Abstract
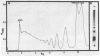
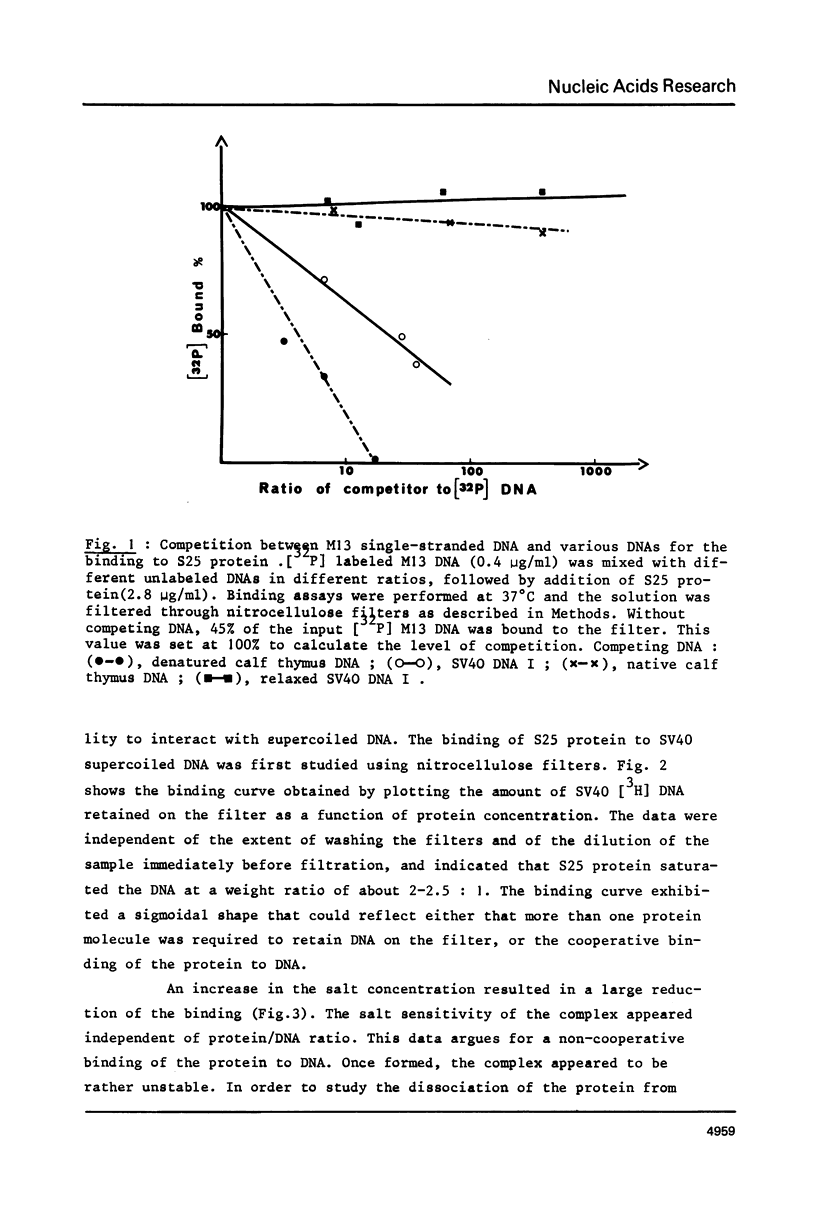
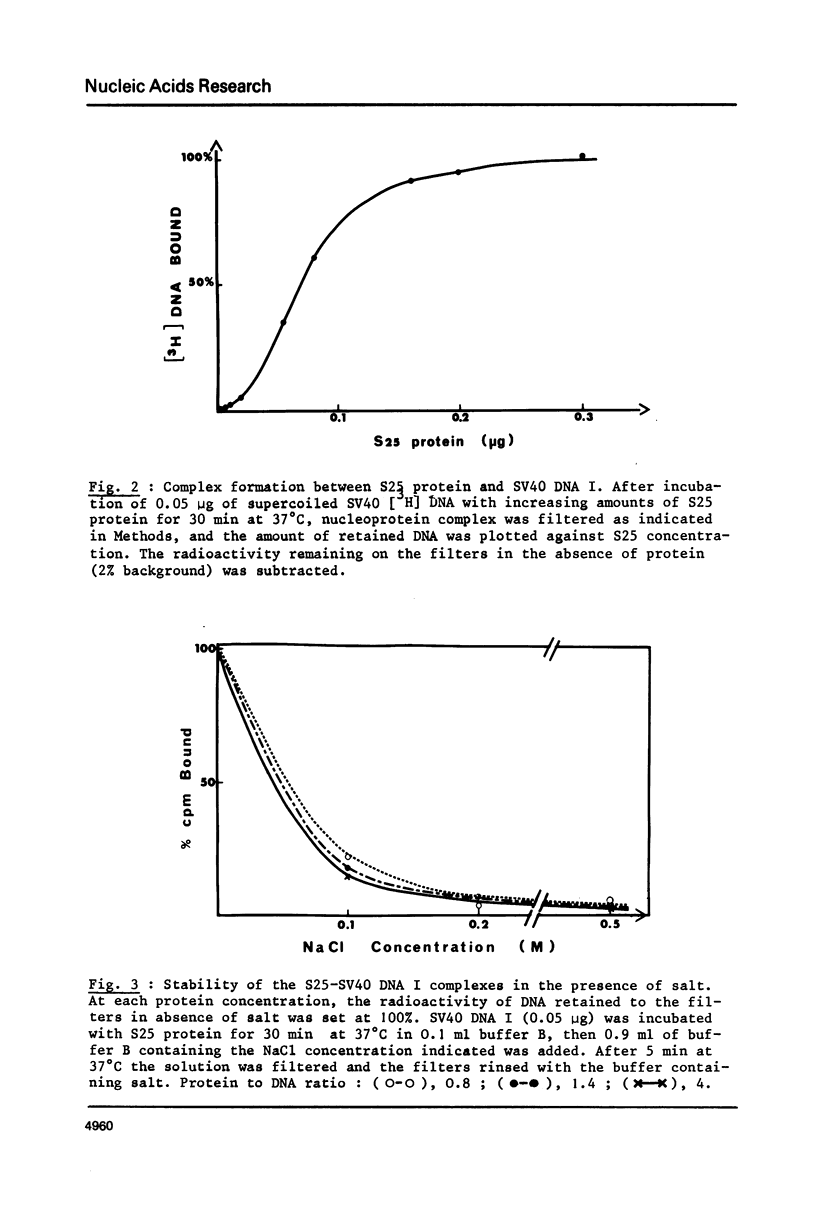
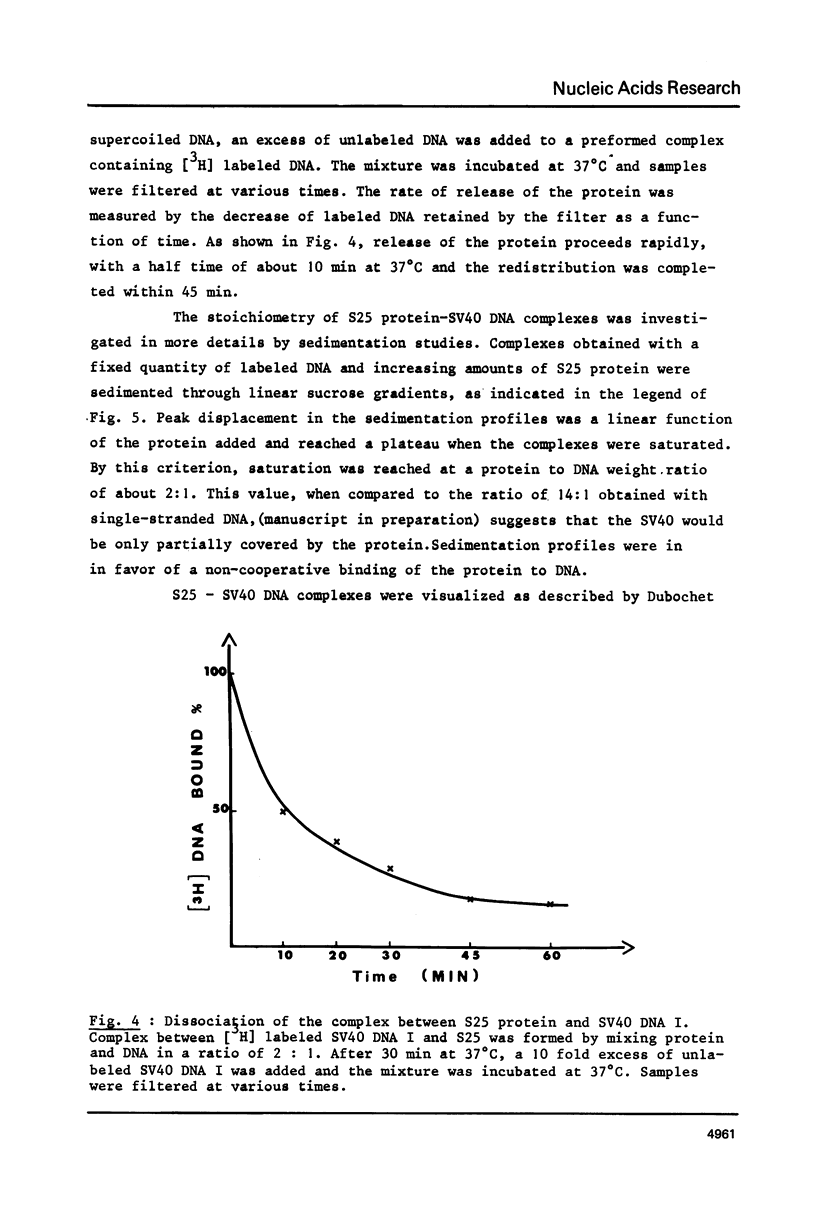
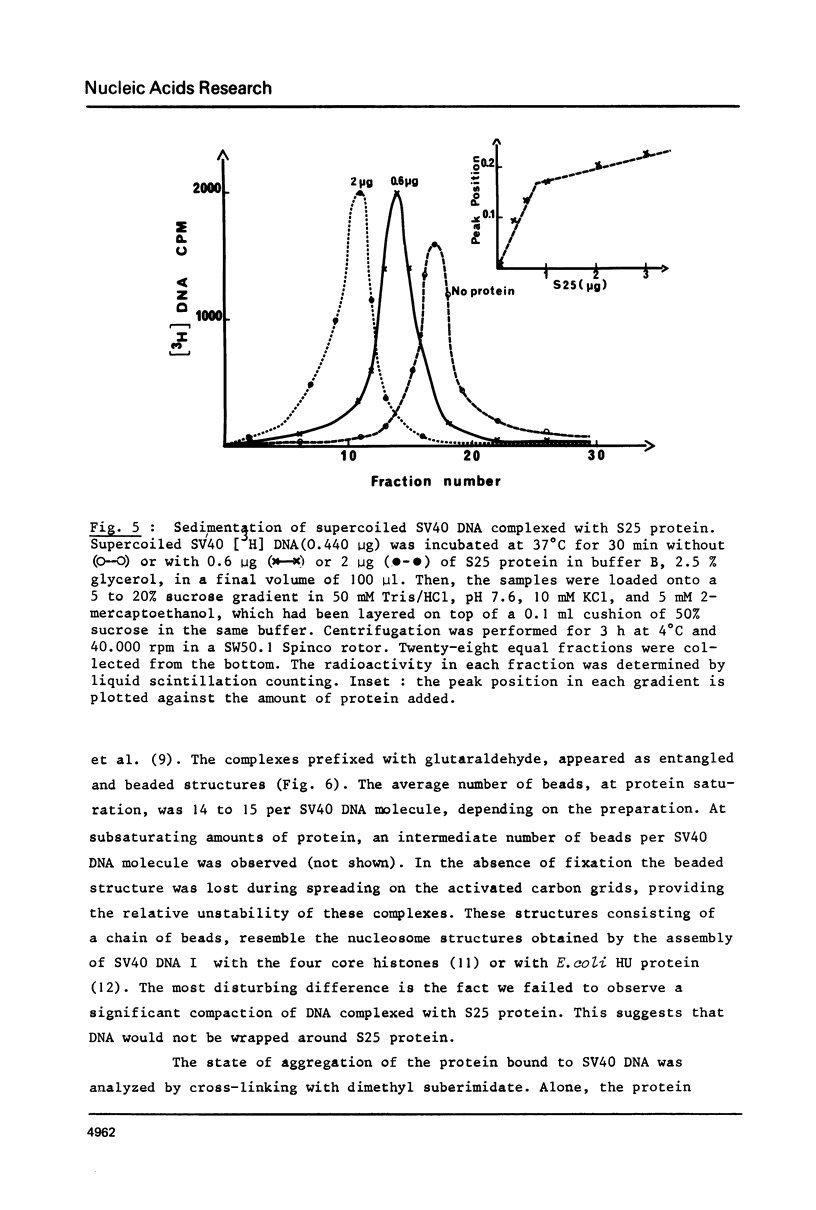
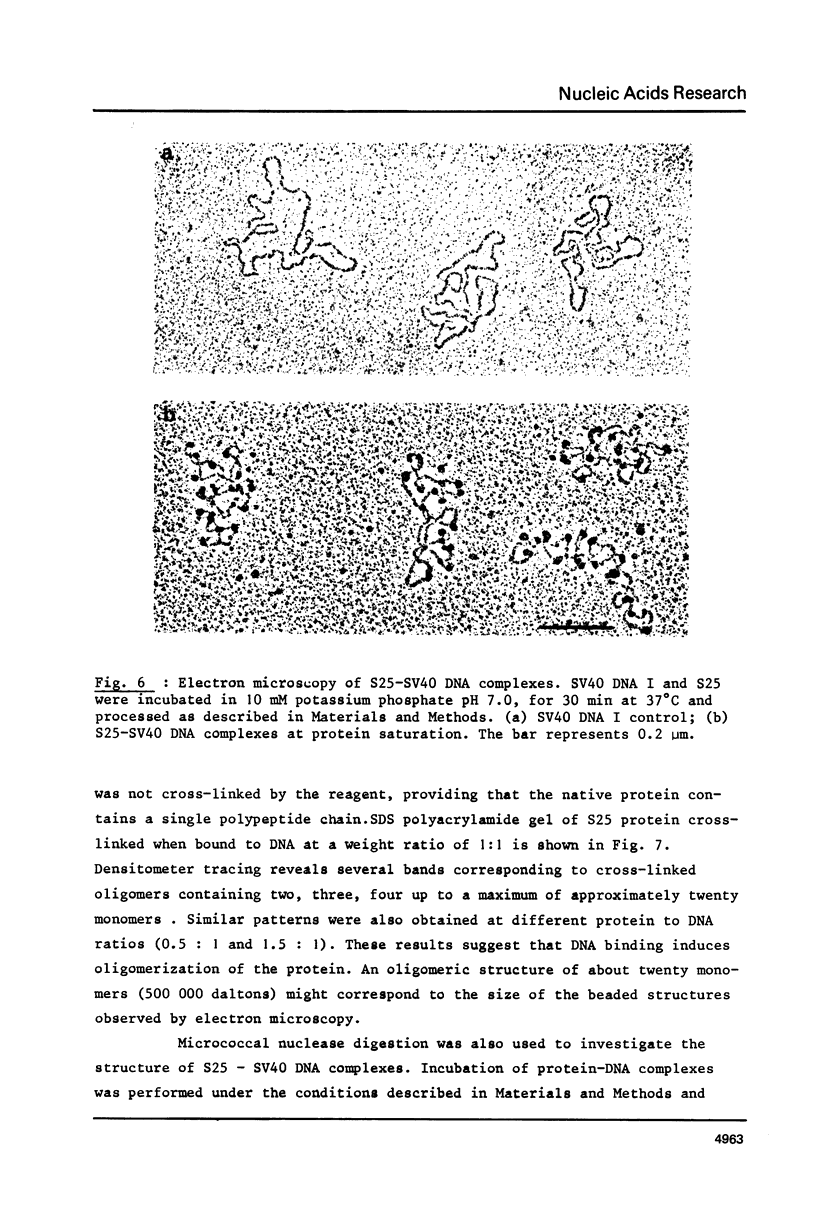
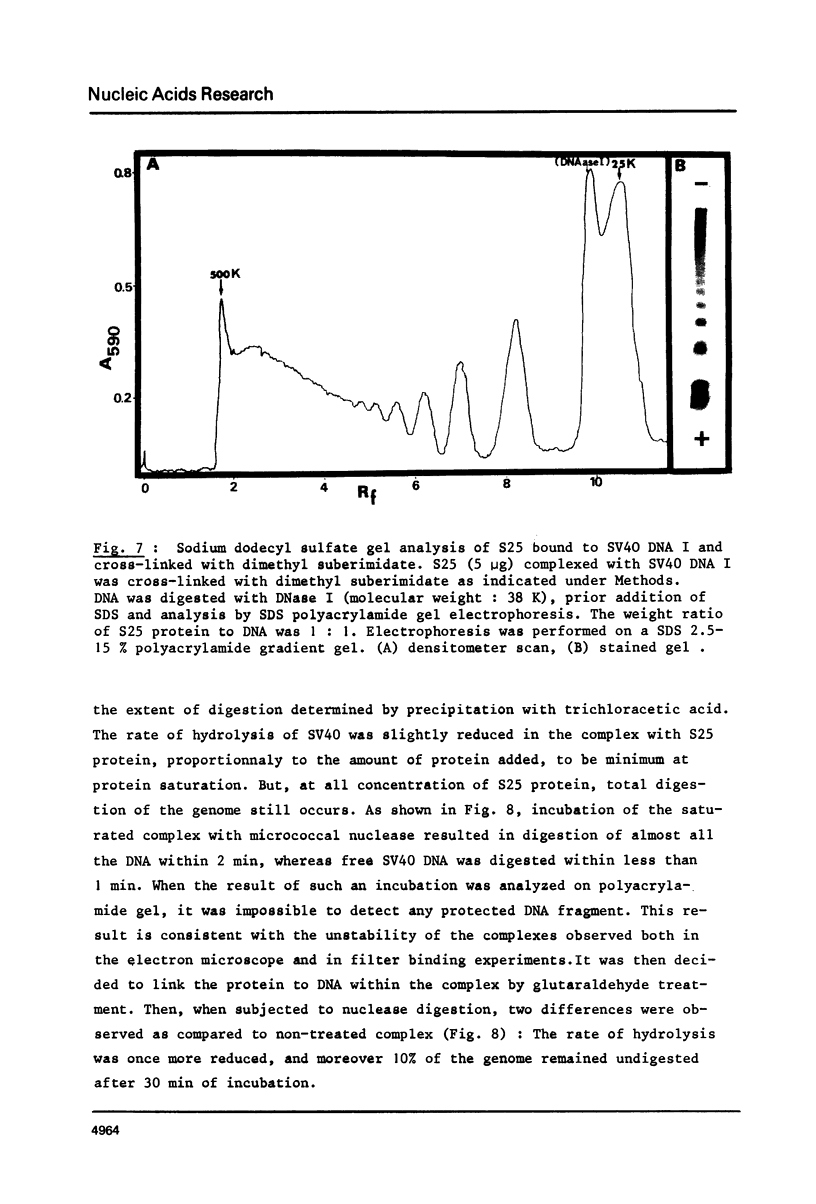
As shown by competition experiments, the single-strand DNA binding protein from normal rat liver (S25) interacts preferentially with supercoiled DNA compared to relaxed DNA duplexes. When followed both by sedimentation analysis and by nitrocellulose filter assay, the binding of S25 to SV40 supercoiled DNA (FI) appears to be non-cooperative. Saturation is reached at a protein to DNA weight ratio of about 2. The S25-DNA complexes prefixed with glutaraldehyde appear as beaded structures having an average of 14 to 16 beads per SV40 DNA molecules. Cross-linking of S25 bound to SV40 DNA by dimethyl suberimidate allows to detect oligomeric structures containing a maximum of twenty monomers of S25. When complexes are treated by glutaraldehyde, 10% of the genome become resistant against micrococcal nuclease. Moreover, S25 affects the DNA helical structure. Superhelical forms are generated by the association of S25 with SV40 DNA, in the presence of nicking-closing enzyme.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonne C., Duguet M., de Recondo A. M. Rat liver DNA binding proteins: physiological variations. FEBS Lett. 1979 Oct 15;106(2):292–296. doi: 10.1016/0014-5793(79)80517-7. [DOI] [PubMed] [Google Scholar]
- Champoux J. J., McConaughy B. L. Purification and characterization of the DNA untwisting enzyme from rat liver. Biochemistry. 1976 Oct 19;15(21):4638–4642. doi: 10.1021/bi00666a014. [DOI] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubochet J., Ducommun M., Zollinger M., Kellenberger E. A new preparation method for dark-field electron microscopy of biomacromolecules. J Ultrastruct Res. 1971 Apr;35(1):147–167. doi: 10.1016/s0022-5320(71)80148-x. [DOI] [PubMed] [Google Scholar]
- Duguet M., Soussi T., Rossignol J. M., Méchali M., De Recondo A. M. Stimulation of rat liver alpha- and beta-type DNA polymerases by an homologous DNA-unwinding protein. FEBS Lett. 1977 Jul 1;79(1):160–164. doi: 10.1016/0014-5793(77)80374-8. [DOI] [PubMed] [Google Scholar]
- Duguet M., de Recondo A. M. A deoxyribonucleic acid unwinding protein isolated from regenerating rat liver. Physical and functional properties. J Biol Chem. 1978 Mar 10;253(5):1660–1666. [PubMed] [Google Scholar]
- Germond J. E., Hirt B., Oudet P., Gross-Bellark M., Chambon P. Folding of the DNA double helix in chromatin-like structures from simian virus 40. Proc Natl Acad Sci U S A. 1975 May;72(5):1843–1847. doi: 10.1073/pnas.72.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Javaherian K., Liu J. F., Wang J. C. Nonhistone proteins HMG1 and HMG2 change the DNA helical structure. Science. 1978 Mar 24;199(4335):1345–1346. doi: 10.1126/science.628842. [DOI] [PubMed] [Google Scholar]
- Javaherian K., Sadeghi M., Liu L. F. Nonhistone proteins HMG1 and HMG2 unwind DNA double helix. Nucleic Acids Res. 1979 Aug 10;6(11):3569–3580. doi: 10.1093/nar/6.11.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones O. W., Berg P. Studies on the binding of RNA polymerase to polynucleotides. J Mol Biol. 1966 Dec 28;22(2):199–209. doi: 10.1016/0022-2836(66)90126-4. [DOI] [PubMed] [Google Scholar]
- Rouvière-Yaniv J., Yaniv M., Germond J. E. E. coli DNA binding protein HU forms nucleosomelike structure with circular double-stranded DNA. Cell. 1979 Jun;17(2):265–274. doi: 10.1016/0092-8674(79)90152-1. [DOI] [PubMed] [Google Scholar]
- Tsai R. L., Green H. Studies on a mammalian cell protein (P8) with affinity for DNA in vitro. J Mol Biol. 1973 Feb 19;73(3):307–316. doi: 10.1016/0022-2836(73)90344-6. [DOI] [PubMed] [Google Scholar]