Abstract
Recent studies have underscored physiological and pathophysiological roles for the tryptophan-degrading enzyme indolamine 2,3-dioxygenase (IDO) in immune counterregulation. However, IDO was first recognized as an antimicrobial effector, restricting tryptophan availability to Toxoplasma gondii and other pathogens in vitro. The biological relevance of these findings came under question when infectious phenotypes were not forthcoming in IDO-deficient mice. The recent discovery of an IDO homolog, IDO-2, suggested that the issue deserved reexamination. IDO inhibition during murine toxoplasmosis led to 100% mortality, with increased parasite burdens and no evident effects on the immune response. Similar studies revealed a counterregulatory role for IDO during leishmaniasis (restraining effector immune responses and parasite clearance), and no evident role for IDO in herpes simplex virus type 1 (HSV-1) infection. Thus, IDO plays biologically important roles in the host response to diverse intracellular infections, but the dominant nature of this role—antimicrobial or immunoregulatory—is pathogen-specific.
Oxidative degradation of tryptophan to kynurenine is catalyzed by at least 2 structurally distinct classes of enzymes in mammals: a homeostatic enzyme, tryptophan 2,3-dioxygenase, expressed by the liver; and indolamine 2,3-dioxygenase (IDO), whose expression is regulated in diverse cell types by pathogen- and host-derived inflammatory signals, including proinflammatory cytokines (paradigmatically, interferon-γ [IFN-γ]), Toll-like receptor ligands (eg, lipopolysaccharide), and interactions between immune cells (eg, the engagement of costimulatory molecules on antigen-presenting cells by cytotoxic T-lymphocyte antigen-4) [1]. IDO has recently been shown to serve important immunoregulatory functions. Tryptophan catabolism by IDO functions as a counterregulatory pathway, mediating potent modulation of T-cell responses in vitro and in vivo [1]. The molecular mechanisms remain to be fully defined. Both localized tryptophan deprivation (inhibiting mammalian target of rapamycin signaling and upregulating the GCN2 kinase stress response pathway, leading to arrest of T-cell proliferation and anergy) and the production of bioactive tryptophan metabolites (facilitating the generation of regulatory T cells, inhibiting the generation of T-helper 17 cells, and driving T-cell apoptosis) have been implicated in various systems [1–7]. Modulation of T-cell responses by IDO-expressing dendritic cells is thought to play an important physiological role in suppressing the development expression of autoimmune and allergic diseases [1]. Similarly, inhibition of allogeneic T-cell responses by IDO-expressing trophoblast cells is thought to facilitate maternal/fetal tolerance [1]. Pathophysiological roles for IDO-mediated immunosuppression have also been described. IDO is expressed by many tumors, as well as by a subpopulation of dendritic cells in tumor-draining lymph nodes; IDO inhibition can rescue anergic, tumor antigen–specific T-cell effector function, inhibiting tumor growth in mouse models [8–10]. Sustained IDO activation is also thought to be an important cause of immunosuppression in human immunodeficiency virus (HIV) infection [11]. The potential for therapeutic exploitation of physiological IDO activity (in autoimmune disease and transplantation) and therapeutic targeting of pathophysiological IDO activity (in cancer and HIV) are currently under active exploration.
More than 2 decades ago, however, IDO was described as an antimicrobial defense mechanism. The original reports were with Toxoplasma gondii, a ubiquitous protozoan that causes severe disease in immunocompromised hosts. Treatment of T. gondii–infected human cells with IFN-γ upregulated IDO expression and activity, leading to restriction of parasite replication—something reversible by the addition of exogenous tryptophan [12–15]. The tryptophan auxotrophy of T. gondii provided a clear biological rationale for the targeting of this organism by immune-driven IDO activity. Subsequently, similar studies reported that IFN-γ induction of IDO played a role in restricting the replication of a range of intracellular pathogens, including bacterial tryptophan auxotrophs (notably, Chlamydiae species [14, 16, 17]) and diverse viruses [18–22]). Despite this extensive in vitro literature, the generation of IDO-deficient mice was not followed by published evidence of an important in vivo role for IDO in host defense—something that cast doubt on the biological relevance of these in vitro observations. Indeed, despite the fact that acute murine toxoplasmosis leads to induction of IDO expression and activity [23, 24], we found that the course of infection with T. gondii was unaltered in IDO-deficient mice, on either C57BL/6 or BALB/c backgrounds, compared with wild-type controls (data not shown). The recent discovery of a gene closely related to IDO(-1), IDO-2 [25, 26], suggested an obvious potential reason for this apparent lack of phenotype in IDO-1 knockout mice. This, together with the practical availability of 1-methyl tryptophan (1-MT), an in vivo inhibitor of IDO-1 and -2, led to us to reexamine the role of IDO in the host response to infection with intracellular pathogens.
MATERIALS AND METHODS
Infection Models
Toxoplasmosis
Female C57BL/6 mice were infected by the intraperitoneal injection of 20 T. gondii cysts (ME49 strain) recovered from brain homogenates of chronically infected mice [27]. T. gondii cysts were counted in brain homogenates by microscopy [27]. Systemic cytokine production over 18 hours was quantified by the cytokine capture assay (CCA) assay [28].
Cutaneous Leishmaniasis
Infective-stage metacyclic promastigotes (105) of Leishmania major clone V1 (MHOM/IL/80/Friedlin) were inoculated intradermally into the ears of female C57BL/6 mice [29]. Lesion size was quantified with vernier calipers [29]. Parasite burden was quantified as described [29]. Intradermal lymphocytes were isolated as described [29]. Cells were subsequently analyzed by flow cytometry for surface markers and intracellular forkhead box p3 (Foxp3) expression. Cells isolated from lymph nodes draining lesions were stimulated with L. major promastigote lysates; cytokines were quantified by enzyme-linked immunosorbent assay (ELISA) in culture supernatants harvested 48 hours later. Systemic cytokine production over 18 hours was quantified by the CCA assay. Lesional mRNA expression was quantified by quantitative reverse transcription–polymerase chain reaction (qRT-PCR).
Herpes Simples Virus Type 1 Infection
Male Swiss Webster or C57BL/6 mice were inoculated on scarified corneas with 2 × 105 plaque-forming units (pfu) of wild-type herpes simplex virus type 1 (HSV-1) strain 17syn+ [30]. The infectious viral burden in eyes and trigeminal ganglia was quantified 4 days after infection by plaque assay [30]. The burden of latent infection was measured by real-time PCR [31]. The reactivation competency of latent genomes was tested by explant of trigeminal ganglia in the presence of acyclovir for 3 days, followed by quantification of lytic viral proteins by immunohistochemistry. During infection, animals were observed for signs of encephalitis, death, and reactivation of virus in eye swabs. Standard histological analysis of the central nervous system and periocular areas was performed.
Animal care was provided in accordance with National Institutes of Health guidelines; studies were approved by the Cincinnati Children’s Hospital Medical Center Institutional Animal Care and Use Committee.
IDO Inhibition
Mice were treated orally with the D isomer of 1-MT (D1-MT; Sigma-Aldrich), prepared as described [2], in their drinking water (2 mg/mL solution, sweetened with Nutrasweet; controls: Nutrasweet alone). To analyze effects of 1-MT on acute infection, mice were pretreated for 1 week prior to infectious challenge. The volume of water consumed by the animals was monitored. No difference in the volume of water consumed was observed between 1-MT-treated and control-treated animals. Consistent with the lack of toxicity reported in preclinical toxicological studies [32], administration of D1-MT for 101 days to uninfected mice led to no evident adverse effects (data not shown). In some experiments, the effects of treating mice with the L isomer of 1-MT (Sigma-Aldrich), handled in the same fashion, was compared with that of treating mice with D1-MT.
Reagents
The following PCR primers were used: IFN-γ 5' TGGCTGTTTCTGGCTGTTACTG, 3' ACGCTTATGTTGTTGCTGATGG; tumor necrosis factor (TNF)–α 5' CCAGACCCTCACACTCAGATCA, 3' CACTTGGTGGTTTGCTACGAC; interleukin (IL)–12/23p40 5' GGAAGCACGGCAGCAGAATA, 3' GAACTTGAGGGAGAAGTAGGAATGG; IL-10 5' GAAGCATGGCCCAGAAATCA, 3' TGCTCCACTGCCTTGCTCTT; IDO-1 5' GTGGGCAGCTTTTCAACTTC, 3' GGGCTTTGCTCTACCACATC; IDO-2 5' TGCCTGATGGCCTATAACCAGTGT, 3' TGCAGGATGTGAACCTCTAACGCT; β-actin 5' GGCCCAGAGCAAGAGAGGTA, 3' GGTTGGCCTTAGGGTTCAGG; HSV-1 5' CTTAACAGCGTCAACAGCGTGCCG; 3' CAAAGGTGCGGGAGT.
Antibodies for flow cytometric analysis were from eBiosciences. Immunohistochemical analysis of IDO-1 in formalin-fixed tissue was performed with rabbit anti-IDO-1 polyclonal antibodies, as previously described [8]. ELISA reagents for CCA analysis (of IL-10, TNF, IFN-γ and IL-4) and for IL-17A/F were from eBiosciences; those for quantification of in vitro IL-10 and IFN-γ production were from BD Bioscience.
Statistical Analysis
Data were analyzed by Mantel–Cox test (survival analysis) and analysis of variance (ANOVA), followed by Tukey multiple comparison test or unpaired Student t test, as appropriate. For kinetic lesion size analysis during Leishmania infection, the assumption of normality was tested using a normal quantile-quantile (Q-Q) plot or by multivariate analysis of variation (MANOVA), to reject the null hypothesis. Leishmania numbers were log-transformed before analysis.
RESULTS
IDO Is Necessary for Control of Acute and Latent Toxoplasmosis
We infected mice intraperitoneally with T. gondii in the presence and absence of IDO inhibition with D1-MT. As shown in Figure 1A, IDO inhibition rendered mice incapable of surviving T. gondii infection. Similar to previous reports in NOS2-deficient mice [33, 34], death did not occur during early infection, but at a time when latent infection has been normally achieved in wild-type mice in this model. T. gondii is able to replicate in most nucleated cells. In the immunocompetent, serious disease is averted by a vigorous immune response, dominated by IFN-γ production, which leads to parasite killing and the transformation of remaining parasites into the dormant (bradyzoite; cyst) form, largely in the central nervous system [35]. This systemic immune response is, perforce, kept under tight control. Neither genetic deficiency of mediators driving the protective immune response (eg, IFN-γ) nor of mediators that restrain this response (eg, IL-10) is compatible with survival during toxoplasmosis [35]. Mice with the former deficiency die with increased parasite burdens; with the latter, of unrestrained production of proinflammatory mediators and decreased parasite burdens [35]. Thus, the mortality observed with IDO inhibition during toxoplasmosis was compatible with an inability to control either the parasite or the immune response to the parasite. Of note, IDO inhibition was associated with significantly increased parasite burdens (Figure 1B). Such inhibition did not alter serum levels of critical pro- and antiinflammatory cytokines (IFN-γ, TNF-α, IL-10; Figure 1C–E), something mirrored by a lack of biologically important changes in the expression levels of mRNA for IL-12/23p40, IFN-γ, TNF-α or IL-10 in the central nervous system (Figure 1F–I), Thus, IDO inhibition throughout the course of toxoplasmosis is associated with a late failure to control parasite replication, in the absence of evident effects on the immune response.
Figure 1.
IDO inhibition during acute toxoplasmosis leads to an inability to control infection. C57BL/6 mice were infected intraperitoneally with T. gondii (ME49 strain), in the presence and absence of IDO inhibition with D1-MT. A, Survival analysis (n = 8 mice/group; closed symbols, 1-MT; open symbols, control). **P < .0001 (Mantel-Cox test). Representative of 3 independent experiments (100% vs 0% mortality in the 2 not pictured). B, Parasite burden. T. gondii cysts were counted in brain homogenates of mice sacrificed 30 days after infection. Values shown are means + standard error (SE) of 22 mice/group, pooled from 2 separate experiments. *P < .03 (Student t test). C–E, Systemic cytokine production was quantified by the CCA assay [28] 30 days after infection. Values shown are means + SE of 8 mice/group. C, Serum IFN-γ. D, Serum TNF-α. E, Serum IL-10. F–I, Cytokine mRNA was quantified in brain by qRT-PCR 30 days after infection. Values shown are means + SE of data normalized for β-actin mRNA expression of 8 infected mice/treatment, and 3 uninfected mice. F, IL-12/23p40 mRNA. G, IFN-γ mRNA. H, TNF-α mRNA. I, IL-10 mRNA.
T. gondii infection leads to sustained upregulation of IDO-1 and IDO-2 mRNA expression in the brain (Figure 2A and 2B). Immunohistochemical analysis revealed expression of IDO-1 by glial and other cells (Figure 2C–F). In order to analyze the effect of IDO inhibition on latent T. gondii infection, we treated mice with D1-MT beginning 30 days after infection. Notably, IDO inhibition led to reactivation of disease and mortality (Figure 3A), associated with increased parasite burdens (Figure 3B), but without evident effects on either local or systemic production of inflammatory mediators (Figure 3C–I).
Figure 2.
Toxoplasmosis leads to sustained central nervous system expression of IDO. A and B, mRNA was quantified in brain by qRT-PCR 30 days after infection. Values shown are means + SE of data normalized for β-actin mRNA expression of 8 infected mice/treatment, and 3 uninfected mice. *ANOVA P = .0002, Tukey’s correction; P < .05; **ANOVA P = .0254, Tukey’s correction P < .05, compared with uninfected mice. A, IDO-1 mRNA expression. B, IDO-2 mRNA expression. IDO-1 and -2 mRNA expression in the brain was similarly elevated 40 days and 70 days after infection (data not shown). C–F, Immunohistochemical analysis of IDO-1 protein expression in the brains of T. gondii–infected mice. C and D, T. gondii–infected mice develop foci of nonsuppurative encephalitis (arrowhead) and gliosis. Individual glial cells often have cytoplasmic expression of IDO (black arrows). E and F, T. gondii cysts (arrowheads) within the neuropil show no significant inflammatory response or IDO immunoreactivity. Intense IDO reactivity is often associated with endothelial cells that line small capillaries. Magnification: 400× (bar = 46 μm).
Figure 3.
IDO inhibition during latent infection with T. gondii induces disease reactivation. A, Survival analysis (n = 8 mice/group). Treatment with D1-MT was begun 30 days after infection (dotted line). **P < .01 (Mantel–Cox test). Representative of 3 independent experiments. B, Parasite burden. T. gondii cysts were counted in brain homogenates of mice sacrificed 70 days after infection. Closed symbols, 1-MT; open symbols, control. Values shown are means + SE of 7–8 mice/group *P < .01 (Student t test) compared with control treatment. C–E, Systemic cytokine production was quantified by the CCA assay at the times indicated; values shown are means + SE of 5–6 mice/group. Closed symbols, 1-MT; open symbols, control. *P < .01 (Student t test) compared with control treatment. C, Serum IFN-γ. D, Serum TNF-α. E, Serum IL-10. F–I, Cytokine mRNA was quantified in brain by qRT-PCR at the times indicated. Hatched bars, uninfected; white bars, infected/control treatment; black bars, infected/1-MT treatment. Values shown are means + SE of data normalized for β-actin mRNA expression of 6 mice/group (3 for uninfected mice). F, IL-12/23p40 mRNA. G, IFN-γ mRNA. H, TNF-α mRNA. I, IL-10 mRNA.
IDO Plays a Role in Immune Counterregulation During Cutaneous Leishmaniasis
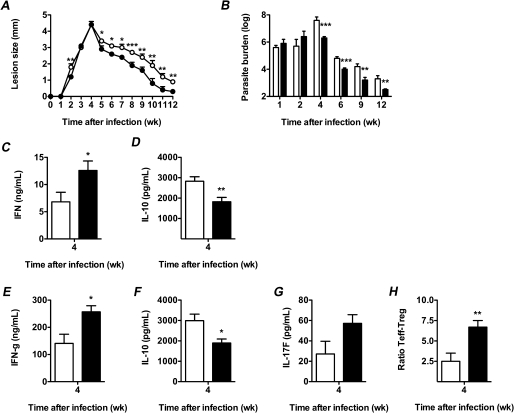
Like Toxoplasma, Leishmania are tryptophan auxotrophs [36]. Cutaneous leishmaniasis has additional similarities with toxoplasmosis. Parasite replication is restricted by an immune response in which IFN-γ plays a central role, and immune counterregulation is key to the outcome of infection. In the case of L. major infection, IL-10 production is critical to the negotiation of latency between host and parasite; neutralization of IL-10 leads not to latency, but to sterile cure [29, 37]. Given these similarities, we analyzed the effect of IDO inhibition on the course of cutaneous leishmaniasis. We infected C57BL/6 mice intradermally with L. major (V1 strain), in the presence and absence of IDO inhibition with D1-MT. As expected, L. major infection induced lesional expression of IDO-1 and IDO-2 mRNA (Figure 4A and 4B). Immunohistochemical analysis revealed that the majority of lesional IDO-1 immunoreactivity was associated with granulocytes, while lymph-node immunoreactivity was restricted to mononuclear inflammatory cells (likely, both macrophages and dendritic cells) (Figure 4C–F). Inhibition of IDO led to significant decreases in lesion size during the phase of parasite clearance (Figure 5A), along with a significantly better control of parasite load (Figure 5B). During the early phase of parasite clearance, D1-MT treatment was associated with significantly increased serum IFN-γ and decreased serum IL-10 concentrations, (Figure 5C and 5D), without changes in serum IL-4 concentrations (data not shown)—findings that mirrored Leishmania antigen-driven IFN-γ and IL-10 production by T cells isolated from lymph nodes draining the sites of cutaneous infection (Figure 5E and 5F). D1-MT treatment was also associated with a trend toward increased lymph node T-cell production of IL-17F (Figure 5G) and IL-17A production (data not shown) in response to leishmanial antigens. At this same time, IDO inhibition was associated with a significant increase in the lesional ratio of effector to regulatory CD4+ T cells (Figure 5H). Thus, in contrast to its role in toxoplasmosis, IDO plays a modest role in immune counterregulation in leishmaniasis, restraining both the immune response and pathogen clearance.
Figure 4.
Cutaneous leishmaniasis leads to IDO expression in lesions and lymph nodes. A and B, mRNA was quantified in ears by qRT-PCR at the indicated times after infection. Values shown are means + SE of 3–4 mice/group, of data normalized for β-actin mRNA expression. *P < .05 (Student t test) compared with uninfected mice. A, IDO-1 mRNA. B, IDO-2 mRNA. C–F, Immunohistochemical analysis of IDO-1 protein expression in L. major–infected mice. C, Ear lesions: mixed inflammation of the ear skin dermis (D) extends to either side of auricular cartilage (AC) and is associated with intense cytoplasmic staining of inflammatory cells with IDO. D, Ear lesions: higher magnification of mixed dermal inflammation reveals that the majority of cytoplasmic IDO immunoreactivity is in granulocytes with characteristic segmented nuclei (arrowheads). E, Draining lymph nodes: cytoplasmic expression of IDO is restricted to mononuclear inflammatory cells (macrophages and dendritic cells) within the subcapsular sinus (SS), and within the superficial and mid cortex (arrowheads). F, Draining lymph nodes: higher magnification of lymph node cortex containing mononuclear cells with cytoplasmic IDO immunoreactivity (arrowhead). Magnification: A, C 100× (bar = 180 μm); B, D 400× (bar = 46 μm).
Figure 5.
IDO inhibition during cutaneous leishmaniasis leads to enhanced parasite clearance, associated with decreased immune counterregulation. C57BL/6 mice were infected intradermally with L. major (V1 strain) in the presence and absence of IDO inhibition with D1-MT. A, Lesion size. n = 36 mice/group to begin with (with subsequent harvest of 6 mice/group at 1, 2, 4, 6, 9, and 12 weeks after infection). Representative of 2 independent experiments. B, Parasite burden. Quantification of parasite outgrowth from titrations of ear tissue lysates was performed at the indicated times. Values shown are means + SE of 6 mice/group. C and D, Systemic cytokine production was quantified by the CCA assay 4 weeks after infection. Values shown are means + SE of 6 mice/group. C, Serum IFN-γ. D, Serum IL-10. E–G, L. major antigen-driven cytokine production. Cytokines were quantified by ELISA in the supernatants of cells isolated from draining lymph nodes (4 weeks after infection) that were restimulated in vitro with L. major promastigote lysates. Values shown are means + SE of 6 mice/group. E, IFN-γ. F, IL-10. G, IL-17F. H, Lesional effector/regulatory T-cell ratio. Effector (TCR+CD4+CD25+FOXP3−) and regulatory (TCR+CD4+FOXP3+) T cells were quantified by flow cytometry 4 weeks after infection. Values shown are means + SE of 6 mice/group. *P < .05, **P < .01, ***P < .005 (Student t test) compared with control treatment. Closed symbols, 1-MT; open symbols, control.
IDO Inhibition Fails to Alter the Pathobiology of HSV-1 Infection
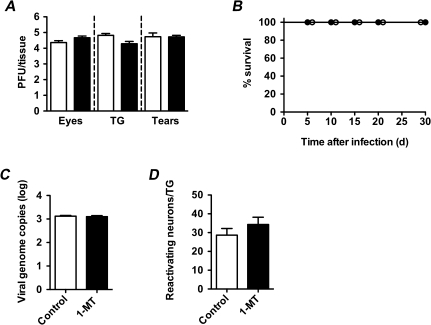
Protective immunity against the neurotropic virus HSV-1 depends on IFN-γ [38, 39]. HSV-1 is also prototypical of viruses reported to undergo IDO-mediated restriction of replication in vitro [19]. We thus inoculated C57BL/6 mice with 2 × 105 pfu of HSV-1 strain 17syn+ [30] on scarified corneas, in the presence and absence of IDO inhibition with D1-MT (begun 3 days prior to infection). The acute phase of infection was monitored by evaluating viral titers in the eyes and trigeminal ganglia 4 days after inoculation—the time of peak viral replication [19]—in a subset of mice. As shown in Figure 6A, IDO inhibition had no effect on acute viral replication. Further, IDO inhibition had no effect on mortality (Figure 6B), did not alter the burden of latent infection (Figure 6C), and failed to affect the reactivation competency of latent genomes (Figure 6D). Thus, inhibition of IDO during HSV-1 infection failed to alter viral replication, virulence, or the induction of latency. Similar results were seen with IDO inhibition during HSV-1 infection of Swiss Webster mice (data not shown).
Figure 6.
IDO inhibition during HSV-1 infection fails to alter disease or viral dynamics in C57BL/6 mice. Mice were inoculated with 2 × 105 pfu of HSV-1 strain 17syn+ on scarified corneas, 3 days after beginning treatment with D1-MT or control. A, Acute viral replication (n = 3–7 mice/group). Viral titers in eyes, trigeminal ganglia, and tears were quantified by plaque assay 4 days after infection. B, Survival analysis (n = 9 mice/group). C, Burden of latent viral infection (n = 3–8 mice/group). Latent viral genomes were quantified by quantitative polymerase chain reaction 30 days after infection. D, Reactivation competence of latent HSV-1 genomes (n = 6 mice/group). Immunohistochemical analysis of lytic protein expression in trigeminal ganglia (TG), performed 3 days after explant (on day 45 of infection) in the presence of acyclovir. Representative of 2 independent experiments. Values shown are means + SE; closed symbols, 1-MT; open symbols, control.
We next analyzed whether IDO inhibition perturbed the viral/host balance of established HSV-1 latent infection. Forty-five days after inoculation with HSV-1, latently infected Swiss Webster mice (8/group) were treated with D1-MT or vehicle control. Treatment was continued for an additional 45 days, during which time animals were observed for signs of encephalitis, death, or reactivation of virus in eye swabs. Of note, no animals died, none became sick, and no infectious virus was detected in eye swabs (data not shown). Histological examination of the central nervous system and trigeminal ganglia showed no differences between groups (data not shown). Thus, IDO inhibition during latent infection does not result in uncontrolled reactivation and spread of latent HSV in the nervous system. Taken together, these studies indicate that IDO does not play a biologically important role in restricting HSV-1 replication or latency.
1-MT Isomers Have Similar Effects on Intracellular Infection
The stereoisomers of 1-MT may have somewhat different biological properties. The L isomer has been reported to be more potent at inhibiting purified IDO-1 enzyme in cell-free systems or recombinant IDO-1 expressed in cell lines, and may preferentially inhibit IDO in tumor cells [40]. However, the D isomer appears more effective in reversing the suppression of T cells by IDO-1-expressing dendritic cells in both human and mouse systems [40]. This may be due to lower nonspecific toxicity, since the DL mixture appears to have off-target toxic effects on antigen-presenting cells when used at high concentrations [41]. It is also possible that the D isomer is more effective at inhibiting the activity of IDO-2 [26]. Whatever the mechanism, the D isomer has been shown to be effective in vivo in mice [2, 40], and in vitro using IDO-expressing antigen-presenting cells from both mice and humans [8, 42, 43].
Notably, parallel experiments employing D1-MT and L1-MT led to identical results in mouse models of experimental toxoplasmosis (mortality; Figure 7A), cutaneous leishmaniasis (lesion size; Figure 7B), and HSV-1 infection (acute viral replication, mortality, viral latency, and viral reactivation competence—data not shown).
Figure 7.
1-MT isomers have similar biological effects on experimental toxoplasmosis and leishmaniasis. A, T. gondii infection, survival analysis. C57BL/6 mice were infected intraperitoneally with T. gondii (ME49 strain) in the presence and absence of IDO inhibition with D1-MT (black circles), L1-MT (black squares), or vehicle control (open circles). n = 6 mice/group; **P < .0001 (Mantel–Cox test). B, L. major infection, lesion size. C57BL/6 mice were infected intradermally with L. major (V1 strain), in the presence and absence of IDO inhibition with D1-MT, L1-MT or vehicle control (symbols as in A). Values shown represent means + SE of 8 mice/group. MANOVA P < .025; *P < .05, **P < .01, ***P < .001 (Student t test).
DISCUSSION
The data presented here demonstrate that IDO, recently recognized to play key physiological and pathophysiological roles in immune counterregulation, play biologically important, contradictory roles during intracellular protozoal infection—facilitating (T. gondii) or suppressing (L. major) microbial clearance in a pathogen-specific manner. Our finding of an important antimicrobial role for IDO in toxoplasmosis, in the absence of demonstrable effects on the immune response, provides in vivo validation of an extensive in vitro literature going back more than 20 years. As for other protozoa, IDO has also recently been shown to play a critical role in host resistance against Trypanosoma cruzi in mouse models. In the case of T. cruzi, the antimicrobial effects of IDO appear to be mediated by kynurenines [44]. On the other hand, IDO inhibition has been reported to lead to increased effector T-cell responses in the absence of any robust effects on disease course in a mouse model of malaria [45].
Despite an extensive in vitro literature on viruses and IDO, viruses are not marked by tryptophan auxotrophy. The mechanisms by which tryptophan restriction leads to suppression of in vitro viral replication have thus not been entirely straightforward. Tryptophan starvation stresses mammalian cells and inhibits their proliferation [46]. It is perhaps not surprising that such stress renders cells less efficient as hosts for viral replication, whether or not this is actually exploited as an antiviral defense mechanism in vivo. The lack of an evident role for IDO in restricting HSV-1 replication in vivo suggests that such in vitro studies may be misleading and that in vivo studies will be needed to determine whether IDO modulates the course of infection with particular viruses. Indeed, consonant with the hypothesis that IDO activation plays an immunosuppressive role in HIV infection, blockade of IDO during retroviral infection of mice (LP-BM5) and rhesus macaques (simian immunodeficiency virus) led to decreased viral burdens [47, 48].
As for other classes of pathogens, despite an in vitro literature on IDO-mediated restriction of Chlamydiae replication, our preliminary experiments have suggested that IDO inhibition fails to alter pulmonary bacterial burden during lung infection with Chlamydophila pneumoniae (data not shown). In the case of fungi, the immunoregulatory properties of IDO appear to be essential to limit inflammatory responses to Aspergillus and Candida, which, in the absence of such restraint, compromise the hosts’ ability to eradicate infection [6]. Similar findings have been reported in mouse models of tuberculosis [49]. Immunohistochemical analysis revealed that infection of mice with Mycobacterium tuberculosis is associated with dramatic upregulation of IDO-1 expression [49], something that we have replicated and found to be true in human disease as well (data not shown). Elegant studies employing bone marrow chimeric mice indicate that IDO expression by nonhematopoietic cells in the lung is necessary to limit IL-17 production and harmful neutrophilic inflammation during experimental tuberculosis [49].
It will be noted that the biologically antagonistic, pathogen-specific roles played by IDO during infection (restraint of microbial replication, restraint of the host response) are remarkably similar, at least superficially, to the activities of another enzyme exploited by both the innate and adaptive immune systems—inducible nitric oxide synthase [50]. Critical issues remain to be addressed in these and other infection models, including: (1) the relative role of IDO-1 and -2 in immune counterregulation and antimicrobial activity, (2) which IDO-expressing cell types are responsible for these diverse activities, (3) the relative role of tryptophan starvation and tryptophan metabolite production in these activities, and (4) the potential contribution of tryptophan catabolic enzyme expression by pathogens themselves to disease pathogenesis. With regard to the first of these issues, the current studies do not provide much in the way of insight. The fact that IDO-1 and -2 are differentially expressed by distinct cell types at baseline and undergo differential regulation of stimulated expression in vivo suggests that the enzymes are unlikely to be fully functionally redundant [51]. That said, despite the fact that IDO enzymes play opposing biological roles in experimental toxoplasmosis and leishmaniasis, the similar fold-induction of IDO-1 and -2 mRNA expression in these infections in the face of very different levels of baseline IDO-2 mRNA expression in ear and brain do not suggest an obvious hypothesis for enzyme specificity of the observed dominant biological effect. As a caveat, it should be noted that regulation of IDO is quite complex, including the use of alternate promoters and the generation of diverse splice forms, as well as posttranslational modification of enzyme activity [51, 52]. As for the last of these issues, while Basic Local Alignment Search Tool (BLAST) searches suggest that it is unlikely that T. gondii and L. major express IDO homologs, such searches indicate that several bacterial pathogens of human importance—including Pseudomonas aeruginosa and Burkholderia cepacia—do encode IDO homologs (not shown), something that may well be exploitable therapeutically.
While these studies suggest appropriate caution during sustained therapeutic inhibition of IDO (eg, giving secondary chemoprophylaxis to those latently infected with T. gondii), both counterregulatory and antimicrobial activities may provide potential novel therapeutic targets during chronic infection. For infections in which IDO plays a counterregulatory role such as leishmaniasis and HIV, IDO inhibition may be useful as an adjunct to antimicrobial therapy. On the other hand, for latent infections in which IDO plays an antimicrobial role, IDO may also provide a therapeutic target. Eradication of latent infection is hampered by the fact that, in the latent state, pathogens are metabolically inert and thus insensitive to the activity of current antimicrobials. For a latent pathogen against which IDO is an important antimicrobial effector mechanism, IDO inhibition may facilitate pathogen eradication through controlled reactivation, under cover of antibiotics.
Notes
Acknowledgments.
The authors thank M. Rao for statistical assistance.
Financial support.
This work was supported by grants from the National Institute of Allergy and Infectious Disease to C. L. K. (AI057992), A. L. M. (AI063402), and N. M. S. (AI032121); the National Institute of Child Health and Development to A. L. M. (HD041187); the National Cancer Institute to D. L. M. (CA096651, CA103320, CA112431); and the National Institute of Arthritis and Musculoskeletal and Skin Diseases to S. D. (AR007594).
Potential conflicts of interest.
D. H. M., A. L. M., J. B. D, G. C. P., C. L. K., and S. D. have intellectual property interests in the therapeutic use of IDO inhibitors; D. H. M., A. L. M., J. B. D, and G. C. P. receive consulting income from NewLink Genetics Inc., a company of which G. C. P. and J. B. D are major shareholders and from which G. C. P has received a grant. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–74. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 2.Munn DH, Sharma MD, Baban B, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–42. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Fallarino F, Grohmann U, Vacca C, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069–77. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 4.Baban B, Chandler PR, Sharma MD, et al. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol. 2009;183:2475–83. doi: 10.4049/jimmunol.0900986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–8. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romani L, Zelante T, De Luca A, Fallarino F, Puccetti P. IL-17 and therapeutic kynurenines in pathogenic inflammation to fungi. J Immunol. 2008;180:5157–62. doi: 10.4049/jimmunol.180.8.5157. [DOI] [PubMed] [Google Scholar]
- 7.Sharma MD, Hou DY, Liu Y, et al. Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood. 2009;113:6102–1. doi: 10.1182/blood-2008-12-195354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munn DH, Sharma MD, Hou D, et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114:280–90. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uyttenhove C, Pilotte L, Theate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–74. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 10.Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11:312–9. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- 11.Boasso A, Shearer GM. How does indoleamine 2,3-dioxygenase contribute to HIV-mediated immune dysregulation. Curr Drug Metab. 2007;8:217–23. doi: 10.2174/138920007780362527. [DOI] [PubMed] [Google Scholar]
- 12.Pfefferkorn ER. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Nat Acad Sci USA. 1984;81:908–12. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlin JM, Borden EC, Sondel PM, Byrne GI. Interferon-induced indoleamine 2,3-dioxygenase activity in human mononuclear phagocytes. J Leuk Biol. 1989;45:29–34. doi: 10.1002/jlb.45.1.29. [DOI] [PubMed] [Google Scholar]
- 14.Murray HW, Szuro-Sudol A, Wellner D, et al. Role of tryptophan degradation in respiratory burst-independent antimicrobial activity of gamma interferon-stimulated human macrophages. Infect Immun. 1989;57:845–9. doi: 10.1128/iai.57.3.845-849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmitz JL, Carlin JM, Borden EC, Byrne GI. Beta interferon inhibits Toxoplasma gondii growth in human monocyte-derived macrophages. Infect Immun. 1989;57:3254–6. doi: 10.1128/iai.57.10.3254-3256.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlin JM, Borden EC, Byrne GI. Interferon-induced indoleamine 2,3-dioxygenase activity inhibits Chlamydia psittaci replication in human macrophages. J Interferon Res. 1989;9:329–37. doi: 10.1089/jir.1989.9.329. [DOI] [PubMed] [Google Scholar]
- 17.Pantoja LG, Miller RD, Ramirez JA, Molestina RE, Summersgill JT. Inhibition of Chlamydia pneumoniae replication in human aortic smooth muscle cells by gamma interferon-induced indoleamine 2, 3-dioxygenase activity. Infect Immun. 2000;68:6478–81. doi: 10.1128/iai.68.11.6478-6481.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams O, Besken K, Oberdorfer C, MacKenzie CR, Russing D, Daubener W. Inhibition of human herpes simplex virus type 2 by interferon gamma and tumor necrosis factor alpha is mediated by indoleamine 2,3-dioxygenase. Microbes Infect. 2004;6:806–12. doi: 10.1016/j.micinf.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Adams O, Besken K, Oberdorfer C, MacKenzie CR, Takikawa O, Daubener W. Role of indoleamine-2,3-dioxygenase in alpha/beta and gamma interferon-mediated antiviral effects against herpes simplex virus infections. J Virol. 2004;78:2632–6. doi: 10.1128/JVI.78.5.2632-2636.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bodaghi B, Goureau O, Zipeto D, Laurent L, Virelizier JL, Michelson S. Role of IFN-gamma-induced indoleamine 2,3 dioxygenase and inducible nitric oxide synthase in the replication of human cytomegalovirus in retinal pigment epithelial cells. J Immunol. 1999;162:957–64. [PubMed] [Google Scholar]
- 21.Obojes K, Andres O, Kim KS, Daubener W, Schneider-Schaulies J. Indoleamine 2,3-dioxygenase mediates cell type-specific anti-measles virus activity of gamma interferon. J Virol. 2005;79:7768–6. doi: 10.1128/JVI.79.12.7768-7776.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terajima M, Leporati AM. Role of indoleamine 2,3-dioxygenase in antiviral activity of interferon-gamma against vaccinia virus. Viral Immunol. 2005;18:722–9. doi: 10.1089/vim.2005.18.722. [DOI] [PubMed] [Google Scholar]
- 23.Fujigaki S, Saito K, Takemura M, et al. L-tryptophan-L-kynurenine pathway metabolism accelerated by Toxoplasma gondii infection is abolished in gamma interferon-gene-deficient mice: cross-regulation between inducible nitric oxide synthase and indoleamine-2,3-dioxygenase. Infect Immun. 2002;70:779–86. doi: 10.1128/iai.70.2.779-786.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silva NM, Rodrigues CV, Santoro MM, Reis LF, Alvarez-Leite JI, Gazzinelli RT. Expression of indoleamine 2,3-dioxygenase, tryptophan degradation, and kynurenine formation during in vivo infection with Toxoplasma gondii: induction by endogenous gamma interferon and requirement of interferon regulatory factor 1. Infect Immun. 2002;70:859–68. doi: 10.1128/iai.70.2.859-868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ball HJ, Sanchez-Perez A, Weiser S, et al. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;396:203–13. doi: 10.1016/j.gene.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Metz R, Duhadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, Prendergast GC. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007;67:7082–7. doi: 10.1158/0008-5472.CAN-07-1872. [DOI] [PubMed] [Google Scholar]
- 27.Aliberti J, Serhan C, Sher A. Parasite-induced lipoxin A4 is an endogenous regulator of IL-12 production and immunopathology in Toxoplasma gondii infection. J Exp Med. 2002;196:1253–62. doi: 10.1084/jem.20021183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finkelman FD, Morris SC. Development of an assay to measure in vivo cytokine production in the mouse. Int Immunol. 1999;11:1811–8. doi: 10.1093/intimm/11.11.1811. [DOI] [PubMed] [Google Scholar]
- 29.Belkaid Y, Hoffmann KF, Mendez S, et al. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J Exp Med. 2001;194:1497–506. doi: 10.1084/jem.194.10.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sawtell NM. The probability of in vivo reactivation of herpes simplex virus type 1 increases with the number of latently infected neurons in the ganglia. J Virol. 1998;72:6888–92. doi: 10.1128/jvi.72.8.6888-6892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sawtell NM, Thompson RL, Haas RL. Herpes simplex virus DNA synthesis is not a decisive regulatory event in the initiation of lytic viral protein expression in neurons in vivo during primary infection or reactivation from latency. J Virol. 2006;80:38–50. doi: 10.1128/JVI.80.1.38-50.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia L, Schweikart K, Tomaszewski J, et al. Toxicology and pharmacokinetics of 1-methyl-d-tryptophan: absence of toxicity due to saturating absorption. Food Chem Toxicol. 2008;46:203–11. doi: 10.1016/j.fct.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scharton-Kersten TM, Yap G, Magram J, Sher A. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med. 1997;185:1261–73. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schluter D, Deckert-Schluter M, Lorenz E, Meyer T, Rollinghoff M, Bogdan C. Inhibition of inducible nitric oxide synthase exacerbates chronic cerebral toxoplasmosis in Toxoplasma gondii–susceptible C57BL/6 mice but does not reactivate the latent disease in T. gondii–resistant BALB/c mice. J Immunol. 1999;162:3512–8. [PubMed] [Google Scholar]
- 35.Yap GS, Shaw MH, Ling Y, Sher A. Genetic analysis of host resistance to intracellular pathogens: lessons from studies of Toxoplasma gondii infection. Microbes Infect. 2006;8:1174–8. doi: 10.1016/j.micinf.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 36.O'Daly JA, Rodriguez MB. Differential growth requirements of several Leishmania spp. in chemically defined culture media. Acta Trop. 1988;45:109–26. [PubMed] [Google Scholar]
- 37.O'Garra A, Vieira P. T(H)1 cells control themselves by producing interleukin-10. Nat Rev Immunol. 2007;7:425–8. doi: 10.1038/nri2097. [DOI] [PubMed] [Google Scholar]
- 38.Bouley DM, Kanangat S, Wire W, Rouse BT. Characterization of herpes simplex virus type-1 infection and herpetic stromal keratitis development in IFN-gamma knockout mice. J Immunol. 1995;155:3964–71. [PubMed] [Google Scholar]
- 39.Geiger KD, Nash TC, Sawyer S, et al. Interferon-gamma protects against herpes simplex virus type 1-mediated neuronal death. Virology. 1997;238:189–97. doi: 10.1006/viro.1997.8841. [DOI] [PubMed] [Google Scholar]
- 40.Hou DY, Muller AJ, Sharma MD, et al. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res. 2007;67:792–801. doi: 10.1158/0008-5472.CAN-06-2925. [DOI] [PubMed] [Google Scholar]
- 41.Agaugue S, Perrin-Cocon L, Coutant F, Andre P, Lotteau V. 1-Methyl-tryptophan can interfere with TLR signaling in dendritic cells independently of IDO activity. J Immunol. 2006;177:2061–71. doi: 10.4049/jimmunol.177.4.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boasso A, Herbeuval JP, Hardy AW, et al. HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood. 2007;109:3351–9. doi: 10.1182/blood-2006-07-034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Munn DH, Sharma MD, Lee JR, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–70. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 44.Knubel CP, Martinez FF, Fretes RE, et al. Indoleamine 2,3-dioxygenase (IDO) is critical for host resistance against Trypanosoma cruzi. FASEB J. 2010;24:2689–701. doi: 10.1096/fj.09-150920. [DOI] [PubMed] [Google Scholar]
- 45.Tetsutani K, To H, Torii M, Hisaeda H, Himeno K. Malaria parasite induces tryptophan-related immune suppression in mice. Parasitology. 2007;134:923–30. doi: 10.1017/S0031182007002326. [DOI] [PubMed] [Google Scholar]
- 46.Takikawa O, Kuroiwa T, Yamazaki F, Kido R. Mechanism of interferon-gamma action. Characterization of indoleamine 2,3-dioxygenase in cultured human cells induced by interferon-gamma and evaluation of the enzyme-mediated tryptophan degradation in its anticellular activity. J Biol Chem. 1988;263:2041–8. [PubMed] [Google Scholar]
- 47.Boasso A, Vaccari M, Fuchs D, et al. Combined effect of antiretroviral therapy and blockade of IDO in SIV-infected rhesus macaques. J Immunol. 2009;182:4313–20. doi: 10.4049/jimmunol.0803314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoshi M, Saito K, Hara A, et al. The absence of IDO upregulates type I IFN production, resulting in suppression of viral replication in the retrovirus-infected mouse. J Immunol. 2010;185:3305–12. doi: 10.4049/jimmunol.0901150. [DOI] [PubMed] [Google Scholar]
- 49.Desvignes L, Ernst JD. Interferon-gamma-responsive nonhematopoietic cells regulate the immune response to Mycobacterium tuberculosis. Immunity. 2009;31:974–85. doi: 10.1016/j.immuni.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Ann Rev Immunol. 1997;15:323–50. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 51.Ball HJ, Yuasa HJ, Austin CJ, Weiser S, Hunt NH. Indoleamine 2,3-dioxygenase-2; a new enzyme in the kynurenine pathway. Int J Biochem Cell Biol. 2009;41:467–71. doi: 10.1016/j.biocel.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Thomas SR, Terentis AC, Cai H, et al. Post-translational regulation of human indoleamine 2,3-dioxygenase activity by nitric oxide. J Biol Chem. 2007;282:23778–87. doi: 10.1074/jbc.M700669200. [DOI] [PubMed] [Google Scholar]