Abstract
Background
We examined the association between substance use (SU) disorder and mortality among HIV-infected patients in a large, private medical care program.
Methods
In a retrospective cohort design, HIV-infected patients (≥14 years old) from a large health plan (Northern California) were studied to examine mortality associated with diagnosis of SU dependence or abuse over an 11-year period.
Results
At study entry or during follow-up, 2,279 (25%) of 9,178 HIV-infected patients had received a diagnosis of SU disorder. Diagnoses were categorized as alcohol dependence/abuse only, illicit drugs only, or both. Cause of death differed by the category of SU diagnosis. Mortality rates ranged from 35.5 deaths per 1,000 person-years in patients with an SU disorder to 17.5 deaths among patients without an SU disorder. Regression results indicated mortality risk was significantly higher in all categories of SU disorder compared to no SU diagnosis (hazard ratios ranging from 1.65 to 1.67) after adjustment for SU treatment and confounders.
Conclusions
A diagnosis of SU dependence/abuse is associated with higher mortality among HIV-infected patients for whom access to medical services is not a significant factor.
Keywords: HIV, Mortality, Substance Dependence and Abuse
Trends in survival after a diagnosis of human immunodeficiency virus-1 (HIV-1) infection have been well documented. While the introduction of combination antiretroviral (ARV) therapy (Detels et al., 1998; Hogg et al., 1999; Murphy et al., 2001) has resulted in a substantial decrease in mortality for the HIV-infected population, vulnerable subgroups have different survival patterns (Fonseca et al., 2007; Nash et al., 2005; Sackoff et al., 2006; Woldemichael et al., 2009) and have experienced a smaller decline in death rates (Lucas et al., 2006). Vulnerable patients include those with illicit drug or alcohol use problems, highly prevalent among patients treated for HIV/AIDS (Conigliaro et al., 2004; Lucas et al., 2002). Several mortality studies have focused on survival trends among HIV-infected injection drug users (IDUs) (Estrada, 2005; Garcia de la Hera et al., 2004; Goedert et al., 2001; Kohli et al., 2005; Mocroft et al., 2004; Pezzotti et al., 1999; Poundstone et al., 2001; Wood et al., 2008) or hard drug users (Thorpe et al., 2004), who may have been homeless (Pezzotti et al., 1999) or receiving publicly funded medical care services (Muga et al., 2007; Pezzotti et al., 1999). Few studies (Lucas et al., 2006; Walley et al., 2008) have examined survival patterns for HIV-infected individuals who use alcohol or illicit drugs, but are generally not IDUs, and have been diagnosed with substance use dependence or abuse from a private health plan. With the advent of health insurance parity for substance use (SU) dependence and abuse and given the larger context of health reform, it is important to investigate survival patterns of HIV-infected individuals within a health plan with characteristics similar to those plans that may result from health reform.
The current study examines survival patterns in a large cohort of HIV-infected patients who are members of a privately insured, integrated health care system. The study compares mortality in patients diagnosed with SU dependence or abuse to that in patients without SU diagnoses.
METHODS
Study Population
We conducted a retrospective observational cohort study for years 1996 to 2005 among HIV-infected patients who were members of the Kaiser Permanente Northern California (KPNC) health plan. The KPNC is an integrated health care system with a membership of 3.5 million members, representing 34% of the insured population in Northern California. HIV-infected patients are seen at medical centers throughout the KPNC 17-county catchment region. The membership is representative of the Northern California population with respect to race/ethnicity, gender, and socioeconomic status, except for some under-representation of both extremes of the economic spectrum (Krieger, 1992).
The base study population consisted of 10,199 HIV-infected patients who received health care at KPNC at some time between 1996 and 2005. The study sample included all HIV-infected patients who were 14 years of age or older (n = 10,169) on or after January 1, 1996, and had at least 6 months of membership during the first year of study observation (n = 9,245). Patients could enter the study until December 31, 2005. This resulted in a study sample of 9,245 patients.
Data Sources
Since 1988, the KPNC Division of Research (DOR) has maintained a surveillance system of patients who are HIV-1 seropositive, ascertained through monitoring electronic inpatient, outpatient, laboratory testing, and pharmacy dispensing databases for sentinel indicators of probable HIV infection. HIV-1 seropositivity then is confirmed through review of patient medical records. Ascertainment of HIV-infected patients by the KPNC HIV registry has been shown to be at least 95% complete. The HIV registry contains information on patient demographics (e.g., sex, birth date, race/ethnicity), HIV transmission risk group (men who have sex with men, injection drug use, heterosexual sex, other, unknown), dates of known HIV infection, and AIDS diagnoses. KPNC also maintains complete and historical electronic databases of patient demographics, hospital admission/discharge/transfer data, prescription dispensing, outpatient visits, and laboratory tests results, including CD4 T-cell counts and HIV-1 RNA levels. Chronic hepatitis B and C infections were ascertained from laboratory testing results and by linkage to the KPNC Viral Hepatitis Registry. Chronic hepatitis cases were defined by either repeated positive HBV surface antigen (e.g., HBsAg) test results or by detectable virus through DNA testing. All treatment information on ARV medications was obtained from the pharmacy dispensing database. Mortality information including date and cause of death is obtained from hospitalization records, membership files, California death certificates, and Social Security Administration databases.
Substance Use Dependence or Abuse Diagnosis and Treatment
A diagnosis of ICD-9 SU dependence or abuse can be made by the patient’s clinician in primary care, substance use treatment, or psychiatry as a primary or secondary diagnosis (Mertens et al., 2005; Ray et al., 2007). Diagnostic categories include all alcoholic psychoses, drug psychoses, alcohol dependence syndrome, drug dependence (including opioid, barbiturate, sedative/tranquilizer, cocaine, cannabis, amphetamine, and hallucinogen dependence, but excluding tobacco dependence), alcohol abuse, cannabis abuse, hallucinogen abuse, barbiturate abuse, sedative/tranquilizer abuse, opioid abuse, cocaine abuse, and amphetamine abuse.
KPNC provides comprehensive outpatient SU services available to all members of the health plan. Services include both day hospital and traditional outpatient programs (Weisner et al., 2001), both of which include 8 weeks of individual group therapy, education, relapse prevention, family therapy, with aftercare visits once a week for 10 months. Random drug testing is conducted during treatment in both day hospital and outpatient settings. Clinical staffs include licensed clinicians (social workers and psychologists), certified alcohol and drug counselors, and medical staff (psychiatrists, internists, and nurses). In addition to these primary services, ambulatory (and inpatient) detoxification and residential services are available, as needed. A small proportion of patients engage in residential SU treatment, conducted by contractual agreement with outside institutions. These data are available in the KPNC referrals and claims databases.
Study Outcome
The primary outcome examined in this study was all-cause mortality, with cohort follow-up through December 31, 2006.
Statistical Methods
The study analysis focused on diagnosis of SU dependence or abuse as the primary predictor of interest. Patients with an SU diagnosis were further classified as either alcohol dependence or abuse only, or drug use dependence or abuse only, or both. The distribution of demographic, clinical, and behavioral characteristics was compared between patients with and without an SU diagnosis using Pearson’s chi-square statistic. The distribution of cause of death was examined by the type of SU diagnosis (alcohol dependence or abuse only, illicit drug use dependence or abuse only, both alcohol and illicit drug use diagnoses, and no SU diagnosis). Age-adjusted all-cause mortality rates and associated 95% confidence intervals (CI) were calculated for each level of SU diagnosis using the direct method with the 2000 U.S census as the standard population (Breslow and Day, 1987). Mortality hazard ratios (HR) were estimated for each level of SU diagnosis using Cox proportional hazards regression, with the occurrence of first SU diagnosis treated as a time-dependent covariate. Model selection included covariates with univariate tests of association that were significant at p < 0.25 as well as variables of known clinical importance including SU treatment (yes vs. no). Potential confounders included age at entry into study, race/ethnicity, gender, HIV transmission risk group, CD4 T-cell counts and HIV RNA levels and ARV treatment (naïve or experienced) modeled as time-dependent covariates, years of known HIV infection, AIDS diagnosis prior to entry into study, and chronic hepatitis B and C viral infections modeled as time-dependent covariates. All data analyses were conducted using SAS software, version 9.1 (SAS, Inc., Cary, NC).
RESULTS
The initial study sample included 9,245 HIV-infected patients. We excluded 67 patients who had visited a SU treatment clinic but who lacked a SU diagnosis, yielding a final study sample of 9,178 patients (patients are sometimes referred for assessment and do not receive diagnoses). At study entry or during study follow-up, 2,279 (25%) of the 9,178 HIV-infected patients were diagnosed with SU problems. Among these 2,279 patients, 23% were diagnosed with alcohol problems only, 41% with illicit drug problems only, and 36% with both alcohol and illicit drug problems. The most prevalent, individual SU diagnoses were multiple drugs’ dependence/abuse (35%) and alcohol alone (33%). Less prevalent diagnoses were cannabis (8%), amphetamines (8%), cocaine (4%), opioids (2%), and other drugs (sedatives, hallucinogens, alcohol/drug psychoses) (10%). Approximately 47% of the 2,279 patients had received a single substance dependence/abuse diagnosis.
Patients with a SU diagnosis had distributions for gender, ARV therapy experience, AIDS diagnosis, and time from known HIV infection to baseline, which were similar to patients with no SU diagnosis (Table 1). Statistically significant testing results indicated that SU-diagnosed patients were somewhat more likely to: be younger at baseline, be white, be IDU or both IDUs and having sex with men, have higher (≥103.3) HIV RNA levels at baseline, and be infected with hepatitis B or C virus, when compared to patients without a SU diagnosis. Although the test result was statistically significant, the categories of CD4 T-lymphocyte cell counts measured at baseline were still similar in distribution in both SU-diagnosed patients and those without a SU diagnosis. Finding significant results for small differences in distributions is likely the consequence of having a very large sample size in this study. The majority of patients were 30 to 49 years of age at entry into the study, and men represented 90% of patients in both groups. Of the 2,279 patients with a SU diagnosis, 35% received SU treatment.
Table 1.
Distribution of Study Characteristics by Diagnosis of Substance Use (SU) Dependence or Abuse Among KPNC HIV-Infected Patients, 1996 to 2005
| Characteristic | SU dependence/abuse diagnosis
|
χ2 | p-Value | |||
|---|---|---|---|---|---|---|
| No
|
Yes
|
|||||
| N | % | N | % | |||
| Age | ||||||
| <30 years | 803 | 11.6 | 235 | 10.3 | 26.2 | <0.001 |
| 30 to 39 | 2,807 | 40.7 | 1,023 | 44.9 | ||
| 40 to 49 | 2,226 | 32.3 | 741 | 32.5 | ||
| 50 to 59 | 845 | 12.2 | 240 | 10.5 | ||
| 60+ | 218 | 3.2 | 40 | 1.8 | ||
| Race/ethnicity | ||||||
| White | 4,046 | 58.6 | 1,447 | 63.5 | 46.7 | <0.001 |
| Black | 1,176 | 17.0 | 425 | 18.6 | ||
| Hispanic | 920 | 13.3 | 248 | 10.9 | ||
| Other | 362 | 5.2 | 87 | 3.8 | ||
| Unknown | 395 | 5.7 | 72 | 3.2 | ||
| Gender | ||||||
| Male | 6,219 | 90.1 | 2,070 | 90.8 | 0.9 | 0.33 |
| Female | 680 | 9.9 | 209 | 9.2 | ||
| Risk group | ||||||
| Heterosexual contact | 952 | 13.8 | 272 | 11.9 | 397.4 | <0.001 |
| Men who have sex with men (MSM) | 4,617 | 66.9 | 1,348 | 59.1 | ||
| Injection drug use (IDU) | 154 | 2.2 | 216 | 9.5 | ||
| MSM and IDU | 178 | 2.6 | 189 | 8.3 | ||
| Coagulation disorder/transfusion | 113 | 1.6 | 26 | 1.1 | ||
| Unknown | 885 | 12.8 | 228 | 10.0 | ||
| CD4 cell count at baseline | ||||||
| ≥500 | 1,298 | 18.8 | 460 | 20.2 | 18.0 | <0.001 |
| 350 to 499 | 1,235 | 17.9 | 439 | 19.3 | ||
| 201 to 349 | 1,319 | 19.1 | 390 | 17.1 | ||
| 100 to 200 | 879 | 12.7 | 309 | 13.6 | ||
| <100 | 935 | 13.6 | 338 | 14.8 | ||
| Unknown | 1,233 | 17.9 | 343 | 15.1 | ||
| HIV RNA level at baseline | ||||||
| <102.7 | 1,584 | 23.0 | 456 | 20.0 | 21.6 | <0.001 |
| 102.7 to <103.3 | 469 | 6.8 | 147 | 6.5 | ||
| 103.3 to <104.7 | 1,965 | 28.5 | 717 | 31.5 | ||
| ≥104.7 | 1,371 | 19.9 | 511 | 22.4 | ||
| Unknown | 1,510 | 21.9 | 448 | 19.7 | ||
| Antiretroviral therapy experience at baseline | ||||||
| Never | 2,421 | 35.1 | 832 | 36.5 | 1.5 | 0.22 |
| Ever | 4,478 | 64.9 | 1,447 | 63.5 | ||
| AIDS diagnosis at baseline | ||||||
| No | 4,988 | 72.3 | 1,684 | 73.9 | 2.2 | 0.14 |
| Yes | 1,911 | 27.7 | 595 | 26.1 | ||
| Hepatitis B viral infection | ||||||
| No | 6,646 | 96.3 | 2,154 | 94.5 | 14.3 | <0.001 |
| Yes | 253 | 3.7 | 125 | 5.5 | ||
| Hepatitis C viral infection | ||||||
| No | 6,469 | 93.8 | 1,952 | 85.7 | 149.1 | <0.001 |
| Yes | 430 | 6.2 | 327 | 14.3 | ||
| Time from known HIV-infection to baseline | ||||||
| <1 year | 2,667 | 38.7 | 919 | 40.3 | 2.1 | 0.35 |
| 1 to 5 years | 2,010 | 29.1 | 653 | 28.7 | ||
| 6+ years | 2,222 | 32.2 | 707 | 31.0 | ||
| SU treatment | ||||||
| No | 6,899 | 100.0 | 1,474 | 64.7 | 2,671.2 | <0.001 |
| Yes | 0 | 0.0 | 805 | 35.3 | ||
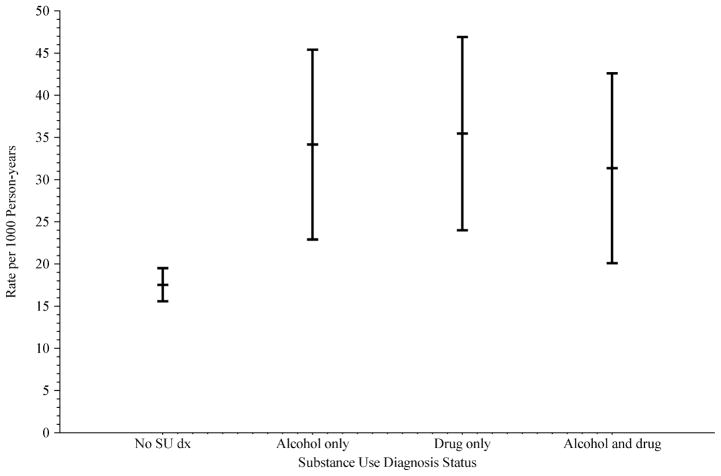
Table 2 describes the distribution of cause of death by categories of SU diagnosis. The majority of deaths in each type of SU diagnosis category were attributed to HIV/AIDS. However, deaths in patients with a diagnosis of alcohol use dependence or abuse only were less likely to have HIV/AIDS as a cause of death when compared to patients diagnosed with illicit drug use dependence or abuse only, both alcohol and illicit drug use diagnoses, as well as those without a SU diagnosis ( , p = 0.04). Other causes of death such as cancer ( , p = 0.21) and cardiovascular ( , p = 0.17) disease were not differentially distributed across categories of SU diagnosis. Examining deaths from all causes, 620 deaths occurred among patients without an SU problem diagnosis, yielding an age-adjusted death rate of 17.5 per 1,000 person-years (Fig. 1). In comparison, patients diagnosed with alcohol use dependence or abuse had an age-adjusted death rate of 34.1 per 1,000 person-years; age-adjusted rates of similar magnitude were observed among patients diagnosed with illicit drug use dependence or abuse only, and among patients diagnosed with both alcohol and illicit drug use dependence or abuse. The age-adjusted death rate 95% CI for the 3 categories of SU dependence or abuse diagnoses does not overlap with the CIs for patients without a SU diagnosis.
Table 2.
Cause of Death by Type of Substance Use (SU) Dependence/Abuse Diagnosis Among KPNC HIV-Infected Patients, 1996 to 2006
| Cause of death | SU dependence/abuse diagnosis
|
|||||||
|---|---|---|---|---|---|---|---|---|
| No SU Dx
|
Alcohol Dx only
|
Illicit drug Dx only
|
Alcohol and illicit drug Dx
|
|||||
| N | % | N | % | N | % | N | % | |
| HIV/AIDS | 389 | 62.7 | 38 | 46.3 | 65 | 59.1 | 58 | 61.7 |
| Cancer | 71 | 11.5 | 14 | 17.1 | 8 | 7.3 | 10 | 10.6 |
| Cardiovascular | 39 | 6.3 | 10 | 12.2 | 10 | 9.1 | 5 | 5.3 |
| Liver disease | 4 | 0.6 | 6 | 7.3 | 2 | 1.8 | 4 | 4.3 |
| Bacterial, viral, parasitic infection | 17 | 2.7 | 4 | 4.9 | 3 | 2.7 | 3 | 3.2 |
| Blood (anemia, hemophilia) disease | 6 | 1.0 | 0 | 0 | 1 | 0.9 | 0 | 0 |
| Digestive disease | 7 | 1.1 | 0 | 0 | 3 | 2.7 | 2 | 2.1 |
| Metabolic disorder | 6 | 1.0 | 2 | 2.4 | 1 | 0.9 | 0 | 0 |
| Respiratory disease | 10 | 1.6 | 0 | 0 | 5 | 4.5 | 1 | 1.1 |
| Accident, violence | 7 | 1.1 | 2 | 2.4 | 3 | 2.7 | 2 | 2.1 |
| Suicide | 13 | 2.1 | 1 | 1.2 | 3 | 2.7 | 3 | 3.2 |
| Other | 5 | 0.8 | 0 | 0 | 0 | 0 | 3 | 3.2 |
| Unknown | 46 | 7.4 | 5 | 6.1 | 6 | 5.5 | 3 | 3.2 |
| All causes | 620 | 100.0 | 82 | 100.0 | 110 | 100.0 | 94 | 100.0 |
Dx, diagnosis.
Fig. 1.
Age-adjusted mortality rates and 95% confidence intervals by substance use diagnosis status among KPNC HIV-infected patients, 1996 to 2006.
Mortality HR comparing patients with a SU diagnosis to patients with no SU diagnosis was estimated using Cox proportional hazards regression (Table 3). Patients with a diagnosis in any of the 3 categories of alcohol only, drug only, and combined alcohol drug dependence or abuse had a significantly elevated risk of dying when compared to patients without a SU diagnosis, with unadjusted HR ranging from 2.02 to 2.21 (model 1). The HRs for each category of SU dependence or abuse diagnoses were somewhat increased after adjustment for substance dependence/abuse treatment (model 2); however, the 95% CIs of those HRs were in the same range as those estimated for model 1. Further adjustment for demographic and clinical confounders somewhat diminished the magnitude of the HR for each SU disorder diagnosis in comparison with no SU diagnosis; however, these estimates remained statistically significant (model 3). While most of the potential confounders included in model 3 were associated with time to death, subanalyses revealed that mostly the CD4 cell counts at baseline were responsible for the attenuation of the HRs for patients with SU dependence or abuse diagnoses. Additional analyses did not reveal any evidence of SU diagnosis effect modification by other model covariates.
Table 3.
Mortality Hazard Ratios (HR) for SU Diagnosis Status via Cox Proportional Hazards Regression, Adjusted for Potential Confounders, Among KPNC HIV-Infected Patients, 1996 to 2006
| Characteristic | Model 1
|
Model 2
|
Model 3
|
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| SU Dx | |||
| None | 1.00 | 1.00 | 1.00 |
| Alcohol only | 2.21 (1.75, 2.78) | 2.29 (1.81, 2.89) | 1.65 (1.30, 2.09) |
| Illicit drug only | 2.10 (1.71, 2.57) | 2.21 (1.79, 2.72) | 1.67 (1.34, 2.09) |
| Alcohol and illicit drug | 2.02 (1.62, 2.51) | 2.31 (1.79, 2.98) | 1.67 (1.28, 2.19) |
| SU treatment | |||
| No | 1.00 | 1.00 | |
| Yes | 0.77 (0.58, 1.02) | 1.06 (0.79, 1.41) | |
| Age | |||
| <30 years | 1.00 | ||
| 30 to 39 | 1.28 (0.91, 1.82) | ||
| 40 to 49 | 1.94 (1.37, 2.75) | ||
| 50 to 59 | 2.92 (2.02, 4.21) | ||
| ≥60 | 6.25 (4.18, 9.36) | ||
| Race/ethnicity | |||
| White | 1.00 | ||
| Black | 0.80 (0.67, 0.95) | ||
| Hispanic | 0.72 (0.57, 0.91) | ||
| Other | 1.05 (0.76, 1.44) | ||
| Unknown | 0.37 (0.16, 0.82) | ||
| Gender | |||
| Male | 1.00 | ||
| Female | 0.86 (0.64, 1.14) | ||
| HIV risk transmission | |||
| Heterosexual contact | 1.00 | ||
| MSM | 0.91 (0.72, 1.16) | ||
| IDU | 0.69 (0.47, 1.00) | ||
| MSM and IDU | 0.93 (0.62, 1.37) | ||
| Coagulation disorder/transfusion | 0.96 (0.63, 1.46) | ||
| Unknown | 1.15 (0.86, 1.53) | ||
| CD4 cell count at baseline | |||
| ≥500 cells/μl | 1.00 | ||
| 350 to 499 | 1.59 (1.16, 2.19) | ||
| 201 to 349 | 2.26 (1.69, 3.03) | ||
| 100 to 200 | 5.34 (3.98, 7.17) | ||
| <100 | 22.3 (16.9, 29.6) | ||
| Unknown | 0.64 (0.27, 1.51) | ||
| HIV RNA level at baseline | |||
| <102.7 | 1.00 | ||
| 102.7 to <103.3 | 0.84 (0.61, 1.17) | ||
| 103.3 to <104.7 | 0.82 (0.67, 0.99) | ||
| ≥104.7 | 1.25 (1.03, 1.52) | ||
| Unknown | 1.65 (1.26, 2.16) | ||
| Antiretroviral therapy | |||
| Never | 1.00 | ||
| HAART | 0.56 (0.42, 0.74) | ||
| Mono- or dual therapy | 1.48 (1.13, 1.93) | ||
| Past use | 2.33 (1.81, 2.99) | ||
| AIDS diagnosis at baseline | |||
| No | 1.00 | ||
| Yes | 1.34 (1.15, 1.55) | ||
| Hepatitis B infection | |||
| No | 1.00 | ||
| Yes | 2.14 (1.72, 2.66) | ||
| Hepatitis C infection | |||
| No | 1.00 | ||
| Yes | 1.62 (1.32, 1.99) | ||
CI, confidence intervals; SU, substance use; MSM, men who have sex with men; IDU injection drug use; mono, single NRTI or NNRTI therapy; dual, 2-drug (NRTI and/or NNRTI) therapy.
DISCUSSION
During 11 years of follow-up (1996 to 2006), we observed a higher mortality risk for HIV-infected patients who were diagnosed with SU dependence or abuse in comparison with HIV-infected patients without an SU diagnosis. This elevated risk was found in all subcategories of SU dependence/abuse diagnosis, i.e., alcohol use only, illicit drug use only, and combined alcohol and illicit drug use, and did not substantively vary across these subcategories. That effect was not diminished by adjustment for SU treatment and remained statistically significant even after adjustment for confounders such as age, race, immune status, HIV viral load, history of ARV therapy use, hepatitis B or hepatitis C viral infection. Receiving SU treatment was marginally associated with a lower risk of mortality in a bivariate model that included SU diagnosis subcategories. However, SU treatment was not statistically significant in the fully adjusted Cox model. Adjustment for immune status through measurement of CD4 cell counts accounted for most of the attenuation in the SU treatment effect. These differences in mortality risk occurred in a cohort of patients who received comprehensive HIV/AIDS medical services that were administered largely by HIV/AIDS care specialists in the KPNC fully integrated health plan. It is perhaps that patients who accessed SU treatment did so after their dependence or abuse had become severe. While that may have motivated them to seek SU treatment, it may have been too late to reverse trends in outcomes, particularly because their mortality was often attributable to alcohol-related health problems rather than HIV.
By comparison, in a study of HIV-infected intermittent and persistent users of illicit drugs and alcohol in comparison to nonusers, Lucas et al. (2006) observed significantly increased rates of opportunistic infections and decreased survival time among intermittent and persistent users. HIV-infected patients from the HIV-Alcohol Longitudinal Cohort (HIV-ALC) or the HIV-Longitudinal Interrelationships of Viruses and Ethanol cohort (HIV-LIVE) who either had received a SU diagnosis or responded as heavy alcohol users demonstrated that illicit drug use (heroin and/or cocaine) was associated with increased mortality risk, while recent (previous 30 days) heavy alcohol use was not (Walley et al., 2008). The Women’s Interagency HIV Study (WIHS) did not observe any increased mortality risk associated with heavy alcohol consumption, crack, cocaine, or heroin use (French et al., 2009). HIV-infected patients receiving a diagnosis of SU dependence/abuse are more likely the most serious substance users in comparison with patients who self-report substance use (no diagnoses), which is the measurement method in most studies of disease outcomes among HIV-infected patients. This might account for a lack of consistency in findings of association between SU and HIV-related outcomes.
Distribution of causes of death varied by SU diagnosis subcategory in our study. In particular, patients diagnosed with alcohol dependence/abuse only were less likely to die of HIV/AIDS compared with patients diagnosed in other subcategories. In the aforementioned HIV-ALC/HIV-LIVE study, Walley and colleagues (2008) observed that >80% of deaths were not HIV-related in an analysis of both short-term (within 6 months following study interview) or long-term (1996 to 2005) mortality. Although the number of deaths was small, the alcohol disorder only group in our study appeared to have proportionately more deaths owing to liver disease and infection, which may have been preventable and may have been directly related to substance abuse. To address this problem, the best solution may be integrating SU treatment with HIV/AIDS medical care, which has been shown to produce beneficial results (Basu et al., 2006; Lum and Tulsky, 2006; Sullivan et al., 2006).
A limitation of our study may have been the differences in timing of the SU diagnoses. Some patients in our sample may have received their SU diagnosis in the initial phase of SU dependence or abuse, and other patients in a more advanced stage. Some substance users may have met the criteria for an SU diagnosis without receiving a diagnosis. In addition, some study subjects may have received informal SU services (e.g., Alcoholics Anonymous) or self-pay services outside of the KPNC health plan, and our study does not have information about those services. We did not control for number or type of HIV-related care/treatment visits. While differential utilization of HIV/AIDS care may be associated with HIV/AIDS disease progression, it is beyond the scope of the current study to investigate how patterns of HIV/AIDS care affect mortality; however, we will be conducting a future study that examines the potential associations of patterns of care with HIV-related adverse outcomes.
Our study did not control for adherence to ARV regimens in the regression models. In our study cohort, 35% of patients were ARV-naïve at entry into study. The majority of those patients (>25% of all study subjects) remained ARV-naïve throughout study follow-up and thus would be missing or excluded from a measurement of ARV adherence. However, adherence is an important predictor of clinical outcomes among HIV-infected patients receiving ARV therapy, and lack of adjustment for that covariate in the regression analysis is a limitation of this study. We did not control for number of HIV-related opportunistic infections in our modeling analyses; however, we did adjust for CD4 cell counts (time-dependent covariate) throughout study follow-up, which can serve as a measure of HIV disease progression and immune status. We were not able to control for level of comorbidity (e.g., Charlson index) for other diseases and conditions at baseline, because many patients had insufficient prior membership time.
This study is to our knowledge the first to examine mortality among HIV-infected patients with private health insurance who received medical care in an integrated health plan and were diagnosed with substance use dependence or abuse by a health care professional. Our study comprised one of the largest clinical cohorts of HIV-infected patients in the United States.
In conclusion, we observed that excess mortality does occur in HIV-infected patients diagnosed with SU dependence or abuse for whom access to medical services and ability to pay for care are not significant factors. The fact that excess mortality does exist for these patients may indicate that even when HIV/AIDS and SU treatment care is available and accessed, individuals with SU problems remain vulnerable to less-than-optimal outcomes—often from non-HIV-related causes. Our study findings suggest that screening for alcohol and drug problems during the initial phase of HIV/AIDS treatment (and throughout the course of HIV/AIDS patient care) and providing SU treatment when SU problems are less severe may prove beneficial and extend life for these vulnerable patients.
Acknowledgments
The authors thank Felica Chi who developed computer algorithms used to assign ICD-9 codes to patients with SU diagnoses and Agatha Hinman for editorial assistance in the preparation of the manuscript. This study was funded by the National Institute on Drug Abuse (grant R37 DA10572).
Footnotes
CONFLICT OF INTEREST
This study was approved by the Institutional Review Boards of Kaiser Permanente Northern California and the University of California, San Francisco.
References
- Basu S, Smith-Rohrberg D, Bruce RD, Altice FL. Models for integrating buprenorphine therapy into the primary HIV care setting. Clin Infect Dis. 2006;42:716–721. doi: 10.1086/500200. [DOI] [PubMed] [Google Scholar]
- Breslow NE, Day NE. Statistical methods in cancer research. Volume II – the design and analysis of cohort studies. IARC Sci Publ. 1987;82:1–406. [PubMed] [Google Scholar]
- Conigliaro J, Madenwald T, Bryant K, Braithwaite S, Gordon A, Fultz SL, Maisto S, Samet J, Kraemer K, Cook R, Day N, Roach D, Richey S, Justice A. The Veterans Aging Cohort Study: observational studies of alcohol use, abuse, and outcomes among human immunodeficiency virus-infected veterans. Alcohol Clin Exp Res. 2004;28:313–321. doi: 10.1097/01.alc.0000113414.73220.21. [DOI] [PubMed] [Google Scholar]
- Detels R, Munoz A, McFarlane G, Kingsley LA, Margolick JB, Giorgi J, Schrager LK, Phair JP. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. Multicenter AIDS Cohort Study Investigators. JAMA. 1998;280:1497–1503. doi: 10.1001/jama.280.17.1497. [DOI] [PubMed] [Google Scholar]
- Estrada AL. Health disparities among African-American and Hispanic drug injectors – HIV, AIDS, hepatitis B virus and hepatitis C virus: a review. AIDS. 2005;19(Suppl 3):S47–S52. doi: 10.1097/01.aids.0000192070.95819.7c. [DOI] [PubMed] [Google Scholar]
- Fonseca MG, de Lucena FF, Sousa A, Bastos FI. AIDS mortality, “race or color,” and social inequality in a context of universal access to highly active antiretroviral therapy (HAART) in Brazil, 1999–2004. Cad Saude Publica. 2007;23(Suppl 3):S445–S455. doi: 10.1590/s0102-311x2007001500012. [DOI] [PubMed] [Google Scholar]
- French AL, Gawel SH, Hershow R, Benning L, Hessol NA, Levine AM, Anastos K, Augenbraun M, Cohen MH. Trends in mortality and causes of death among women with HIV in the United States: a 10-year study. J Acquir Immune Defic Syndr. 2009;51:399–406. doi: 10.1097/QAI.0b013e3181acb4e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia de la Hera M, Ferreros I, del Amo J, Garcia de Olalla P, Perez Hoyos S, Muga R, del Romero J, Guerrero R, Hernandez-Aguado I. Gender differences in progression to AIDS and death from HIV seroconversion in a cohort of injecting drug users from 1986 to 2001. J Epidemiol Community Health. 2004;58:944–950. doi: 10.1136/jech.2003.017475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert JJ, Fung MW, Felton S, Battjes RJ, Engels EA. Cause-specific mortality associated with HIV and HTLV-II infections among injecting drug users in the USA. AIDS. 2001;15:1295–1302. doi: 10.1097/00002030-200107060-00012. [DOI] [PubMed] [Google Scholar]
- Hogg RS, Yip B, Kully C, Craib KJ, O’Shaughnessy MV, Schechter MT, Montaner JS. Improved survival among HIV-infected patients after initiation of triple-drug antiretroviral regimens. CMAJ. 1999;160:659–665. [PMC free article] [PubMed] [Google Scholar]
- Kohli R, Lo Y, Howard AA, Buono D, Floris-Moore M, Klein RS, Schoenbaum EE. Mortality in an urban cohort of HIV-infected and at-risk drug users in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;41:864–872. doi: 10.1086/432883. [DOI] [PubMed] [Google Scholar]
- Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas GM, Gebo KA, Chaisson RE, Moore RD. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS. 2002;16:767–774. doi: 10.1097/00002030-200203290-00012. [DOI] [PubMed] [Google Scholar]
- Lucas GM, Griswold M, Gebo KA, Keruly J, Chaisson RE, Moore RD. Illicit drug use and HIV-1 disease progression: a longitudinal study in the era of highly active antiretroviral therapy. Am J Epidemiol. 2006;163:412–420. doi: 10.1093/aje/kwj059. [DOI] [PubMed] [Google Scholar]
- Lum PJ, Tulsky JP. The medical management of opioid dependence in HIV primary care settings. Curr HIV/AIDS Rep. 2006;3:195–204. doi: 10.1007/s11904-006-0016-z. [DOI] [PubMed] [Google Scholar]
- Mertens JR, Weisner C, Ray GT, Fireman B, Walsh K. Hazardous drinkers and drug users in HMO primary care: prevalence, medical conditions, and costs. Alcohol Clin Exp Res. 2005;29:989–998. doi: 10.1097/01.alc.0000167958.68586.3d. [DOI] [PubMed] [Google Scholar]
- Mocroft A, Gatell J, Reiss P, Ledergerber B, Kirk O, Vella S, Blaxhult A, Phillips AN, Lundgren J. Causes of death in HIV infection: the key determinant to define the clinical response to anti-HIV therapy. AIDS. 2004;18:2333–2337. doi: 10.1097/00002030-200411190-00018. [DOI] [PubMed] [Google Scholar]
- Muga R, Langohr K, Tor J, Sanvisens A, Serra I, Rey-Joly C, Munoz A. Survival of HIV-infected injection drug users (IDUs) in the highly active antiretroviral therapy era, relative to sex- and age-specific survival of HIV-uninfected IDUs. Clin Infect Dis. 2007;45:370–376. doi: 10.1086/519385. [DOI] [PubMed] [Google Scholar]
- Murphy EL, Collier AC, Kalish LA, Assmann SF, Para MF, Flanigan TP, Kumar PN, Mintz L, Wallach FR, Nemo GJ. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med. 2001;135:17–26. doi: 10.7326/0003-4819-135-1-200107030-00005. [DOI] [PubMed] [Google Scholar]
- Nash D, Katyal M, Shah S. Trends in predictors of death due to HIV-related causes among persons living with AIDS in New York City: 1993–2001. J Urban Health. 2005;82:584–600. doi: 10.1093/jurban/jti123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzotti P, Galai N, Vlahov D, Rezza G, Lyles CM, Astemborski J. Direct comparison of time to AIDS and infectious disease death between HIV seroconverter injection drug users in Italy and the United States: results from the ALIVE and ISS studies. AIDS Link to Intravenous Experiences. Italian Seroconversion Study. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:275–282. doi: 10.1097/00042560-199903010-00010. [DOI] [PubMed] [Google Scholar]
- Poundstone KE, Chaisson RE, Moore RD. Differences in HIV disease progression by injection drug use and by sex in the era of highly active anti-retroviral therapy. AIDS. 2001;15:1115–1123. doi: 10.1097/00002030-200106150-00006. [DOI] [PubMed] [Google Scholar]
- Ray GT, Mertens JR, Weisner C. The excess medical cost and health problems of family members of persons diagnosed with alcohol or drug problems. Med Care. 2007;45:116–122. doi: 10.1097/01.mlr.0000241109.55054.04. [DOI] [PubMed] [Google Scholar]
- Sackoff JE, Hanna DB, Pfeiffer MR, Torian LV. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145:397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- Sullivan LE, Barry D, Moore BA, Chawarski MC, Tetrault JM, Pantalon MV, O’Connor PG, Schottenfeld RS, Fiellin DA. A trial of integrated buprenorphine/naloxone and HIV clinical care. Clin Infect Dis. 2006;43(Suppl 4):S184–S190. doi: 10.1086/508182. [DOI] [PubMed] [Google Scholar]
- Thorpe LE, Frederick M, Pitt J, Cheng I, Watts DH, Buschur S, Green K, Zorrilla C, Landesman SH, Hershow RC. Effect of hard-drug use on CD4 cell percentage, HIV RNA level, and progression to AIDS-defining class C events among HIV-infected women. J Acquir Immune Defic Syndr. 2004;37:1423–1430. doi: 10.1097/01.qai.0000127354.78706.5d. [DOI] [PubMed] [Google Scholar]
- Walley AY, Cheng DM, Libman H, Nunes D, Horsburgh CR, Jr, Saitz R, Samet JH. Recent drug use, homelessness and increased short-term mortality in HIV-infected persons with alcohol problems. AIDS. 2008;22:415–420. doi: 10.1097/QAD.0b013e3282f423f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisner C, Mertens J, Parthasarathy S, Moore C, Lu Y. Integrating primary medical care with addiction treatment: a randomized controlled trial. JAMA. 2001;286:1715–1723. doi: 10.1001/jama.286.14.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldemichael G, Christiansen D, Thomas S, Benbow N. Demographic characteristics and survival with AIDS: health disparities in Chicago, 1993–2001. Am J Public Health. 2009;99(Suppl 1):S118–S123. doi: 10.2105/AJPH.2007.124750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood E, Hogg RS, Lima VD, Kerr T, Yip B, Marshall BD, Montaner JS. Highly active antiretroviral therapy and survival in HIV-infected injection drug users. JAMA. 2008;300:550–554. doi: 10.1001/jama.300.5.550. [DOI] [PubMed] [Google Scholar]