Abstract
Young children and allogeneic HCT recipients respond poorly to polysaccharide antigens rendering them susceptible to severe infections due to encapsulated bacteria. This study evaluated the responses of 127 HCT patients, median age: 23.0 years, vaccinated with PnCRM7 and HIB, two conjugate vaccines highly immunogenic in healthy children. Median time to vaccination was 1.1 years after HCT. Sixty-two percent of patients responded to PNCRM7 (45/51 children, 34/76 adults, p<0.001). Overall response to HIB was 86% including 77% of PNCRM7 non-responders. Although PNCRM7 response was adversely affected by older age (p<0.001), individuals ≥50 years old responded significantly better if vaccinated following acquisition of specific minimal milestones of immune competence, CD4 >200/ul, IgG >500 mg/dl, PHA within 60% lower limit of normal (11/19 vs 0/8, p<0.006). A similar trend was observed in patients with limited chronic GVHD. In all patients, higher levels of circulating CD4+CD45RA cells correlated with improved PNCRM7 response. These data demonstrate that PNCRM7 is immunogenic in allogeneic HCT patients, including older adults, but suggest that vaccination at fixed intervals after HCT, irrespective of immune competence, may limit its effectiveness. Prospective, multi-center trials assessing the best strategy to administer this vaccine and its impact on pneumococcal infections following transplantation are warranted.
Keywords: vaccination, pneumococcus, Haemophilus Influenza
Introduction
Invasive pneumococcal infections (IPI) remain a significant, potentially vaccine preventable cause of morbidity and mortality late after successful allogeneic transplantation (1–4). Although IPI are highest in patients with chronic GVHD (1–5), the incidence in patients without GVHD is over 20 times that reported in the general population (1–5). Using data prospectively collected by the Toronto Invasive Bacterial Diseases Network from 1995 through 2005, Kumar et al. compared the incidence of IPI in adults 18–65 years of age who did or did not receive a hematopoietic cell transplant (6). The calculated incidence of IPI was 590 per 100,000 allogeneic transplant recipients per year compared with 11.5/100, 000 persons per year in non-transplanted individuals 18–65 years of age (p<0.0001). All pneumococcal strains isolated from transplant patients were serotypes included in the pure pneumococcal polysaccharide vaccine, PPV23. Despite recommendations to use this vaccine after HCT (7, 8), it is poorly immunogenic in transplant recipients (9–11), contributing to a false sense of security in PPV23 recipients of the vaccine and inconsistent prescribing by health care professionals (5,6).
In 2000, the FDA licensed the 7-valent protein-conjugated pneumococcal vaccine (Prevnar (PNCRM7®)) containing 7 serotypes (4, 6B, 9V, 14, 18C, 19F, 23F) that cause 70–80% of invasive pneumococcal infections in North America (12,13). Five of the serotypes (6B, 9V, 14, 19F, 23F) are responsible for the majority of antibiotic resistant pneumococcal infections worldwide (12–14). Conjugation of pneumococcal saccharides to a carrier protein (CRM197, a non-toxic variant of diphtheria toxin) results in T cell-dependent B cell generation of memory B cells that respond to the attached sugars (12,13). In contrast, PPV23, elicits a T cell independent B cell response and fails to induce antigenic memory, precluding the development of an anamnestic response following antigenic re-exposure (14). PNCRM7 vaccination has dramatically decreased the incidence of pneumococcal disease in healthy infants and children (15), populations who possess circulating B cells that are phenotypically (CD20+CD5+CD38+CD1c+) and functionally similar to those of HCT recipients (16).
To determine the response of allogeneic HCT recipients to PNCRM7 compared with HIB, the immunization records and pre and post vaccine titers of all patients transplanted at Memorial Sloan Kettering Cancer Center between 2002 and 2005, were analyzed for immunization against pneumococcus. The effect of stem cell and donor type, use and method of T cell depletion, presence of graft versus host disease, and receipt of donor leukocyte infusion (DLI) on vaccine responses were assessed. The effect of PPV23 administration following the conjugate vaccine was also analyzed.
Patients, materials, and methods
A waiver of authorization to conduct this study was approved by the Memorial Sloan Kettering Cancer Center Institutional Review Board. The medical records of all patients who remained disease-free for 1 year after an allogeneic transplant performed at this center from 1/1/02 through 12/31/05 were reviewed for immunization against pneumococcus. Dates of vaccination and pre and post vaccine titers were obtained from a prospectively maintained database and confirmed by retrospective chart review. Of 189 patients who survived disease-free ≥1 year after HCT, 80% were vaccinated against pneumococcus. Due to preliminary data demonstrating poor vaccine responses in patients immunized prior to the development of a CD4 cell count >200 cells/ul or IgG level >500 mg/dl, 75% of patients were vaccinated after achieving these milestones. One hundred and thirty-two patients received a series of 3 PNCRM7 immunizations administered 4–8 weeks apart. Due to PNCRM7 shortages during the study period, 21 patients (median age: 43.1 yr) received PPV23 as their primary pneumococcal vaccine. Pre and post vaccine titers were available in 127 of 132 PNCRM7 recipients (Table 1) and 18 of 21 patients PPV23 recipients. Pre and post vaccine titers were also analyzed in 36 of the 127 recipients of PNCRM7 who received PPV23 as their secondary pneumococcal vaccine. Ninety-four percent of patients vaccinated with PNCRM7 received a concomitant series of Haemophilus influenza b conjugate vaccines. Ninety percent of patients received at least 2 vaccines at MSKCC. All patients were evaluated at MSKCC before and after completing vaccinations, including assessment of acute and chronic GVHD using established criteria (17, 18).
Table 1.
Patient Characteristics
| All | Matched Related | Unrelated | Mismatched Related | |
|---|---|---|---|---|
| N=127 | n=67 | n=53 | n=7 | |
| Sex, % female | 58(45.7) | 29(43.3) | 26(48.2) | 3(42.9)) |
| Age at transplant, | 23(0.1–64) | 32(0.1–64) | 28(0.2–62) | 6(0.8–9) |
| median, y(range) | ||||
| Diagnosis (%) | ||||
| Acute Leukemia | 64(50.4) | 38(56.7) | 20(38.5) | 2(28.6) |
| Chronic Leukemia | 6(4.72) | 4(6.0) | 2(3.9) | 0 |
| Aplastic | 26(20.5) | 8(11.9) | 11(21.2) | 3(42.9) |
| Anemia/MDS | ||||
| NHL/HD | 13(10.2) | 6(9.0) | 7(13.5) | 0 |
| Others* | 18(14.2) | 11(16.4) | 3(5.8) | 2(28.6) |
| Stem-Cell product | ||||
| (%) | ||||
| Unmodified | 56(44.1) | 35(52.2) | 20(38.5) | 1(14.3) |
| Transplant | ||||
| T cell depleted | 71(55.9) | 32(47.8) | 32(61.5) | 6(85.7) |
| transplant | ||||
| TBI (%) | 59(46.5) | 33(49.3) | 24(46.2) | 2(28.6) |
| Acute GVHD (%) | 10(7.9) | 5(7.5) | 5(9.6) | 0 |
| Chronic GVHD (%) | 25(19.7) | 14(20.9) | 11(20.7) | 0 |
| Donor Leukocyte | 12(9.4) | 11(16.4) | 1(1.9) | 0 |
| Infusion (%) | ||||
| CD4+ CD45RA+ | ||||
| Median, (range) | 119(0–1199) | 110(0.3–1029) | 115(0–1077) | 360(128–1199) |
Serotype-specific pneumococcal capsular polysaccharide IgG was measured by enzyme-linked immunsorbant assay (ELISA). The FDA standard reference serum 89-S was used as the calibration standard. Titers against pneumococcal serotypes 1,3,14,19F, 23F, and 7F were measured in order to distinguish responses specific to PPV23 (1,3, 7F) from responses common to both vaccines (14, 19F, 23F). Response to PNCRM7 or PPV23 was defined as seroconversion or >3 fold rise in titer against serotypes 14, 19F, and 23F, three significant causes of antibiotic resistant pneumococcal infections in HCT recipients (2, 5). Initial antibody response was measured at a median of 3.0 months (range: 2–12 months) after completion of PNCRM7 and HIB immunization. PPV23 response was measured at a median of 3.2 months (range 1.5–12 months) following immunization. HIB response, measured by ELISA, was defined as seroconversion (> 1 ug/ml) or >3 fold increase in antibody titer. The lower limits of quantification for pneumococcal and H flu antibody levels were 0.5 ug/ml and 0.15 ug/ml, respectively.
Immunofluorescence studies. Two color immunofluorescence was performed on the circulating lymphocytes all patients within three months of vaccine initiation. Directly fluoresceinated (FITC) antibodies, including CD45 (common leukocyte antigen, positive control), MsIgG, CD3 (pan T-cell), CD20 (pan-B cell), CD14 (monocyte marker), and phycoerythrin-conjugated (PE) antibodies MsIgG, CD8 (cytotoxic or suppressor T cell marker), CD4 (helper T cell), CD45RA (naïve T cell subset), and CD56 (natural killer cells) were purchased from Becton Dickinson (Mountain View, CA). Immunofluorescence was performed on whole blood as previously described (19). Immunofluorescence samples were analyzed on a FACScan (Becton Dickinson). The lymphoid populations to be analyzed were gated on using log 90 degree and forward angle scatter characteristics. The leukocyte-specific monoclonal antibody CD45 was used to gate out any residual red cells. Monocyte contamination was ruled out by lack of reactivity (<1%) of the gated lymphoid cells with the monocyte specific marker CD14.
Patient and transplant characteristics
The demographics of the 127 patients vaccinated with PNCRM7 are shown in Table 1. The median age at HCT was 23.0 years (range: 0.1–64.0 years). Thirty-three percent of the population was ≥ 40 years old at HCT. The stem cell donor was an HLA-A, B, DRβ1 identical sibling, an HLA-mismatched family member, or unrelated donor in 53%, 5.5%, and 42% of cases, respectively. Fifty-six percent of patients received a T cell depleted bone marrow or peripheral blood stem cell product. T cells were depleted from bone marrow by soybean lectin agglutination, followed by rosetting with sheep erythrocytes (n=16) (20), and from peripheral blood by CD34 positive selection followed by rosetting with sheep erythrocytes (n=55) (21). The majority (84%) of recipients of an unmodified HCT received either short course methotrexate combined with tacrolimus or cyclosporine (n=33) or cyclosporine and alemtuzumab (n=14) for GVHD prophylaxis. Of the 56 patients who received an unmodified HCT, 26% developed chronic GVHD, of whom 32% were on immunosuppression at the time of revaccination.
Twelve patients received adoptive immunotherapy in the form of unfractionated donor lymphocytes for the treatment (n=2) or prevention (n=1) of an EBV-LPD, to prevent recurrent malignancy (n=6), or to treat mixed T cell chimerism (n=3). Twelve patients received post transplant rituximab for the treatment or prevention of an EBV-LPD (n=9), treatment of an auto-immune cytopenia (n=2), or prevention of recurrence of a CD20+ malignancy (1). Twelve patients received the CD20 monoclonal antibody, rituximab, for the treatment of an auto-immune hemolytic anemia (n=1), treatment (n=2) or prevention (n=7) of an EBV lymphoproliferative disorder, or prevention of recurrence of a CD20+ malignancy (n=2). Rituximab was administered at a median (range) of 252 (135–728) days prior to revaccination.
Biostatistics
Frequency distributions for characteristics of patients by donor type and response to vaccination was summarized in Table 1– Table 3. Fisher’s exact test and the Wilcoxon rank sum test were used to examine covariate differences between responders and non-responders. Subsequent to the univariate analyses, a logistic regression model was developed to determine the set of factors that independently predicted vaccine response. The Hosmer and Lemeshow goodness-of-fit statistic was used to assess how close the model-predicted values were to the corresponding observed values (22). The statistical packages SAS (9.1) and R (2.3.1) were used to generate the test statistics and build the regression model.
Table 3.
Characteristics of Hib responders and nonresponder
| Characteristic | Responder | Nonresponder | P VALUE |
|---|---|---|---|
| N=99 | N=16 | ||
| Median age (range) | 18 (0.1–64) | 34.5(0.7–62) | 0.07 |
| Sex, male, no. (%) | 54 (54.6) | 10(62.5) | 0.60 |
| Diagnosis, no. (%) | 0.22 | ||
| Acute Leukemia | 51(51.5) | 6(37.5) | |
| Chronic Leukemia | 4(4.0) | 2(12.5) | |
| Aplastic Anemia/MDS | 21(21.2) | 4(25) | |
| NHL/HD | 8(8.1) | 3(18.8) | |
| Graft type, no. (%) | 0.78 | ||
| Unmodified Transplant | 43(43.4) | 8(30.0) | |
| T Cell depleted Transplant | 56(56.6) | 8(50.0) | |
| Donor, no. (%) | 0.75 | ||
| Matched related | 49(49.5) | 9(56.2) | |
| Mismatched related | 7(7.1) | 0 | |
| Unrelated | 43(43.4) | 7(43.7) | |
| TBI regimen, no. (%) | 45(45.5) | 8(50.0) | 0.79 |
| DLI, no. (%) | 7(7.1) | 1(6.3) | 1.0 |
| Acute GVHD, no (%) | 8(8.1) | 1(6.3) | 1.0 |
| Chronic GVHD, no. (%) | 20(20.2) | 3(18.8) | 1.0 |
Results
There were no serious adverse reactions in any patient attributable to vaccination with PNCRM7. PPV23, or HIB. Prior to revaccination, over 80% of patients lacked detectable titers against any pneumococcal serotype and Haemophilus influenza. The median time to initiate vaccination with PNCRM7 and HIB was 1.1 years post HCT. Forty percent of patients were immunized within one year and 81% within two years of transplant. There was no significant difference in the time to PNCRM7 vaccination in recipients of a T-cell depleted versus an unmodified (T-cell replete) transplant (median 1.0 year versus 1.14 years, p=0.3). The median time to vaccination in children and adults was 1 year and 1.3 years, respectively (p<.001). The shorter interval between HCT and vaccination of children was in part due to more rapid immune reconstitution in patients <18 years of age combined with the tendency of pediatricians to vaccinate immediately after the milestones were met. In the 25 patients with chronic GVHD, the median time to vaccination was 1.8 years compared to 1.1 years in patients without GVHD (p<0.001).
Sixty-two percent (79/127) of patients responded to PNCRM7 (Table 2a, 2b). A dichotomy existed between the response observed in children (median age: 9 years (0.1–17.9) and adults (median age: 41 years(18–64). Eighty-eight percent of children responded to the heptavalent pneumococcal conjugate vaccine compared to 44% of adults (p<0.001). In contrast only 1 of 18 patients, median age: 42.5 years, immunized with PPV23 responded to serotypes 14, 19F, and 23F. Of the 16 adult recipients of PPV23, only 1 of 16 responded compared to 34 of 76 adults given the conjugated vaccine (p=0.003). PNCRM7 response in children and adults was not affected by the use of T cell depletion or donor type (Table 2b). Five of 12 patients who received rituximab responded to PNCRM7, including 2 of 4 children and 3 of 8 adults. For PNCRM7 vaccination, results from multivariate logistic regression model indicated that age at transplant significantly predicted response (p=0.0002). DLI and disease diagnosis were associated with response, but did not significantly predict response after adjusting for age at transplant (Table 2a).
Table 2.
Characteristics of Prevnar responders and nonresponder
| Characteristics | Responder | Nonresponder | Univariate | Multivariate |
|---|---|---|---|---|
| N=79 | N=48 | |||
| Median age (range) | 15(0.1–59) | 40.5(0.7–64) | <0.001 | 0.001 |
| Sex, male, no. (%) | 39(49.4) | 30(62.5) | 0.20 | |
| *Diagnosis, no. (%) | 0.03 | 0.27 | ||
| Acute Leukemia | 37(46.8) | 27(56.3) | ||
| Chronic Leukemia | 4(5.1) | 2(4.2) | ||
| Aplastic Anemia/MDS | 19(24.1) | 7(14.6) | ||
| NHL/HD | 4(5.1) | 9(18.8) | ||
| Others | 15(19.0) | 3(6.3) | ||
| Graft type, no. (%) | 0.46 | |||
| Unmodified Transplant | 37(46.8) | 19(39.6) | ||
| T Cell depleted Transplant | 42(53.2) | 29(60.4) | ||
| Donor, no. (%) | 0.09 | |||
| HLA-matched related | 39(49.4) | 28(58.3) | ||
| HLA-mismatched related | 7(8.8) | 0 | ||
| unrelated | 33(41.8) | 20(41.7) | ||
| TBI regimen, no. (%) | 36(45.6) | 23(47.9) | 0.85 | |
| DLI, no. (%) | 2(2.6) | 10(20.9) | 0.001 | 0.06 |
| Acute GVHD, no (%) | 9(11.4) | 1(2.1) | 0.08 | |
| Chronic GVHD, no. (%) | 18(22.8) | 7(14.6) | 0.35 | |
| Natural Log | ||||
| CD4+/CD45RA+ | ||||
| Median(range) | 5.0(−4.6–7.1) | 4.4(−4.6–6.4) | 0.004 | 0.21 |
| Table 2b. PNCRM7 response in children (upper) and adults (lower) | ||
|---|---|---|
| Children <18 yrs | ||
| Median age: 9 years | ||
| Range: 0.1–17.9 | ||
| Overall Response | 45/51 (88%) | |
| Transplant type | 23/27 (85%) | p=0.67 |
| Unmodified | 22/24 ((92%) | |
| T-cell depleted | ||
| Donor Type | p=0.40 | |
| HLA-matched Related | 22/24 (92% | |
| Unrelated | 16/20 (80%) | |
| HLA-MM related | 7/7 (100%) | |
| HLA-matched related | p=0.53 | |
| Unmodified | 14/16 (88%) | |
| T- cell depleted | 8/8 (100%) | |
| Unrelated donor | p=1.00 | |
| Unmodified | 8/10 (80%) | |
| T-cell depleted | 8/10 (80%) | |
|
Adults ≥18 yrs, Median 41 yrs Range: 18.0–64.0 |
||
| Overall response | 34/76 (44%) | |
| Transplant type | p=0.63 | |
| Unmodified | 14/29 (48%) | |
| T-cell depleted | 20/47 (43%) | |
| Donor Type | p=0.35 | |
| Related | 17/43 (40%) | |
| Unrelated | 17/33 (52%) | |
| HLA-matched related | p=0.20 | |
| Unmodified | 10/19 (53%) | |
| T-cell depleted | 7/23 (30%) | |
| Unrelated donor | p=0.46 | |
| Unmodified | 4/10 (44%) | |
| T cell depleted | 13/23 (57%) | |
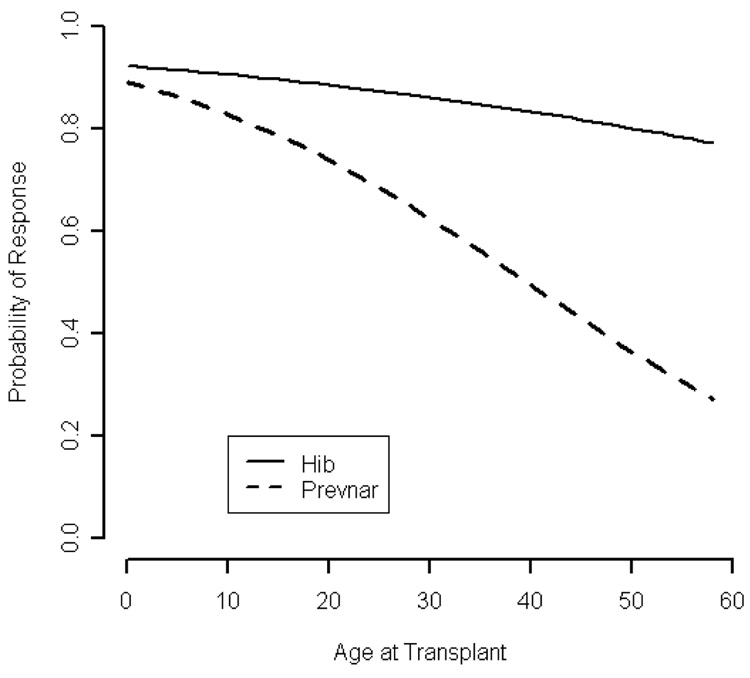
HIB response was evaluable in 115 of 127 recipients of PNCRM7 and 13 of 18 recipients of PPV23. Overall, 86% of PNCRM7 recipients responded to HIB, including 96% of children (48/50) and 79% (51/65) of adults (p=0.006). Response to the Hib conjugate vaccine was observed in 77% and 100% of patients who failed PNCRM7 or PPV23, respectively. Since age was the only prognostic factor associated with response to the HIB conjugate vaccine, a single variable logistic regression model was used to predict the probability of response based on age at transplant (Table 3). A graph depicting the probability of response to HIB and PNCRM7 based on the age at transplant is shown in Figure 1.
Figure 1.
Predicted probability of response to PNCRM7 and HIB from logistic regression model by age at transplant
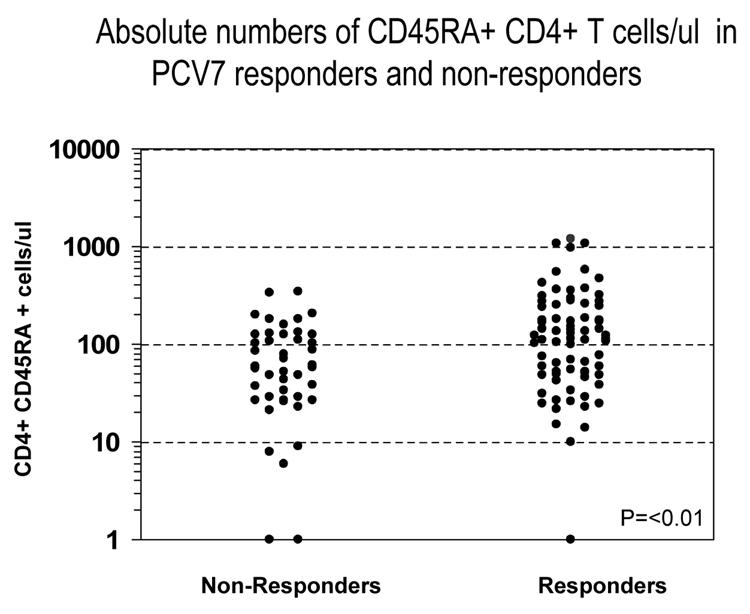
Correlation of PNCRM7 response with in vitro parameters of immune reconstitution including the PHA response and circulating numbers of CD3, CD4, CD4+CD45RA naïve T cells, CD8, and CD19 cells/ul was evaluated. In patients >50 years of age, 11 of 19 patients vaccinated after reaching minimal milestones of immune reconstitution (CD4 >200/ul, IgG >500 mg/dl, PHA within 60% lower limit of normal) responded to PNCRM7, compared with 0 of 8 vaccinated prior to reaching these milestones (p=0.006). Among patients vaccinated after achieving minimal milestones of immune reconstitution, there was no correlation between response and the absolute level of CD3 T cells, CD8+ T cells, CD4, or CD19+ B cells (data not shown). In contrast, the level of circulating CD4+CD45RA+ cells/ul, p<0.01, correlated with response (figure 2).
Figure 2.
The figure demonstrates the relationship between circulating numbers of CD4+CD45RA+ cell counts and PNCRM7 response in 127 recipients of an allogeneic HCT.
Twenty seven patients who did not respond to an initial series of PNCRM7 were subsequently vaccinated with PPV23 (n=16) or received a second series of PNCRM7 (n=11). Patients were vaccinated at a median of 270 days following their last pneumococcal vaccine. Four (25%) patients responded to PPV23 and 7 (44%) to a second series of PNCRM7 (p=0.06). Ten patients who failed PPV23 as their initial pneumococcal vaccine received a second PPV23 (n=4) or were immunized with a series of PNCRM7 (n=6). Five of six patients responded to PNCRM7 compared with 0 of 4 given a second PPV23. Response to the PPV23 specific serotypes (1, 3, 7F) was observed in <20% of patients who received PPV23 either as a primary or secondary vaccine (data not shown).
Discussion
The development of more tolerable and effective cytoreductive regimens and better supportive care has increased not only the number of surviving transplant patients returning to school and the workplace, but the age of surviving population (21, 23–30), individuals particularly vulnerable to severe invasive pneumococcal infections even in the absence of HCT (14). Although the current CDC and EBMT vaccination guidelines recommend the Hib conjugate vaccine following HCT (7,8), both recommend the use of PPV23 either as a single dose of PPV23 at 12 months (EBMT) or sequential doses at 12 months and 24 months (CDC). Nevertheless, several studies have shown a dichotomy between the responses of allogeneic transplant recipients to pure polysaccharide vaccines compared with protein-conjugated vaccines. Barra et al. demonstrated that only 4 of 20 (25%) adult allogeneic transplant recipients given the pure polysaccharide H flu vaccine developed a specific IgG response compared with 11 of 20 (55%) patients given the H flu conjugate vaccine (p<0.05) (31). Guinan et al. studied the responses of 21 allogeneic and 14 autologous HCT recipients immunized with H flu conjugate vaccine and the polysaccharide pneumococcal vaccine, Pnu-immune, (Lederle laboratory Division, American Cyanamid Co., Pearl River, NY) (9). Following the 24 month immunization, only 19% of patients developed protective titers against the 6 measured pneumococcal serotypes (1, 3, 6A, 7F, 8, 9N), while 80% responded the HIB-conjugate vaccine. Our study similarly demonstrates that the majority of patients who fail the pneumococcal polysaccharide vaccine are capable of responding to HIB. In addition, we show that 77% of patients unable to respond to PNCRM7 responded to the conjugated HIB vaccine. The greater response to the HIB conjugate versus the pneumococcal conjugate vaccine may reflect the fact that the former vaccine contains a single protein-saccharide conjugate, is inherently more immunogenic, and/or expands memory T and B cell populations persisting from prior childhood immunization or natural infection with Haemophilus influenza.
To date, this is the largest study evaluating PNCRM7 responses following allogeneic HCT and the first to evaluate PNCRM7 responses in adult recipients of an unmodified or T cell depleted unrelated HCT or adults following a T cell depleted HCT from any donor type. Although retrospective, it includes 80% of all patients transplanted during a defined period (2002–2005) who survived disease-free >1 year. Molrine et al. evaluated PNCRM7 response in 65 unmodified HLA-matched related HCT recipients (median age: 40 years) immunized with PNCRM7 at 3, 6, and 12 months, 30 of whom received grafts from donors immunized before bone marrow harvest (32). After the first immunization, antibody responses in the immunized donor group were significantly higher for 5 of the 7 vaccine serotypes. Although response after the 3rd PNCRM7 immunization was not evaluable in approximately 30% of the starting population due to relapse (n=8), death from other causes (n=5), thrombocytopenia (n=3), missed vaccines (n=2), or DLI (n=1), 60% of the remaining patients in both groups demonstrated significant pneumococcal titers to all 7 serotypes. In a prospective, randomized double blind trial in adult recipients of an HLA-matched related HCT, Kumar and colleagues compared the effect of vaccinating donor-recipient pairs with a single PPV23 or PNCRM7 (33). Donors were vaccinated at least two weeks prior to stem cell harvest; patients at 6 months post HCT. At 12 months post HCT, the mean number of serotypes for which a response was observed was 1.8 in the PPV23 group and 3.1 in the PNCRM7 group. Similar to the study of Storek et al., no advantage to pre-HCT immunization of the donor and/ or host with PPV23 was observed (34). Meisel et al. studied the response of 43 patients < 17 years of age after a related (45%) or unrelated (55%) HCT. demonstrating that 74% of 43 patients responded to all 7 vaccine serotypes following the third immunization (35). In a study of 30 children vaccinated according to the Royal College of Paediatrics and Child Health guidelines, Patel and colleagues demonstrated that 80% responded to each of the serotypes contained in PNCRM7 when given two monthly doses initiated at 15 months or 21 months following an HLA-matched sibling (n=10) or unrelated HCT (n=20), respectively (36, 37). Considering the poor response of transplant recipients to PPV23 (9–11, 31, 33, 34) and its failure to reduce the incidence of pneumococcal pneumonia even in healthy middle-aged and elderly adults (38), this and the above studies (33,35) support further trials of PNCRM7 in adults and children following HCT.
The EBMT (4) and CDC guidelines (2) advocate revaccination at fixed intervals following transplant, irrespective of patient age, donor or hematopoietic cell type, intensity of conditioning, presence of chronic graft versus host disease, or use of post transplant rituximab. Nevertheless, many of these variables affect the kinetics of T and B cell reconstitution following HCT (19, 39), and likely the ability of patients to respond and maintain protective titers following vaccination (40). Only the Royal College of Paediatrics and Child Health guidelines stratify the timing of vaccination on the basis of donor type (autologous and HLA matched sibling HCT versus all other donors) due to the delayed recovery of immune competence often associated with receipt of an unrelated or HLA mis-matched donor transplant (19, 39). Our study demonstrates that in older patients response to PNCRM7 is significantly better in those vaccinated after acquisition of minimal milestones of immune reconstitution, milestones reached at different time points post HCT, depending on age and presence of chronic GVHD (19, 39). It also shows that even within a population of patients who have achieved minimal milestones of immune reconstitution, not all patients respond suggesting that qualitative and/or quantitative differences in effector and memory helper T and B cells are likely to differentiate responders and non-responders. It is possible that certain vaccines such as tetanus and polio may elicit responses in the majority of patients early post HCT due to expansion of memory T and B cells transferred with the graft. In contrast, vaccines such as recombinant Hepatitis B (41) and pneumococcus may constitute neoantigens requiring generation of T and B cells from donor lymphoid progenitors developing within the host thymus and other tissues of the lymphoid system. Our study demonstrated a relationship between higher numbers of circulating CD4+CD45RA+ T cell numbers and improved PNCRM7 response suggesting that responders had achieved a significant level of reconstitution from precursor populations developing within the host. The correlation; however, was not absolute. Thus, impaired reconstitution of B cell precursors or antigen presenting cells may persist in some patients despite de novo T cell reconstitution.
Although invasive pneumococcal infections occur more frequently in patients with chronic GVHD, deaths from these infections occur in patients with and without chronic GVHD (1–5) as well as in autologous transplant recipients (5). This study, as well as the majority of studies evaluating PNCRM7 response to date, have not included large numbers of patients with chronic GHVD, either by design or early death from chronic GVHD in enrolled patients (32, 33,35, 36). Clearly, there is a need to design multi-center prospective trials which do include patients with limited and potentially extensive chronic GVHD in order to identify the most effective vaccination strategy to elicit protection against pneumococcal disease in the highest proportion of patients at the earliest time possible. Inclusion in such trials of correlative studies analyzing in vitro immune reconstitution should identify immune characteristics most predictive of early and durable immune responses.
Prior to the introduction of the conjugated pneumococcal vaccine, 3,000 cases of meningitis, 50,000 cases of bacteremia, 500,000 cases of pneumonia, and 7 million cases of otitis media due to pneumococcus occurred annually in this country, causing 40,000 deaths per year (reviewed in 7). Following widespread vaccination of infants with PNCRM7, rates of invasive pneumococcal disease due to penicillin resistant strains decreased from 6.3 cases in 1999 to 2.7 cases/100,000 in 2004 (42). The reduction in pneumococcal infections in children vaccinated with PNCRM7 (13) and the seroconversion rates noted in this and other studies, suggest that the impact of PNCRM7 should be prospectively studied in a large multi-center trial. To improve responses in older patients, strategies such as a primary series of 4 doses of PNCRM7, the use of B and T cell immunomodulatory agents, such as interleukin-7 (43) and/or the addition of an adjuvant as incorporated in the recombinant Hepatitis B vaccine, Fendrix, should be explored (44). To provide even more comprehensive coverage in this at-risk population, future studies assessing the PCV13 vaccine, currently being tested in healthy adults and children (45) should be undertaken.
Acknowledgment
We wish to thank the invaluable nursing support of Joanne Taylor, Ruth Ford, Anne Casson, Joanne Torok-Castanza, Bernadette Cuelo, Catherine Copeland, and Heidi Abendroth without whom this study could not have been completed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kulkarni S, Powles R, Treleaven J, et al. Chronic graft versus host disease is associated with long-term risk for pneumococcal infections in recipients of bone marrow transplants. Blood. 2000;95:3683–3686. [PubMed] [Google Scholar]
- 2.Engelhard D, Cordonnier C, Shaw PJ, et al. Early and late invasive pneumococcal infection following stem cell transplantation: a European Bone Marrow Transplantation survey. Br J Haematol. 2002;117:444–450. doi: 10.1046/j.1365-2141.2002.03457.x. [DOI] [PubMed] [Google Scholar]
- 3.Socie G, Salooja N, Cohen A, et al. Non-malignant late effects after allogeneic stem cell transplantation. Blood. 2003;101:3373–3385. doi: 10.1182/blood-2002-07-2231. [DOI] [PubMed] [Google Scholar]
- 4.Chen CS, Boeckh M, Seidel K, et al. Incidence, risk factors, and mortality from pneumonia developing late after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2003;32:515–522. doi: 10.1038/sj.bmt.1704162. [DOI] [PubMed] [Google Scholar]
- 5.Youssef S, Rodriguez G, Rolston KV, et al. Streptococcus pneumoniae infections in 47 hematopoietic stem cell transplantation recipients: clinical characteristics of infections and vaccine-breakthrough infections, 1989–2005. Medicine. 2007;86:69–77. doi: 10.1097/md.0b013e31803eb176. [DOI] [PubMed] [Google Scholar]
- 6.Kumar D, Humar A, Plevneshi A, et al. Invasive pneumococcal disease in adult hematopoietic stem cell transplant recipients: a decade of prospective population-based surveillance. Bone Marrow Transplant. 2008 doi: 10.1038/sj.bmt.1705964. e pub. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Guidelines for Preventing Opportunistic Infections Among Hematopoietic Stem Cell Transplant Recipients: Recommendations of CDC, the Infectious Disease Society of America, and the American Society of Blood and Marrow Transplantation. MMWR. 2000;49:1–128. doi: 10.1016/S1083-8791(00)70002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ljungman P, Engelhard D, de la Cámara R, et al. Vaccination of stem cell transplant recipients: recommendations of the Infectious Diseases Working Party of the EBMT. Bone Marrow Transplant. 2005;35:737–746. doi: 10.1038/sj.bmt.1704870. [DOI] [PubMed] [Google Scholar]
- 9.Guinan EC, Molrine DC, Antin JH, et al. Polysaccharide conjugate vaccine responses in bone marrow transplant patients. Transplantation. 1994;57:677–684. doi: 10.1097/00007890-199403150-00009. [DOI] [PubMed] [Google Scholar]
- 10.Parkkali T, Kayhty H, Ruutu T, et al. A comparison of early and late vaccination with Haemophilus influenzae type b conjugate and pneumococcal polysaccharide vaccines after allogeneic BMT. Bone Marrow Transplant. 1996;18:961–967. [PubMed] [Google Scholar]
- 11.Pao MK, Papadopoulos EB, Kernan NA, et al. Immunogenicity of haemophilus influenza and pneumococcal vaccines in related and unrelated transplant recipients. Blood. 2006;108:178a. [Google Scholar]
- 12.Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J. 2000;10:187–195. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Hsu K, Pelton S, Karumuri S, Heisey-Grove D, Klein J. Population-based surveillance for childhood invasive pneumococcal disease in the era of conjugate vaccine. Pediatr Infect Dis J. 2005;24:17–23. doi: 10.1097/01.inf.0000148891.32134.36. [DOI] [PubMed] [Google Scholar]
- 14.Prevention of Pneumococcal Disease. Recommendations of the Advisory Committee on on Immunization Practices (ACIP) MMWR. 1997;46(RR-8):1–24. [PubMed] [Google Scholar]
- 15.Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Eng J Med. 2003;348:1737–1746. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 16.Small TN, Keever CA, Weiner-Fedus S, et al. B cell differentiation following autologous, conventional, or T-cell depleted bone marrow transplantation: A recapitulation of normal B cell ontogeny. Blood. 1990;76:1647–1656. [PubMed] [Google Scholar]
- 17.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR severity index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855–864. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan KM, Shulman HM, Storb R, et al. Chronic graft-versus-host disease in 52 patients: Adverse natural course and successful treatment with combination immunosuppression. Blood. 1981;57:267–276. [PubMed] [Google Scholar]
- 19.Small TN, Papadopoulos EB, Boulad F, et al. Comparison of immune reconstitution following unrelated and related T cell depleted bone marrow transplantation: Effect of patient age and donor leukocyte infusions. Blood. 1999;93:467–480. [PubMed] [Google Scholar]
- 20.Reisner Y, Kapoor N, Kirkpatrick D, et al. Transplantation for severe combined immunodeficiency disease with HLA-A,B,D,DR incompatible parental marrow cells fractionated by soybean agglutination and sheep red blood cells. Blood. 1983;61:341–348. [PubMed] [Google Scholar]
- 21.Jakubowski AA, Small TN, Young JW, et al. T cell–depleted stem-cell transplantation for adults with hematologic malignancies: sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007;110:4552–4559. doi: 10.1182/blood-2007-06-093880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosmer DW, Lemeshow S. A goodness-of-fit test for the multiple logistic regression model. Communications in Statistics. 1980;A10:1043–1069. [Google Scholar]
- 23.Papdopoulos EB, Carabasi MH, Castro-Malaspina H, et al. T-cell-depleted allogeneic bone marrow transplantation as postremission therapy for acute myelogenous leukemia: Freedom from relapse in the absence of graft-versus-host disease. Blood. 1998;91:1083–1090. [PubMed] [Google Scholar]
- 24.Weisdorf DJ, Anasetti C, Antin JH, et al. Allogeneic bone marrow transplantation for chronic myelogenous leukemia: comparative analysis of unrelated versus matched sibling donor transplantation. Blood. 2002;99:1971–1977. doi: 10.1182/blood.v99.6.1971. [DOI] [PubMed] [Google Scholar]
- 25.Alyea EP, Weller E, Fisher DC, et al. Comparable outcome with T-cell-depleted unrelated-donor versus related-donor allogeneic bone marrow transplantation. Biol Blood Marrow Transplant. 2002;8:601–607. doi: 10.1053/bbmt.2002.v8.abbmt080601. [DOI] [PubMed] [Google Scholar]
- 26.Moore J, Nivison-Smith I, Goh K, et al. Equivalent survival for sibling and unrelated donor allogeneic stem cell transplantation for acute myelogenous leukemia. Biol Blood Marrow Transplant. 2007;13:601–607. doi: 10.1016/j.bbmt.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 27.Weisser M, Schleuning M, Haferlach C, Schwerdtfeger R, Kolb HJ. Allogeneic stem-cell transplantation provides excellent results in advanced stage chronic myeloid leukemia with major cytogenetic response to pre-transplant Imatinib therapy. Leuk Lymphoma. 2007;48:295–301. doi: 10.1080/10428190601078464. [DOI] [PubMed] [Google Scholar]
- 28.Oran B, Giralt S, Saliba R, et al. Allogeneic hematopoietic stem cell transplantation for the treatment of high-risk acute myelogenous leukemia and myelodysplastic syndrome using reduced-intensity conditioning with fludarabine and melphalan. Biol Blood Marrow Transplant. 2007;13:454–462. doi: 10.1016/j.bbmt.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giles FJ, Borthakur B, Ravandi F, et al. The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. Br J Haematol. 2007;136:624–627. doi: 10.1111/j.1365-2141.2006.06476.x. [DOI] [PubMed] [Google Scholar]
- 30.Escalón MP, Champlin RE, Saliba RM, et al. Nonmyeloablative allogeneic hematopoietic transplantation: a promising salvage therapy for patients with non-Hodgkin's lymphoma whose disease has failed a prior autologous transplantation. J Clin Onc. 2004;22:2419–2423. doi: 10.1200/JCO.2004.09.092. [DOI] [PubMed] [Google Scholar]
- 31.Barra A, Cordonnier C, Preziosi M-P, et al. Immunogenicity of Haemophilus Influenza Type b Conjugate Vaccine in Allogeneic Bone Marrow Recipients. J Infect Dis. 1992;166:1021–1028. doi: 10.1093/infdis/166.5.1021. [DOI] [PubMed] [Google Scholar]
- 32.Molrine DC, Antin JH, Guinan EC, et al. Donor immunization with pneumococcal conjugate vaccine and early protective antibody responses following allogeneic hematopoietic cell transplantation. Blood. 2003;101:831–836. doi: 10.1182/blood-2002-03-0832. [DOI] [PubMed] [Google Scholar]
- 33.Kumar D, Chen MH, Welsh B, et al. A randomized, double-blind trial of pneumococcal vaccination in adult allogeneic stem cell transplant donors and recipients. CID. 2007;45:1576–1582. doi: 10.1086/523583. [DOI] [PubMed] [Google Scholar]
- 34.Storek J, Dawson MA, Lim LC. Efficacy of donor vaccination before hematopoietic cell transplantation and recipient vaccination both before and early after transplantation. Bone Marrow Transpl. 2004;33:337–346. doi: 10.1038/sj.bmt.1704336. [DOI] [PubMed] [Google Scholar]
- 35.Meisel R, Kuypers L, Dirksen U, et al. Pneumococcal conjugate vaccine provides early protective antibody responses in children after related and unrelated allogeneic hematopoietic stem cell Transplantation. Blood. 2007;109:2322–2326. doi: 10.1182/blood-2006-06-032284. [DOI] [PubMed] [Google Scholar]
- 36.Patel SR, Ortín M, Cohen BJ, et al. Revaccination with measles, tetanus, poliovirus, Haemophilus influenzae type B, meningococcus C, and pneumococcus vaccines in children after hematopoietic stem cell transplantation. Clin Infect Dis. 2007;44:625–634. doi: 10.1086/511641. [DOI] [PubMed] [Google Scholar]
- 37.Royal College of Paediatrics and Child Health (RCPCH) Immunisation of the immunocompromised child: best practice statement. RCPCH. 2002:10–13. [Google Scholar]
- 38.Örtqvist A, Hedlund J, Burman L-A, et al. Randomised trial of 23-valent pneumococcal capsular polysaccharide vaccine in prevention of pneumonia in middle-aged and elderly people. Lancet. 1998;351:399–403. doi: 10.1016/s0140-6736(97)07358-3. [DOI] [PubMed] [Google Scholar]
- 39.Storek J, Joseph A, Dawson MA, Douek DC, Storer B, Maloney DG. Factors influencing T-lymphopoiesis after allogeneic hematopoietic cell transplantation. Transplantation. 2002;73:1154–1158. doi: 10.1097/00007890-200204150-00026. [DOI] [PubMed] [Google Scholar]
- 40.Avanzini M, Locatelli F, Santos C, et al. B lymphocyte reconstitution after hematopoietic stem cell transplantation: functional immaturity and slow recovery of memory CD27 B cells. Experimental Hematology. 2005;33:480–486. doi: 10.1016/j.exphem.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Jaffe D, Papadopoulos EB, Young JW, et al. Immunogenicity of recombinant hepatitis B vaccine (rHBV) in recipients of unrelated or related allogeneic hematopoietic cell transplantation (HCT) Blood. 2006;108:2470–2475. doi: 10.1182/blood-2006-04-006981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kyaw MH, Lynfield R, Schaffner W. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Eng J Med. 2006;354:1455–1463. doi: 10.1056/NEJMoa051642. [DOI] [PubMed] [Google Scholar]
- 43.Alpdogan O, van den Brink MR. IL-7 and IL-15: therapeutic cytokines for immunodeficiency. Trends Immunol. 2005;26:56–64. doi: 10.1016/j.it.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 44.Kundi M. New hepatitis B vaccine formulated with an improved adjuvant system. Expert Rev Vaccines. 2007;6:133–140. doi: 10.1586/14760584.6.2.133. [DOI] [PubMed] [Google Scholar]
- 45.Scott DA, Komjathy SF, Hu BT, et al. Phase 1 trial of a 13-valent pneumococcal conjugate vaccine in healthy adults. Vaccine. 2007;25:6164–6166. doi: 10.1016/j.vaccine.2007.06.004. [DOI] [PubMed] [Google Scholar]