Abstract
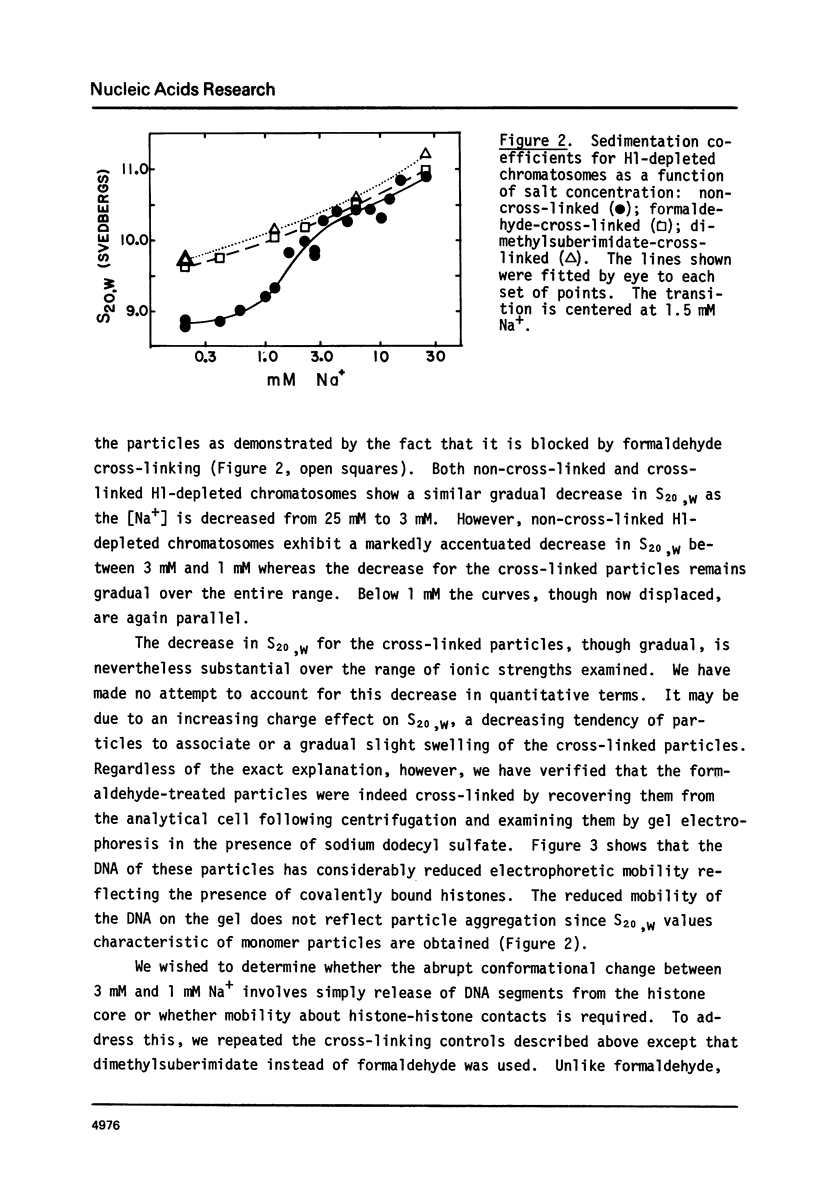
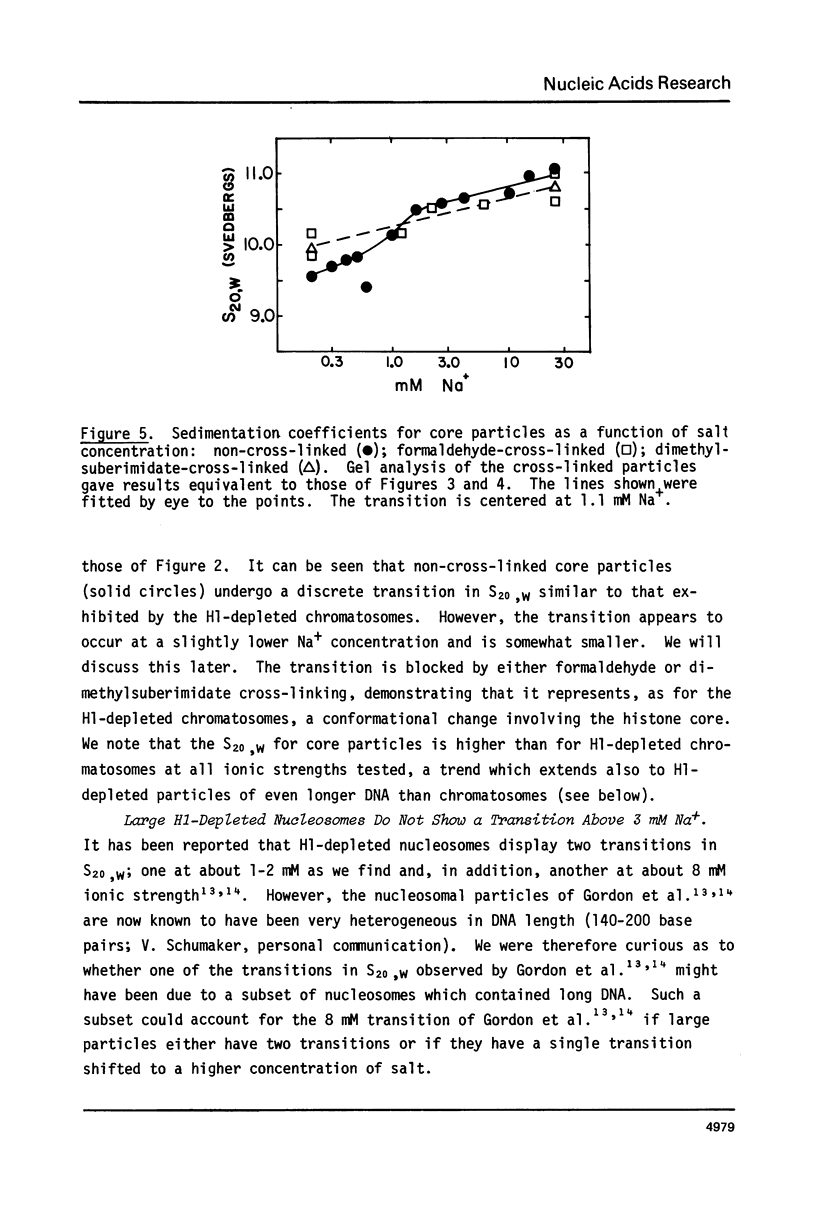
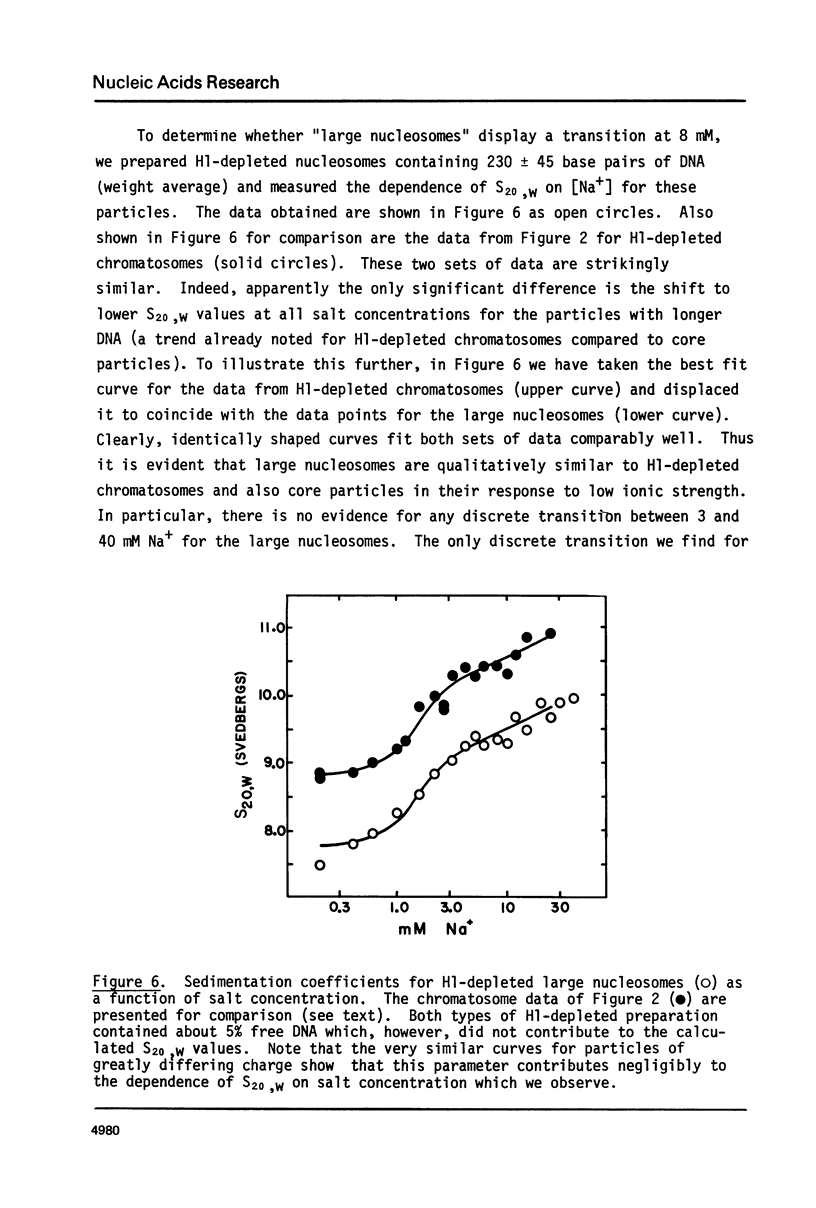
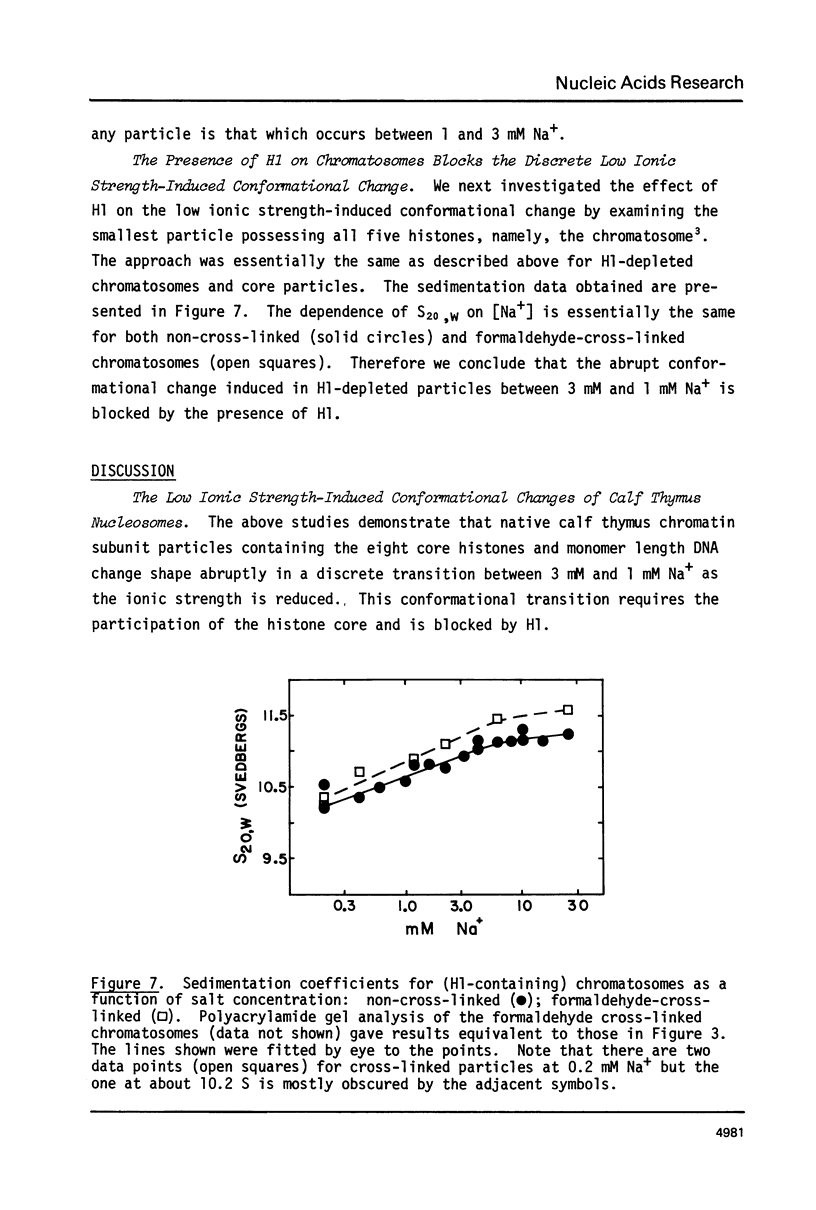
Calf thymus nucleosomes exhibit two different and independent hydrodynamic responses to diminishing salt concentration. One change is gradual over the range 40 to 0.2 mM Na+ and is accompanied by decreases in contact-site cross-linking efficiency. The other change is abrupt, being centered between 1 and 2 mM Na+. We found only one abrupt change in sedimentation rate for particles ranging in DNA content fom 144 to 230 base pairs. This response to decreasing ionic strength is similar for particles of both 169 and 230 base pairs. Core particles (144 base pairs) exhibit a somewhat diminished response. The abrupt change is blocked by formaldehyde or dimethylsuberimidate cross-linking. The blockage by dimethylsuberimidate demonstrates that the abrupt conformational change requires the participation of the core histones. H1 completely blocks the abrupt but not the gradual conformational change. Thus H1 uncouples the different responses to low ionic strength and exerts an important constraint on the conformational states available to the nucleosome core.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Stedman J. D. Histone 1 is proximal to histone 2A and to A24. Proc Natl Acad Sci U S A. 1979 May;76(5):2190–2194. doi: 10.1073/pnas.76.5.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulikas T., Wiseman J. M., Garrard W. T. Points of contact between histone H1 and the histone octamer. Proc Natl Acad Sci U S A. 1980 Jan;77(1):127–131. doi: 10.1073/pnas.77.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao M. V., Martinson H. G., Gralla J. D. lac Operator nucleosomes. 2. lac Nucleosomes can change conformation to strengthen binding by lac repressor. Biochemistry. 1980 Jul 8;19(14):3260–3269. doi: 10.1021/bi00555a025. [DOI] [PubMed] [Google Scholar]
- Dieterich A. E., Axel R., Cantor C. R. Salt-induced structural changes of nucleosome core particles. J Mol Biol. 1979 Apr 25;129(4):587–602. doi: 10.1016/0022-2836(79)90470-4. [DOI] [PubMed] [Google Scholar]
- Dieterich A. E., Eshaghpour H., Crothers D. M., Cantor C. R. Effect of DNA length on the nucleosome low salt transition. Nucleic Acids Res. 1980 Jun 11;8(11):2475–2487. doi: 10.1093/nar/8.11.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin. Nature. 1978 Jan 12;271(5641):115–122. doi: 10.1038/271115a0. [DOI] [PubMed] [Google Scholar]
- Fulmer A. W., Fasman G. D. Ionic strength-dependent conformational transitions of chromatin. Circular dichroism and thermal denaturation studies. Biopolymers. 1979 Nov;18(11):2875–2891. doi: 10.1002/bip.1979.360181115. [DOI] [PubMed] [Google Scholar]
- Gariglio P., Llopis R., Oudet P., Chambon P. The template of the isolated native simian virus 40 transcriptional complexes is a minichromosome. J Mol Biol. 1979 Jun 15;131(1):75–105. doi: 10.1016/0022-2836(79)90302-4. [DOI] [PubMed] [Google Scholar]
- Giri C. P., Gorovsky M. A. DNase I sensitivity of ribosomal genes in isolated nucleosome core particles. Nucleic Acids Res. 1980 Jan 11;8(1):197–214. doi: 10.1093/nar/8.1.197-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon V. C., Knobler C. M., Olins D. E., Schumaker V. N. Conformational changes of the chromatin subunit. Proc Natl Acad Sci U S A. 1978 Feb;75(2):660–663. doi: 10.1073/pnas.75.2.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon V. C., Schumaker V. N., Olins D. E., Knobler C. M., Horwitz J. The temperature and pH dependence of conformational transitions of the chromatin subunit. Nucleic Acids Res. 1979 Aug 24;6(12):3845–3858. doi: 10.1093/nar/6.12.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson V. Studies on histone organization in the nucleosome using formaldehyde as a reversible cross-linking agent. Cell. 1978 Nov;15(3):945–954. doi: 10.1016/0092-8674(78)90278-7. [DOI] [PubMed] [Google Scholar]
- Lilley D. M., Jacobs M. F., Houghton M. The nature of the interaction of nucleosomes with a eukaryotic RNA polymerase II. Nucleic Acids Res. 1979 Sep 25;7(2):377–399. doi: 10.1093/nar/7.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter L. C. Kinetic analysis of deoxyribonuclease I cleavages in the nucleosome core: evidence for a DNA superhelix. J Mol Biol. 1978 Sep 15;124(2):391–420. doi: 10.1016/0022-2836(78)90306-6. [DOI] [PubMed] [Google Scholar]
- Martinson H. G., True R. J., Burch J. B. Specific histone-histone contacts are ruptured when nucleosomes unfold at low ionic strength. Biochemistry. 1979 Mar 20;18(6):1082–1089. doi: 10.1021/bi00573a023. [DOI] [PubMed] [Google Scholar]
- Martinson H. G., True R. J. On the mechanism of nucleosome unfolding. Biochemistry. 1979 Mar 20;18(6):1089–1094. doi: 10.1021/bi00573a024. [DOI] [PubMed] [Google Scholar]
- Martinson H. G., True R., Burch J. B., Kunkel G. Semihistone protein A24 replaces H2A as an integral component of the nucleosome histone core. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1030–1034. doi: 10.1073/pnas.76.3.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. D., Felsenfeld G. Nucleosome structure. Annu Rev Biochem. 1980;49:1115–1156. doi: 10.1146/annurev.bi.49.070180.005343. [DOI] [PubMed] [Google Scholar]
- Noll M., Kornberg R. D. Action of micrococcal nuclease on chromatin and the location of histone H1. J Mol Biol. 1977 Jan 25;109(3):393–404. doi: 10.1016/s0022-2836(77)80019-3. [DOI] [PubMed] [Google Scholar]
- Peters K., Richards F. M. Chemical cross-linking: reagents and problems in studies of membrane structure. Annu Rev Biochem. 1977;46:523–551. doi: 10.1146/annurev.bi.46.070177.002515. [DOI] [PubMed] [Google Scholar]
- Ring D., Cole R. D. Chemical cross-linking of H1 histone to the nucleosomal histones. J Biol Chem. 1979 Nov 25;254(22):11688–11695. [PubMed] [Google Scholar]
- Simon R. H., Camerini-Otero R. D., Felsenfeld G. An octamer of histones H3 and H4 forms a compact complex with DNA of nucleosome size. Nucleic Acids Res. 1978 Dec;5(12):4805–4818. doi: 10.1093/nar/5.12.4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. T. Mechanism of a reversible, thermally induced conformational change in chromatin core particles. J Biol Chem. 1979 Oct 25;254(20):10123–10127. [PubMed] [Google Scholar]
- Simpson R. T. Structure of the chromatosome, a chromatin particle containing 160 base pairs of DNA and all the histones. Biochemistry. 1978 Dec 12;17(25):5524–5531. doi: 10.1021/bi00618a030. [DOI] [PubMed] [Google Scholar]
- Simpson R. T., Whitlock J. P. Mapping DNAase l-susceptible sites in nucleosomes labeled at the 5' ends. Cell. 1976 Oct;9(2):347–353. doi: 10.1016/0092-8674(76)90124-0. [DOI] [PubMed] [Google Scholar]
- Stein A., Bina-Stein M., Simpson R. T. Crosslinked histone octamer as a model of the nucleosome core. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2780–2784. doi: 10.1073/pnas.74.7.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein A. DNA folding by histones: the kinetics of chromatin core particle reassembly and the interaction of nucleosomes with histones. J Mol Biol. 1979 May 15;130(2):103–134. doi: 10.1016/0022-2836(79)90421-2. [DOI] [PubMed] [Google Scholar]
- Tack L. O., Simpson R. T. Characterization of chromatin modified with ethyl acetimidate. Biochemistry. 1977 Aug 23;16(17):3746–3753. doi: 10.1021/bi00636a003. [DOI] [PubMed] [Google Scholar]
- Thoma F., Koller T., Klug A. Involvement of histone H1 in the organization of the nucleosome and of the salt-dependent superstructures of chromatin. J Cell Biol. 1979 Nov;83(2 Pt 1):403–427. doi: 10.1083/jcb.83.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd R. D., Garrard W. T. Two-dimensional electrophoretic analysis of polynucleosomes. J Biol Chem. 1977 Jul 10;252(13):4729–4738. [PubMed] [Google Scholar]
- Wasylyk B., Chambon P. Studies on the mechanism of transcription of nucleosomal complexes. Eur J Biochem. 1980 Jan;103(2):219–226. doi: 10.1111/j.1432-1033.1980.tb04306.x. [DOI] [PubMed] [Google Scholar]
- Wasylyk B., Thevenin G., Oudet P., Chambon P. Transcription of in vitro assembled chromatin by Escherichia coli RNA polymerase. J Mol Biol. 1979 Mar 5;128(3):411–440. doi: 10.1016/0022-2836(79)90095-0. [DOI] [PubMed] [Google Scholar]
- Weisbrod S., Groudine M., Weintraub H. Interaction of HMG 14 and 17 with actively transcribed genes. Cell. 1980 Jan;19(1):289–301. doi: 10.1016/0092-8674(80)90410-9. [DOI] [PubMed] [Google Scholar]
- Weischet W. O., Allen J. R., Riedel G., Van Holde K. E. The effects of salt concentration and H-1 depletion on the digestion of calf thymus chromatin by micrococcal nuclease. Nucleic Acids Res. 1979;6(5):1843–1862. doi: 10.1093/nar/6.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock J. P., Jr, Simpson R. T. Localization of the sites along nucleosome DNA which interact with NH2-terminal histone regions. J Biol Chem. 1977 Sep 25;252(18):6516–6520. [PubMed] [Google Scholar]
- Wu H. M., Dattagupta N., Hogan M., Crothers D. M. Structural changes of nucleosomes in low-salt concentrations. Biochemistry. 1979 Sep 4;18(18):3960–3965. doi: 10.1021/bi00585a018. [DOI] [PubMed] [Google Scholar]






