Abstract
Study Objectives:
To investigate the relationship between oxygen desaturation index (ODI), body mass index (BMI), and apnea-hypopnea index (AHI) in a large sleep clinic population.
Design:
Retrospective observational.
Setting:
Sleep disorders clinic.
Patients or Participants:
11,448 individuals undergoing diagnostic polysomnography (PSG) at a sleep disorders clinic.
Measurements and Results:
Polysomnography were scored using Chicago criteria. ODI at 2%, 3%, and 4% threshold levels were derived. The study population was subdivided into BMI categories in steps of 5 kg/m2. Mean ODI and the accuracy of ODI for detecting an AHI ≥ 15 (moderate-severe OSA) or ≥ 30 (severe OSA) were examined by BMI category, using the area under the curve (AUC) of receiver operator characteristic (ROC) curves for the 3 ODI thresholds. Based on AUC, ODI-3% performed best overall, achieving a significantly higher AUC than ODI-2% and ODI-4% for the diagnosis of moderate-severe OSA, and a higher AUC than ODI-2% for the diagnosis of severe OSA. When examining the effect of BMI, ODI-3% achieved a significantly higher AUC than ODI-2% in all BMI categories, and ODI-4% in non-obese subjects. The sensitivity of ODI for detecting OSA increased with BMI, while specificity decreased.
Conclusions:
ODI-3% performed best overall, and when combined with appropriate clinical assessment, could be considered as an initial diagnostic test for OSA. OSA is more frequently associated with oxygen desaturation in obese subjects. BMI influences the accuracy of ODI for the diagnosis of OSA, and ODI should not be used in isolation as a test for OSA in subjects with a BMI below 25kg/m2.
Citation:
Ling IT; James AL; Hillman DR. Interrelationships between body mass, oxygen desaturation, and apnea-hypopnea indices in a sleep clinic population. SLEEP 2012;35(1):89-96.
Keywords: Oxygen desaturation index, obstructive sleep apnea, hypopnea, diagnostic accuracy, obesity
INTRODUCTION
Obstructive sleep apnea (OSA) is characterized by recurrent upper airway obstruction resulting in cessation (apnea) or reduction (hypopnea) of airflow during sleep. Technician attended laboratory polysomnography (PSG) remains the gold standard for the diagnosis of OSA, however is limited in its availability.1 It remains to be determined if the level of detail and complexity provided by attended laboratory PSG is required for the routine diagnosis of OSA.2
With estimates that up to 82% of males and 93% of females with OSA remain undiagnosed,3 there is interest in developing cost-effective screening tests that help predict the presence of OSA. Clinical methods that have been evaluated include questionnaires, prediction algorithms, cephalometry, and artificial neural networks. A recent meta-analysis of clinical screening tests resulted in pooled sensitivities of 52% to 86% and pooled specificities of 58% to 80%, and concluded that the false negative rates of most tests are likely to result in failure to identify a significant proportion of patients with OSA.4 An earlier meta-analysis of diagnostic tests for OSA found a high degree of heterogeneity between studies, with data on portable monitors and radiologic or morphologic features too heterogeneous to combine.5 Overnight oximetry with the oxygen desaturation index (ODI) has been investigated as a diagnostic test for OSA, although its accuracy remains largely undefined.6
Obesity is a major risk factor for OSA, with occurrence of OSA strongly associated with excess body weight.7 The severity of oxygen desaturation during sleep disordered breathing (SDB) is correlated with the degree of obesity expressed as the body mass index (BMI).8 It follows therefore that the sensitivity of ODI for detecting OSA should increase with BMI and, conversely, that there may be a lower level of BMI below which ODI may not be sufficiently sensitive to detect breathing disturbances.
In this study, we utilized data from a large prospectively accumulated database of diagnostic PSGs to investigate the relationships between BMI, ODI, and apnea-hypopnea index (AHI) in a sleep clinic population. Our purpose was to investigate the impact of BMI on the diagnostic accuracy of ODI for detecting OSA. We also sought to determine which ODI threshold (of ODI-2%, ODI-3%, or ODI-4%) best characterized the magnitude of nocturnal breathing disturbance determined from PSG analysis.
METHODS
Sample
Patients referred for diagnostic PSG at a sleep disorders clinic (the West Australian Sleep Disorders Research Institute [WASDRI]) between February 2001 and June 2010 had PSG data collected and entered into a database. All participants had given informed consent for clinical and PSG data to be collected for research purposes, and the study was approved by the local human research ethics committee. Patient data included study date, age, sex, height, weight, total study time, total sleep time, AHI, number of arousals, and number of desaturations at 2%, 3%, and 4% thresholds. Data were extracted, and all PSGs where BMI and ODI could be derived were included for analysis. Where individuals had more than one PSG performed, only the initial diagnostic study was included. Studies in which positive airway pressure therapy was used were excluded.
Polysomnography
All PSGs were technician-attended laboratory studies and used a polygraph system (Compumedics E-Series, Abbotsford, Victoria, Australia) that included electroencephalogram, electro-oculogram, submental and anterior tibialis electromyogram, and electrocardiogram. Airflow was monitored using both an oronasal thermistor and a nasal pressure transducer, and thoracoabdominal motion was monitored by respiratory inductive plethysmography. Oxygen saturation was monitored using the integrated oximeter (Nonin Xpod 3011, Nonin Medical, Plymouth, Minnesota, USA). All signals were stored digitally for interpretation. The study period was usually between 21:00 and 06:00.
Sleep staging was performed manually using conventional criteria established by Rechtschaffen and Kales.9 Respiratory events were scored using Chicago criteria,10 with hypopneas defined as > 50% reduction in a valid measure of airflow, or a lesser discernable reduction in airflow associated with either ≥ 3% oxygen desaturation or an arousal. Duration criteria for both apneas and hypopneas were ≥ 10 sec. Arousals were defined as an abrupt change in electroencephalogram frequency lasting > 3 sec with ≥ 10 sec of stable sleep preceding the change. The number of desaturations at the 3 predefined threshold levels was derived using an automatic desaturation detection algorithm (Profusion PSG 2, Compumedics Ltd, Abbotsford, Victoria, Australia). After scoring was completed, PSG variables and clinical information were exported to the WASDRI database.
Analysis
The population was subdivided into BMI categories in steps of 5 kg/m2 and the difference in subject characteristics between BMI categories analyzed using unpaired 2-tailed t-tests or one-way ANOVA as indicated. For detailed analysis, individuals with BMI < 20 and > 40 kg/m2 were excluded as these categories contained substantially lower numbers than those between these thresholds (176 subjects had a BMI < 20 kg/m2, 806 a BMI of 40-45 kg/m2, and 732 a BMI > 45 kg/m2).
Moderate to severe OSA was defined as AHI ≥ 15 events/h by Chicago criteria. ODI was calculated as the number of desaturations per sleep hour. Within each BMI category, the diagnostic sensitivity and specificity of ODI-2%, 3%, and 4% for the diagnosis of moderate-severe OSA were calculated for cut-off values of 10, 15, and 20 desaturation events/h. Receiver operator characteristic (ROC) curves were constructed for each ODI threshold and BMI category. The area under the curve (AUC) of each ROC curve was calculated. Comparisons between AUC for the different ODI thresholds within each BMI category were performed using the Hanley and McNeil method.11 Severe OSA was defined as AHI ≥ 30 events/h. Within each BMI category, sensitivities and specificities of the 3 ODI measures for the diagnosis of severe OSA were calculated for cut-off values of 10, 20, and 30 desaturation events/h. As for moderate-severe OSA, ROC curves were constructed and the AUC of each curve was calculated and compared.
The arousal index (AI) was calculated as the number of arousals per sleep hour. ROC curves were also constructed for the 3 ODI thresholds for the detection of an AI ≥ 20, and the AUC of each curve calculated and compared. Lastly, the effect of BMI on the relationship between ODI and AHI was also studied by expressing ODI as a ratio of AHI, and examining this ratio relative to BMI (value rounded to the nearest 1 kg/m2) in all subjects with an AHI ≥ 15.
RESULTS
Subject Characteristics
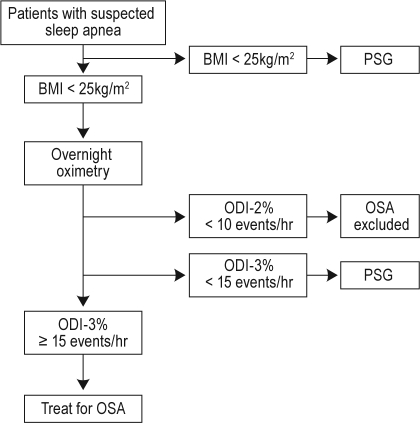
Subject selection is summarized in Figure 1. Data on 21,313 PSGs were available. After excluding studies where positive airway pressure was in use, studies where there was insufficient information in the database to determine BMI or ODI, and repeat studies on the same subjects, there were 11,448 subjects with PSG results and accompanying clinical information available for analysis (54% of the total). Subject characteristics of this population and its BMI categories are presented in Table 1.
Figure 1.
Flow of subjects through the study. WASDRI, West Australian Sleep Disorders Research Institute.
Table 1.
Descriptive characteristics of the sample population and across representative BMI categories
| Variable | All | BMI 20-25 | BMI 25-30 | BMI 30-35 | BMI 35-40 |
|---|---|---|---|---|---|
| N | 11,448 | 1,309 | 3,420 | 3,181 | 1,824 |
| Age | 51.2 ± 14.0 | 48.1 ± 15.9 | 51.8 ± 14.0 | 52.7 ± 13.5 | 51.5 ± 13.1 |
| Male (%) | 64.5 | 57.5 | 72.3 | 71.5 | 60.4 |
| BMI (kg/m2) | 32.5 ± 9.0 | 23.1 ± 1.3 | 27.7 ± 1.4 | 32.4 ± 1.4 | 37.2 ± 1.4 |
| Total sleep time (h) | 5.7 ± 1.5 | 6.0 ± 1.5 | 5.9 ± 1.4 | 5.8 ± 1.5 | 5.6 ± 1.6 |
| AHI ≥ 15 (%) | 64.0 | 36.0 | 57.7 | 69.2 | 75.4 |
| AHI ≥ 30 (%) | 38.6 | 13.8 | 28.1 | 42.6 | 52.9 |
| AHI (/h) | 31.3 ± 29.3 | 15.9 ± 16.7 | 23.5 ± 20.7 | 32.1 ± 26.0 | 40.6 ± 32.9 |
| ODI-2% (/h) | 36.6 ± 39.2 | 20.5 ± 9.6 | 28.5 ± 30.3 | 37.8 ± 36.3 | 46.2 ± 42.9 |
| ODI-3% (/h) | 22.3 ± 30.1 | 9.6 ± 19.8 | 14.9 ± 19.7 | 22.9 ± 26.4 | 30.3 ± 34.4 |
| ODI-4% (/h) | 15.1 ± 25.3 | 5.6 ± 15.1 | 9.0 ± 15.3 | 15.1 ± 21.5 | 21.4 ± 29.7 |
Values are given as mean ± SD or as a proportion (%). BMI, body mass index; AHI, apnea-hypopnea index; ODI, oxygen desaturation index.
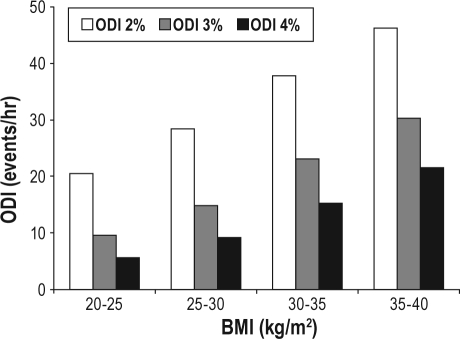
The mean ODI-2%, 3%, and 4% in each BMI category is shown in Figure 2. Mean ODI at each desaturation threshold level rose consistently, with increasing BMI, being highest in the group with the greatest BMI (> 45 kg/m2). ODI of each BMI category was significantly different from ODI in all other categories (P < 0.001 for all comparisons by one-way ANOVA).
Figure 2.
Mean oxygen desaturation index (ODI)-2%, ODI-3%, and ODI-4% across the study population divided into body mass index (BMI) categories. ODI at each level was significantly different to ODI in all other categories (P < 0.05).
Sensitivity and Specificity
In general, the diagnostic sensitivity of each of the 3 ODI thresholds for OSA increased with BMI, while the specificity of ODI for OSA fell with increasing BMI. Diagnostic sensitivity fell with increasing ODI thresholds, and was lowest in the 20-25 kg/m2 BMI category at 17.8% when using ODI-4% ≥ 20 for diagnosis of moderate-severe OSA, and 26.1% when using ODI-4% ≥ 30 for the diagnosis of severe OSA.
Diagnostic sensitivity and specificity of ODI-2%, ODI-3%, and ODI-4% at various cut-off levels for the diagnosis of moderate-severe and severe OSA are summarized in Tables 2, 3, and 4. Tables 2–4 show that, in general, sensitivity increased (decreasing false negative rate) with increased severity of OSA, increased BMI, decreased ODI threshold, and decreased ODI cut-off. Conversely, specificity decreased (increasing false positive rate) under the same conditions.
Table 2.
Sensitivity and specificity of ODI-2% for the diagnosis of moderate-severe and severe OSA
| BMI | Moderate-Severe OSA (AHI ≥ 15) |
Severe OSA (AHI ≥ 30) |
|||||
|---|---|---|---|---|---|---|---|
| ODI-2% ≥ 10 | ODI-2% ≥ 15 | ODI-2% ≥ 20 | ODI-2% ≥ 10 | ODI-2% ≥ 20 | ODI-2% ≥ 30 | ||
| 20-25 | Sens | 80.3 | 70.2 | 61.7 | 92.8 | 86.7 | 70.0 |
| Spec | 61.8 | 71.9 | 79.8 | 52.9 | 73.0 | 84.8 | |
| 25-30 | Sens | 85.6 | 78.3 | 69.1 | 91.3 | 84.6 | 71.4 |
| Spec | 53.4 | 63.6 | 70.6 | 39.6 | 60.3 | 75.9 | |
| 30-35 | Sens | 87.2 | 82.0 | 75.2 | 91.1 | 87.1 | 77.9 |
| Spec | 54.6 | 63.6 | 70.2 | 38.1 | 58.1 | 71.6 | |
| 35-40 | Sens | 87.9 | 83.3 | 78.4 | 89.1 | 85.9 | 79.1 |
| Spec | 50.8 | 59.5 | 65.7 | 33.8 | 53.1 | 67.4 | |
| ALL | Sens | 86.3 | 80.5 | 73.7 | 90.3 | 86.1 | 77.2 |
| Spec | 55.3 | 65.3 | 72.2 | 40.6 | 61.0 | 74.8 | |
ODI, oxygen desaturation index; OSA, obstructive sleep apnea; BMI, body mass index; AHI, apnea-hypopnea index.
Table 3.
Sensitivity and specificity of ODI-3% for the diagnosis of moderate-severe and severe OSA
| BMI | Moderate-Severe OSA (AHI ≥ 15) |
Severe OSA (AHI ≥ 30) |
|||||
|---|---|---|---|---|---|---|---|
| ODI-3% ≥ 10 | ODI-3% ≥ 15 | ODI-3% ≥ 20 | ODI-3% ≥ 10 | ODI-3% ≥ 20 | ODI-3% ≥ 30 | ||
| 20-25 | Sens | 56.9 | 43.3 | 30.4 | 82.8 | 60.6 | 46.7 |
| Spec | 90.4 | 96.7 | 98.3 | 82.3 | 95.7 | 98.7 | |
| 25-30 | Sens | 65.7 | 52.2 | 40.3 | 80.4 | 63.8 | 44.0 |
| Spec | 83.1 | 92.5 | 95.7 | 68.7 | 90.0 | 97.0 | |
| 30-35 | Sens | 73.3 | 63.1 | 54.5 | 84.3 | 72.8 | 59.3 |
| Spec | 77.9 | 89.3 | 93.3 | 62.4 | 84.8 | 94.7 | |
| 35-40 | Sens | 78.2 | 70.3 | 62.7 | 85.7 | 75.5 | 63.1 |
| Spec | 74.6 | 87.5 | 94.7 | 57.2 | 81.7 | 92.2 | |
| ALL | Sens | 71.9 | 61.7 | 52.6 | 83.8 | 72.1 | 58.3 |
| Spec | 82.1 | 91.6 | 95.3 | 67.2 | 87.7 | 95.6 | |
ODI, oxygen desaturation index; OSA, obstructive sleep apnea; BMI, body mass index; AHI, apnea-hypopnea index.
Table 4.
Sensitivity and specificity of ODI-4% for the diagnosis of moderate-severe and severe OSA
| BMI | Moderate-Severe OSA (AHI ≥ 15) |
Severe OSA (AHI ≥ 30) |
|||||
|---|---|---|---|---|---|---|---|
| ODI-4% ≥ 10 | ODI-4% ≥ 15 | ODI-4% ≥ 20 | ODI-4% ≥ 10 | ODI-4% ≥ 20 | ODI-4% ≥ 30 | ||
| 20-25 | Sens | 31.9 | 21.6 | 17.8 | 60.0 | 43.3 | 26.1 |
| Spec | 98.6 | 99.4 | 99.6 | 95.1 | 99.2 | 99.6 | |
| 25-30 | Sens | 41.9 | 29.3 | 20.5 | 63.3 | 39.0 | 24.8 |
| Spec | 95.9 | 98.4 | 98.9 | 88.7 | 98.2 | 99.5 | |
| 30-35 | Sens | 55.5 | 44.5 | 35.5 | 72.7 | 54.1 | 36.9 |
| Spec | 94.0 | 98.2 | 99.4 | 83.8 | 97.0 | 99.3 | |
| 35-40 | Sens | 62.9 | 52.2 | 43.7 | 75.2 | 58.7 | 42.9 |
| Spec | 93.8 | 97.6 | 98.9 | 80.6 | 95.3 | 99.0 | |
| ALL | Sens | 53.7 | 42.6 | 34.4 | 71.8 | 53.7 | 38.6 |
| Spec | 95.6 | 98.3 | 99.1 | 86.6 | 97.4 | 99.4 | |
ODI, oxygen desaturation index; OSA, obstructive sleep apnea; BMI, body mass index; AHI, apnea-hypopnea index.
ROC AUC Comparisons
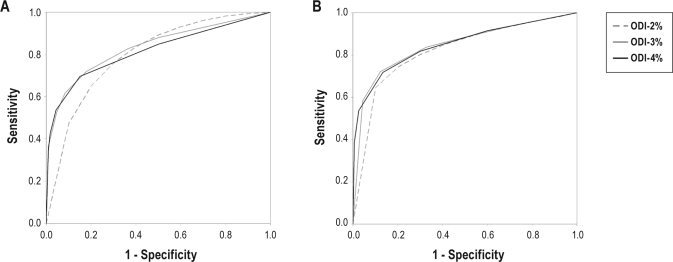
ROC curves of ODI for the diagnosis of moderate-severe and severe OSA are shown in Figure 3. Calculated AUC of ROC curves across the range of BMI categories and ODI thresholds demonstrated a range from 0.732 to 0.880 (Table 5). Overall, ODI-3% achieved a significantly higher AUC than ODI-2% and ODI-4% for the diagnosis of moderate-severe OSA. ODI-3% also had a significantly higher AUC than ODI-2%, but not ODI-4%, for the diagnosis of severe OSA.
Figure 3.
Receiver-operator characteristic (ROC) curves of oxygen desaturation index (ODI)-2%, ODI-3%, and ODI-4% for the diagnosis of moderate-severe (A) and severe (B) obstructive sleep apnea (OSA). Moderate-severe OSA was defined as an apnea-hypopnea index (AHI) ≥ 15. Severe OSA was defined as an AHI ≥ 30.
Table 5.
Area under curve of receiver-operator characteristic curves across BMI categories and ODI thresholds for the diagnosis of moderate-severe and severe OSA
| BMI | Moderate-Severe OSA (AHI ≥ 15) |
Severe OSA (AHI ≥ 30) |
||||
|---|---|---|---|---|---|---|
| ODI-2% | ODI-3% | ODI-4% | ODI-2% | ODI-3% | ODI-4% | |
| 20-25 | 0.748 | 0.779* | 0.739† | 0.836 | 0.880* | 0.849† |
| 25-30 | 0.732 | 0.795* | 0.761*† | 0.768 | 0.825* | 0.810*† |
| 30-35 | 0.751 | 0.811* | 0.802* | 0.764 | 0.835* | 0.832* |
| 35-40 | 0.736 | 0.831* | 0.832* | 0.735 | 0.826* | 0.834* |
| ALL | 0.754 | 0.821* | 0.804*† | 0.776 | 0.843* | 0.842* |
Significantly different from ODI-2% (P < 0.05).
Significantly different from ODI-3% (P < 0.05).
BMI, body mass index; ODI, oxygen desaturation index; OSA, obstructive sleep apnea; AHI, apnea-hypopnea index.
When examining the effect of BMI, ODI-3% demonstrated a significantly higher AUC than ODI-2% in all BMI categories, and ODI-4% in non-obese subjects. ODI-4% demonstrated a higher AUC than ODI-2% in all except in the 20-25 kg/m2 category. Both ODI-3% and ODI-4% achieved an “adequate” AUC > 0.80 for the diagnosis of severe OSA in all BMI categories, but for moderate-severe OSA only in obese BMI categories.
AUC of ROC curves of ODI for the detection of AI ≥ 20 are summarized in Table 6. Consistent with findings relating to OSA diagnosis, ODI-3% performed best overall, achieving a significantly higher AUC than ODI-2% and ODI-4%. When examined by BMI category, ODI-3% demonstrated a higher AUC than ODI-2% in all except in the 20-25 kg/m2 BMI category. While the AUC was greater for ODI-3% than for ODI-4% in all BMI categories, this difference was not statistically significant.
Table 6.
Area under curve of receiver-operator characteristic curves across BMI categories and ODI thresholds for the detection of an arousal index ≥ 20
| BMI | Arousal Index ≥ 20 |
||
|---|---|---|---|
| ODI-2% | ODI-3% | ODI-4% | |
| 20-25 | 0.656 | 0.668 | 0.659 |
| 25-30 | 0.690 | 0.702* | 0.694 |
| 30-35 | 0.724 | 0.738* | 0.730 |
| 35-40 | 0.725 | 0.746* | 0.742* |
| ALL | 0.712 | 0.726* | 0.719*† |
Significantly different from ODI-2% (P < 0.05).
Significantly different from ODI-3% (P < 0.05).
BMI, body mass index; ODI, oxygen desaturation index.
ODI/AHI Ratio
The ratios of ODI-2% to AHI, ODI-3% to AHI, and ODI-4% to AHI relative to BMI in all subjects with AHI ≥ 15 are shown in Figure 4. These ratios all increased with increasing BMI. ODI-3%/AHI approached 0.8, and ODI-4%/AHI approached 0.5 at higher BMI categories. ODI-2%/AHI exceeded 1.0 across all BMI categories, and was greater than 1.15 at BMI > 30 kg/m2.
Figure 4.
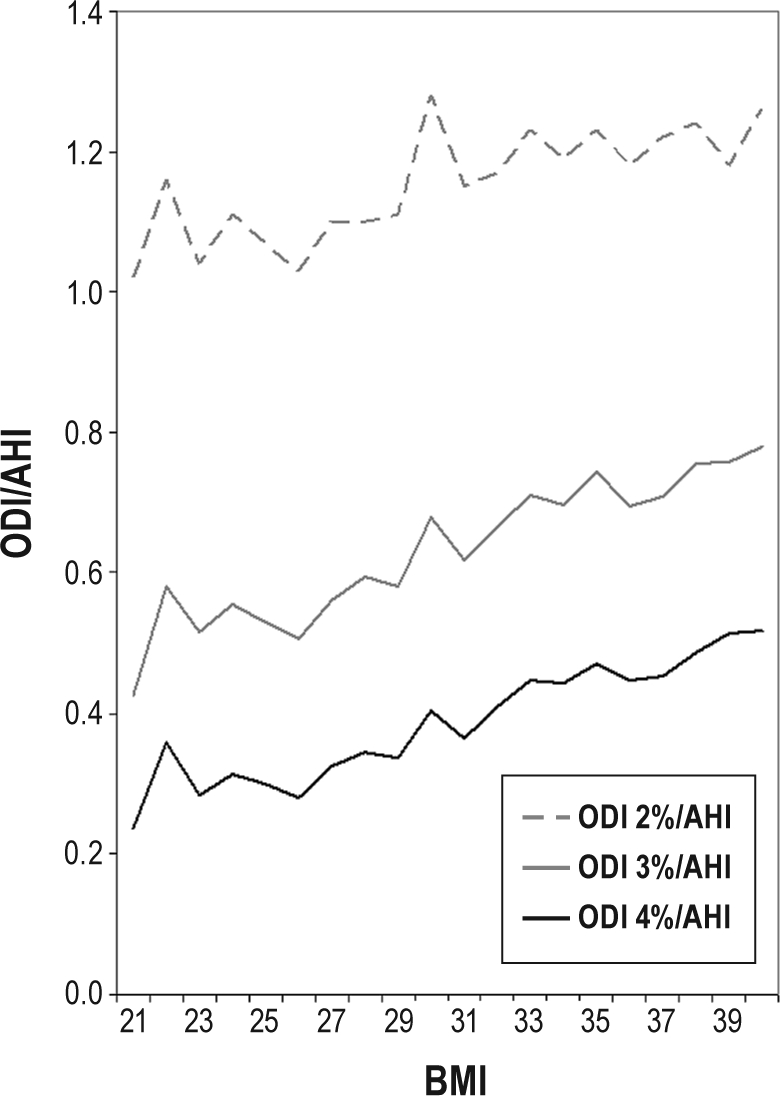
Ratios of oxygen desaturation index (ODI) at 2% level, 3% level, and 4% level to apnea-hypopnea index (ODI-2%/AHI, ODI-3%/AHI, and ODI-4%/AHI) plotted against body mass index (BMI) in all subjects with AHI ≥ 15.
DISCUSSION
Our findings demonstrate that the interactions between BMI, ODI, and AHI are complex. The accuracy, as reflected by AUC of ROC curves, of ODI for the diagnosis of moderate-severe OSA increases with BMI when using ODI-3% and ODI-4%, and is greater when using ODI-3% than with ODI-2% and ODI-4%. The relationship between ODI and AHI changes with increasing BMI, with the ratio of ODI to AHI being consistently lower in non-obese subjects than in obese subjects.
This BMI dependence reflects the influence of obesity on pulmonary oxygen stores and gas exchange when recumbent and asleep. Peppard and colleagues demonstrated that BMI is a significant independent predictor of the degree of oxygen desaturation during sleep disordered breathing.8 It is likely that the effect of excess weight on functional residual capacity when asleep12 is the primary mechanism by which obesity accentuates oxygen desaturation during obstructive events. The degree of desaturation for a given length of apnea is, apart from rate of oxygen consumption, related to the magnitude of alveolar oxygen stores13 (and therefore alveolar volume) and the baseline oxygen saturation,14 which defines the portion of the oxygen dissociation curve and therefore the relationship between changing arterial oxygen pressure and saturation. This baseline oxygen saturation is in turn determined by adequacy of alveolar gas exchange, which is aggravated by obesity-related atelectasis as functional residual capacity falls below closing volume in the recumbent sleeping individual.15,16 Therefore, it can be expected that obese subjects will experience greater degrees oxygen desaturation in association with apneas and hypopneas during sleep.
The ODI/AHI ratios increased with increasing BMI, indicating that SDB is more often associated with oxygen desaturation in those with higher body mass. Hence, it is to be expected that the sensitivity of any given ODI threshold for the detection of OSA will improve in higher BMI categories. Using lower desaturation criteria increases the sensitivity of the test at the cost of specificity, for example, when using ODI-2%. This fall in specificity is more notable when considering the detection of AHI ≥ 30. Obese individuals have lower lung volumes12 and greater whole-body oxygen demand,17 and it is likely that such individuals experience more frequent episodes of desaturation without meeting the defined criteria for hypopnea. Furthermore, pulse oximetry is known to have a margin of error of approximately 2%,18 and variability in this measurement very likely contributes to low specificity when using ODI-2%. Notably, the ODI/AHI ratio exceeded unity at all BMI levels for ODI-2% (Figure 4). Conversely, when using ODI-4% for the detection of AHI ≥ 30, specificity falls only marginally with increasing BMI, while sensitivity rises, resulting in preserved AUC in higher BMI categories.
In an early study, Cooper and colleagues19 found that qualitatively analyzed overnight oximetry was more sensitive for the detection of more severe OSA compared with milder OSA. Our findings confirm this observation with the use of quantitative ODI values, demonstrating that the sensitivity of any particular ODI cut-off value is appreciably higher for the detection of severe OSA compared with moderate-severe OSA (see Tables 3 to 5). Additionally, we show that in the non-obese categories, the performance of ODI for diagnosing severe OSA is considerably better than for moderate-severe OSA. The reasons for this are unclear, although the results suggest that oxygen desaturation with SDB occurs more frequently in the non-obese with severe OSA compared with moderate OSA.
In a study on a population of 424 patients, Nakano and colleagues20 demonstrated that the diagnostic characteristics of ODI for OSA varied with BMI, and suggested that the performance of the test could be enhanced by selecting cut-off values appropriate to subject BMI. Despite the use of different definitions for hypopnea, they similarly found that ODI-3% produced the highest AUC, except in the overweight group where ODI-4% had a marginally higher AUC. The findings of the present study are largely in agreement, although the sensitivities we obtained are considerably lower, most likely due to the use of a broader definition for hypopnea.
Strengths and Limitations
The strength of this study lies primarily in the large numbers of observations that have been systematically collected over an 8-year period. Standardized methods and equipment were used over the study period, allowing for consistency in the data. The main limitations include a study population confined to patients referred specifically for assessment of sleep disorders, which could limit the ability to generalize the results to an unselected population. Additionally, only ODI was derived from the results of overnight oximetry, where other studies have found utility in other more complicated indices21,22 or in combination with a clinical questionnaire.23 With regard to latter, it should be noted that the population of the present study was referred on the basis of clinical suspicion as assessed by other medical practitioners. Referred patients have therefore already been subjected to a form of clinical screening, which has been effective in increasing the prevalence of OSA from approximately 2% to 4% in the general population24 to the 64% that we observe in our sleep clinic population (Table 1).
Hypopneas in our study were scored using Chicago criteria, an aspect of which incorporates a desaturation requirement of ≥ 3%.10 While it is possible that this influences the performance of ODI at the various desaturation thresholds we have reported, it is important to note that the ODI to AHI ratios reported indicate that oxygen desaturation is only present in a certain proportion of events when using these criteria. In those with BMI < 25 kg/m2 in particular, the ODI-3% to AHI ratio is below 0.60, indicating that greater than 40% of events were scored due to the presence of other criteria without oxygen desaturation of ≥ 3%. These would include hypopneas with a reduction in airflow > 50%, hypopneas associated with EEG arousal, and apneas. The ROC curves of ODI for the detection of an AI ≥ 20 and the AUC comparisons formally quantify the performance of ODI for detecting an increased arousal index, an accepted marker of sleep disruption which is determined by EEG criteria alone, without recourse to respiratory variables.25,26 The fact that ODI-3% also performed best overall by this analysis further supports the argument that, as a solitary measure, this desaturation threshold more accurately characterises sleep disordered breathing and its accompaniments than ODI-2% or ODI-4%. This conclusion is supported by the study of Nakano and colleagues,20 who reported ODI-3% as having the highest AUC in two of three BMI categories when using a hypopnea definition that was independent of oxygen desaturation.
These supporting features do not exclude the possibility that a degree of mathematical coupling and auto-correlation have influenced this conclusion, given the use of an AHI definition that incorporates one of the metrics being assessed.27 However, the correlations between ODI-3% and AHI and ODI-4% and AHI were similar, being marginally stronger for ODI-4% (r = 0.745 and 0.751, respectively). This suggests that auto-correlation is unlikely to have significantly biased conclusions towards ODI-3%, when assessing the diagnostic utility of ODI for respiratory events.
Clinical Implications
Overnight oximetry has been proposed and used as a screening test for OSA.6 There have been several published studies assessing its sensitivity and specificity; however, methods have been heterogeneous and limit the ability to pool results. Study populations have often been small, and definitions of hypopnea and methods of analysis of overnight oximetry have been variable.20–22,28–30
The American Academy of Sleep Medicine (AASM) 2001 position statement recommended that a hypopnea be defined as an abnormal respiratory event lasting ≥ 10 seconds with ≥ 30% reduction in respiratory effort or airflow and ≥ 4% desaturation.31 This recommendation largely derives from data from the Sleep Heart Health Study32,33 and has become the approved definition in use by Medicare and Medicaid in the United States for the purposes of treatment funding. Nonetheless, the AASM practice parameters for polysomnography published in 2005 acknowledged that several definitions of hypopnea are in clinical use, without a clear consensus.34 It has recently been shown that use of the current AASM criteria results in an AHI that is approximately 30% of that obtained by use of the Chicago criteria.35
The present study is the first to assess the performance of ODI for the diagnosis of OSA in a large sleep clinic population. We demonstrate that ODI-3% performed best and is most likely to correctly identify patients with both moderate and severe OSA in the overall sample. ODI-3% is better than ODI-2% in all BMI categories presented, and better than ODI-4% when examining non-obese subjects. The findings suggest that use of a desaturation threshold of ≥ 3% is a viable alternative to ≥ 4% in hypopnea definition, as is the case for the alternative AASM criteria.36
When considering the isolated use of ODI as a diagnostic test for OSA, different ODI thresholds might be selected in order to use ODI to rule in or rule out OSA. For example, an ODI-3% ≥ 15/h or ODI-4% ≥ 10/h (Table 3 and 4) may be used to rule in OSA, and subjects with a positive test may proceed on to treatment. Subjects with a negative test should proceed to PSG. Conversely, an ODI-2% < 10 events/h (Table 2) may be used to help rule out OSA for subjects with a BMI above 25 kg/m2. However for subjects with a BMI < 25 kg/m2 the sensitivity of ODI-2%, (or ODI-3% or ODI-4%) is suboptimal, and these subjects are better served by investigation with PSG in the first instance. A suggested pathway for the use of ODI in clinical practice is given in Figure 5.
Figure 5.
Suggested clinical pathway incorporating body mass index (BMI), oxygen desaturation index (ODI), and polysomnography (PSG) for the evaluation of patients with suspected obstructive sleep apnea (OSA).
Patients are usually referred for sleep clinic assessment on the basis of symptoms such as loud snoring, daytime sleepiness, and tiredness,37 as well as clinical features such as obesity,38 increased neck circumference,39 and predisposing craniofacial structure.40 Our data, derived from a sleep clinic population, are most applicable to such preselected patients, and should not be applied to an unselected community population.
CONCLUSION
The present study has demonstrated that complex interrelationships exist between BMI, ODI, and AHI in a large sleep clinic population. Sleep disordered breathing is more frequently associated with oxygen desaturation in obese subjects, with BMI influencing the accuracy of ODI for the diagnosis of OSA. The sensitivity of ODI in subjects with a BMI < 25 kg/m2 is suboptimal, and ODI should not be used as a test for OSA in such subjects. Of the three ODI thresholds considered, ODI-3% performed best, achieving an AUC > 0.80 for the diagnosis of severe OSA in all BMI subcategories, and for moderate-severe OSA in obese categories. Furthermore, it is shown that ODI is considerably more sensitive for detecting severe OSA than moderate-severe OSA. These results suggest that an oxygen desaturation criteria of ≥ 3% can be appropriately used in hypopnea definition, and that ODI can be incorporated into a clinical pathway as a valuable initial diagnostic test in the evaluation of many patients referred for sleep clinic assessment.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Hillman conducts funded research for Apnex Medical and has received honoraria for speaking engagements from ResMed Limited. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank and acknowledge the work of the dedicated sleep technologists and administrative staff of the West Australian Sleep Disorders Research Institute.
Footnotes
A commentary on this article appears in this issue on page 13.
REFERENCES
- 1.Flemons WW, Douglas NJ, Kuna ST, Rodenstein DO, Wheatley J. Access to diagnosis and treatment of patients with suspected sleep apnea. Am J Respir Crit Care Med. 2004;169:668–72. doi: 10.1164/rccm.200308-1124PP. [DOI] [PubMed] [Google Scholar]
- 2.Eastwood PR, Malhotra A, Palmer LJ, et al. Obstructive sleep apnoea: from pathogenesis to treatment: current controversies and future directions. Respirology. 2010;15:587–95. doi: 10.1111/j.1440-1843.2009.01699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–6. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 4.Ramachandran SK, Josephs LA. A meta-analysis of clinical screening tests for obstructive sleep apnea. Anesthesiology. 2009;110:928–39. doi: 10.1097/ALN.0b013e31819c47b6. [DOI] [PubMed] [Google Scholar]
- 5.Ross SD, Sheinhait IA, Harrison KJ, et al. Systematic review and meta-analysis of the literature regarding the diagnosis of sleep apnea. Sleep. 2000;23:519–32. [PubMed] [Google Scholar]
- 6.Netzer N, Eliasson AH, Netzer C, Kristo DA. Overnight pulse oximetry for sleep-disordered breathing in adults: a review. Chest. 2001;120:625–33. doi: 10.1378/chest.120.2.625. [DOI] [PubMed] [Google Scholar]
- 7.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–9. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 8.Peppard PE, Ward NR, Morrell MJ. The impact of obesity on oxygen desaturation during sleep-disordered breathing. Am J Respir Crit Care Med. 2009;180:788–93. doi: 10.1164/rccm.200905-0773OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rechtshaffen A, Kales A. A manual of standardized terminology, technique and scoring system for sleep stages of human sleep. Los Angeles: Brain Information Service, Brain Information Institute, University of California; 1968. [Google Scholar]
- 10.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 11.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–43. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 12.Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest. 2006;130:827–33. doi: 10.1378/chest.130.3.827. [DOI] [PubMed] [Google Scholar]
- 13.Findley LJ, Ries AL, Tisi GM, Wagner PD. Hypoxemia during apnea in normal subjects: mechanisms and impact of lung volume. J Appl Physiol. 1983;55:1777–83. doi: 10.1152/jappl.1983.55.6.1777. [DOI] [PubMed] [Google Scholar]
- 14.Bradley TD, Martinez D, Rutherford R, et al. Physiological determinants of nocturnal arterial oxygenation in patients with obstructive sleep apnea. J Appl Physiol. 1985;59:1364–8. doi: 10.1152/jappl.1985.59.5.1364. [DOI] [PubMed] [Google Scholar]
- 15.Lehrman SG, Limann B, Koshy A, Aronow WS, Ahn C, Maguire G. Association of lung volumes with nocturnal oxygen saturation in obese persons: a possible role for therapeutic continuous positive airway pressure. Am J Ther. 2008;15:221–4. doi: 10.1097/MJT.0b013e3180a721f7. [DOI] [PubMed] [Google Scholar]
- 16.Pelosi P, Croci M, Ravagnan I, et al. The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesia. Anesth Analg. 1998;87:654–60. doi: 10.1097/00000539-199809000-00031. [DOI] [PubMed] [Google Scholar]
- 17.Crummy F, Piper AJ, Naughton MT. Obesity and the lung: 2. Obesity and sleep-disordered breathing. Thorax. 2008;63:738–46. doi: 10.1136/thx.2007.086843. [DOI] [PubMed] [Google Scholar]
- 18.Jubran A. Pulse oximetry. Crit Care. 1999;3:R11–R7. doi: 10.1186/cc341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper BG, Veale D, Griffiths CJ, Gibson GJ. Value of nocturnal oxygen saturation as a screening test for sleep apnoea. Thorax. 1991;46:586–8. doi: 10.1136/thx.46.8.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakano H, Ikeda T, Hayashi M, et al. Effect of body mass index on overnight oximetry for the diagnosis of sleep apnea. Respir Med. 2004;98:421–7. doi: 10.1016/j.rmed.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Levy P, Pepin JL, Deschaux-Blanc C, Paramelle B, Brambilla C. Accuracy of oximetry for detection of respiratory disturbances in sleep apnea syndrome. Chest. 1996;109:395–9. doi: 10.1378/chest.109.2.395. [DOI] [PubMed] [Google Scholar]
- 22.Olson LG, Ambrogetti A, Gyulay SG. Prediction of sleep-disordered breathing by unattended overnight oximetry. J Sleep Res. 1999;8:51–5. doi: 10.1046/j.1365-2869.1999.00134.x. [DOI] [PubMed] [Google Scholar]
- 23.Schafer H, Ewig S, Hasper E, Luderitz B. Predictive diagnostic value of clinical assessment and nonlaboratory monitoring system recordings in patients with symptoms suggestive of obstructive sleep apnea syndrome. Respiration. 1997;64:194–9. doi: 10.1159/000196670. [DOI] [PubMed] [Google Scholar]
- 24.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 25.Guilleminault C, Partinen M, Quera-Salva MA, Hayes B, Dement WC, Nino-Murcia G. Determinants of daytime sleepiness in obstructive sleep apnea. Chest. 1988;94:32–7. doi: 10.1378/chest.94.1.32. [DOI] [PubMed] [Google Scholar]
- 26.Carskadon MA, Dement WC. Nocturnal determinants of daytime sleepiness. Sleep. 1982;5(Suppl 2):S73–81. doi: 10.1093/sleep/5.s2.s73. [DOI] [PubMed] [Google Scholar]
- 27.Walsh TS, Lee A. Mathematical coupling in medical research: lessons from studies of oxygen kinetics. Br J Anaesth. 1998;81:118–20. doi: 10.1093/bja/81.2.118. [DOI] [PubMed] [Google Scholar]
- 28.Golpe R, Jimenez A, Carpizo R, Cifrian JM. Utility of home oximetry as a screening test for patients with moderate to severe symptoms of obstructive sleep apnea. Sleep. 1999;22:932–7. [PubMed] [Google Scholar]
- 29.Ryan PJ, Hilton MF, Boldy DA, et al. Validation of British Thoracic Society guidelines for the diagnosis of the sleep apnoea/hypopnoea syndrome: can polysomnography be avoided? Thorax. 1995;50:972–5. doi: 10.1136/thx.50.9.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vazquez JC, Tsai WH, Flemons WW, et al. Automated analysis of digital oximetry in the diagnosis of obstructive sleep apnoea. Thorax. 2000;55:302–7. doi: 10.1136/thorax.55.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meoli AL, Casey KR, Clark RW, et al. Hypopnea in sleep-disordered breathing in adults. Sleep. 2001;24:469–70. [PubMed] [Google Scholar]
- 32.Redline S, Sanders MH, Lind BK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21:759–67. [PubMed] [Google Scholar]
- 33.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 34.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28:499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 35.Ruehland WR, Rochford PD, O'Donoghue FJ, Pierce RJ, Singh P, Thornton AT. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep. 2009;32:150–7. doi: 10.1093/sleep/32.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iber C, Ancoli-Israel S, Chesson A, Quan S. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 37.Chervin RD. Sleepiness, fatigue, tiredness, and lack of energy in obstructive sleep apnea. Chest. 2000;118:372–9. doi: 10.1378/chest.118.2.372. [DOI] [PubMed] [Google Scholar]
- 38.Tishler PV, Larkin EK, Schluchter MD, Redline S. Incidence of sleep-disordered breathing in an urban adult population: the relative importance of risk factors in the development of sleep-disordered breathing. JAMA. 2003;289:2230–7. doi: 10.1001/jama.289.17.2230. [DOI] [PubMed] [Google Scholar]
- 39.Stradling JR, Crosby JH. Predictors and prevalence of obstructive sleep apnoea and snoring in 1001 middle aged men. Thorax. 1991;46:85–90. doi: 10.1136/thx.46.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cakirer B, Hans MG, Graham G, Aylor J, Tishler PV, Redline S. The relationship between craniofacial morphology and obstructive sleep apnea in whites and in African-Americans. Am J Respir Crit Care Med. 2001;163:947–50. doi: 10.1164/ajrccm.163.4.2005136. [DOI] [PubMed] [Google Scholar]