Abstract
Study Objectives:
To determine whether cerebral metabolite changes may underlie abnormalities of neurocognitive function and respiratory control in OSA.
Design:
Observational, before and after CPAP treatment.
Setting:
Two tertiary hospital research institutes.
Participants:
30 untreated severe OSA patients, and 25 age-matched healthy controls, all males free of comorbidities, and all having had detailed structural brain analysis using voxel-based morphometry (VBM).
Measurements and Results:
Single voxel bilateral hippocampal and brainstem, and multivoxel frontal metabolite concentrations were measured using magnetic resonance spectroscopy (MRS) in a high resolution (3T) scanner. Subjects also completed a battery of neurocognitive tests. Patients had repeat testing after 6 months of CPAP. There were significant differences at baseline in frontal N-acetylaspartate/choline (NAA/Cho) ratios (patients [mean (SD)] 4.56 [0.41], controls 4.92 [0.44], P = 0.001), and in hippocampal choline/creatine (Cho/Cr) ratios (0.38 [0.04] vs 0.41 [0.04], P = 0.006), (both ANCOVA, with age and premorbid IQ as covariates). No longitudinal changes were seen with treatment (n = 27, paired t tests), however the hippocampal differences were no longer significant at 6 months, and frontal NAA/Cr ratios were now also significantly different (patients 1.55 [0.13] vs control 1.65 [0.18] P = 0.01). No significant correlations were found between spectroscopy results and neurocognitive test results, but significant negative correlations were seen between arousal index and frontal NAA/Cho (r = −0.39, corrected P = 0.033) and between % total sleep time at SpO2 < 90% and hippocampal Cho/Cr (r = −0.40, corrected P = 0.01).
Conclusions:
OSA patients have brain metabolite changes detected by MRS, suggestive of decreased frontal lobe neuronal viability and integrity, and decreased hippocampal membrane turnover. These regions have previously been shown to have no gross structural lesions using VBM. Little change was seen with treatment with CPAP for 6 months. No correlation of metabolite concentrations was seen with results on neurocognitive tests, but there were significant negative correlations with OSA severity as measured by severity of nocturnal hypoxemia.
Citation:
O'Donoghue FJ; Wellard RM; Rochford PD; Dawson A; Barnes M; Ruehland WR; Jackson ML; Howard ME; Pierce RJ; Jackson GD. Magnetic resonance spectroscopy and neurocognitive dysfunction in obstructive sleep apnea before and after CPAP treatment. SLEEP 2012;35(1):41-48.
Keywords: Neuroimaging, sleep disordered breathing, neuropsychological, hypoxia
INTRODUCTION
The pathogenesis of Obstructive sleep apnea (OSA) remains incompletely defined, and is likely multifactorial. Although upper airway anatomy and collapsibility are important, it is clear that central control of upper airway musculature and of ventilation play a role.1 Changes in the regulation of autonomic function2,3 and neuroendocrine function4,5 have been described in OSA, and there has been a consistent association with neurocognitive dysfunction.6–8 These changes are partially reversible following effective treatment8,9 To date there has been little exploration of the pathologic basis for these differences in CNS function. There are no published pathological studies of brain alterations in the disorder. There have been a number of neuroimaging studies examining brain structure, but the data are conflicting.10–16 Recently, our group published the largest study to date, showing left cerebellar and right middle temporal gyrus decreases in gray matter volume in 60 OSA subjects compared to 60 healthy controls.17
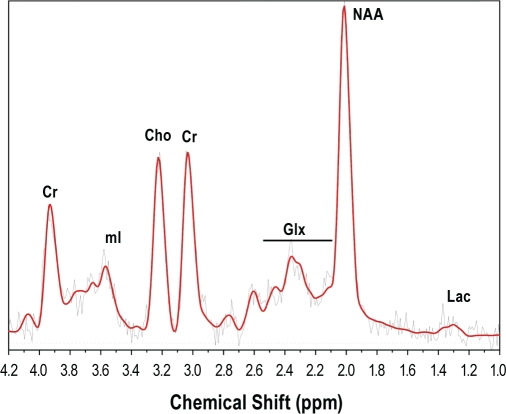
Proton magnetic resonance spectroscopy (MRS) measures tissue concentrations of known metabolites (Figure 1). N-acetylaspartate (NAA), located mainly in neuronal mitochondria, is a marker of neuronal viability and functional integrity. Choline (Cho) containing compounds increase with myelin breakdown and membrane turnover. Cho may increase in some pathologies18 and decrease in others.19 Creatine (Cr), which is involved in energy cycling, is relatively constant in concentration, and is often used as an internal standard.20 MRS abnormalities have been demonstrated in many neurological conditions,21–24 even in the absence of overt structural damage,25,26 and may be reversible after treatment of the disease.25,26 The technique depends on the different resonance frequencies associated with the change in the effective magnetic field surrounding protons due to differences in electron shielding in the immediate vicinity.20 Fourier transformation of the signal produces a spectrum of resonance frequencies, with peak intensities proportional to metabolite concentrations (Figure 1). Due to calibration difficulties, particularly over protracted periods, metabolite concentrations are often expressed as ratios, relative to Cr.
Figure 1.
Magnetic resonance spectrum from the hippocampus of a normal subject. The spectrum is a Fourier transformation of resonance frequencies in the voxel of interest. Peaks correspond to concentrations of known metabolites. Cr, total creatine (creatine plus phosphocreatine); mI, myoinositol; Cho, choline containing compounds; Glx, glutamate plus glutamine; Lac, lactate; NAA, N-acetylaspartate plus N-acetylaspartylglutamate.
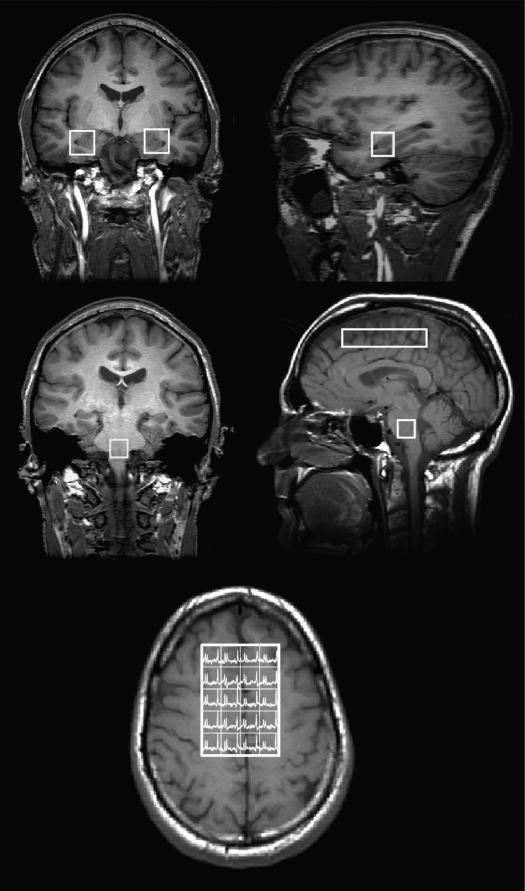
Figure 2.
Position of acquired voxels, superimposed on T1-weighted images. Top row, coronal (left) and sagittal (right) images showing placement of hippocampal voxels. Middle row, left panel: coronal image showing position of brainstem voxel. Middle row, right panel: sagittal image showing brainstem voxel and frontal CSI voxel array. Bottom panel, axial image showing voxel array analyzed from acquired CSI region, (a further outer rim of voxels were discarded to avoid edge artifact).
Chemical shift imaging (CSI) is a magnetic resonance spectroscopy technique that enables the recording of signals from an array of voxels within a volume of tissue. It has the advantage of providing spectral information from multiple voxels, rather than the localized information provided by a single voxel. The additional information obtained with the CSI method comes at the expense of reduced signal-to-noise ratio for a given acquisition time and requires good magnetic field homogeneity over a greater area than that necessary for single voxel spectroscopy. Thus high-resolution scanners are preferred for this form of imaging.
Previous studies have found abnormalities on MRS in OSA,27–32 but the results have varied both in type and location of abnormalities,27–32 and only single selected voxels were studied. No study has evaluated MRS in an OSA group previously shown to be free of structural changes, based on voxel-based morphometry (VBM), in the MRS regions of interest. It therefore remains possible that the changes seen on these previous studies were due to subtle structural abnormalities. In addition, no study has been conducted using high magnetic field strength, giving greater spatial and spectral resolution.33
We hypothesized that MRS abnormalities would exist in frontal lobes, hippocampus and/or brainstem in untreated severe OSA patients. We chose these sites because (1) frontal lobes are a primary site of neurocognitive dysfunction in OSA6; (2) the hippocampus has been demonstrated to be sensitive to intermittent hypoxia, at least in rodents34,35; and (3) the brainstem is the major site of ventilatory control. Of note, no structural changes were seen in these areas in either of our previous publications using VBM,12,17 and, in the case of the hippocampus, using region of interest analysis.12 Second, we hypothesized that abnormalities would be at least partially reversible after treatment of OSA. Third, we hypothesized that metabolite concentrations measured by MRS would correlate with severity of OSA and with results of neurocognitive tests.
Preliminary results have been published in abstract form.36,37
METHODS
Subjects
Thirty male, severe untreated OSA patients (apnea-hypopnea index [AHI] > 30, > 15% total sleep time [TST] on polysomnography at SpO2 < 90%) were recruited from the clinics of the Department of Respiratory and Sleep Medicine, Austin Health, and 25 age-matched healthy male control subjects by newspaper advertisement. Twenty-seven (26 for the pooled analysis17) of the patients, and 24 control subjects, were included in our previous VBM (structural) papers.12,17 Exclusion criteria were: clinical diagnosis or history of respiratory disease, cerebrovascular or ischemic heart disease, diabetes mellitus, central nervous system disorders (neurodegenerative diseases, epilepsy, head injury, psychosis, hypothyroidism, current depression), alcohol or illicit drug abuse, or current intake of psychoactive medications. Additionally, no control subject had a history of snoring or other sleep complaint. All subjects had in-laboratory polysomnography, staged according to standard criteria.38,39 Scoring criteria for respiratory events for both OSA subjects and healthy controls were according to the 1999 AASM recommendations.40
All subjects gave informed written consent. The study was approved by the institutional ethics committee.
Protocol
Subjects presented at 08:30, having had a normal sleep the previous night. They completed a battery of neurocognitive tests assessing subjective sleepiness (Epworth Sleepiness Scale, [ESS]),41 premorbid IQ (National Adult Reading Task, [NART]), vigilance and reaction time (Psychomotor Vigilance Task, [PVT]), working memory (digits forwards and backwards), executive function (Trails A and B, Controlled Oral Word Association Task [COWAT] and Stroop Color Word Interference Task [Stroop]), mood (Beck Depression Inventory [BDI]), and sleep-related quality of life (Functional Outcomes of Sleep Questionnaire, [FOSQ]). Patients then proceeded to scanning, scheduled for 10:30.
Image Acquisition and Processing
MR imaging was performed on a 3T LX Horizon scanner (GE Healthcare, Milwaukee). A coronal Fast spoiled gradient-recalled echo acquisition (FSPGR) (TR: 9, TE: 1.9, TI: 500, flip angle: 20°; FOV: 25x17.5 cm, matrix size; 512x512) was acquired for the localization of MRS voxels.
Isotropic single voxel proton MR spectra were acquired using a pulse sequence of point-resolved spectroscopy (PRESS) with 2 chemical shift selective imaging pulses for water suppression. Spectra (2048 data points, spectral width of 5000 Hz, TE: 144 ms, TR: 3.0 sec) were recorded from isotropic voxels of 2.0 cm and 1.5 cm in bilateral hippocampi and brain stem, respectively. A frontal lobe CSI region of interest was acquired using a 24 cm field of view and 16 phase encoding steps (nominal isotropic voxel size of 1.5 cm). Acquisition parameters were: tip angle 90°, TR 2.0s, TE 144 ms, 512 data points, and 1000 Hz spectral width. Metabolite concentrations were determined with LC model (version 6.1)42 and a basis set of 15 metabolites recorded on the same magnet, and expressed as ratios.
Measurements of NAA, Cho, and Cr were assessed. The mean metabolite ratio for both hippocampi was used for analysis. However post hoc analysis was performed on data from each hippocampus separately. For CSI data, average spectra were compared from the 4x5 central voxels. Outer voxels were disregarded in order to avoid edge artifacts caused by magnetic field inhomogeneity.
In 27 OSA patients, testing and scans were repeated after 6 months of CPAP treatment (Autoset Spirit, ResMed, Sydney, Australia), with compliance and estimated residual AHI monitored using the pump's internal recording systems. The other 3 OSA patients declined to return for a second scan.
Statistics
Statistical analysis was conducted with SPSS version 18 (SPSS, Chicago, IL). Univariate analysis of variance was conducted to identify relationships between group (OSA or healthy control subjects) and metabolite concentration ratios at each location. Subsequently, all comparisons with a univariate P value < 0.1 were entered into an analysis of covariance with age and premorbid IQ (NART) as covariates. Both age and IQ have previously been associated with changes in MRS parameters.43–45 Post hoc testing was performed in similar manner on lateralized hippocampal data.
Paired Student t-tests, with correction for multiple comparisons, were used to compare metabolites and neurocognitive test results pre- and post-treatment in OSA patients.
The relationship between MRS parameters and both OSA severity and the results of neurocognitive testing was explored using Pearson correlations, with Dunn-Sidak correction of P-values for multiple comparisons. This analysis was conducted on the pooled group of untreated OSA patients and control subjects. Because of the large number of potential variables to analyze, and therefore the requirement to correct for a large number of comparisons, this analysis was restricted to those metabolite variables that showed a significant difference between untreated OSA patients and control subjects.
Results are presented as mean (SD) unless otherwise stated. P < 0.05 was accepted as significant.
RESULTS
Table 1 contains details of demographic, anthropometric, premorbid IQ and PSG data for each group. Of the 25 enrolled healthy control subjects, one was excluded due to a small calcified lesion in the falx cerebri, leading to imaging artifact, and another due to claustrophobia preventing completion of the imaging sequence. Patients were more obese and also had slightly lower premorbid IQ (NART) than healthy controls.
Table 1.
Baseline characteristics of the two study groups
| OSA (n = 30) | Control (n = 23) | P* | |
|---|---|---|---|
| Age (yrs) | 45.2 (9.6) | 41.3 (9.3) | 0.14 |
| BMI (kg/m2) | 33.3 (4.6) | 25.6 (2.9) | < 0.01 |
| NART | 109.0 (8.0) | 115.5 (8.3) | < 0.01 |
| AHI | 71.5 (16.2) | 5.3 (3.9) | < 0.01 |
| % SpO2 < 90% | 38.6 (19.3) | 0.02 (0.07) | < 0.01 |
| % SpO2 < 80% | 9.6 (14.4) | 0.0 (0.0) | < 0.01 |
| % Stage 1/2 | 68.9 (15.8) | 60.1 (10.5) | 0.02 |
| % Stage 3/4 | 17.7 (14.7) | 21.1 (9.9) | 0.32 |
| % REM | 13.4 (5.5) | 17.7 (4.9) | < 0.01 |
| Arousal Index | 55.1 (18.4) | 15.7 (9.6) | < 0.01 |
| ESS | 12.9 (4.3) | 5.2 (3.4) | < 0.01 |
All values are expressed as mean (SD). Definition of abbreviations: NART, National Adult Reading Task (full scale) score, a measure of premorbid IQ; AHI, apnea/hypopnea index; % SpO2 < 90% (80%), percent of total sleep time at diagnostic polysomnography spent at oxygen saturations less than 90% (80%); % Stage 1/2 (3/4)(REM), percent of total sleep time at diagnostic polysomnography spent in relevant stages; ESS, Epworth sleepiness score, a measure of subjective daytime sleepiness.
Independent samples t-tests.
Statistically significant differences were found at baseline between OSA patients and healthy control subjects in frontal NAA/Cho (4.56 [0.41] vs 4.92 [0.44], P = 0.001), and in hippocampal Cho/Cr (0.38 [0.04] vs 0.41 [0.04], P = 0.006) ratios (both ANCOVA, with age and premorbid IQ as covariates; Table 2). No significant differences were found in other ratios. Post hoc testing determined right (0.38 [0.05] vs 0.42 [0.05], P = 0.006) but not left (0.39 [0.04] vs 0.41 [0.05], P = 0.09) Cho/Cr differences reached statistical significance, with no between group differences in other MRS parameters on either side.
Table 2.
Magnetic resonance spectroscopy metabolite ratios in control subjects and in OSA patients before and after CPAP treatment (6 months)
| Variable | Control (n = 23) | OSApre (n = 30) | Univar P* | ANCOVA P* | OSApost (n = 27) | Univar P‡ | ANCOVA P‡ |
|---|---|---|---|---|---|---|---|
| Frontal NAA/Cho | 4.92 (0.44) | 4.56 (0.41) | < 0.01 | < 0.01 | 4.47 (0.46) | < 0.01 | < 0.01 |
| Frontal NAA/Cr | 1.65 (0.18) | 1.59 (0.13) | 0.20 | 1.55 (0.13) | 0.04 | 0.01 | |
| Frontal Cho/Cr | 0.33 (0.03) | 0.35 (0.02) | 0.06 | 0.05 | 0.35 (0.03) | 0.14 | |
| Hipp NAA/Cho | 4.04 (0.45) | 4.15 (0.52) | 0.43 | 4.11 (0.47) | 0.59 | ||
| Hipp NAA/Cr | 1.66 (0.15) | 1.58 (0.23) | 0.16 | 1.61 (0.19) | 0.29 | ||
| Hipp Cho/Cr | 0.41 (0.04) | 0.38 (0.04) | < 0.01 | < 0.01 | 0.39 (0.05) | 0.11 | |
| Brainstem NAA/Cho | 3.44 (0.69) | 3.35 (0.58) | 0.63 | 3.14 (0.51) | 0.10 | 0.31 | |
| Brainstem NAA/Cr | 2.03 (0.46) | 2.14 (0.54) | 0.46 | 2.12 (0.66) | 0.57 | ||
| Brainstem Cho/Cr | 0.59 (0.11) | 0.65 (0.14) | 0.16 | 0.67 (0.15) | 0.06 | 0.09 |
Explanation of terms: Frontal, Frontal lobe Chemical Shift Imaging (CSI) region of interest predominantly composed of white matter; Hipp, Mean of 2 single voxels centered on left and right hippocampi; Brainstem, Single brainstem voxel at the level of the pontomedullary junction; NAA, N-acetylaspartate plus N-acetylaspartylglutamate (a marker of neuronal viability); Cho, Choline containing compounds (a marker of membrane turnover); Cr, Creatine, an internal standard; OSApre, OSA patients at baseline; OSApost, OSA patients after 6 months CPAP; Univar, univariate ANOVA.
Comparisons of OSApre and control.
Comparison of OSApost and control. Comparisons with a P value < 0.1 on univariate ANOVA were entered into an ANCOVA with age and NART (premorbid IQ) as covariates.
In the 27 patients who had repeat testing after CPAP treatment, “time at pressure” compliance was 5.95 (1.77) h, and residual machine-estimated AHI 6.8 (2.9) events/h. Two patients had a mean compliance < 2 h per night, with all others > 5 h per night. After 6 months of treatment, no significant change in the metabolite ratios was seen at any site in OSA patients (paired t-tests, with correction for multiple comparisons, data not shown). When comparing OSA subjects post treatment with controls at baseline, differences in frontal NAA/Cho remained (Table 2). Differences in frontal NAA/Cr were now also significant (OSA 1.55 [0.13] vs control 1.65 [0.18], P = 0.01, ANCOVA). The differences in hippocampal Cho/Cr ratios were no longer significant (OSA 0.39 [0.05] control 0.41 [0.04] univariate P = 0.11). Exclusion of the 2 subjects with average CPAP compliance of < 4 h did not change the results of this analysis, nor was there any significant correlation between CPAP usage expressed as mean hours per night and change in any MRS parameter (data not shown). Post hoc testing did not reveal any between-group differences in right or left hippocampi after CPAP therapy.
As expected, there were differences between OSA subjects and controls across multiple domains of neurocognitive testing (Table 3). Improvements were seen in a number of domains after 6 months of CPAP treatment (Table 4). Again, exclusion of the 2 partially treatment-compliant patients did not alter these results. There were no differences between OSA patients after treatment and healthy control subjects at baseline in any neurocognitive parameter. Metabolite ratios correlated poorly with neurocognitive parameters, and no correlation was significant after correction was made for multiple comparisons. However, significant negative correlations were found between frontal lobe NAA/Cr ratios and arousal index, and between hippocampal Cho/Cr and % TST spent at oxygen saturations < 90% (Table 5). It could be argued that, by design, the groups differed in these variables. However, when these variables were entered into a forward stepwise multiple regression with group (OSA or control) as a variable, the relationship with the sleep variables was stronger, and group was not included in the final equation.
Table 3.
Neurocognitive test results at baseline with P < 0.1 for differences between OSA patients and controls on univariate analysis
| OSA (n = 30) | Control (n = 23) | Univariate P | Multivariate P | ||
|---|---|---|---|---|---|
| Subjective Sleepiness | ESS | 12.9 (4.3) | 5.2 (3.4) | < 0.01 | < 0.01 |
| Vigilance/Reaction time | PVT LAPSE | 3.1 (2.8) | 1.8 (1.1) | 0.04 | 0.01 |
| Memory | DIG FOR* | 6.4 (1.5) | 7.2 (0.9) | 0.03 | 0.28 |
| DIG BACK* | 5.1 (1.4) | 5.7 (1.7) | 0.3 | ||
| Executive Function | TRAILS B | 79.1 (35.7) | 58.5 (18.6) | < 0.01 | 0.44 |
| AB DIFF | 49.0 (31.1) | 32.5 (19.6) | 0.03 | 0.74 | |
| COWAT* | 39.2 (12.7) | 47.9 (11.1) | 0.02 | 0.08 | |
| STROOP* | 2.7 (7.7) | 10.8 (8.2) | < 0.01 | 0.02 | |
| Mood | BDI | 7.5 (5.0) | 2.7 (2.7) | < 0.01 | < 0.01 |
| QOL | FOSQ Mean | 3.1 (0.5) | 3.8 (0.3) | < 0.01 | < 0.01 |
Explanation of terms: PVT Lapse, Lapses on Psychomotor Vigilance Task; Dig For/Back, digits forwards/backwards test; AB Diff, difference between times for TRAILS test parts A and B; COWAT, Controlled Oral Word Association Task; Stroop, interference parameter on Stroop Colour Word Interference Test; BDI, Beck Depression Inventory; FOSQ, Functional Outcomes of Sleep Questionnaire. Comparisons with a P value < 0.1 on univariate ANOVA were entered into an ANCOVA with age and NART (premorbid IQ) as covariates.
n = 19 for OSA subjects, 21 for control subjects for these tests.
Table 4.
Neurocognitive test results in OSA subjects pre and post 6 months of CPAP treatment (n = 27)
| Pre | Post | P* | ||
|---|---|---|---|---|
| Subjective Sleepiness | ESS | 13.2 (3.8) | 6.0 (4.4) | < 0.01 |
| Vigilance/Reaction time | PVT LAPSE | 3.1 (2.9) | 2.2 (1.5) | 0.91 |
| Memory | DIG FOR# | 6.7 (1.4) | 6.4 (1.8) | 0.99 |
| DIG BACK# | 5.3 (1.4) | 5.2 (1.6) | 0.99 | |
| Executive Function | TRAILS B | 74.0 (33.9) | 69.3 (32.1) | 0.99 |
| AB DIFF | 45.1 (29.0) | 42.0 (27.1) | 0.99 | |
| COWAT# | 41.8 (12.1) | 44.9 (12.8) | 0.89 | |
| STROOP# | 3.3 (7.8) | 8.4 (8.30) | 0.04 | |
| Mood | BDI | 7.8 (5.2) | 3.5 (3.3) | < 0.01 |
| QOL | FOSQ Mean | 3.1 (0.5) | 3.7 (0.3) | < 0.01 |
Abbreviations as in Table 3.
Paired samples t-tests, with Dunn-Sidak correction for multiple comparisons. #n = 16 for these tests.
Table 5.
Pearson correlations of metabolite ratios with OSA severity parameters
| %TST < 90% | AHI | AI | ||
|---|---|---|---|---|
| Frontal NAA/Cho | R | −0.30 | −0.28 | −0.40 |
| corrected P | 0.21 | 0.28 | 0.03 | |
| Hippocampal Cho/Cr | R | −0.40 | −0.30 | −0.30 |
| corrected P | 0.02 | 0.16 | 0.16 |
Explanation of terms: NAA/Cho, N-acetylaspartate/Choline ratio; Cho/Cr, Choline/Creatine ratio; %TST < 90%, percentage of total sleep time spent with oxygen saturation < 90%; AHI, apnea-hypopnea index; ArI, arousal index. P values were corrected for multiple comparisons using the Dunn-Sidak method.
DISCUSSION
This study identified significant differences in metabolite ratios in the frontal lobe white matter and in the hippocampus between OSA subjects and controls. By way of comparison, the 9% change in frontal NAA/Cho ratios approximates to the change in normal-appearing brain after traumatic brain injury,46 in medial temporal lobe in Alzheimer disease,44 or the maximal changes found with aging from the second to the eighth decade.44 The frontal lobe differences were not reversed after treatment for 6 months with CPAP, but there was no longer a significant difference from controls in the hippocampus. As expected, OSA patients showed impairment in a range of neurocognitive tests, and performance was improved on treatment. Cerebral metabolite concentrations correlated poorly with results of neurocognitive testing but did correlate with indices of severity of OSA.
Previous studies have evaluated MRS in OSA subjects (Table 6). The results of the current study are in broad agreement with these.27–29,31,32 The current study is the largest to date, and our patients were more rigorously screened for cardiovascular and neuropsychiatric morbidity than in some of the earlier studies.27–29 Furthermore, our statistical analysis controlled for age and premorbid IQ—factors reported to influence MRS parameters.43–45 Thus we believe our results strengthen the evidence for frontal metabolic change in OSA, independent of the influence of cardiovascular disease. The observation that NAA/Cr ratios were also reduced (significantly in the post-treatment analysis), suggests that the changes seen in NAA/Cho were due primarily to a decrease in NAA, indicative of impaired neuronal viability and/or integrity in this region.
Table 6.
Previous studies of magnetic resonance spectroscopy in OSA
| Reference | N OSA, control | ROI | Findings | Other results | Comment |
|---|---|---|---|---|---|
| Kamba27 | 15,23 | Medial parietooccipital cortex, parietoccipital PWM | Decreased NAA/Cho in WM not GM in severe OSA vs mild OSA and control | Multiple comorbidities. OSA patients older. | |
| Kamba28 | 55,0 | Medial parietooccipital cortex, parietoccipital PWM | Decreased NAA/Cho proportional to AHI, independent of age and comorbidity. | No relationship to indices of oxygenation | |
| Bartlett30 | 8,5 | Left hippocampus | Increased NAA/Cr | No correlation with PVT but correlated with arousal index | Felt primarily due to decreased Cr |
| Alchanatis29 | 22,10 | Frontal PWM, prefrontal GM, parietoccipital PWM. | Decreased NAA/Cr and Cho/Cr frontal white matter | No differences other locations. No correlation with OSA severity. | Some cardiovascular disease. Results the same if these excluded. |
| Tonon31 | 14,10 | Medial parietoccipital cortex | Decreased NAA, unchanged after 6 months CPAP | Correlation with nadir SpO2 and with MSLT but not neuropsych testing | Careful screening for comorbidity. Patient reported compliance only. |
| Sarchielli32 | 20, 20 | Frontal and temporal voxels including both GM and WM | Decreased frontal NAA/Cr, increased temporal Cho/Cr | Frontal changes correlated with AHI |
Explanation of terms: ROI, region of interest; PWM, periventricular white matter; GM, gray matter; PVT, psychomotor vigilance task; MSLT, mean latency on multiple sleep latency test.
A key novel aspect of this study is that these patients have been the subject of previous reports,12,17 in which detailed anatomical analysis of brain structure in OSA patients was conducted using voxel based morphometry. Those studies12,17 did not show changes in brain structure in OSA patients in the areas reported in the current study. Thus, the differences reported in the current paper cannot be ascribed to subtle differences in macroscopic brain structure. A further important aspect of the current study is that patients were assessed both before and after CPAP. While this has been previously reported in the study of Tonon et al.,31 these authors limited their analysis to a medial parietooccipital voxel, a region not normally associated with abnormalities in OSA. This region was chosen in order to optimize MRS data quality, because the occipital region has the lowest magnetic susceptibility. In the current study, conducted in a high magnetic field strength scanner, we were able to evaluate areas more usually linked to neurocognitive dysfunction in OSA.6,8
A surprising finding was the significant difference in Cho/Cr ratios in the hippocampal area (OSA 0.38 [0.04] vs control 0.41 [0.04], P = 0.005) at baseline. Choline containing compounds are normally associated with membrane turnover and are increased in conditions such as inflammation and neoplasia.20 Furthermore concentrations in the frontal lobe white matter, while not significantly different, showed a trend for differences in the opposite direction (OSA 0.35 [0.02], control 0.33 [0.02]; P = 0.057). Temporal lobe MRS measurements tend to be associated with greater susceptibility to artifact and to be more variable than those in the frontal lobe.33 Therefore these differences should be treated with some caution, Nevertheless, when right and left hippocampi were compared separately between groups, results were very similar, although only significant on the right side. Furthermore the %TST spent at oxygen saturations < 90% correlated negatively with hippocampal Cho/Cr, and animal studies have suggested that the hippocampus is particularly sensitive to the effects of intermittent hypoxia.35,47 Indeed an MRS paper has shown spectroscopic abnormalities in this area in mouse pups exposed to intermittent, but not continuous, hypoxia.34 Thus changes in this area in severe OSA might be expected. Alchanatis and coworkers29 found decreased Cho/Cr in OSA patients in the frontal lobes at a field strength of 1.5T; and Shim et al. found decreased NAA, Cho, and Cr in parietal white matter of COPD subjects. If confirmed by future studies, we speculate that the apparent differing directions of change in Cho/Cr in frontal lobes and hippocampus may represent different stages in evolution of the same pathological process, with increased lipid turnover (as suggested by increased Cho) later followed by decreased turnover and possible apoptosis (suggested by decreased Cho).
We examined reversibility of brain metabolite abnormalities after 6 months of CPAP treatment in our OSA patients. Reversibility has previously been demonstrated in epilepsy,25 Cushing syndrome,48 and chronic alcoholism.49 In the study of Douglas et al.,34 the metabolite abnormalities induced in neonatal mice exposed to 4 weeks of intermittent hypoxia were reversed after 4 weeks of normoxia. However our study has failed to show any change in metabolite concentrations after 6 months of treatment with CPAP, despite excellent objectively recorded compliance. Mean CPAP machine reported AHI over this period was 6.8 (2.9) events/h. Given the small but significant overreporting bias in ResMed CPAP machine estimated AHI when compared to simultaneous PSG,50 this suggests excellent control of OSA. We did see a small nonsignificant change in hippocampal Cho/Cr ratios, such that the differences between OSA subjects and controls were no longer statistically significant, and a nonsignificant change in NAA/Cr in the frontal lobes, such that differences between OSA patients and controls reached statistical significance post CPAP. However, in part these changes may have been due to the smaller numbers in the post-CPAP analysis (n = 27 vs 30). Studies examining the effect of CPAP treatment on neurocognitive dysfunction in OSA have frequently failed to show complete reversal of abnormalities.8 Evoked potential studies have also shown abnormalities of N2 and P3 latencies in OSA, which improved but failed to normalize after CPAP treatment.51 The results of the current study suggest that this may be due to irreversible, or at least slowly reversible, differences in brain metabolism, particularly in the frontal lobes. Whether these abnormalities are primary, or secondary to the disease process, is open to conjecture.
We assessed a battery of neuropsychological tests, performed on the same days as MRS imaging of the specific brain areas involved in performance of these tests. Only weak, nonsignificant correlations were seen between metabolite concentrations and neurocognitive test results, either before or after treatment (when the concurrent effect of chronic sleep deprivation would be expected to have dissipated). This may indicate that the performance in these tests is due to complex interactions of many brain areas, which cannot be represented by a limited linear analysis such as that used in the current study. Ultimately a larger group with measurements from more areas of brain would be necessary to more fully answer this question.
We did find a relationship between OSA severity and both frontal and hippocampal metabolite concentrations. It could be argued that this was predictable, given there were differences in metabolite ratios in these areas, and by design there were differences in OSA severity between the groups. However, using multiple regression analysis, the relationships appeared to be more strongly associated with the sleep variables than with group (OSA or control). Our study did not address other potential pathways by which OSA may cause cerebral injury (e.g., metabolic syndrome, cardiovascular disease, hypertension, impaired cerebral autoregulation). Such relationships would be of interest in future research.
The only objective test to show significant improvement with treatment (after correction for multiple comparisons) was the Stroop color-word association test. As these patients had very severe OSA (AHI 71) and were unusually compliant with CPAP, it could be argued that our subjects were not representative of the general OSA population, and perhaps more changes could have been seen with treatment in a milder group, where damage was not so well established. On the other hand the Stroop test was also the only objective test to differ between OSA subjects and healthy controls at baseline, and therefore the failure to change significantly could be due to the lack of initial impairment. Nevertheless these possibilities cannot be discounted. Further studies including a treatment arm in a wider range of OSA severity are clearly needed to clarify these issues.
In summary, we have demonstrated abnormalities in metabolite ratios in frontal lobe white matter and in the hippocampus of untreated severe OSA subjects, compared to healthy controls. Frontal, but not hippocampal changes persisted after 6 months of treatment with CPAP, with excellent objectively measured compliance. Metabolite concentrations were poorly correlated with both neurocognitive test results, but significant correlations were found with severity of OSA.
DISCLOSURE STATEMENT
This study was funded in part by ResMed Australia. Dr. Rochford has received research support from Compumedics and ResMed Australia. Dr. Ruehland has received research support from ResMed Australia, Fisher and Paykel Healthcare, and Philips Respironics. Dr. Howard has received loans of equipment from Sleep Diagnostics and Edansafe. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Supported by National Health and Medical Research Council of Australia grants number 226200 and 430300, by ResMed Pty and the Austin Hospital Medical Research Foundation. The authors are grateful to the sleep physicians at Austin Health and to Dr. James Jamieson for help in recruiting OSA patients.
Rob Pierce died tragically in the Victorian bushfires on February 7th 2009. This paper is dedicated to him.
Footnotes
A commentary on this article appears in this issue on page 9.
REFERENCES
- 1.Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:144–53. doi: 10.1513/pats.200707-114MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harper RM, Macey PM, Henderson LA, et al. fMRI responses to cold pressor challenges in control and obstructive sleep apnea subjects. J Appl Physiol. 2003;94:1583–95. doi: 10.1152/japplphysiol.00881.2002. [DOI] [PubMed] [Google Scholar]
- 3.Narkiewicz K, van de Borne PJ, Cooley RL, Dyken ME, Somers VK. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation. 1998;98:772–6. doi: 10.1161/01.cir.98.8.772. [DOI] [PubMed] [Google Scholar]
- 4.Grunstein RR, Handelsman DJ, Lawrence SJ, Blackwell C, Caterson ID, Sullivan CE. Neuroendocrine dysfunction in sleep apnea: reversal by continuous positive airways pressure therapy. J Clin Endocrinol Metab. 1989;68:352–8. doi: 10.1210/jcem-68-2-352. [DOI] [PubMed] [Google Scholar]
- 5.Meston N, Davies RJ, Mullins R, Jenkinson C, Wass JA, Stradling JR. Endocrine effects of nasal continuous positive airway pressure in male patients with obstructive sleep apnoea. J Intern Med. 2003;254:447–54. doi: 10.1046/j.1365-2796.2003.01212.x. [DOI] [PubMed] [Google Scholar]
- 6.Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002;11:1–16. doi: 10.1046/j.1365-2869.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- 7.Beebe DW, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep. 2003;26:298–307. doi: 10.1093/sleep/26.3.298. [DOI] [PubMed] [Google Scholar]
- 8.Engleman HM, Douglas NJ. Sleep. 4: Sleepiness, cognitive function, and quality of life in obstructive sleep apnoea/hypopnoea syndrome. Thorax. 2004;59:618–22. doi: 10.1136/thx.2003.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferini-Strambi L, Baietto C, Di Gioia MR, et al. Cognitive dysfunction in patients with obstructive sleep apnea (OSA): partial reversibility after continuous positive airway pressure (CPAP) Brain Res Bull. 2003;61:87–92. doi: 10.1016/s0361-9230(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 10.Macey PM, Henderson LA, Macey KE, et al. Brain morphology associated with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;166:1382–7. doi: 10.1164/rccm.200201-050OC. [DOI] [PubMed] [Google Scholar]
- 11.Morrell MJ, McRobbie DW, Quest RA, Cummin AR, Ghiassi R, Corfield DR. Changes in brain morphology associated with obstructive sleep apnea. Sleep Med. 2003;4:451–4. doi: 10.1016/s1389-9457(03)00159-x. [DOI] [PubMed] [Google Scholar]
- 12.O'Donoghue FJ, Briellmann RS, Rochford PD, et al. Cerebral structural changes in severe obstructive sleep apnea. Am J Respir Crit Care Med. 2005;171:1185–90. doi: 10.1164/rccm.200406-738OC. [DOI] [PubMed] [Google Scholar]
- 13.Macey PM, Kumar R, Woo MA, Valladares EM, Yan-Go FL, Harper RM. Brain structural changes in obstructive sleep apnea. Sleep. 2008;31:967–77. [PMC free article] [PubMed] [Google Scholar]
- 14.Yaouhi K, Bertran F, Clochon P, et al. A combined neuropsychological and brain imaging study of obstructive sleep apnea. J Sleep Res. 2009;18:36–48. doi: 10.1111/j.1365-2869.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- 15.Canessa N, Castronovo V, Cappa SF, et al. Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care Med. 2011;183:1419–26. doi: 10.1164/rccm.201005-0693OC. [DOI] [PubMed] [Google Scholar]
- 16.Torelli F, Moscufo N, Garreffa G, et al. Cognitive profile and brain morphological changes in obstructive sleep apnea. Neuroimage. 2011;54:787–93. doi: 10.1016/j.neuroimage.2010.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrell MJ, Jackson ML, Twigg GL, et al. Changes in brain morphology in patients with obstructive sleep apnoea. Thorax. 2010;65:908–14. doi: 10.1136/thx.2009.126730. [DOI] [PubMed] [Google Scholar]
- 18.McBride DQ, Miller BL, Nikas DL, et al. Analysis of brain tumors using 1H magnetic resonance spectroscopy. Surg Neurol. 1995;44:137–44. doi: 10.1016/0090-3019(95)00139-5. [DOI] [PubMed] [Google Scholar]
- 19.Molina JA, Garcia-Segura JM, Benito-Leon J, et al. Proton magnetic resonance spectroscopy in dementia with Lewy bodies. Eur Neurol. 2002;48:158–63. doi: 10.1159/000065520. [DOI] [PubMed] [Google Scholar]
- 20.Danielsen ER, Ross B. Magnetic resonance spectroscopy of neurological diseases. New York: Marcel Dekker; 1999. [Google Scholar]
- 21.Connelly A, Jackson GD, Duncan JS, King MD, Gadian DG. Magnetic resonance spectroscopy in temporal lobe epilepsy. Neurology. 1994;44:1411–7. doi: 10.1212/wnl.44.8.1411. [DOI] [PubMed] [Google Scholar]
- 22.Firbank MJ, Harrison RM, O'Brien JT. A comprehensive review of proton magnetic resonance spectroscopy studies in dementia and Parkinson's disease. Dement Geriatr Cogn Disord. 2002;14:64–76. doi: 10.1159/000064927. [DOI] [PubMed] [Google Scholar]
- 23.Ross B, Bluml S. Magnetic resonance spectroscopy of the human brain. Anat Rec. 2001;265:54–84. doi: 10.1002/ar.1058. [DOI] [PubMed] [Google Scholar]
- 24.Stanley JA, Pettegrew JW, Keshavan MS. Magnetic resonance spectroscopy in schizophrenia: methodological issues and findings--part I. Biol Psychiatry. 2000;48:357–68. doi: 10.1016/s0006-3223(00)00949-5. [DOI] [PubMed] [Google Scholar]
- 25.Hugg JW, Kuzniecky RI, Gilliam FG, Morawetz RB, Fraught RE, Hetherington HP. Normalization of contralateral metabolic function following temporal lobectomy demonstrated by 1H magnetic resonance spectroscopic imaging. Ann Neurol. 1996;40:236–9. doi: 10.1002/ana.410400215. [DOI] [PubMed] [Google Scholar]
- 26.De Stefano N, Narayanan S, Matthews PM, Francis GS, Antel JP, Arnold DL. In vivo evidence for axonal dysfunction remote from focal cerebral demyelination of the type seen in multiple sclerosis. Brain. 1999;122(Pt 10):1933–9. doi: 10.1093/brain/122.10.1933. [DOI] [PubMed] [Google Scholar]
- 27.Kamba M, Suto Y, Ohta Y, Inoue Y, Matsuda E. Cerebral metabolism in sleep apnea. Evaluation by magnetic resonance spectroscopy. Am J Respir Crit Care Med. 1997;156:296–8. doi: 10.1164/ajrccm.156.1.9611063. [DOI] [PubMed] [Google Scholar]
- 28.Kamba M, Inoue Y, Higami S, Suto Y, Ogawa T, Chen W. Cerebral metabolic impairment in patients with obstructive sleep apnoea: an independent association of obstructive sleep apnoea with white matter change. J Neurol Neurosurg Psychiatry. 2001;71:334–9. doi: 10.1136/jnnp.71.3.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alchanatis M, Deligiorgis N, Zias N, et al. Frontal brain lobe impairment in obstructive sleep apnoea: a proton MR spectroscopy study. Eur Respir J. 2004;24:980–6. doi: 10.1183/09031936.04.00127603. [DOI] [PubMed] [Google Scholar]
- 30.Bartlett DJ, Rae C, Thompson CH, et al. Hippocampal area metabolites relate to severity and cognitive function in obstructive sleep apnea. Sleep Med. 2004;5:593–6. doi: 10.1016/j.sleep.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Tonon C, Vetrugno R, Lodi R, et al. Proton magnetic resonance spectroscopy study of brain metabolism in obstructive sleep apnoea syndrome before and after continuous positive airway pressure treatment. Sleep. 2007;30:305–11. doi: 10.1093/sleep/30.3.305. [DOI] [PubMed] [Google Scholar]
- 32.Sarchielli P, Presciutti O, Alberti A, et al. A 1H magnetic resonance spectroscopy study in patients with obstructive sleep apnea. Eur J Neurol. 2008;15:1058–64. doi: 10.1111/j.1468-1331.2008.02244.x. [DOI] [PubMed] [Google Scholar]
- 33.Wellard RM, Briellmann RS, Jennings C, Jackson GD. Physiologic variability of single-voxel proton MR spectroscopic measurements at 3T. AJNR Am J Neuroradiol. 2005;26:585–90. [PMC free article] [PubMed] [Google Scholar]
- 34.Douglas RM, Miyasaka N, Takahashi K, Latuszek-Barrantes A, Haddad GG, Hetherington HP. Chronic intermittent but not constant hypoxia decreases NAA/Cr ratios in neonatal mouse hippocampus and thalamus. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1254–9. doi: 10.1152/ajpregu.00404.2006. [DOI] [PubMed] [Google Scholar]
- 35.Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci. 2001;21:2442–50. doi: 10.1523/JNEUROSCI.21-07-02442.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Briellmann RS, O'Donoghue FJ, Wellard RM, et al. Frontal lobe CSI abnormalities in obstructive sleep apnea. Proc 11th ISMRM; 2003; 2003. p. 2061. [Google Scholar]
- 37.O'Donoghue FJ, Briellmann RS, Rochford P, et al. Magnetic resonance spectroscopy and neuropsychological dysfunction in OSA, before and after CPAP. Am J Resp Crit Care Med. 2007:A670. [Google Scholar]
- 38.Rechtschafen A, Kales A, editors. A manual of standardised terminology, techniques and scoring system for sleep stages of human sleep. Bethesda, Maryland: National Institute of Neurological Disease and blindness; 1968. NIH publication no 204. [Google Scholar]
- 39.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 40.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 41.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 42.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–9. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 43.Schuff N, Amend DL, Knowlton R, Norman D, Fein G, Weiner MW. Age-related metabolite changes and volume loss in the hippocampus by magnetic resonance spectroscopy and imaging. Neurobiol Aging. 1999;20:279–85. doi: 10.1016/s0197-4580(99)00022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Minati L, Grisoli M, Bruzzone MG. MR spectroscopy, functional MRI, and diffusion-tensor imaging in the aging brain: a conceptual review. J Geriatr Psychiatry Neurol. 2007;20:3–21. doi: 10.1177/0891988706297089. [DOI] [PubMed] [Google Scholar]
- 45.Jung RE, Haier RJ, Yeo RA, et al. Sex differences in N-acetylaspartate correlates of general intelligence: an 1H-MRS study of normal human brain. Neuroimage. 2005;26:965–72. doi: 10.1016/j.neuroimage.2005.02.039. [DOI] [PubMed] [Google Scholar]
- 46.Garnett MR, Blamire AM, Rajagopalan B, Styles P, Cadoux-Hudson TA. Evidence for cellular damage in normal-appearing white matter correlates with injury severity in patients following traumatic brain injury: a magnetic resonance spectroscopy study. Brain. 2000;123(Pt 7):1403–9. doi: 10.1093/brain/123.7.1403. [DOI] [PubMed] [Google Scholar]
- 47.Row BW, Kheirandish L, Neville JJ, Gozal D. Impaired spatial learning and hyperactivity in developing rats exposed to intermittent hypoxia. Pediatr Res. 2002;52:449–53. doi: 10.1203/00006450-200209000-00024. [DOI] [PubMed] [Google Scholar]
- 48.Khiat A, Bard C, Lacroix A, Boulanger Y. Recovery of the brain choline level in treated Cushing's patients as monitored by proton magnetic resonance spectroscopy. Brain Res. 2000;862:301–7. doi: 10.1016/s0006-8993(00)02147-8. [DOI] [PubMed] [Google Scholar]
- 49.Bartsch AJ, Homola G, Biller A, et al. Manifestations of early brain recovery associated with abstinence from alcoholism. Brain. 2007;130(Pt 1):36–47. doi: 10.1093/brain/awl303. [DOI] [PubMed] [Google Scholar]
- 50.Ueno K, Kasai T, Brewer G, et al. Evaluation of the apnea-hypopnea index determined by the S8 auto-CPAP, a continuous positive airway pressure device, in patients with obstructive sleep apnea-hypopnea syndrome. J Clin Sleep Med. 2010;6:146–51. [PMC free article] [PubMed] [Google Scholar]
- 51.Rumbach L, Krieger J, Kurtz D. Auditory event-related potentials in obstructive sleep apnea: effects of treatment with nasal continuous positive airway pressure. Electroencephalogr Clin Neurophysiol. 1991;80:454–7. doi: 10.1016/0168-5597(91)90094-e. [DOI] [PubMed] [Google Scholar]