patients on opioids (120-420 mg/d) for chronic pain who had developed CSAS and were non-responsive to CPAP. Bilevel PAP therapy for 6 months in 4 patients decreased the AHI from 60.2 ± 30.9 at baseline to 16.6 ± 12.3. Central apneas were eliminated in 3 of the 4 patients. In addition, the ESS scores improved, there was correction of nocturnal hypoxemia, and sleep fragmentation was reduced. Additional oxygen therapy was required in 3 of the 4 patients due to continuing nocturnal hypoxemia that was attributed to a history of smoking. Allam et al.23 showed that ASV is a potential treatment as well (see section 5.2.6 for more details), but details were not given on the breakdown of patients with CSAS secondary to CHF versus those with CSAS secondary to opioids. From the reported data, only 5 patients on opioids were included in the results. In another small non-randomized trial, Javaheri et al.104 reported that ASV improved sleep disordered breathing better than CPAP in 5 patients on chronic opioid treatment (252 ± 150 mg/d). The AHI decreased from 70 ± 19 at baseline to 55 ± 25 with CPAP and to 20 ± 18 with ASV. The CAI decreased from 26 ± 27 at baseline to 0 ± 0 on ASV, while actually increasing with CPAP. There appeared to be no difference in the effect on hypopneas between CPAP and ASV. Lastly, Farney et al.105 retrospectively studied 22 consecutive patients who had been using opioid therapy for at least 6 months who were referred for suspected sleep apnea (but not CSR). Neither CPAP nor ASV significantly changed the average AHI after 1 night of treatment (baseline, 66.6 ± 37.3; CPAP 70.1 ± 32.6; ASV 54.2 ± 33.0). In addition, ASV did not significantly reduce the mean CAI (baseline 26.4 ± 25.1 to 15.6 ± 23.0), while CPAP significantly increased it to 48.1 ± 27.1. The discrepancy between the results obtained by Javaheri et al.104 and Farney et al.105 may be related to the methodology and the method of titration of ASV. At this time, the amount of evidence is very low with respect to therapy for patients with CSAS associated with opioid use and precludes the formation of a recommendation. Further studies are clearly needed to corroborate or refute the conclusions and to determine if CPAP is less effective than BPAP or ASV in these patients. Assessment for discontinuation of opioid use and substitution of other forms of pain relief seems prudent.
5.0 FUTURE DIRECTIONS
The existing literature highlights the paucity of data available on the treatment of central sleep apnea syndromes. The most extensive literature for CSAS therapy pertains to the use of positive airway pressure therapy devices. Even here, the overall quality of evidence is moderate at best, and further investigations are clearly needed to consider additional outcome measures, identify factors that predict response to PAP therapy, and estimate cost effectiveness. The SERVE-HF106 is a large, multicenter RCT that began recruiting participants in 2008. The objectives of this study include evaluating the long-term effects and cost effectiveness of ASV on mortality and morbidity in patients with CHF with CSAS. Results are expected in 2012 at the earliest. Another upcoming and notable study is the “Effect of Adaptive Servo Ventilation (ASV) on Survival and Hospital Admissions in Heart Failure (ADVENT-HF).” The primary objective of this study is to determine if ASV can reduce the rate of cardiovascular related hospital admissions and mortality in individuals with heart failure and sleep apnea (both obstructive and central sleep apnea). Trial completion is anticipated for 2015. It is also worth mention that the use of multimodality titration studies akin to that described by Kuzniar et al.107 is a potential investigative front that could identify successful PAP treatment more effectively and efficiently. Accordingly, there is much to look forward to in terms of advancing the current literature on PAP therapy for CSAS.
The level of evidence looking at therapeutic modalities other than PAP therapies is even more limited both in quality and quantity. Specifically, data on medications and the possible use of carbon dioxide are sparse and inexact. Recommendation levels on pharmacological therapies were generally an option level, as evidence was overall low or very low quality, and the benefits and harms are unclear. Further elucidation of the potential role of carbon dioxide and pharmacological interventions for the treatment of CSAS is necessary.
Also, the level of evidence on CSAS not due to CHF (i.e., primary, in conjunction with ESRD or other medical conditions, or due to drug or substance) is very low, and more research is needed to enable recommendations to be more made with more certainty.
Controversy remains as to whether complex sleep apnea represents an independent and sustained sleep related breathing disorder, or whether it is a temporary occurrence108 that eventually abates with continued PAP therapy. Further studies to resolve this debate are warranted.
Other novel therapies that are being examined include positional therapy for patients with CSAS and heart failure,109,110 exercise therapy,111 as well as new ventilation/positive airway pressure treatments for patients with and without heart disease.112 Research exploring alternate therapeutic options such as these and others is essential.
While significant progress has been made in developing therapies for CSAS, the current review underscores the need to enhance the quality, quantity, as well as the scope of future studies to optimize patient care strategies for the treatment of these disorders.
FOOTNOTE A
ESTIMATE OF EFFECT: The observed relationship between an intervention and an outcome expressed as, for example, a number needed to treat, odds ratio, risk difference, risk ratio, relative risk reduction, standardized mean difference, or weighted mean difference.12
DISCLOSURE STATEMENT
This is not an industry supported study. The authors have indicated no financial conflicts of interest.
APPENDIX—Supplemental information and data used in meta-analyses
LVEF Data for CPAP
| Author, Year | Duration of CPAP | Δ LVEF, CPAP | Δ LVEF, Control | n (treatment / control) |
|---|---|---|---|---|
| Dohi, 2008 - Responders | 6 mo. | +9.3 ± 9.7 | N/A | 9 |
| Arzt, 2007 - Suppressed | 3 mo. | +3.6 [2.1 to 5.1] | +0.4 [-0.6 to 1.5] | 57/110 |
| Arzt, 2007 - Unsuppressed | 3 mo. | +0.3 [-1.0 to 1.6] | 43/110 | |
| Bradley, 2005 | 3 mo. | +2.2 ± 5.4 | +0.4 ± 5.3 | 128/130 |
| Yasuma, 2005 | 3 mo. | +9.9 ± 2.5 | N/A | 5 |
| 12 mo. | +7.3 ± 5.5 | |||
| Sin, 2000 – only patients with CSAS | 3 mo. | +7.8 ± 2.2* | −0.5 ± 1.5* | 14/15 |
| Tkacova, 1997 | 3 mo. | +8.0 ± 13.5 | −0.5 ± 8.0 | 9/8 |
| Granton, 1996 | 3 mo. | +8.6 ± 15.4 | −1.1 ± 7.9 | 9/8 |
| Naughton, 1995a | 1 mo. | +3.7 ± 13.3 | +0.1 ± 8.8 | 12/12 |
| 3 mo. | +7.7 ± 15.1 | −0.5 ± 8.4 | 12/12 | |
| Naughton, 1995b | 1 mo. | +6.5 ± 12.0 | −1.0 ± 10.5 | 9/9 |
| Naughton, 1994 | 1 mo. | +4.6 ± 7.9 | −1.5 ± 6.5 | 10/4 |
| Takasaki, 1989 | 3 mo. | +7 ± 18 | N/A | 5 |
Read off Figure 1 of paper.
AHI Data: RCTs + 1 NRT for CPAP
| Author, Year | Duration of CPAP | ΔCPAP | ΔControl | n (treatment / control) | Titrated |
|---|---|---|---|---|---|
| Bradley, 2005 / Ruttanaumapawan, 2009 | 3 mo.* | −21.3 ± 15.6 | −0.2 ± 21.0 | 97/108 | N |
| 2 yrs | −23.2 ± 15.9 | −1.3 ± 16.0 | 33/38 | N | |
| Granton, 1996 | 3 mo. | −32 ± 26 | −16 ± 25 | 9/8 | N |
| Naughton, 1995a | 1 mo. | −28.5 ± 16.1 | −6.1 ± 20.8 | 12/12 | N |
| Naughton, 1995b | 1 mo. | −29.6 ± 15.5 | −8.7 ± 20.9 | 9/9 | N |
| Naughton, 1994 | 1 mo. | −35.3 ± 15.5 | −13.3 ± 25.4 | 12/6 | N |
The 3-month data was used in the meta-analysis since it has the same time-frame as the other studies and had more subjects.
AHI Data: Before-after CPAP data
| Author, Year | Duration of CPAP | AHI baseline (± SD) | AHI after CPAP (± SD) | n | Titrated |
|---|---|---|---|---|---|
| Bradley, 2005 / Ruttanaumapawan, 2009 | 3 mo. | 38.9 ± 15.0 | 17.6 ± 16.3 | 97 | N |
| Granton, 1996 | 3 mo. | 49 ± 33 | 17 ± 21 | 9 | N |
| Naughton, 1995a | 1 mo. | 43.2 ± 17.0 | 14.7 ± 16.6 | 12 | N |
| Naughton, 1995b | 1 mo. | 48.1 ± 14.7 | 18.5 ± 18.0 | 9 | N |
| Naughton, 1994 | 1 mo. | 54.0 ± 14.9 | 18.7 ± 17.3 | 12 | N |
| Yasuma, 2005 | 3 mo. | 34.7 ± 21.4 | 6.0 ± 7.0 | 5 | N |
| 6 mo. | 34.7 ± 21.4 | 1.2 ± 1.4 | |||
| Krachman, 2003 | 1 night | 44 ± 27 | 15 ± 24 | 9 | N |
| Takasaki, 1989 | 10 d to 4 wks | 69 ± 20 | 15 ± 16 | 5 | Y |
LVEF Data: BPAP-ST devices
| Author, Year | Duration of Treatment | Δ LVEF, Fixed pressure device (%) | Δ LVEF, Control (%) | n (treatment / control) |
|---|---|---|---|---|
| Dohi, 2008 | 6 mo. | +12.7 ± 10.0 | N/A | 7/0 |
| Kasai, 2005 | 3 mo. | +9.9 ± 8.6 | −1.4 ± 8.5 | 7/7 |
AHI Data: BPAP-ST (before-after treatment)
| Author, Year | Duration of Treatment | AHI baseline (± SD) | AHI after Fixed pressure device (± SD) | n |
|---|---|---|---|---|
| Dohi, 2008 | 1 night | 54.4 ± 7.8 | 8.4 ± 4.7 | 9 |
| Kasai, 2005 | 1 night | 49.4 ± 16.1 | 10 ± 8* | 7 |
| Willson, 2001 | 1 night | 49 ± 10 | 6 ± 5 | 9 |
From Figure 1 in study.
LVEF Data ASV
| Author, Yr | Control / Comparison Treatment | Duration | N (treatment / control) | Δ LVEF Test Treatment | Δ LVEF Control / Comparison Treatment |
|---|---|---|---|---|---|
| Kasai 2010 | CPAP | 3 mo. | 15/15 | +9.1 ± 4.7 | +1.9 ± 10.9 |
| Pepperell 2003 | Sub-therapeutic ASV | 4 wks | 15/11 | +1.8 ± 10.8 | +0.6 ± 13.2 |
| Oldenburg 2008 | Baseline | 6 mo. | 29 | +7.0 ± 9.1 | N/A |
| Zhang 2006 | Oxygen | 2 wks | 14 | +7.0 ± 4.2 | +2.4 ± 4.5 |
| Philippe 2006 | CPAP | 6 mo. | 7/6 | +7 ± 5* | −2 ± 13* |
| Fietze 2008 | BPAP-ST | 6 wks | 15/15 | +1.9 ± 8.1 | +5.6 ± 9.5 |
estimated from graph.
AHI Data: ASV
| Author, Yr | Control Treatment | Duration | n (treatment / control) | Test Treatment |
Control Treatment |
||
|---|---|---|---|---|---|---|---|
| Baseline | After | Baseline | After | ||||
| Pepperell 2003 | Sub-therapeutic ASV | 1 night | 15/15 | 24.7 ± 11.3 | 5.0 ± 5.4 | 23.3 ± 13.3 | 20.6 ± 8.9 |
| 4 wks | 15/11 | 24.7 ± 11.3 | 5.4 ± 7.4 | 23.3 ± 13.3 | 14.7 ± 10.6 | ||
| Oldenburg 2008 | Baseline | 6 mo. | 29/0 | 37.4 ± 9.4 | 3.8 ± 4.1 | N/A | |
| Szollosi 2006 | Baseline | 1 night | 10/0 | 30.0 ± 20.9 | 14.0 ± 12.0 | N/A | |
| Zhang 2006 | Oxygen | 2 weeks | 14/14 | 34.5 ± 6.1 | 6.5 ± 0.8 | 34.5 ± 6.1 | 27.8 ± 8.2 |
| Kasai 2010 | CPAP | 3 mo. | 15/15 | 37.4 ± 19.5 | 1.9 ± 2.1 | 38.6 ± 13.9 | 15.4 ± 12.8 |
| −35.4 ± 19.5 | −23.2 ± 12.0 | ||||||
| Arzt 2008 | CPAP / BPAP | 1 night | 14/0 | 46.4 ± 15§ | 4 ± 3.7§ | 46.4 ± 15§ | 22 ± 15§ |
| BPAP Auto-SV | |||||||
| Morgenthaler 2007 | CPAP | 1 night | 6/0 | 46.0 ± 22.7 | 0 ± 0 | 46.0 ± 22.7 | 22.8 ± 18.2 |
| BPAP-ST | 1 night | 6/0 | 46.0 ± 22.7 | 0 ± 0 | 46.0 ± 22.7 | 1.5 ± 1.5 | |
| Philippe 2006 | CPAP | 3 mo. | 12/** | 47 ± 18 | 4 ± 5* | 40.5 ± 13.5 | ** |
| 6 mo. | 9/8 | 3 ± 4* | 21 ± 25* | ||||
| Fietze 2008 | BPAP-ST | 6 wks | 15/15 | 31.0 ± 10.0† | 11.1 ± 9.9† | 33.4 ± 20.5† | 16.1 ± 16.2† |
Estimated from graph.
Data do not appear to be last-brought-forward in Figure1B for CPAP; appears to overestimate CPAP effectiveness at 3 months (dropouts appear to be put to 0 AHI).
CSRI = CSAI+PBI (periodic breathing index).
Data given in paper were assumed to be SE and not SD as stated.
Oxygen LVEF Data
| Author, Year | Duration of Trial | LVEF baseline (± SD) | LVEF after Oxygen (± SD) | LVEF baseline (± SD) | LVEF control (± SD) | n (treatment / control) |
|---|---|---|---|---|---|---|
| Toyama, 2009 | 3 mo. | 27 ± 9 | 37 ± 10 | 26 ± 8 | 27 ± 9 | 10/10 |
| Sasayama, 2009 | 12 mo. | 33.0 ± 8.9 | 38.5 ± 15.5 | 31.8 ± 7.6 | 33.0 ± 11.2 | 21 / 21 |
| Shigemetsu, 2007 | 4 mo. | 44.7 ± 11.9 | 46.4 ± 14.8 | N/A | N/A | 18 |
| Sasayama, 2006* | 12 wks | 34.7 ± 10.4 | 38.2 ± 13.6 | 32.8 ± 8.8 | 34.4 ± 10.9 | 24/29 |
| Krachman, 2005 | 1 mo. | 22 ± 11 | 19 ± 9 | N/A | N/A | 10 |
| Zhang, 2006 | 2 wks | 30.2 ± 4.6 | 33.2 ± 5.1 | N/A | N/A | 14 |
This data is updated by Sasayama 2009 and not used in the meta-analysis.
Oxygen AHI Data
| Author, Year | Duration of Trial | AHI baseline or control* (± SD) | AHI after Oxygen (± SD) | AHI baseline (± SD) | AHI control (± SD) | n (treatment / control) |
|---|---|---|---|---|---|---|
| Toyama, 2009 | 3 mo. | 26.1 ± 9.1 | 5.1 ± 3.4 | 19.9 ± 13.0 | 21.0 ± 12.0 | 10/10 |
| Sasayama, 2009 | 12 mo. | 19.0 ± 12.3 | 9.0 ± 8.4 | 20.0 ± 11.4 | 20.8 ± 13.5 | 21/21 |
| Shigemetsu, 2007 | 4 mo. | 33.7 ± 11.1 | 6.2 ± 3.2 | N/A | N/A | 18 |
| Sasayama, 2006‡ | 12 wks | 21.0 ± 10.8 | 10.0 ± 11.6 | 18.0 ± 10.7 | 17.1 ± 11.4 | 25/31 |
| Staniforth, 1998 | 4 wks | 37.8 ± 12.9 | 24.9 ± 12.3 | 37.8 ± 12.9 | 38.0 ± 16.6 | 11 |
| Zhang, 2006 | 2 wks | 34.5 ± 6.1 | 27.8 ± 8.2 | N/A | N/A | 14 |
| Andreas, 1996* | 1 wk | 26 ± 24 | 10 ± 9 | N/A | N/A | 22 |
| Hanly, 1989* | 1 night | 30.0 ± 14.1 | 18.9 ± 7.2 | N/A | N/A | 9 |
| Krachman, 2005 | 1 mo. | 57 ± 61 | 12 ± 17 | N/A | N/A | 10 |
| Broström, 2005 | 3 mo. | 34 (24-46)** | 22 (17-42)** | N/A | N/A | 9 |
| Javaheri, 1999 | Total† | 49 ± 19 | 29 ± 29 | N/A | N/A | 36 |
| 1 night | Responders | 36 ± 11 | 6 ± 4 | N/A | N/A | 14 |
| Partial responders | 57 ± 19 | 44 ± 29 | N/A | N/A | 22 | |
| Franklin, 1997 | 1 night | 31.3 ± 13.3 | 8.7 ± 9.1 | N/A | N/A | 20 |
This data is updated by Sasayama 2009 and not used in the meta-analysis.
Control was room air or compressed air.
Data not normally distributed, cannot use in meta-analysis.
Used total data in meta-analysis.
Oxygen vs. CPAP Data
| Author, Year | Duration of Trial | Oxygen |
CPAP |
N (trial / control) | ||
|---|---|---|---|---|---|---|
| Baseline (± SD) | After (± SD) | Baseline (± SD) | After (± SD) | |||
|
AHI data | ||||||
| Arzt, 2005 | 12 wks | 28.8 ± 10.1 | 8.7 ± 13.0 | 35.9 ± 16.0 | 12.2 ± 14.4 | 10/16 |
| Krachman, 1999 | 1 night | 44 ± 27 | 18 ± 15 | N/A | 15 ± 24 | 9 |
|
LVEF data | ||||||
| Arzt, 2005 | 12 wks | 30.9 ± 7.6 | 32.5 ± 7.3 | 31.7 ± 10.4 | 35.7 ± 10.8 | 10/16 |
AHI data for pharmacological therapies for CSAS related to CHF
| Author, Year | Duration of Treatment | n | AHI baseline (± SD) | AHI after placebo (± SD) | AHI after Tx (± SD) |
|---|---|---|---|---|---|
| Javaheri, 2006 | 6 nights | 12 | 55 ± 24 | 57 ± 28 | 34 ± 20 |
| Hu, 2006 | 5-7 days | 13 | 42.6 ± 15.5 | N/A | 20.8 ± 13.2 |
| Javaheri, 1996 | 5 days | 15 | 47 ± 21 | 37 ± 23 | 18 ± 17 |
| Tamura, 2009 | 6 mo | 16 | 34 ± 13 | N/A | 14 ± 13 |
| Walsh, 1995 | 1 month | 8 | 35 ± 20 | N/A | 20 ± 14 |
| Zilberman, 2007 | 3 months | 35 | 26.5 ± 14.6 | N/A | 18.1 ± 12.7 |
| Andreas, 1998 | 1 night | 9 | 36.7 ± 21.9 | N/A | 5.4 ± 3.6 |
| Steens, 1994 | 1 night | 6 | 41.1 ± 28.9 | N/A | 1.0 ± 1.7 |
CRT LVEF Data, %
| Author, Year | Duration of Trial | CRT off or pre-CRT (± SD) | CRT on or post-CRT (± SD) | n |
|---|---|---|---|---|
| Lüthje, 2009 | 3 months | 20.7 ± 4.6 | 28.4 ± 7.7* | 18 |
| Oldenburg, 2007 | 5.0 ± 2.6 mo. | 25.2 ± 6.1 | 29.1 ± 7.3 | 36 |
| Yiu, 2008 | 3 months | 28.8 ± 9.7** | 38.1 ± 8.9** | 15 |
| Sinha, 2004 | 17 ± 7 wks | 25 ± 5 | 35 ± 9 | 14 |
| Gabor, 2005 | 6 months | 19.0 ± 4.2 | 24.2 ± 7.8 | 10 |
| Skobel, 2005 | 18 ± 7 wks | 19 ± 5 | 33 ± 8 | 18 |
SD calculated from data given (as a change in LVEF) in reference.
Data in paper assumed to be SEM and converted to SD.
CRT AHI Data
| Author, Year | Duration of Trial | CRT off or pre-CRT (± SD) | CRT on or post-CRT (± SD) | n |
|---|---|---|---|---|
| Oldenburg, 2007 | 5.0 ± 2.6 mo. | 31.2 ± 15.5 | 17.3 ± 13.7 | 36 |
| Kara, 2008 | 1 night | 14.3 ± 10.0 | 8.1 ± 5.2 | 12 |
| Lüthje, 2009 | 3 months | 37.1 ± 13.4 | 25.7 ± 17.5 | 18 |
| Sinha, 2004 | 17 ± 7 wks | 19.2 ± 10.3 | 4.6 ± 4.4 | 14 |
| Yiu, 2008 | 3 months | 27.5 ± 4.7 | 18.0 ± 3.0 | 15 |
| Gabor, 2005 | 6 months | 42.7 ± 9.1 | 30.8 ± 18.7 | 10 |
| Skobel, 2005* | 18 ± 7 wks | 18 ± 8 | 3 ± 2 | 18 |
7/18 patients with mixed sleep apnea included in data.
REFERENCES
- 1.Balshem H, Helfand M, Schunemann H, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–6. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Sleep Medicine. International classification of sleep disorders, second edition: diagnostic and coding manual. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 3.Mezon B, West P, Israels J, Kryger M. Sleep breathing abnormalities in kyphoscoliosis. Am Rev Respir Dis. 1980;122:617–21. doi: 10.1164/arrd.1980.122.4.617. [DOI] [PubMed] [Google Scholar]
- 4.Bitter T, Faber L, et al. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur Heart J. 2009;11:602–8. doi: 10.1093/eurjhf/hfp057. [DOI] [PubMed] [Google Scholar]
- 5.Javaheri S, Parker TJ, Liming JD, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure: types and their prevalences, consequences, and presentations. Circulation. 1998;97:2154–9. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 6.Alattar M, Scharf S. Opioid-associated central sleep apnea: a case series. Sleep Breath. 2009;13:201–6. doi: 10.1007/s11325-008-0221-7. [DOI] [PubMed] [Google Scholar]
- 7.Wang D, Teichtahl H, Drummer O, et al. Central sleep apnea in stable methadone maintenance treatment patients. Chest. 2005;128:1348–56. doi: 10.1378/chest.128.3.1348. [DOI] [PubMed] [Google Scholar]
- 8.Marin J, Carrizo S, Vicente E, Agusti A. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 9.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young T, Finn L, Peppard P, et al. Sleep disordered breathing and mortality: Eighteen-year follow-up of the Wisconsin Sleep Cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol. 2007;49:2028–34. doi: 10.1016/j.jacc.2007.01.084. [DOI] [PubMed] [Google Scholar]
- 12. GRADEPro [computer program]2008.
- 13.Guyatt G, Oxman A, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–94. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 14.Zaza S, Wright-DeAguaero LK, Briss P, et al. Data collection instrument and procedure for systematic reviews in the Guide to Community Preventive Services. Am J Prev Med. 2000;18:44–74. doi: 10.1016/s0749-3797(99)00122-1. [DOI] [PubMed] [Google Scholar]
- 15.Atkins D, Best D, Briss P, et al. Grading and quality of evidence and strength of recommendations. BMJ. 2004;328:1490–4. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schunemann H, Jaeschke R, Cook D, et al. An official ATS statement: Grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. Am J Respir Crit Care Med. 2006;174:605–14. doi: 10.1164/rccm.200602-197ST. [DOI] [PubMed] [Google Scholar]
- 17.Bax L, Yu L, Ikeda N, Tsuruta H, Moons K. Development and validation of MIX: Comprehensive free software for meta-analysis of causal research data. BMC Med Res Methodol. 2006;6:50. doi: 10.1186/1471-2288-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bax L, Yu L, Ikeda N, Tsuruta H, Moons K. MIX: Comprehensive free software for meta-analysis of causal research data. Version 1.7. 2008. http://mix-for-meta-analysis.info. [DOI] [PMC free article] [PubMed]
- 19.Aurora R, Morgentahaler T. On the goodness of recommendations: the changing face of practice parameters. Sleep. 2010;33:1273–6. doi: 10.1093/sleep/33.10.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Javaheri S, Smith J, Chung E. The prevalence and natural history of complex sleep apnea. J Clin Sleep Med. 2009;5:205–11. [PMC free article] [PubMed] [Google Scholar]
- 21.Kuzniar TJ, Pusalavidyasagar S, Gay PC, Morgenthaler TI. Natural course of complex sleep apnea: a retrospective study. Sleep Breath. 2008;12:135–9. doi: 10.1007/s11325-007-0140-z. [DOI] [PubMed] [Google Scholar]
- 22.Morgenthaler TI, Gay PC, Gordon N, Brown LK. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep. 2007;30:468–75. doi: 10.1093/sleep/30.4.468. [DOI] [PubMed] [Google Scholar]
- 23.Allam J, Olson E, Gay P, Morgentahaler T. Efficacy of adaptive servoventilation in treatment of complex and central sleep apnea syndromes. Chest. 2007;132:1839–46. doi: 10.1378/chest.07-1715. [DOI] [PubMed] [Google Scholar]
- 24.Xie A, Rankin F, Rutherford R, Bradley T. Effects of inhaled CO2 and added dead space on idiopathic central sleep apnea. J Appl Physiol. 1997;82:918–26. doi: 10.1152/jappl.1997.82.3.918. [DOI] [PubMed] [Google Scholar]
- 25.DeBacker W, Verbraecken J, Willemen M, Wittesaele W, DeCock W, Van de Heyning P. Central apnea index decreases after prolonged treatment with acetazolamide. Am J Respir Crit Care Med. 1995;151:87–91. doi: 10.1164/ajrccm.151.1.7812578. [DOI] [PubMed] [Google Scholar]
- 26.White D, Zwillich C, Pickett C, Douglas N, Findley L, Weil J. Central sleep apnea: improvement with acetazolamide therapy. Arch Intern Med. 1982;142:1816–9. [PubMed] [Google Scholar]
- 27.Quadri S, Drake C, Hudgel DW. Improvement of idiopathic central sleep apnea with zolpidem. J Clin Sleep Med. 2009;5:122–9. [PMC free article] [PubMed] [Google Scholar]
- 28.Bonnet M, Dexter J, Arand D. The effect of triazolam on arousal and respiration in central sleep apnea patients. Sleep. 1990;13:31–41. doi: 10.1093/sleep/13.1.31. [DOI] [PubMed] [Google Scholar]
- 29.Arzt M, Floras J, Logan A, et al. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian continuous positive airway pressure of patients with central sleep apnea and heart failure trial (CANPAP) Circulation. 2007;115:3173–80. doi: 10.1161/CIRCULATIONAHA.106.683482. [DOI] [PubMed] [Google Scholar]
- 30.Bradley T, Logan A, Kimoff R, et al. Continuous positive airway pressure for central sleep apnea and heart failure. New Engl J Med. 2005;353:2025–33. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 31.Krachman S, Crocetti J, Berger T, Chatila W, Eisen H, D'Alonzo G. Effects of nasal continuous positive airway pressure on oxygen body stores in patients with Cheyne-Stokes respiration and congestive heart failure. Chest. 2003;123:59–66. doi: 10.1378/chest.123.1.59. [DOI] [PubMed] [Google Scholar]
- 32.Naughton M, Benard D, Liu P, Rutherford R, Rankin F, Bradley T. Effects of nasal CPAP on sympathetic activity in patients with heart failure and central sleep apnea. Am J Respir Crit Care Med. 1995;152:473–9. doi: 10.1164/ajrccm.152.2.7633695. [DOI] [PubMed] [Google Scholar]
- 33.Sin D, Logan A, Fitzgerald F, Liu P, Bradley T. Effects of continuous positive airway pressure on cardiovascular outcomes in heart failure patients with and without Cheyne-Stokes respiration. Circulation. 2000;102:61–6. doi: 10.1161/01.cir.102.1.61. [DOI] [PubMed] [Google Scholar]
- 34.Tkacova R, Liu P, Naughton M, Bradley T. Effect of continuous positive airway pressure on mitral regurgitant fraction and atrial natriuretic peptide in patients with heart failure. J Am Coll Cardiol. 1997;30:739–45. doi: 10.1016/s0735-1097(97)00199-x. [DOI] [PubMed] [Google Scholar]
- 35.Ruttanaumpawan P, Logan A, Floras J, Bradley T, Investigators C. Effect of continuous positive airway pressure on sleep structure in heart failure patients with central sleep apnea. Sleep. 2009;32:91–98. [PMC free article] [PubMed] [Google Scholar]
- 36.Dohi T, Kasai T, Narui K, et al. Bi-level positive airway pressure ventilation for treating heart failure with central sleep apnea that is unresponsive to continuous postitive airway pressure. Circ J. 2008;72:1100–5. doi: 10.1253/circj.72.1100. [DOI] [PubMed] [Google Scholar]
- 37.Yasuma F. Cheyne-Stokes respiration in congestive heart failure: Continuous positive airway pressure of 5-8 cm H2O for 1 year in five cases. Respiration. 2005;72:198–201. doi: 10.1159/000084053. [DOI] [PubMed] [Google Scholar]
- 38.Granton J, Naughton M, Benard D, Liu P, Goldstein R, Bradley T. CPAP improves inspiratory muscle strength in patients with heart failure and central sleep apnea. Am J Respir Crit Care Med. 1996;153:277–82. doi: 10.1164/ajrccm.153.1.8542129. [DOI] [PubMed] [Google Scholar]
- 39.Naughton M, Liu P, Benard D, Goldstein R, Bradley T. Treatment of congestive heart failure and Cheyne-Stokes respiration during sleep by continuous positive airway pressure. Am J Respir Crit Care Med. 1995;151:92–97. doi: 10.1164/ajrccm.151.1.7812579. [DOI] [PubMed] [Google Scholar]
- 40.Guilleminault C, Clerk A, Labanowski M, Simmons J, Stoohs R. Cardiac failure and benzodiazepines. Sleep. 1993;16:524–8. [PubMed] [Google Scholar]
- 41.Davies R, Harrington K, Ormerod O, Stradling J. Nasal continuous positive airway pressure in chronic heart failure with sleep-disordered breathing. Am J Respir Crit Care Med. 1993;147:630–4. doi: 10.1164/ajrccm/147.3.630. [DOI] [PubMed] [Google Scholar]
- 42.Takasaki Y, Orr D, Popkin J, Rutherford R, Liu P, Bradley T. Effect of nasal continuous positive airway pressure on sleep apnea in congestive heart failure. Am Rev Respir Dis. 1989;140:1578–84. doi: 10.1164/ajrccm/140.6.1578. [DOI] [PubMed] [Google Scholar]
- 43.Naughton M, Benard D, Rutherford R, Bradley T. Effect of continuous positive airway pressure on central sleep apnea and nocturnal PCO2 in heart failure. Am J Respir Crit Care Med. 1994;1509:1598–604. doi: 10.1164/ajrccm.150.6.7952621. [DOI] [PubMed] [Google Scholar]
- 44.Javaheri S. Effects of continuous positive airway pressure on sleep apnea and ventricular irritability in patients with heart failure. Circulation. 2000;101:392–7. doi: 10.1161/01.cir.101.4.392. [DOI] [PubMed] [Google Scholar]
- 45.Noda A, Izawa H, Asano H, et al. Beneficial effect of bilevel positive airway pressure on left ventricular function in ambulatory patients with idiopathic dilated cardiomyopathy and central sleep apnea-hypopnea: a preliminary study. Chest. 2007;131:1694–701. doi: 10.1378/chest.06-2271. [DOI] [PubMed] [Google Scholar]
- 46.Kasai T, Narui K, Dohi T, et al. Efficacy of nasal bi-level positive airway pressure in congestive heart failure patients with Cheyne-Stokes respiration and central sleep apnea. Circ J. 2005;69:913–21. doi: 10.1253/circj.69.913. [DOI] [PubMed] [Google Scholar]
- 47.Willson G, Wilcox I, Piper A, et al. Noninvasive pressure preset ventilation for the treatment of Cheyne-Stokes respiration during sleep. Eur Respir J. 2001;17:1250–7. doi: 10.1183/09031936.01.99086101. [DOI] [PubMed] [Google Scholar]
- 48.Willson G, Wilcox I, Piper A, Flynn W, Grunstein R, Sullivan C. Treatment of central sleep apnoea in congestive heart failure with nasal ventilation. Thorax. 1998;53(Suppl 3):S41–46. doi: 10.1136/thx.53.2008.s41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kohnlein T, Welte T, Tan L, Elliott M. Assisted ventilation for heart failure patients with Cheyne-Stokes respiration. Eur Respir J. 2002;20:934–41. doi: 10.1183/09031936.00.02622001. [DOI] [PubMed] [Google Scholar]
- 50.Kuzniar T, Morgentahaler T. Treatment of complex sleep apnea syndrome. Curr Treat Opt Neurol. 2008;10:336–41. doi: 10.1007/s11940-008-0036-7. [DOI] [PubMed] [Google Scholar]
- 51.Pepperell J, Maskell N, Jones D, et al. A randomized controlled trial of adaptive ventilation for Cheyne-Stokes breathing in heart failure. Am J Respir Crit Care Med. 2003;168:1109–14. doi: 10.1164/rccm.200212-1476OC. [DOI] [PubMed] [Google Scholar]
- 52.Oldenburg O, Schmidt A, Lamp B, et al. Adaptive servoventilation improves cardiac function in patients with chronic heart failure and Cheyne-Stokes respiration. Eur J Heart Fail. 2008;10:581–6. doi: 10.1016/j.ejheart.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 53.Szollosi I, O'Driscoll D, Dayer M, Coats A, Morrell M, Simonds A. Adaptive servo-ventilation and deadspace: effects on central sleep apnoea. J Sleep Res. 2006;14:199–205. doi: 10.1111/j.1365-2869.2006.00515.x. [DOI] [PubMed] [Google Scholar]
- 54.Fietze I, Blau A, Glos M, Theres H, Baumann G, Penzel T. Bi-level positive pressure ventilation and adaptive servo ventilation in patients with heart failure and Cheyne-Stokes respiration. Sleep Med. 2008;9:652–9. doi: 10.1016/j.sleep.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 55.Morgentahaler T, Gay P, Gordon N, Brown L. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep. 2007;30:468–75. doi: 10.1093/sleep/30.4.468. [DOI] [PubMed] [Google Scholar]
- 56.Arzt M, Wensel R, Montalvan S, et al. Effects of dynamic bilevel positive airway pressure support on central sleep apnea in men with heart failure. Chest. 2008;134:61–6. doi: 10.1378/chest.07-1620. [DOI] [PubMed] [Google Scholar]
- 57.Zhang X-L, Yin K-S, Jia E-Z, Su M. Efficacy of adaptive servoventilation in patients with congestive heart failure and Cheyne-Stokes respiration. Chin Med J. 2006;119:622–7. [PubMed] [Google Scholar]
- 58.Koyama T, Watanabe H, Kobukai Y, et al. Beneficial effects of adaptive servo ventilation in patients with chronic heart failure. Circ J. 2010;74:2118–24. doi: 10.1253/circj.cj-10-0082. [DOI] [PubMed] [Google Scholar]
- 59.Oldenburg O, Bitter T, Lehmann R, et al. Adaptive servoventilation improves cardiac function and respiratory stability. Clin Res Cardiol. 2011;100:107–15. doi: 10.1007/s00392-010-0216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kasai T, Usui Y, Yoshioka T, et al. Effect of flow-triggered adaptive servo-ventilation compared with continuous positive airway pressure in patients with chronic heart failure with coexisting obstructive sleep apnea and Cheyne-Stokes respiration. Circ Heart Fail. 2010;3:140–8. doi: 10.1161/CIRCHEARTFAILURE.109.868786. [DOI] [PubMed] [Google Scholar]
- 61.Philippe C, Stoica-Herman M, Drouot X, et al. Compliance with and effectiveness of adaptive servoventilation versus continuous positive airway pressure in the treatment of Cheyne-Stokes respiration in heart failure over a six month period. Heart. 2006;92:337–42. doi: 10.1136/hrt.2005.060038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brown L. Adaptive servo-ventilation for sleep apnea: technology, titration protocols, and treatment efficacy. Sleep Med Clin. 2010;5:419–37. [Google Scholar]
- 63.Chowdhuri S, Shanidze I, Pierchala L, Belen D, Mateika J, Badr M. Effect of episodic hypoxia on the susceptibility to hypocapnic central apnea during NREM sleep. J Appl Physiol. 2010;108:368–77. doi: 10.1152/japplphysiol.00308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xie A, Skatrud J, Puleo D, Dempsey J. Influence of arterial O2 on the susceptibility to posthyperventilation apnea during sleep. J Appl Physiol. 2006;100:171–7. doi: 10.1152/japplphysiol.00440.2005. [DOI] [PubMed] [Google Scholar]
- 65.Sasayama S, Izumi T, Seino Y, Ueshima K, Asanoi H. Effects of nocturnal oxygen therapy on outcome measures in patients with chronic heart failure and cheyne-stokes respiration. Circ J. 2006;70:1–7. doi: 10.1253/circj.70.1. [DOI] [PubMed] [Google Scholar]
- 66.Hanly P, Millar T, Steljes D, Baert R, Frais M, Kryger M. The effect of oxygen on respiration and sleep in patients with congestive heart failure. Ann Intern Med. 1989;111:777–82. doi: 10.7326/0003-4819-111-10-777. [DOI] [PubMed] [Google Scholar]
- 67.Staniforth A, Kinnear W, Starling R, Hetmanski D, Cowley A. Effect of oxygen on sleep quality, cognitive function, and sympathetic activity in patients with chronic heart failure and Cheyne-Stokes respiration. Eur Heart J. 1998;19:922–8. doi: 10.1053/euhj.1997.0861. [DOI] [PubMed] [Google Scholar]
- 68.Andreas S, Clemens C, Sandholzer H, Figulla H, Kreuzer H. Improvement of exercise capacity with treatment of Cheyne-Stokes respiration in patients with congestive heart failure. J Am Coll Cardiol. 1996;27:1486–90. doi: 10.1016/0735-1097(96)00024-1. [DOI] [PubMed] [Google Scholar]
- 69.Toyama T, Seki R, Kasama S, et al. Effectiveness of nocturnal home oxygen therapy to improve exercise capacity, cardiac function and cardiac sympathetic nerve activiy in patients with chronic heart failure and central sleep apnea. Circ J. 2009;73:299–304. doi: 10.1253/circj.cj-07-0297. [DOI] [PubMed] [Google Scholar]
- 70.Sasayama S, Izumi T, Matsuzaki M, et al. Improvement of quality of life with nocturnal oxygen therapy in heart failure patients with central sleep apnea. Circ J. 2009;73:1255–62. doi: 10.1253/circj.cj-08-1210. [DOI] [PubMed] [Google Scholar]
- 71.Shigemitsu M, Nishio K, Kusuyama T, Itoh S, Konno N, Katagiri T. Nocturnal oxygen therapy prevents progress of ongestive heart failure with central sleep apnea. Int J Cardiol. 2007;115:354–60. doi: 10.1016/j.ijcard.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 72.Franklin K, Eriksson P, Sahlin C, Lundgren R. Reversal of central sleep apnea with oxygen. Chest. 1997;111:163–9. doi: 10.1378/chest.111.1.163. [DOI] [PubMed] [Google Scholar]
- 73.Javaheri S, Ahmed M, Parker T, Brown C. Effects of nasal O2 on sleep-related disordered breathing in ambulatory patients with stable heart failure. Sleep. 1999;22:1101–6. doi: 10.1093/sleep/22.8.1101. [DOI] [PubMed] [Google Scholar]
- 74.Krachman S, Nugent T, Crocetti J, D'Alonzo G, Chatila W. Effects of oxygen therapy on left ventricular function in patients with Cheyne-Stokes respiration and congestive heart failure. J Clin Sleep Med. 2005;1:271–6. [PubMed] [Google Scholar]
- 75.Brostrom A, Hubbert L, Jakobsson P, Johansson P, Fridlund B, Dahlstrum U. Effects of long-term nocturnal oxygen treatment in patients with severe heart failure. J Cardiovasc Nurs. 2005;20:385–96. doi: 10.1097/00005082-200511000-00005. [DOI] [PubMed] [Google Scholar]
- 76.Arzt M, Schulz M, Wensel R, et al. Nocturnal continuous positive airway pressure improves ventilatory efficiency during exercise in patients with chronic heart failure. Chest. 2005;127:794–802. doi: 10.1378/chest.127.3.794. [DOI] [PubMed] [Google Scholar]
- 77.Krachman S, D'Alonzo G, Berger T, Eisen H. Comparison of oxygen therapy with nasal continuous positive airway pressure on Cheyne-Stokes respiration during sleep in congestive heart failure. Chest. 1999;116:1550–7. doi: 10.1378/chest.116.6.1550. [DOI] [PubMed] [Google Scholar]
- 78.Hu K, Li Q, Yang J, Chen X, Hu S, Wu X. The role of high-frequency jet ventilation in the treatment of Cheyne-Stokes respiration in patients with chronic heart failure. Int J Cardiol. 2006;106:224–31. doi: 10.1016/j.ijcard.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 79.Teschler H, Dohring J, Wang Y, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164:614–9. doi: 10.1164/ajrccm.164.4.9908114. [DOI] [PubMed] [Google Scholar]
- 80.Javaheri S. Acetazolamide improves central sleep apnea in heart failure: a double-blind, prospective study. Am J Respir Crit Care Med. 2006;173:234–7. doi: 10.1164/rccm.200507-1035OC. [DOI] [PubMed] [Google Scholar]
- 81.Javaheri S, Parker T, Wexler L, Liming J, Lindower P, Roselle G. Effect of theophylline on sleep-disordered breathing in heart failure. N Engl J Med. 1996;335:562–7. doi: 10.1056/NEJM199608223350805. [DOI] [PubMed] [Google Scholar]
- 82.Hu K, Li Q, Yang J, Hu S, Chen X. The effect of theophylline on sleep-disordered breathing in patients with stable chronic congestive heart failure. Chin Med J. 2003;116:1711–6. [PubMed] [Google Scholar]
- 83.Tamura A, Kawano Y, Kadota J. Carvedilol reduces the severity of central sleep apnea in chronic heart failure. Circ J. 2009;73:295–8. doi: 10.1253/circj.cj-08-0678. [DOI] [PubMed] [Google Scholar]
- 84.Tamura A, Kawano Y, Naono S, Kotoku M, Kadota J-i. Relationship between beta-blocker treatment and the severity of central sleep apnea in chronic heart failure. Chest. 2007;131:130–5. doi: 10.1378/chest.06-0919. [DOI] [PubMed] [Google Scholar]
- 85.Walsh J, Andrews R, Starling R, Cowley A, Johnston I, Kinnear W. Effects of captopril and oxygen on sleep apnoea in patients with mild to moderate congestive cardiac failure. Br Heart J. 1995;73:237–41. doi: 10.1136/hrt.73.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zilberman M, Silverberg D, Bits I, et al. Improvement of anemia with erythropoietin and intravenous iron reduces sleep-related breathing disorders and improves daytime sleepiness in anemic patients with congestive heart failure. Am Heart J. 2007;154:870–6. doi: 10.1016/j.ahj.2007.07.034. [DOI] [PubMed] [Google Scholar]
- 87.Andreas S, Weidel K, Hagenah G, Heindl S. Treatment of Cheyne-Stokes respiration with nasal oxygen and carbon dioxide. Eur Respir J. 1998;12:414–9. doi: 10.1183/09031936.98.12020414. [DOI] [PubMed] [Google Scholar]
- 88.Steens R, Millar T, Xiaoling S, et al. Effect of inhaled 3% CO2 on Cheyne-Stokes respiration in congestive heart failure. Sleep. 1994;17:61–8. doi: 10.1093/sleep/17.1.61. [DOI] [PubMed] [Google Scholar]
- 89.Oldenburg O, Faber L, Vogt J, et al. Influence of cardiac resynchronisation therapy on different types of sleep disordered breathing. Eur J Heart Fail. 2007;9:820–6. doi: 10.1016/j.ejheart.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 90.Luthje L, Renner B, Kessels R, et al. Cardiac resynchronization therapy and atrial overdrive pacing for the treatment of central sleep apnoea. Eur J Heart Fail. 2009;11:273–80. doi: 10.1093/eurjhf/hfn042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sinha A-M, Skobel E, Breithardt O-A, et al. Cardiac resynchronization therapy improves central sleep apnea and Cheyne-Stokes respiration in patients with chronic heart failure. J Am Coll Cardiol. 2004;44:68–71. doi: 10.1016/j.jacc.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 92.Yiu K, Lee K, Lau C, et al. Alleviation of pulmonary hypertension by cardiac resynchronization therapy is associated with improvement in central sleep apnea. Pacing Clin Electrophysiol. 2008;31:1522–7. doi: 10.1111/j.1540-8159.2008.01222.x. [DOI] [PubMed] [Google Scholar]
- 93.Gabor J, Newman D, Barnard-Roberts V, et al. Improvement in Cheyne-Stokes respiration following cardiac resynchronization therapy. Eur Respir J. 2005;26:95–100. doi: 10.1183/09031936.05.00093904. [DOI] [PubMed] [Google Scholar]
- 94.Skobel E, Sinha A-M, Randerath W, Breidhardt OBC. Effect of cardiac resynchronization therapy on sleep quality, quality of life, and symptomatic depression in patients with chronic heart failure and Cheyne, Stokes respiration. Sleep Breath. 2005;9:159–66. doi: 10.1007/s11325-005-0030-1. [DOI] [PubMed] [Google Scholar]
- 95.Mansfield D, Solin P, Roebuck T, Bergin P, Kaye D, Naughton M. The effect of successful heart transplant treatment of heart failure on central sleep apnea. Chest. 2003;124:1675–81. doi: 10.1378/chest.124.5.1675. [DOI] [PubMed] [Google Scholar]
- 96.Kumagai T, Ishibashi Y, Kawarazaki H, et al. Effects of nocturnal oxygen therapy on sleep apnea syndrome in peritoneal dialysis patients. Clin Nephrol. 2008;70:332–9. doi: 10.5414/cnp70332. [DOI] [PubMed] [Google Scholar]
- 97.Pressman M, Benz R, Schleifer C, Peterson D. Sleep disordered breathing in ESRD: acute beneficial effects of treatment with nasal continuous positive airway pressure. Kidney Int. 1993;43:1134–9. doi: 10.1038/ki.1993.159. [DOI] [PubMed] [Google Scholar]
- 98.Jean G, Piperno D, Francois B, Charra B. Sleep apnea incidence in maintenance hemodialysis patients: influence of dialysate buffer. Nephron. 1995;71:138–42. doi: 10.1159/000188701. [DOI] [PubMed] [Google Scholar]
- 99.Hanly P, Pierratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med. 2001;344:102–7. doi: 10.1056/NEJM200101113440204. [DOI] [PubMed] [Google Scholar]
- 100.Fischer R, Lang S, Leitl M, Thiere M, Steiner U, Huber R. Theophylline and acetazolamide reduce sleep-disordered breathing at high altitude. Eur Respir J. 2004;23:47–52. doi: 10.1183/09031936.03.00113102. [DOI] [PubMed] [Google Scholar]
- 101.Nickol A, Leverment J, Richards P, et al. Temazepam at high altitude reduces periodic breathing without impairing next-day performance: a randomized cross-over double-blind study. J Sleep Res. 2006;15:445–54. doi: 10.1111/j.1365-2869.2006.00558.x. [DOI] [PubMed] [Google Scholar]
- 102.Beaumont M, Batejat D, Coste O, et al. Effects of Zolpidem and Zaleplon on sleep, respiratory patterns and performance at a simulated altitude of 4,000 m. Neuropsychobiology. 2004;49:154–62. doi: 10.1159/000076723. [DOI] [PubMed] [Google Scholar]
- 103.Imray C, Wright A, Subudhi A, Roach R. Acute mountain sickness: pathophysiology, prevention, and treatment. Prog Cardiovasc Dis. 2010;52:467–84. doi: 10.1016/j.pcad.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 104.Javaheri S, Malik A, Smith J, Chung E. Adaptive pressure support servoventilation: a novel treatment for sleep apnea associated with use of opioids. J Clin Sleep Med. 2008;4:305–10. [PMC free article] [PubMed] [Google Scholar]
- 105.Farney R, Walker J, Boyle K, Cloward T, Shilling K. Adaptive servoventilation (asv) in patients with sleep disordered breathing associated with chronic opioid medications for non-malignant pain. J Clin Sleep Med. 2008;4:311–9. [PMC free article] [PubMed] [Google Scholar]
- 106.SERVE-HF. Treatment of sleep-disordered breathing by adaptive servo-ventilation in HF patients. 2010. [Accessed July 2]. http://www.servehf.com/en/Health-professionals/objective-of-the-study.html.
- 107.Kuzniar T, Golbin J, Morgentahaler T. Moving beyond empiric continuous positive airway pressure (CPAP) trials for central sleep apnea: a multi-modality titration study. Sleep Breath. 2007;11:259–66. doi: 10.1007/s11325-007-0118-x. [DOI] [PubMed] [Google Scholar]
- 108.Javaheri S, Smith J, Chung E. The prevalence and natural history of complex sleep apnea. J Clin Sleep Med. 2009;5:205–11. [PMC free article] [PubMed] [Google Scholar]
- 109.Joho S, Oda Y, Hirai T, Inoue H. Impact of sleeping position on central sleep apnea/Cheyne-Stokes respiration in patients with heart failure. Sleep Med. 2010;11:143–8. doi: 10.1016/j.sleep.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 110.Soll BA, Yeo KK, Davis JW, Seto TB, Schatz IJ, Shen EN. The effect of posture on Cheyne-Stokes respirations and hemodynamics in patients with heart failure. Sleep. 2009;32:1499–506. doi: 10.1093/sleep/32.11.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ueno LM, Drager LF, Rodrigues AC, et al. Effects of exercise training in patients with chronic heart failure and sleep apnea. Sleep. 2009;32:637–47. doi: 10.1093/sleep/32.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Randerath WJ, Galetke W, Kenter M, Richter K, Schafer T. Combined adaptive servo-ventilation and automatic positive airway pressure (anticyclic modulated ventilation) in co-existing obstructive and central sleep apnea syndrome and periodic breathing. Sleep Med. 2009;10:898–903. doi: 10.1016/j.sleep.2008.09.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
LVEF Data for CPAP
| Author, Year | Duration of CPAP | Δ LVEF, CPAP | Δ LVEF, Control | n (treatment / control) |
|---|---|---|---|---|
| Dohi, 2008 - Responders | 6 mo. | +9.3 ± 9.7 | N/A | 9 |
| Arzt, 2007 - Suppressed | 3 mo. | +3.6 [2.1 to 5.1] | +0.4 [-0.6 to 1.5] | 57/110 |
| Arzt, 2007 - Unsuppressed | 3 mo. | +0.3 [-1.0 to 1.6] | 43/110 | |
| Bradley, 2005 | 3 mo. | +2.2 ± 5.4 | +0.4 ± 5.3 | 128/130 |
| Yasuma, 2005 | 3 mo. | +9.9 ± 2.5 | N/A | 5 |
| 12 mo. | +7.3 ± 5.5 | |||
| Sin, 2000 – only patients with CSAS | 3 mo. | +7.8 ± 2.2* | −0.5 ± 1.5* | 14/15 |
| Tkacova, 1997 | 3 mo. | +8.0 ± 13.5 | −0.5 ± 8.0 | 9/8 |
| Granton, 1996 | 3 mo. | +8.6 ± 15.4 | −1.1 ± 7.9 | 9/8 |
| Naughton, 1995a | 1 mo. | +3.7 ± 13.3 | +0.1 ± 8.8 | 12/12 |
| 3 mo. | +7.7 ± 15.1 | −0.5 ± 8.4 | 12/12 | |
| Naughton, 1995b | 1 mo. | +6.5 ± 12.0 | −1.0 ± 10.5 | 9/9 |
| Naughton, 1994 | 1 mo. | +4.6 ± 7.9 | −1.5 ± 6.5 | 10/4 |
| Takasaki, 1989 | 3 mo. | +7 ± 18 | N/A | 5 |
Read off Figure 1 of paper.
AHI Data: RCTs + 1 NRT for CPAP
| Author, Year | Duration of CPAP | ΔCPAP | ΔControl | n (treatment / control) | Titrated |
|---|---|---|---|---|---|
| Bradley, 2005 / Ruttanaumapawan, 2009 | 3 mo.* | −21.3 ± 15.6 | −0.2 ± 21.0 | 97/108 | N |
| 2 yrs | −23.2 ± 15.9 | −1.3 ± 16.0 | 33/38 | N | |
| Granton, 1996 | 3 mo. | −32 ± 26 | −16 ± 25 | 9/8 | N |
| Naughton, 1995a | 1 mo. | −28.5 ± 16.1 | −6.1 ± 20.8 | 12/12 | N |
| Naughton, 1995b | 1 mo. | −29.6 ± 15.5 | −8.7 ± 20.9 | 9/9 | N |
| Naughton, 1994 | 1 mo. | −35.3 ± 15.5 | −13.3 ± 25.4 | 12/6 | N |
The 3-month data was used in the meta-analysis since it has the same time-frame as the other studies and had more subjects.
AHI Data: Before-after CPAP data
| Author, Year | Duration of CPAP | AHI baseline (± SD) | AHI after CPAP (± SD) | n | Titrated |
|---|---|---|---|---|---|
| Bradley, 2005 / Ruttanaumapawan, 2009 | 3 mo. | 38.9 ± 15.0 | 17.6 ± 16.3 | 97 | N |
| Granton, 1996 | 3 mo. | 49 ± 33 | 17 ± 21 | 9 | N |
| Naughton, 1995a | 1 mo. | 43.2 ± 17.0 | 14.7 ± 16.6 | 12 | N |
| Naughton, 1995b | 1 mo. | 48.1 ± 14.7 | 18.5 ± 18.0 | 9 | N |
| Naughton, 1994 | 1 mo. | 54.0 ± 14.9 | 18.7 ± 17.3 | 12 | N |
| Yasuma, 2005 | 3 mo. | 34.7 ± 21.4 | 6.0 ± 7.0 | 5 | N |
| 6 mo. | 34.7 ± 21.4 | 1.2 ± 1.4 | |||
| Krachman, 2003 | 1 night | 44 ± 27 | 15 ± 24 | 9 | N |
| Takasaki, 1989 | 10 d to 4 wks | 69 ± 20 | 15 ± 16 | 5 | Y |
LVEF Data: BPAP-ST devices
| Author, Year | Duration of Treatment | Δ LVEF, Fixed pressure device (%) | Δ LVEF, Control (%) | n (treatment / control) |
|---|---|---|---|---|
| Dohi, 2008 | 6 mo. | +12.7 ± 10.0 | N/A | 7/0 |
| Kasai, 2005 | 3 mo. | +9.9 ± 8.6 | −1.4 ± 8.5 | 7/7 |
AHI Data: BPAP-ST (before-after treatment)
| Author, Year | Duration of Treatment | AHI baseline (± SD) | AHI after Fixed pressure device (± SD) | n |
|---|---|---|---|---|
| Dohi, 2008 | 1 night | 54.4 ± 7.8 | 8.4 ± 4.7 | 9 |
| Kasai, 2005 | 1 night | 49.4 ± 16.1 | 10 ± 8* | 7 |
| Willson, 2001 | 1 night | 49 ± 10 | 6 ± 5 | 9 |
From Figure 1 in study.
LVEF Data ASV
| Author, Yr | Control / Comparison Treatment | Duration | N (treatment / control) | Δ LVEF Test Treatment | Δ LVEF Control / Comparison Treatment |
|---|---|---|---|---|---|
| Kasai 2010 | CPAP | 3 mo. | 15/15 | +9.1 ± 4.7 | +1.9 ± 10.9 |
| Pepperell 2003 | Sub-therapeutic ASV | 4 wks | 15/11 | +1.8 ± 10.8 | +0.6 ± 13.2 |
| Oldenburg 2008 | Baseline | 6 mo. | 29 | +7.0 ± 9.1 | N/A |
| Zhang 2006 | Oxygen | 2 wks | 14 | +7.0 ± 4.2 | +2.4 ± 4.5 |
| Philippe 2006 | CPAP | 6 mo. | 7/6 | +7 ± 5* | −2 ± 13* |
| Fietze 2008 | BPAP-ST | 6 wks | 15/15 | +1.9 ± 8.1 | +5.6 ± 9.5 |
estimated from graph.
AHI Data: ASV
| Author, Yr | Control Treatment | Duration | n (treatment / control) | Test Treatment |
Control Treatment |
||
|---|---|---|---|---|---|---|---|
| Baseline | After | Baseline | After | ||||
| Pepperell 2003 | Sub-therapeutic ASV | 1 night | 15/15 | 24.7 ± 11.3 | 5.0 ± 5.4 | 23.3 ± 13.3 | 20.6 ± 8.9 |
| 4 wks | 15/11 | 24.7 ± 11.3 | 5.4 ± 7.4 | 23.3 ± 13.3 | 14.7 ± 10.6 | ||
| Oldenburg 2008 | Baseline | 6 mo. | 29/0 | 37.4 ± 9.4 | 3.8 ± 4.1 | N/A | |
| Szollosi 2006 | Baseline | 1 night | 10/0 | 30.0 ± 20.9 | 14.0 ± 12.0 | N/A | |
| Zhang 2006 | Oxygen | 2 weeks | 14/14 | 34.5 ± 6.1 | 6.5 ± 0.8 | 34.5 ± 6.1 | 27.8 ± 8.2 |
| Kasai 2010 | CPAP | 3 mo. | 15/15 | 37.4 ± 19.5 | 1.9 ± 2.1 | 38.6 ± 13.9 | 15.4 ± 12.8 |
| −35.4 ± 19.5 | −23.2 ± 12.0 | ||||||
| Arzt 2008 | CPAP / BPAP | 1 night | 14/0 | 46.4 ± 15§ | 4 ± 3.7§ | 46.4 ± 15§ | 22 ± 15§ |
| BPAP Auto-SV | |||||||
| Morgenthaler 2007 | CPAP | 1 night | 6/0 | 46.0 ± 22.7 | 0 ± 0 | 46.0 ± 22.7 | 22.8 ± 18.2 |
| BPAP-ST | 1 night | 6/0 | 46.0 ± 22.7 | 0 ± 0 | 46.0 ± 22.7 | 1.5 ± 1.5 | |
| Philippe 2006 | CPAP | 3 mo. | 12/** | 47 ± 18 | 4 ± 5* | 40.5 ± 13.5 | ** |
| 6 mo. | 9/8 | 3 ± 4* | 21 ± 25* | ||||
| Fietze 2008 | BPAP-ST | 6 wks | 15/15 | 31.0 ± 10.0† | 11.1 ± 9.9† | 33.4 ± 20.5† | 16.1 ± 16.2† |
Estimated from graph.
Data do not appear to be last-brought-forward in Figure1B for CPAP; appears to overestimate CPAP effectiveness at 3 months (dropouts appear to be put to 0 AHI).
CSRI = CSAI+PBI (periodic breathing index).
Data given in paper were assumed to be SE and not SD as stated.
Oxygen LVEF Data
| Author, Year | Duration of Trial | LVEF baseline (± SD) | LVEF after Oxygen (± SD) | LVEF baseline (± SD) | LVEF control (± SD) | n (treatment / control) |
|---|---|---|---|---|---|---|
| Toyama, 2009 | 3 mo. | 27 ± 9 | 37 ± 10 | 26 ± 8 | 27 ± 9 | 10/10 |
| Sasayama, 2009 | 12 mo. | 33.0 ± 8.9 | 38.5 ± 15.5 | 31.8 ± 7.6 | 33.0 ± 11.2 | 21 / 21 |
| Shigemetsu, 2007 | 4 mo. | 44.7 ± 11.9 | 46.4 ± 14.8 | N/A | N/A | 18 |
| Sasayama, 2006* | 12 wks | 34.7 ± 10.4 | 38.2 ± 13.6 | 32.8 ± 8.8 | 34.4 ± 10.9 | 24/29 |
| Krachman, 2005 | 1 mo. | 22 ± 11 | 19 ± 9 | N/A | N/A | 10 |
| Zhang, 2006 | 2 wks | 30.2 ± 4.6 | 33.2 ± 5.1 | N/A | N/A | 14 |
This data is updated by Sasayama 2009 and not used in the meta-analysis.
Oxygen AHI Data
| Author, Year | Duration of Trial | AHI baseline or control* (± SD) | AHI after Oxygen (± SD) | AHI baseline (± SD) | AHI control (± SD) | n (treatment / control) |
|---|---|---|---|---|---|---|
| Toyama, 2009 | 3 mo. | 26.1 ± 9.1 | 5.1 ± 3.4 | 19.9 ± 13.0 | 21.0 ± 12.0 | 10/10 |
| Sasayama, 2009 | 12 mo. | 19.0 ± 12.3 | 9.0 ± 8.4 | 20.0 ± 11.4 | 20.8 ± 13.5 | 21/21 |
| Shigemetsu, 2007 | 4 mo. | 33.7 ± 11.1 | 6.2 ± 3.2 | N/A | N/A | 18 |
| Sasayama, 2006‡ | 12 wks | 21.0 ± 10.8 | 10.0 ± 11.6 | 18.0 ± 10.7 | 17.1 ± 11.4 | 25/31 |
| Staniforth, 1998 | 4 wks | 37.8 ± 12.9 | 24.9 ± 12.3 | 37.8 ± 12.9 | 38.0 ± 16.6 | 11 |
| Zhang, 2006 | 2 wks | 34.5 ± 6.1 | 27.8 ± 8.2 | N/A | N/A | 14 |
| Andreas, 1996* | 1 wk | 26 ± 24 | 10 ± 9 | N/A | N/A | 22 |
| Hanly, 1989* | 1 night | 30.0 ± 14.1 | 18.9 ± 7.2 | N/A | N/A | 9 |
| Krachman, 2005 | 1 mo. | 57 ± 61 | 12 ± 17 | N/A | N/A | 10 |
| Broström, 2005 | 3 mo. | 34 (24-46)** | 22 (17-42)** | N/A | N/A | 9 |
| Javaheri, 1999 | Total† | 49 ± 19 | 29 ± 29 | N/A | N/A | 36 |
| 1 night | Responders | 36 ± 11 | 6 ± 4 | N/A | N/A | 14 |
| Partial responders | 57 ± 19 | 44 ± 29 | N/A | N/A | 22 | |
| Franklin, 1997 | 1 night | 31.3 ± 13.3 | 8.7 ± 9.1 | N/A | N/A | 20 |
This data is updated by Sasayama 2009 and not used in the meta-analysis.
Control was room air or compressed air.
Data not normally distributed, cannot use in meta-analysis.
Used total data in meta-analysis.
Oxygen vs. CPAP Data
| Author, Year | Duration of Trial | Oxygen |
CPAP |
N (trial / control) | ||
|---|---|---|---|---|---|---|
| Baseline (± SD) | After (± SD) | Baseline (± SD) | After (± SD) | |||
|
AHI data | ||||||
| Arzt, 2005 | 12 wks | 28.8 ± 10.1 | 8.7 ± 13.0 | 35.9 ± 16.0 | 12.2 ± 14.4 | 10/16 |
| Krachman, 1999 | 1 night | 44 ± 27 | 18 ± 15 | N/A | 15 ± 24 | 9 |
|
LVEF data | ||||||
| Arzt, 2005 | 12 wks | 30.9 ± 7.6 | 32.5 ± 7.3 | 31.7 ± 10.4 | 35.7 ± 10.8 | 10/16 |
AHI data for pharmacological therapies for CSAS related to CHF
| Author, Year | Duration of Treatment | n | AHI baseline (± SD) | AHI after placebo (± SD) | AHI after Tx (± SD) |
|---|---|---|---|---|---|
| Javaheri, 2006 | 6 nights | 12 | 55 ± 24 | 57 ± 28 | 34 ± 20 |
| Hu, 2006 | 5-7 days | 13 | 42.6 ± 15.5 | N/A | 20.8 ± 13.2 |
| Javaheri, 1996 | 5 days | 15 | 47 ± 21 | 37 ± 23 | 18 ± 17 |
| Tamura, 2009 | 6 mo | 16 | 34 ± 13 | N/A | 14 ± 13 |
| Walsh, 1995 | 1 month | 8 | 35 ± 20 | N/A | 20 ± 14 |
| Zilberman, 2007 | 3 months | 35 | 26.5 ± 14.6 | N/A | 18.1 ± 12.7 |
| Andreas, 1998 | 1 night | 9 | 36.7 ± 21.9 | N/A | 5.4 ± 3.6 |
| Steens, 1994 | 1 night | 6 | 41.1 ± 28.9 | N/A | 1.0 ± 1.7 |
CRT LVEF Data, %
| Author, Year | Duration of Trial | CRT off or pre-CRT (± SD) | CRT on or post-CRT (± SD) | n |
|---|---|---|---|---|
| Lüthje, 2009 | 3 months | 20.7 ± 4.6 | 28.4 ± 7.7* | 18 |
| Oldenburg, 2007 | 5.0 ± 2.6 mo. | 25.2 ± 6.1 | 29.1 ± 7.3 | 36 |
| Yiu, 2008 | 3 months | 28.8 ± 9.7** | 38.1 ± 8.9** | 15 |
| Sinha, 2004 | 17 ± 7 wks | 25 ± 5 | 35 ± 9 | 14 |
| Gabor, 2005 | 6 months | 19.0 ± 4.2 | 24.2 ± 7.8 | 10 |
| Skobel, 2005 | 18 ± 7 wks | 19 ± 5 | 33 ± 8 | 18 |
SD calculated from data given (as a change in LVEF) in reference.
Data in paper assumed to be SEM and converted to SD.
CRT AHI Data
| Author, Year | Duration of Trial | CRT off or pre-CRT (± SD) | CRT on or post-CRT (± SD) | n |
|---|---|---|---|---|
| Oldenburg, 2007 | 5.0 ± 2.6 mo. | 31.2 ± 15.5 | 17.3 ± 13.7 | 36 |
| Kara, 2008 | 1 night | 14.3 ± 10.0 | 8.1 ± 5.2 | 12 |
| Lüthje, 2009 | 3 months | 37.1 ± 13.4 | 25.7 ± 17.5 | 18 |
| Sinha, 2004 | 17 ± 7 wks | 19.2 ± 10.3 | 4.6 ± 4.4 | 14 |
| Yiu, 2008 | 3 months | 27.5 ± 4.7 | 18.0 ± 3.0 | 15 |
| Gabor, 2005 | 6 months | 42.7 ± 9.1 | 30.8 ± 18.7 | 10 |
| Skobel, 2005* | 18 ± 7 wks | 18 ± 8 | 3 ± 2 | 18 |
7/18 patients with mixed sleep apnea included in data.