Abstract
Study Objectives:
Few population-based, longitudinal studies have examined risk factors for persistent insomnia, and the results are inconsistent. Furthermore, none of these studies have examined the role of polysomnographic (PSG) variables such as sleep duration or sleep apnea on the persistence of insomnia.
Design:
Representative longitudinal study.
Setting:
Sleep laboratory.
Participants:
From a random, general population sample of 1741 individuals of the adult Penn State Cohort, 1395 were followed-up after 7.5 years.
Measurements:
Individuals underwent one-night PSG and full medical evaluation at baseline and a telephone interview at follow-up. PSG sleep duration was analyzed as a continuous variable and as a categorical variable: < 6 h sleep (short sleep duration) and ≥ 6 h sleep (longer sleep duration).
Results:
The rates of insomnia persistence, partial remission, and full remission were 44.0%, 30.0%, and 26.0%, respectively. Objective short sleep duration significantly increased the odds of persistent insomnia as compared to normal sleep (OR = 3.19) and to fully remitted insomnia (OR = 4.92). Mental health problems at baseline were strongly associated with persistent insomnia as compared to normal sleep (OR = 9.67) and to a lesser degree compared to fully remitted insomnia (OR = 3.68). Smoking, caffeine, and alcohol consumption and sleep apnea did not predict persistent insomnia.
Conclusions:
Objective short sleep duration and mental health problems are the strongest predictors of persistent insomnia. These data further support the validity and clinical utility of objective short sleep duration as a novel marker of the biological severity of insomnia.
Citation:
Vgontzas AN; Fernandez-Mendoza J; Bixler EO; Singareddy R; Shaffer ML; Calhoun SL; Liao D; Basta M; Chrousos GP. Persistent insomnia: the role of objective short sleep duration and mental health. SLEEP 2012;35(1):61-68.
Keywords: Insomnia, polysomnography, short sleep duration, mental health, persistence
INTRODUCTION
Insomnia is the most common sleep disorder, yet little is known about the etiology, pathophysiology, and clinical course of this highly prevalent and chronic disorder.1–4 In the few published general population longitudinal studies, the estimated rate of persistent insomnia varies from 40% to 70%.5–10 Factors such as gender, age, body weight, physical disorders, depression, or alcohol consumption have been proposed to be associated with persistent insomnia, but the results are inconsistent.5–11 Furthermore, these studies have not included polysomnography (PSG), and, for example, the longitudinal association of sleep duration or sleep disordered breathing (SDB) with persistent insomnia has not yet been studied.
Insomnia is frequently associated with physical and mental health disorders.1–4 Insomnia is a risk factor for the development of depression,12 and depression is a risk factor in the persistence of insomnia.5,7,11 As far as the association of chronic insomnia with the second most common sleep disorder (SDB), the findings have been mixed. Some but not all investigators have suggested that SDB is associated with chronic insomnia.13,14 However, no longitudinal study to date has examined whether SDB is a risk factor for the persistence of insomnia.
Based on previous studies, we have recently suggested that objective sleep duration in chronic insomnia is a marker of the biological severity of the disorder. Previous studies have shown that insomnia with objective short sleep duration is associated with activation of the hypothalamic-pituitary-adrenal (HPA) axis,15–19 hypertension,20 type 2 diabetes,21 neurocognitive deficits,22 and mortality23; whereas insomnia with objective longer sleep duration is associated with normal HPA axis activity,15,16,19 lack of significant medical morbidity,20–22 sleep misperception, anxious-ruminative traits, and poor coping resources.24 However, whether objective short sleep duration plays a role in the persistence of insomnia has not yet been examined.
The aim of this study was to examine the role of objective short sleep duration, SDB, and mental and physical health in the persistence of insomnia in a large general random sample (the Penn State Cohort Study) using PSG and a longitudinal design. We hypothesized that objective short sleep duration would be a significant predictor of persistent insomnia.
METHODS
Population
The data presented here were collected as part of a population-based study of sleep disorders, which used a 2-phase protocol in order to recruit participants from various age groups.25–27 In the first phase of the study, a sample of adult men and women (age ≥ 20 years) was randomly selected from local telephone households in 2 counties of Central Pennsylvania (Dauphin and Lebanon) using the Mitofsky-Waksberg 2-stage random digit dialing procedure.28 A within-household selection procedure described by Kish was used to select the specific man or woman to be interviewed.29 Telephone interviews were conducted with 4,364 age-eligible men and 12,219 age-eligible women residing in the sample households, for a total sample of 16,583 with response rates of 73.5% and 74.1%, respectively. The questionnaire employed in this interview included basic demographic and sleep information. In the second phase of this study, a subsample of 741 men and 1,000 women selected randomly from those subjects previously interviewed by telephone were studied in our sleep laboratory. After giving a complete description of the study to the subjects, written informed consent was obtained. The response rates for this phase were 67.8% and 65.8% for men and women, respectively. We contrasted those subjects who were recorded in the laboratory with those who were selected but not recorded in terms of age, BMI, and prevalence of sleep disorders. There were no significant differences between these 2 groups on any of these variables.
Of the 1741 subjects who completed the comprehensive sleep evaluation, 1395 subjects were followed up after an average duration of 7.5 years (mean duration of 4.5 years for women and 10.5 years for men) by one of the investigators (S.C.) via telephone interview. The response rate of the follow-up study was 79.7%. However, if one considers that 215 subjects died between baseline and follow-up, then the response rate of those alive was 90.9%. In the Penn State Cohort Study, men were recruited first and women 5 years later. This explains the 5-year difference in the follow-up period between men and women. After complete description of the follow-up study to the subjects, verbal informed consent was obtained. The whole study procedure was approved by the university's institutional review board.
Key Measurements
Each subject selected for laboratory evaluation completed a comprehensive sleep history and physical examination. All subjects were evaluated for one night in the sleep laboratory in sound-attenuated, light- and temperature-controlled rooms. During this evaluation, each subject was continuously monitored for 8 h (fixed-time period) using 16-channel polysomnography including electroencephalogram, electrooculogram, and electromyogram. Bedtimes were adjusted to conform to subjects' usual bedtimes, and subjects were recorded between 22:00-23:00 and 06:00-07:00. The sleep recordings were subsequently scored independently, according to Rechtschaffen and Kales criteria.30 Percent of sleep time is total sleep time divided by recorded time in bed and multiplied by 100. From the objectively recorded sleep time data, we regrouped the entire study sample into 2 ordinal groups: the top 50% of persons above the median percent of sleep time (“longer sleep duration group”), and the 50% of persons in the bottom half (“short sleep duration group”). We then rounded the cutoff point to meaningful numbers and thus created the following 2 sleep duration groups: the “longer sleep duration group” consisted of those who slept ≥ 6 h (i.e., percent of sleep time ≥ 75%), and the “short sleep duration group” of those who slept < 6 h (i.e., percent of sleep time < 75%). This cutoff point has been shown in previous studies to be predictive of significant medical morbidity and mortality.20,21–23
Additional information obtained during the PSG included that assessing sleep apnea. Respiration was monitored throughout the night by use of thermocouples at the nose and mouth and thoracic strain gauges. All-night recordings of hemoglobin oxygen saturation (SpO2) were obtained with an oximeter attached to the finger. For the purpose of this study, sleep disordered breathing (SDB) was defined as an apnea or hypopnea index ≥ 5 (AHI ≥ 5). Body mass index was based on measured height (cm) and weight (kg) during the subjects' sleep laboratory visit, and data are presented in terms of mean and percentage within each category.
As part of this protocol we also assessed for the presence of all sleep disorders, based on a standardized questionnaire completed by the subjects on the evening of their sleep laboratory visit. This questionnaire consists of 53 questions (7 demographic, 20 sleep related, and 26 general health questions). In addition, women responded to 8 questions related to menstrual history, menopause, and hormone therapy. Sleep related questions were qualified in terms of severity on a scale of 0-3 (0 = none, 1 = mild, 2 = moderate, 3 = severe) and duration. Health problems were also qualified in terms of severity, type of treatment (on a scale of 0-7), and duration. The presence of sleep difficulty was established on 3 levels of severity. First, insomnia was defined by a complaint of insomnia ≥ 1 year. Second, poor sleep was defined as a moderate to severe complaint (based on a mild to severe scale) of difficulty falling asleep, difficulty staying asleep, early final awakening, or unrefreshing sleep. Finally, normal sleeping was defined as the absence of either of these 2 categories.
To control for possible confounding variables influencing the relationships tested, we ascertained whether the respondent was currently being treated for physical (e.g., hypertension, diabetes, thyroidism) and/or mental (e.g., depression, alcohol use, drug use) health problems. A composite variable for each general category of physical or mental health problems was calculated by indicating a positive response when at least one health problem within each category was present, as we have described elsewhere.22,24,27
From the 1741 subjects, a total of 1300 validly completed a Minnesota Multiphasic Personality Inventory-2 (MMPI-2) at baseline. The MMPI-2 was administered following the standardized rules and scored accordingly.31,32 The scores in 9 clinical scales (Hypochondriasis [1-Hs], Depression [2-D], Hysteria [3-Hy], Psychopathic Deviate [4-Pd], Paranoia [6-Pa], Psychasthenia [7-Pt], Schizophrenia [8-Sc], Hypomania [9-Ma], Social Introversion [0-Si]), and 3 research scales [Depression [D], Anxiety [A], and Ego Strength [ES]) were studied.
Follow-up measures taken through telephone interview included the standardized questionnaire that subjects completed at baseline during their sleep laboratory visit. Sleep related questions were also used to establish the presence of sleep difficulty at follow-up based on 3 levels of severity as defined above (i.e., normal sleeping, poor sleep, and insomnia).
Sample
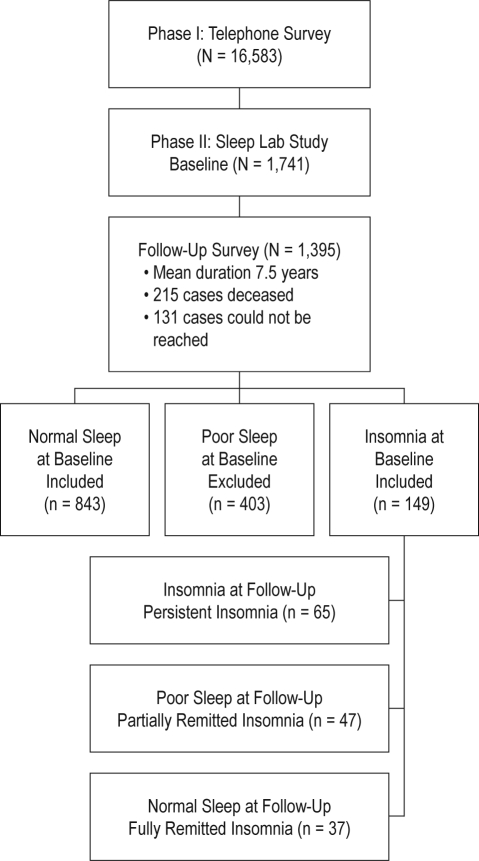
Of the 1395 subjects who were followed up, 149 subjects had insomnia and 403 poor sleep at baseline. Because the focus of the present study was the persistence and remission of insomnia, those individuals with poor sleep at baseline were excluded from further analysis. All subjects selected for the present study were further classified according to their baseline and follow-up insomnia status into 4 groups: normal sleep (individuals with normal sleep at baseline; n = 843), persistent insomnia (individuals with insomnia both at baseline and at follow-up; n = 65), partially remitted insomnia (individuals with insomnia at baseline and with poor sleep at follow-up; n = 47), and fully remitted insomnia (individuals with insomnia at baseline and with normal sleep at follow-up; n = 37). We contrasted those subjects who completed the baseline phase of the study, including the sleep laboratory recording (n = 1741), with those who completed the baseline phase plus the follow-up questionnaire (n = 1395) and the subsample of normal sleepers and insomniacs that we used for the present study (n = 992) in terms of age, gender, race, BMI, and objective short sleep duration at baseline. There were no significant differences between these 3 groups on any of these variables. Figure 1 shows the participant flow in the study.
Figure 1.
Participants' flow in the study.
Statistical Analyses
The design of this study included oversampling of those at higher risk for SDB and women with markedly higher levels of BMI to increase the precision of the risk estimates. Because of this sampling strategy, numeric sampling weights were developed for the analysis so that the estimates could be inferred to the general population. A comprehensive presentation of this sampling strategy has been presented elsewhere,20–27 including the use of the NHANES III laboratory data as the standard33 to adjust both the men and women in terms of sociodemographics to be representative of the national population.
Mean (standard deviation [SD]) and proportions of the demographic characteristics were calculated for the entire population, as well as stratified according to insomnia and objective sleep duration status. Multinomial logistic regression models were used to assess the independent association of objective sleep duration with normal sleep, fully remitted, partially remitted, and persistent insomnia, while controlling for potential confounding factors. Objective sleep duration was entered in the regression models as a continuous variable of hours of sleep. We calculated the odds ratios (OR) and the 95% confidence intervals (95% CI) from the regression models to estimate the risk of fully remitted, partially remitted, and persistent insomnia associated with objective shorter sleep duration, simultaneously adjusting for covariates. The covariates we adjusted for included major confounding factors expected to affect this relationship, i.e., age, race, gender, obesity, SDB, physical health problems, mental health problems, caffeine, cigarettes, alcohol consumption, and sampling weight. From the fully adjusted multinomial regression model, we also calculated the OR and the 95% CI to estimate the risk of fully remitted, partially remitted, and persistent insomnia associated with each covariate. Analysis of covariance (ANCOVA) was used to examine mean differences on MMPI-2 scores between the 4 study groups, while controlling for potential confounding factors. Bonferroni corrections were applied to control for type I errors when performing post hoc multiple comparisons. All analyses were conducted with SPSS version 17.0 for Windows.
RESULTS
The demographic, clinical, and sleep characteristics of the overall study sample and stratified by insomnia status are presented in Table 1. The rates of persistent insomnia, partially remitted insomnia, and fully remitted insomnia were 44.0%, 30.0%, and 26.0%, respectively.
Table 1.
Characteristics of study sample
| All (N = 992) | Normal Sleep (n = 843) | Fully Remitted Insomnia (n = 37) | Partially Remitted Insomnia (n = 47) | Persistent Insomnia (n = 65) | |
|---|---|---|---|---|---|
| Female, % | 47.2 | 44.6 | 84.5 | 73.3 | 65.9 |
| Caucasian, % | 92.5 | 92.8 | 92.3 | 86.7 | 88.9 |
| Age, years | 49.7 (13.1) | 49.6 (13.2) | 51.0 (10.0) | 48.7 (10.8) | 52.4 (13.7) |
| BMI, kg/m2 | 27.2 (5.0) | 27.0 (4.8) | 29.2 (6.6) | 31.0 (7.5) | 28.1 (5.4) |
| Obesity, % | 22.7 | 20.7 | 32.0 | 56.7 | 38.6 |
| Physical health problems, % | 61.8 | 59.5 | 92.3 | 89.7 | 79.5 |
| Mental health problems, % | 15.3 | 12.3 | 34.6 | 43.3 | 54.5 |
| Depression symptoms, % | 11.3 | 8.3 | 32.0 | 36.7 | 50.0 |
| Caffeine, cup/day | 2.2 (2.7) | 2.3 (2.8) | 2.2 (2.6) | 1.8 (2.1) | 1.7 (2.3) |
| ≥ 1 cup/day, % | 64.6 | 65.7 | 53.8 | 53.3 | 55.6 |
| Cigarettes, n/day | 3.9 (10.6) | 3.9 (10.7) | 3.2 (8.9) | 4.0 (10.7) | 4.5 (9.0) |
| ≥ 1/day, % | 20.6 | 20.5 | 15.4 | 20.0 | 25.0 |
| Alcohol, drink/day | 1.3 (6.5) | 1.4 (6.8) | 0.4 (1.5) | 0.2 (0.6) | 0.7 (1.7) |
| ≥ 1 drink/day, % | 28.3 | 29.3 | 11.5 | 10.3 | 25.0 |
| Apnea/Hypopnea Index | 2.8 (8.2) | 2.8 (8.2) | 3.6 (10.0) | 2.3 (5.8) | 2.8 (8.2) |
| SDB, % | 12.7 | 12.9 | 11.5 | 13.3 | 8.9 |
| Objective Sleep Duration, h | 5.8 (1.3) | 5.9 (1.2)a | 6.0 (1.4)a | 5.4 (1.4)a | 5.1 (1.5)a |
| ≥ 6 h, % | 55.5 | 56.8 | 65.4 | 48.3 | 31.8 |
| < 6 h, % | 44.5 | 43.5 | 34.6 | 51.7 | 68.2 |
All data are adjusted for sampling weight. Where appropriate the standard deviation is presented in parenthesis (SD).
AHI = apnea hypopnea index. BMI = body mass index. Obesity = BMI ≥ 30. SDB = sleep disordered breathing (AHI ≥ 5).
Mean (SD) values of objective sleep duration for each group are adjusted for age, race, gender, BMI, SDB, physical health problems, mental health problems, and sampling weight.
We present the 3 sets of multinomial logistic regression models that examined the association of objective sleep duration with insomnia status after progressively adjusting for potential confounders in Table 2. Shorter objective sleep duration was associated with a significantly higher risk for persistent insomnia as compared to the normal sleep group. This association did not change significantly as we increased the number of confounding factors that we adjusted for (OR ranged from 1.55, 95% CI 1.21–1.99, in the first model to OR = 1.68, 95% CI = 1.28–2.21, in the last model). The association of shorter objective sleep duration with persistent insomnia became stronger when fully remitted insomnia was used as the reference group (OR ranged, based on number of adjusted variables, from 1.97 to 2.02).
Table 2.
Multivariable adjusted odds ratios (95% CI) associating normal sleep, fully remitted insomnia, partially remitted insomnia, and persistent insomnia with objective sleep duration
| Outcome Level |
Model 1 |
Model 2 |
Model 3 |
||||
|---|---|---|---|---|---|---|---|
| Comparator | Reference | ORa (95% CI) | P | ORa (95% CI) | P | ORa (95% CI) | P |
| Persistent Insomnia | Normal Sleep | 1.55 (1.21-1.99) | 0.001** | 1.71 (1.31-2.24) | 0.0001** | 1.68 (1.28-2.21) | 0.0001** |
| Partially Remitted | Normal Sleep | 1.32 (0.96-1.82) | 0.083 | 1.38 (0.99-1.92) | 0.058 | 1.37 (0.99-1.91) | 0.061 |
| Fully Remitted | Normal Sleep | 0.79 (0.52-1.20) | 0.263 | 0.83 (0.55-1.25) | 0.371 | 0.83 (0.55-1.26) | 0.386 |
| Persistent Insomnia | Fully Remitted | 1.97 (1.22-3.17) | 0.005** | 2.07 (1.28-3.34) | 0.003** | 2.02 (1.25-3.28) | 0.004** |
| Partially Remitted | Fully Remitted | 1.68 (1.01-2.81) | 0.048* | 1.66 (0.99-2.78) | 0.053 | 1.65 (0.98-2.76) | 0.058 |
| Persistent Insomnia | Partially Remitted | 1.17 (0.80-1.71) | 0.418 | 1.24 (0.84-1.84) | 0.279 | 1.23 (0.83-1.82) | 0.307 |
Model 1: Adjusted for age, race, gender, obesity, SDB, and sampling weight. Model 2: Adjusted for age, race, gender, obesity, SDB, physical health problems, mental health problems, and sampling weight. Model 3: Adjusted for age, race, gender, obesity, SDB, physical health problems, mental health problems, cigarettes, caffeine and alcohol, and sampling weight.
The odds ratio expresses the increased risk associated with a one-hour decrease in objective sleep duration.
P < 0.05;
P < 0.01.
To be consistent with our previous studies, in which objective sleep duration was categorically defined,20–24 we also analyzed the risk associated with short sleep duration (< 6 h) vs. longer sleep duration (≥ 6 h) while controlling for all confounding factors. Objective short sleep duration was associated with a significantly higher risk for persistent insomnia when compared to the normal sleep group (OR = 3.19, 95%CI = 1.55-6.55, P = 0.002) and to the fully remitted insomnia group (OR = 4.92, 95%CI = 1.61-15.0, P = 0.005), whereas this association was not significant for partially remitted insomnia as compared to the normal sleep group (OR = 1.66, 95%CI = 0.74-3.73, P = 0.218) and to the fully remitted insomnia group (OR = 2.56, 95%CI = 0.80-8.26, P = 0.114).
In Table 3 we present the OR and the 95% CI of persistent, partially remitted, and fully remitted insomnia associated with all covariates included in the previous fully adjusted multinomial regression model when compared to normal sleep. Mental health problems at baseline, particularly depression, were very prevalent in the group of persistent insomnia. We observed the highest prevalence of depression at baseline in the group of persistent insomnia (50%), the least in the group of fully remitted insomnia (34.6%), and intermediate in the group of partial remission (43.3%). The strongest association of mental health problems was with the group of persistent insomnia (OR = 9.67, 95% CI = 4.85–19.3) and the least in the group of fully remitted insomnia (OR = 2.63, 95% CI = 1.06–6.54). In contrast, physical health problems were very prevalent in the group of fully remitted insomnia (92.3%), intermediate in the group of partial remission (89.7%), and lowest in the group of persistent insomnia (79.5%). The strongest association of physical health problems was with the group of fully remitted insomnia (OR = 7.93, 95% CI = 1.70–36.9) and the least with the group of persistent insomnia (OR = 1.87, 95% CI = 0.85–4.08). In other words, the presence of physical health problems at baseline predicted a more favorable outcome of the disorder. Furthermore, we examined the factors that predicted who of the insomniacs would move to partial remission or to persistent insomnia as compared to full remission (Table 4). Again, in this comparison mental health problems at baseline were strong predictors of persistent insomnia. Obesity was associated with an increased risk of partial remission.
Table 3.
Multivariable adjusted odds ratio (95% CI) associating fully remitted insomnia, partially remitted insomnia, and persistent insomnia with socio-demographic, medical, and behavioral factors as compared to normal sleep
| Reference |
Comparator |
|||
|---|---|---|---|---|
| Normal Sleep (n = 843) | Fully Remitted Insomnia (n = 37) | Partially Remitted Insomnia (n = 47) | Persistent Insomnia (n = 65) | |
| Age | 1.00 | 0.99 (0.96-1.03) | 1.02 (0.99-1.06) | 1.00 (0.97-1.03) |
| Race | 1.00 | 0.87 (0.16-4.68) | 0.98 (0.28-3.46) | 1.05 (0.33-3.34) |
| Gender | 1.00 | 5.82 (1.88-18.1)** | 2.79 (1.11-6.99)* | 2.29 (1.12-4.70)** |
| Obesity | 1.00 | 1.29 (0.52-3.22) | 3.83 (1.71-8.58)** | 1.89 (0.93-3.85) |
| Physical health problems | 1.00 | 7.93 (1.70-36.9)** | 4.02 (1.26-12.8)* | 1.87 (0.85-4.08) |
| Mental health problems | 1.00 | 2.63 (1.06-6.54)* | 4.24 (1.87-9.64)** | 9.67 (4.85-19.3)** |
| SDB | 1.00 | 1.44 (0.36-5.80) | 1.09 (0.32-3.72) | 0.49 (0.14-1.66) |
| Caffeine | ||||
| ≥ 1 cup/day | 1.00 | 0.80 (0.35-1.83) | 0.82 (0.37-1.81) | 0.82 (0.41-1.62) |
| Cigarettes | ||||
| ≥ 1/day | 1.00 | 0.77 (0.23-2.58) | 1.04 (0.38-2.87) | 0.94 (0.41-2.13) |
| Alcohol | ||||
| ≥ 1 drink/day | 1.00 | 0.68 (0.18-2.55) | 0.71 (0.21-2.38) | 1.79 (0.80-4.02) |
Data are multivariable adjusted OR (95% CI) resulting from Model 3 in Table 2.
P < 0.05;
P < 0.01.
Table 4.
Multivariable adjusted odds ratio (95% CI) associating partially remitted insomnia and persistent insomnia with sociodemographic, medical, and behavioral factors as compared to fully remitted insomnia
| Reference |
Comparator |
||
|---|---|---|---|
| Fully Remitted Insomnia (n = 37) | Partially Remitted Insomnia (n = 47) | Persistent Insomnia (n = 65) | |
| Age | 1.00 | 1.03 (0.99-1.08) | 1.01 (0.96-1.05) |
| Race | 1.00 | 1.13 (0.15-8.50) | 1.21 (0.17-8.65) |
| Gender | 1.00 | 0.48 (0.11-2.02) | 0.39 (0.11-1.47) |
| Obesity | 1.00 | 2.97 (1.02-9.65)* | 1.47 (0.48-4.49) |
| Physical health problems | 1.00 | 0.51 (0.08-3.42) | 0.24 (0.04-1.30) |
| Mental health problems | 1.00 | 1.61 (0.50-5.23) | 3.68 (1.22-11.1)* |
| SDB | 1.00 | 0.76 (0.13-4.56) | 0.34 (0.06-2.07) |
| Caffeine | |||
| ≥ 1 cup/day | 1.00 | 1.02 (0.34-3.10) | 1.02 (0.36-2.88) |
| Cigarettes | |||
| ≥ 1/day | 1.00 | 1.35 (0.30-6.17) | 1.22 (0.30-4.98) |
| Alcohol | |||
| ≥ 1 drink/day | 1.00 | 1.05 (0.18-6.17) | 2.65 (0.58-12.2) |
Data are multivariable adjusted OR (95% CI) resulting from Model 3 in Table 2.
P < 0.05.
Interestingly, behavioral factors (i.e., caffeine, cigarettes, and alcohol consumption) were not significantly associated with the remission or persistence of insomnia. Furthermore, SDB did not predict the persistence of insomnia. These results remained unchanged even if we used a more severe threshold (AHI ≥ 15) to define SDB.
Because of the observed effects of mental health problems on the persistence of insomnia, we further examined in a subsample of subjects that completed the MMPI-2 at baseline whether differences existed in terms of the personality profile of the 4 study groups while controlling for potential confounders. The groups of persistent insomnia and/or partially remitted insomnia showed significantly higher elevations in all MMPI-2 scales when compared to normal sleepers. In contrast, in the group with fully remitted insomnia, only hypochondriasis and depression were significantly higher than the group with normal sleep. Furthermore, the group of persistent insomnia and/or partially remitted insomnia had significantly higher elevations in 2 scales (i.e., 6-Paranoia and 9-Hypomania) compared to the group of fully remitted insomnia.
DISCUSSION
This is the first population-based, longitudinal study that has used polysomnographic variables, including objective sleep duration and SDB, and psychological testing to examine the risk factors of persistent insomnia. The main finding of the present study is that objective sleep duration and mental health problems are the major predictors of the persistence of insomnia over a period of 7.5 years. In fact, objective short sleep duration, as compared to mental health problems, was a stronger predictor of insomnia persistence relative to full remission. This study gives further support to the notion of objective sleep duration as a novel marker of the biological severity of insomnia.
The rate of persistent insomnia in the present study was 44.0%, which is consistent with that of previous population-based studies.5–10 As far as remission, previous studies have reported remission rates between 54% and 56.6%.5,8,10 In our study we demonstrated that the rate of full remission in insomnia was only 26.0%, whereas 30% experienced partial remission. These data suggest that only a minority of chronic insomniacs experience full remission whereas the large majority continue to experience chronic insomnia or poor sleep.
In the present study, we demonstrated that in insomniacs with objective short (< 6 h) sleep duration, as compared to those with longer (≥ 6 h) objective sleep duration, the odds of persistent insomnia are 4.9 times those of full remission. Previous studies by us and others have shown that insomnia with objective short sleep duration is associated with activation of the stress system,15–19,34 the autonomic system,35–37 and with significant medical morbidity, i.e., hypertension,20 type 2 diabetes,21 neurocognitive deficits,22 and mortality23; whereas insomnia with objective longer sleep duration is associated with normal HPA axis activity,15,16,19 lack of significant medical morbidity,20–22 sleep misperception, anxious-ruminative traits, and poor coping resources.24 These findings led us to suggest that objective sleep duration in chronic insomnia is a marker of the biological severity of the disorder and thus potentially useful in the diagnosis and treatment of chronic insomnia. The findings of the present longitudinal study further support this hypothesis, as objective short sleep duration significantly predicted the persistence of insomnia.
Interestingly, although only marginally significant, the risk associated with decreased objective sleep duration for partially remitted insomnia was higher compared to fully remitted insomnia and lower compared to persistent insomnia. This finding is consistent with the clinical experience that partially remitted insomniacs fall between full remission and the most severe group, persistent insomniacs.
The second major finding of our study shows that mental health problems, and especially depression, strongly predict the persistence of insomnia over a period of 7.5 years. The association of insomnia with depression has been well established.1 However, few of the available longitudinal studies examining the persistence and the remission of insomnia have explored this association.5–11 Only three studies in older9,11 and young7 adult populations have shown that the presence of depression was a strong predictor of the persistence of insomnia. Moreover, in our study we had the opportunity to examine for the first time the longitudinal association of MMPI-2 with the persistence of insomnia. In our study, only persistent and partially remitted insomniacs had higher elevations in all MMPI-2 scales. Thus, the insomniacs who will become persistent insomniacs or poor sleepers are more psychologically distressed at baseline than those who fully remit. Our results suggest that mental health problems in insomnia play a significant role in the chronicity of the disorder.
Interestingly, the vast majority of fully remitted insomniacs had physical health problems at baseline. This finding indicates that physical health problems in the absence of high psychological distress do not appear to lead to persistent insomnia. One explanation is that treatment of the physical disorder may lead to remission of insomnia. In the field of insomnia, research on the relative importance of mental vs. physical health as causes of chronic insomnia has been a long enduring debate. Our data suggest that mental health problems are of primary importance in predicting persistent chronic insomnia.
Obesity was associated with partially remitted (significant) and persistent insomnia (nonsignificant). We have previously observed in cross-sectional studies that insomnia and poor sleep are more prevalent in obese individuals compared to non-obese and that obese individuals with sleep complaints are highly distressed as shown using the MMPI-2.38 Our longitudinal data suggest that obesity is a risk factor for insomnia to become chronic. Consistent with our findings, a previous study showed that insomniacs who gain weight were more likely to remain insomniacs at follow-up and that those that lose weight during the follow-up period were more likely to remit.5
Behavioral factors such as caffeine, cigarettes, and alcohol consumption were not significantly associated with the persistence of insomnia. Sleep hygiene instructions are a commonly used treatment option in clinical practice.39 Our results suggest that a moderate use of these substances is not significant in terms of risk of persistent insomnia. However, excessive use of these substances, such as alcohol dependency, may have a negative impact on the chronicity of the disorder.5
Furthermore, the present study shows that SDB at baseline did not predict longitudinally the persistence of insomnia. Based on some cross-sectional studies in clinical samples that have shown a high comorbidity of SDB and insomnia, some investigators have suggested that SDB might play a role in the etiology and/or perpetuation of chronic insomnia.13,14 Interestingly, these studies have not controlled for major confounders such as obesity. Our population-based, longitudinal study does not support that SDB is causal in the persistence of chronic insomnia.
Finally, our study shows that sociodemographic factors such as gender, age, and ethnicity do not predict the persistence of insomnia. Of the available population based, longitudinal studies,5–11 only a few have shown that persistent insomnia is weakly associated with gender10,11 and older age.6,10 Our discrepant findings can be explained by methodological differences such as variance in duration of the insomnia complaint6,10,11 and a focus on specific populations such as elderly.11
Some limitations should be taken into account when interpreting our results. The objective sleep duration in this study was based on one night of PSG, which may not be representative of the subjects' typical or optimal objective sleep duration and may be affected by the so-called “first night effect.” Objective sleep duration was used as an internally valid marker of the severity of insomnia and not as a recommended optimum sleep duration for the general population. It should be noted that our study investigated the relative sleep duration measured objectively (e.g., < 6 h of objective sleep is relatively shorter than ≥ 6 h of sleep). Furthermore, because our cohort study was not designed to include a derivation sample and a validation sample, we cannot assess and confirm the optimal cutoff point of sleep duration in insomnia to predict, for example, persistent insomnia. However, in our previous studies, the association between objective short sleep duration and hyperactivity of the stress system in insomniacs was based on a four consecutive night sleep laboratory protocol, which should better represent the typical sleep profile of the subjects.15,16 The consistency of the findings on the role of objective short sleep duration in predicting insomnia severity between the physiological studies with multiple night recordings15,16 and our previous epidemiological studies based on a single night recording20–24 increases our confidence about the replicability and generalizability of the present findings. Furthermore, in large epidemiologic studies the average objective sleep duration is about 6 hours, which is independent of whether sleep is recorded at home with polysomnography (Sleep Heart Health Study),40 or for three consecutive nights with actigraphy (CARDIA study),41 or in the sleep laboratory (Penn State Cohort).20–24 Thus, the consistency among these three large epidemiological studies in terms of objective sleep duration supports the validity of our analytic strategy of using the median objective sleep duration as a statistical criterion to divide individuals into “longer sleepers” and “shorter sleepers.”
In conclusion, objective short sleep duration and mental health problems play a key role in the persistence of insomnia. From a clinical standpoint, there are two important implications. First, addressing mental health problems in chronic insomnia should be a therapeutic priority. Second, objective short sleep duration in insomnia appears to be a novel, clinically useful marker of the biological severity of chronic insomnia which may transform the way we diagnose and treat chronic insomnia. Currently, the diagnosis of insomnia is based on subjective complaints. The introduction of objective measures of sleep in the evaluation of insomnia may be of relevance for the clinician in terms of prioritizing intervention based on severity. Furthermore, it may lead to the development of more specific treatments, i.e., biological treatment for insomniacs with objective short sleep duration and psychological treatment for insomniacs with normal sleep duration.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This research was funded in part by the National Institutes of Health grants RO1 51931 (Dr. Bixler), RO1 40916 (Dr. Bixler), and RO1 64415 (Dr. Vgontzas). The work was performed at the Sleep Research and Treatment Center at the Penn State University Milton Hershey Hospital, and the staff is especially commended for their efforts. The principal investigator (Dr. Vgontzas) had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.National Institutes of Health. National Institutes of Health State of the Science Conference statement on Manifestations and Management of Chronic Insomnia in Adults. Sleep. 2005;28:1049–57. doi: 10.1093/sleep/28.9.1049. [DOI] [PubMed] [Google Scholar]
- 2.Basta M, Chrousos GP, Vela-Bueno A, Vgontzas AN. Chronic insomnia and the stress system. Sleep Med Clin. 2007;2:279–91. doi: 10.1016/j.jsmc.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: American Psychiatric Press; 2000. Text revision. [Google Scholar]
- 4.American Academy of Sleep Medicine. The International Classification of Sleep Disorders (ICSD-2): diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 5.Janson C, Lindberg E, Gislason T, Elmasry A, Boman G. Insomnia in men: a 10-year prospective population based study. Sleep. 2001;24:425–30. doi: 10.1093/sleep/24.4.425. [DOI] [PubMed] [Google Scholar]
- 6.Morphy H, Dunn KM, Lewis M, Boardman HF, Croft PR. Epidemiology of insomnia: a longitudinal study in a UK population. Sleep. 2007;30:274–80. [PubMed] [Google Scholar]
- 7.Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, Rössler W. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 2008;31:473–80. doi: 10.1093/sleep/31.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jansson-Fröjmark M, Linton SL. The course of insomnia over one year: a longitudinal study in the general population in Sweden. Sleep. 2008;31:881–86. doi: 10.1093/sleep/31.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Yoon JS. Insomnia, depression, and physical disorders in late life: a 2-year longitudinal community study in Koreans. Sleep. 2009;32:1221–8. doi: 10.1093/sleep/32.9.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morin CM, Bélanger L, LeBlanc M, et al. The natural history of insomnia: a population-based 3-year longitudinal study. Arch Intern Med. 2009;169:447–53. doi: 10.1001/archinternmed.2008.610. [DOI] [PubMed] [Google Scholar]
- 11.Quan SF, Katz R, Olson J, et al. Factors associated with incidence and persistence of symptoms of disturbed sleep in an elderly cohort: the Cardiovascular Health Study. Am J Med Sci. 2005;329:163–72. doi: 10.1097/00000441-200504000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011 doi: 10.1016/j.jad.2011.01.011. In press. [DOI] [PubMed] [Google Scholar]
- 13.Krakow B, Melendrez D, Lee SA, Warner TD, Clark JO, Sklar D. Refractory insomnia and sleep-disordered breathing: a pilot study. Sleep Breath. 2004;8:15–29. doi: 10.1007/s11325-004-0015-5. [DOI] [PubMed] [Google Scholar]
- 14.Wickwire EM, Collop NA. Insomnia and sleep-related breathing disorders. Chest. 2010;137:1449–63. doi: 10.1378/chest.09-1485. [DOI] [PubMed] [Google Scholar]
- 15.Vgontzas AN, Tsigos C, Bixler EO, et al. Chronic insomnia and activity of the stress system: a preliminary study. J Psychosom Res. 1998;45:21–31. doi: 10.1016/s0022-3999(97)00302-4. [DOI] [PubMed] [Google Scholar]
- 16.Vgontzas AN, Bixler EO, Lin HM, et al. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2001;86:3787–94. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]
- 17.Rodenbeck A, Cohrs S, Jordan W, Huether G, Rüther E, Hajak G. The sleep-improving effects of doxepin are paralleled by a normalized plasma cortisol secretion in primary insomnia. Psychopharmacology. 2003;170:423–28. doi: 10.1007/s00213-003-1565-0. [DOI] [PubMed] [Google Scholar]
- 18.Backhaus J, Junghanns K, Born J, Hohaus K, Faasch F, Hohagen F. Impaired declarative memory consolidation during sleep in patients with primary insomnia: influence of sleep architecture and nocturnal cortisol release. Biol Psychiatry. 2006;60:1324–30. doi: 10.1016/j.biopsych.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 19.Shaver JL, Johnston SK, Lentz MJ, Landis CA. Stress exposure, psychological distress, and physiological stress activation in midlife women with insomnia. Psychosom Med. 2002;64:793–802. doi: 10.1097/01.psy.0000024235.11538.9a. [DOI] [PubMed] [Google Scholar]
- 20.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491–7. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: a population-based study. Diabetes Care. 2009;32:1–6. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandez-Mendoza J, Calhoun S, Bixler EO, et al. Insomnia with objective short sleep duration is associated with deficits in neuropsychological performance: a general population study. Sleep. 2010;33:459–65. doi: 10.1093/sleep/33.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vgontzas AN, Liao D, Pejovic S, et al. Insomnia with short sleep duration and mortality: the Penn State Cohort. Sleep. 2010;33:1159–64. doi: 10.1093/sleep/33.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez-Mendoza J, Calhoun SL, Bixler EO, et al. Sleep misperception and chronic insomnia in the general population: role of objective sleep duration and psychological profiles. Psychosom Med. 2011;73:88–97. doi: 10.1097/PSY.0b013e3181fe365a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–48. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 26.Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163:608–13. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 27.Bixler EO, Vgontzas AN, Lin HM, Vela-Bueno A, Kales A. Insomnia in central Pennsylvania. J Psychosom Res. 2002;53:589–92. doi: 10.1016/s0022-3999(02)00450-6. [DOI] [PubMed] [Google Scholar]
- 28.Waksberg J. Sampling methods for random digit dialing. J Am Stat Assoc. 1978;73:40–6. [Google Scholar]
- 29.Kish L. Survey sampling. New York: John Wiley & Sons Inc; 1965. [Google Scholar]
- 30.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Bethesda: National Institutes of Health; 1968. [Google Scholar]
- 31.Butcher JN, Graham JR, Ben-Porath YS, Tellegen A, Dahkstrom WG. MMPI-2: Manual for administration, scoring and interpretation. Revised ed. Minneapolis: University of Minnesota Press; 2001. [Google Scholar]
- 32.Nichols DS. Essentials of MMPI-2 assessment. New York: John Wiley & Sons Inc; 2001. [Google Scholar]
- 33.U.S. Department of Health and Human Services (DHHS), National Center for Health Statistics. Hyattsville: Centers for Disease Control and Prevention; 1996. Third National Health and Nutrition Examination Survey, 1988-1994. NHANES III laboratory data file. [Google Scholar]
- 34.Irwin M, Clark C, Kennedy B, Christian Gillin J, Ziegler M. Nocturnal catecholamines and immune function in insomniacs, depressed patients, and control subjects. Brain Behav Immun. 2003;17:365–72. doi: 10.1016/s0889-1591(03)00031-x. [DOI] [PubMed] [Google Scholar]
- 35.Stepanski E, Glinn M, Zorick F, Roehrs T, Roth T. Heart rate changes in chronic insomnia. Stress Med. 1994;10:261–66. [Google Scholar]
- 36.Bonnet MH, Arand DL. 24-hour metabolic rate in insomniacs and matched normal sleepers. Sleep. 1995;18:581–88. doi: 10.1093/sleep/18.7.581. [DOI] [PubMed] [Google Scholar]
- 37.Bonnet MH, Arand DL. Heart rate variability in insomniacs and matched normal sleepers. Psychosom Med. 1998;60:610–15. doi: 10.1097/00006842-199809000-00017. [DOI] [PubMed] [Google Scholar]
- 38.Vgontzas AN, Lin HM, Papaliaga M, et al. Short sleep duration and obesity: the role of emotional stress and sleep disturbances. Int J Obes (Lond) 2008;32:801–9. doi: 10.1038/ijo.2008.4. [DOI] [PubMed] [Google Scholar]
- 39.Stepanski EJ, Wyatt JK. Use of sleep hygiene in the treatment of insomnia. Sleep Med Rev. 2003;7:215–25. doi: 10.1053/smrv.2001.0246. [DOI] [PubMed] [Google Scholar]
- 40.Silva GE, Goodwin JL, Sherrill DL, et al. Relationship between reported and measured sleep times: the sleep heart health study (SHHS) J Clin Sleep Med. 2007;3:622–30. [PMC free article] [PubMed] [Google Scholar]
- 41.Lauderdale DS, Knutson KL, Yan LL, et al. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am J Epidemiol. 2006;164:5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]