Abstract
Background. Most reported human H5N1 viral infections have been severe and were detected after hospital admission. A case ascertainment bias may therefore exist, with mild cases or asymptomatic infections going undetected. We sought evidence of mild or asymptomatic H5N1 infection by examining H5N1-specific T-cell and antibody responses in a high-risk cohort in Vietnam.
Methods. Peripheral blood mononuclear cells were tested using interferon-γ enzyme-linked immunospot T assays measuring the response to peptides of influenza H5, H3, and H1 hemagglutinin (HA), N1 and N2 neuraminidase, and the internal proteins of H3N2. Horse erythrocyte hemagglutination inhibition assay was performed to detect antibodies against H5N1.
Results. Twenty-four of 747 individuals demonstrated H5-specific T-cell responses but little or no cross-reactivity with H3 or H1 HA peptides. H5N1 peptide-specific T-cell lines that did not cross-react with H1 or H3 influenza virus HA peptides were generated. Four individuals also had antibodies against H5N1.
Conclusions. This is the first report of ex vivo H5 HA-specific T-cell responses in a healthy but H5N1-exposed population. Our results indicate that the presence of H5N1-specific T cells could be an additional diagnostic tool for asymptomatic H5N1 infection.
(See the editorial commentary by Epstein, on pages 4–6.)
Influenza H5N1 remains endemic in domestic poultry in large parts of Asia, and although the total number of human infections is relatively small, sporadic human cases with a high risk of death are still being reported [1, 2]. Since 2003, >500 human cases of highly pathogenic influenza A H5N1 have been reported, with 119 cases occurring in Vietnam [3]. At present, H5N1 influenza cannot be transmitted readily between humans, but the possibility remains of a recombination between H5N1 and other influenza viruses, resulting in a virulent and easily transmissible virus [4].
The reported frequency and severity of H5N1 infection in humans is almost certainly biased by the underdetection of mild or asymptomatic cases: leading to an underestimate of the number of cases and an overestimate of the case fatality rate. The extent of this bias is indicated by seroprevalence surveys that have reported anti-H5 antibody prevalence in exposed groups of between 0% and 12% [5–9]. The presence of virus-neutralizing antibody is important for protection against influenza, and antibodies that recognize specific hemagglutinin (HA) subtypes can give an indication of recent infection history [10, 11]. However, measurement of H5N1-specific neutralizing antibodies has been problematic because the traditional hemagglutination inhibition (HAI) assay has low sensitivity for the detection of H5N1 antibodies [12]. Alternative assays that have been used for the detection of H5N1 infection include horse erythrocyte HAI, microneutralization, and pseudoparticle assays [13]. These assays are all subject to false-positive reactions due to the presence of cross-subtype neutralizing antibodies [14]. Measuring interferon (IFN) γ secretion by peripheral blood mononuclear cells (PBMCs) after stimulation with pools of HA peptides from different influenza strains demonstrates the specificity and reactivity of T cells generated by strain-specific vaccination [15–17] or memory cells generated after natural infection [18–21].
In this study, we set out to investigate the rate of infection with H5N1 virus in a community in rural Vietnam that had previously experienced H5N1 cases in both poultry and humans by measuring the prevalence of specific T-cell responses against the HA and neuraminidase (NA) of H5N1 influenza virus. We also compared T-cell responses against the proteome of seasonal H3N2 and the HA and N1 of H5N1 and H1N1 influenza in the community cohort with those in a group of persons who had recovered from H5N1 infection and in healthy controls with no exposure to H5N1.
MATERIALS AND METHODS
Patient Cohorts
For the community cohort, a household-based cohort was established in a community in northern Vietnam that had previously experienced outbreaks of H5N1 in poultry and human H5N1 cases. Human H5N1 infections had occurred in the community in 2004, and additional human cases were identified in 2007 and 2008 in nearby villages. Poultry outbreaks of H5N1 had been detected in the province intermittently since 2004, including an outbreak 1 month before the beginning of the study. Households were randomly selected from a complete household register using a random number table. If a selected household declined to participate, the nearest neighbor was approached for participation. Members of the cohort provided blood for collection of PBMCs and plasma in December 2007.
For the recovered case patients, persons who were convalescent after H5N1 infection (n = 19) were recruited between July 2008 and March 2009, at intervals of 84–1449 days (median, 1300) after H5N1 onset (HHT Trang, A. Fox, T. Dong, LQ Mai, VTK Lien, T. Powell, TN Duong, NTT Yen, PQ Thai, NT Hien, P Horby, authors' unpublished data). For the healthy control group, PBMCs from volunteers (n = 271) from the United Kingdom and Vietnam were tested by enzyme-linked immunospot spot (ELISPOT) assays [19]. All participants provided written informed consent. The study was approved by the ethical review boards of the National Institute of Hygiene and Epidemiology (Hanoi, Vietnam), the University of Oxford (United Kingdom), and the London School of Hygiene & Tropical Medicine (United Kingdom).
Media, Peptides, and ELISPOT Screening
Dulbecco’s phosphate-buffered saline (Sigma–Aldrich) and R10 (Roswell Park Memorial Institute 1640 medium plus 10% vol/vol fetal calf serum, glutamine, and penicillin streptomycin) medium were used as described elsewhere [19]. The sequences of the full influenza proteome from H3N2, A/New York/388/2005 (HA and NA) and A/New York/232/2004 (H3N2) (internal proteins), HA from H1N1 (A/Hong Kong/1134/98 and A/NewYork/228/2003), and H5 and N1 from H5N1 (A/Vietnam/CL26/2004) were split into overlapping peptides of 18–21 residues overlapping by 10, using Peptgen (http://www.hiv.lanl.gov/content/sequence/PEPTGEN/peptgen.html) synthesized by Sigma-Aldrich. Peptides were divided into pools (Table 1). Overlapping peptides spanning the entire length of each HA protein were split up into 2 pools (eg, H5 into H5-1 and H5-2), and the amino acid range is shown for each pool. The exact sequences of the peptides are available on request.
Table 1.
Peptide Pool Identities
| Pool | Peptide Identity (No. of Peptides in Pool)a |
| HA1-1 | H1 HA1 1–294 (37) |
| HA1-2 | H1 HA1 285–565 (37) |
| HA3-1 | H3 HA3 1–298 (37) |
| HA3-2 | H3 HA3 289–556 (37) |
| HA5-1 | H5 HA5 1–281 (38) |
| HA5-2 | H5 HA5 272–565 (39) |
| NA1-1 | N1 NA1 1–227 (30) |
| NA1-2 | N1 NA1 218–442 (31) |
| NA2-1 | N2 NA2 1–303 (40) |
| NA2-2 | N2 NA2 294–467 (24) |
| PB1-1 | H3N2 PB1 1–380 (50) |
| PB1-2 | H3N2 PB1 370–757 (50) |
| PB2-1 | H3N2 PB2 1–384 (52) |
| PB2-2 | H3N3 PB2 375–759 (52) |
| M1/2 | H3N2 M1 1–252 (34); M2 1–97 (13) |
| NP-1 | H3N2 NP 1–261 (34) |
| NP-2 | H3N2 NP 252–498 (34) |
| NS1/2 | H3N2 NS1 1–230(29); NS2 1–121(17) |
| PA-1 | H3N2 PA 1–370 (49) |
| PA-2 | H3N2 PA 361–716 (46) |
Numbers are the range of the AMINO ACIDS contained within the pool.
Blood samples were taken from volunteers at local sites and transported to the National Institute of Hygiene and Epidemiology on the same day, where PBMCs were isolated and 2.5 × 105 were incubated with peptides at 2 μg/mL overnight, as described elsewhere [19]. Plates were read on an ELISPOT plate reader (CTL). Positive pools were defined using established criteria of 3 times average background and/or >10 spots per well [19]. Spot-forming units were the actual number of spots generated from a known number of cells.
Generation of B-Cell Lines and Antigen-Specific T-Cell Lines
Epstein Barr virus (EBV)–transformed B-cell lines (BCLs) were generated by adding EBV supernatant, from a B958 cell line, to 1–2 × 106 PBMCs in a 96-well plate for 3–4 hours, followed by 2 μg/mL cyclosporine (Sandoz Pharmaceuticals) in R15 (RPMI medium with 15% vol/vol fetal calf serum). Antigen-specific short-term T-cell lines (STLs) were generated by pulsing PBMCs with peptide for 90 minutes, washing once, and then culturing in 96-well plates in H10 (10% vol/vol human AB serum; National Blood Service). Three days later, interleukin-2 (PeproTech EC) was added at a final concentration of 200 U/mL. STLs were maintained by restimulating with peptide-pulsed autologous BCLs every 10–15 days. For cultured ELISPOT assays, STLs were rested in H10 for 26–36 hours and then used in an ELISPOT assay. T cells (n = 40 000) were mixed with 10 or 2 μg/mL peptide pools and cultured for 18–20 hours, and then spots were developed using above protocol [22, 23]. T-cell lines that did not expand or were negative at ELISPOT assay were excluded from analysis.
Cloning of Cell Lines
Cell lines were stimulated with peptide-pulsed autologous BCLs for 3–4 hours followed by labeling with human IFN-γ capture kit (Miltenyi Biotec). High IFN-γ producers were sorted on a MoFlow cytometer (DakoCytomation). Clones were restimulated every 14–21 days using phytohemagglutin-treated irradiated allogeneic PBMCs, as described elsewhere [24].
Intracellular Cytokine Staining
Cells were stimulated with 10 μg/mL peptide for 1 hour followed by addition of BFA/Monesin (BD Biosciences). After an additional 12–16 hours, cells were washed, labeled with anti-CD4 Pacific blue (eBiosciences) anti-CD8 fluorescein isothiocyanate (BD Biosciences), permeabilized with FixPerm (BD) labeled with anti–tumor necrosis factor α Allophycocyanin (APC) (eBiosciences) and anti–IFN-γ Phycoerythrin PE (eBiosciences), then washed with Perm/Wash (BD) and fixed. Cells were analyzed on a CyAn flow cytometer (DakoCytomation).
HAI Assay and Antibody ELISPOT Assay
Plasma was tested in a standard HAI assay with antigens representing clade 1 and clade 2.3.4 H5N1 strains circulating in Vietnam and horse red blood cells, as described elsewhere [7, 12]. Donors were considered positive if they had an antibody titer of 1:40 or more [25].
RESULTS
Sufficient blood for ELISPOT assays was obtained from 747 participants in December 2007. Thirty-six participants had responses to H5 HA by ELISPOT assays. Twenty-four participants (3.2%) demonstrated specific responses to the H5 HA peptide pools but far lower (≥2-fold, but the majority of H5-specific responses were 5-fold) or no response to either H1 HA or H3 HA peptide pools. This included 6 donors who only made responses to H5 peptide pools and were negative for H3 and H1 pools (Figure 1A, B). There were 111 participants who had ELISPOT responses to H5 that are less than or equal to the H1 and/or H3 responses (Supplementary Table 1). However, none of 271 healthy controls at low risk of H5N1 exposure showed H5N1-specific responses, and 16% of person who had recovered from H5N1 infection made specific responses to H5 HA pools (Figure 1C). Of these recovered patients, tested during acute infection for H5 HAI, 5 of 14 were positive (HHT Trang, A. Fox, T. Dong, LQ Mai, VTK Lien, T. Powell, TN Duong, NTT Yen, PQ Thai, NT Hien, P Horby, authors' unpublished data). Thirty-seven (5%) of the community cohort participants had positive H5 antibody titers, and 4 also showed H5-specific T-cell responses (Figure 1A, B, denoted by asterisks). Within this group of individuals, 12 had T-cell responses (4 with H5-specific responses, a subset of these) and 25 had no T-cell responses.
Figure 1.
Enzyme-linked immunospot spot (ELISPOT) assay results in cohort volunteers with H5 peptide-specific responses. Peripheral blood mononuclear cells were stimulated with overlapping peptides from H1, H3, and H5 proteins to the first half (A) and second half (B) of the protein. Asterisks denote donors who were also positive for H5 antibody. C, Percentage of each cohort who had H5-specific T-cell responses by interferon-γ ELISPOT assays. Number of donors in each cohort is shown underneath each bar. Abbreviations: HA, hemagglutinin; SFU, spot-forming units.
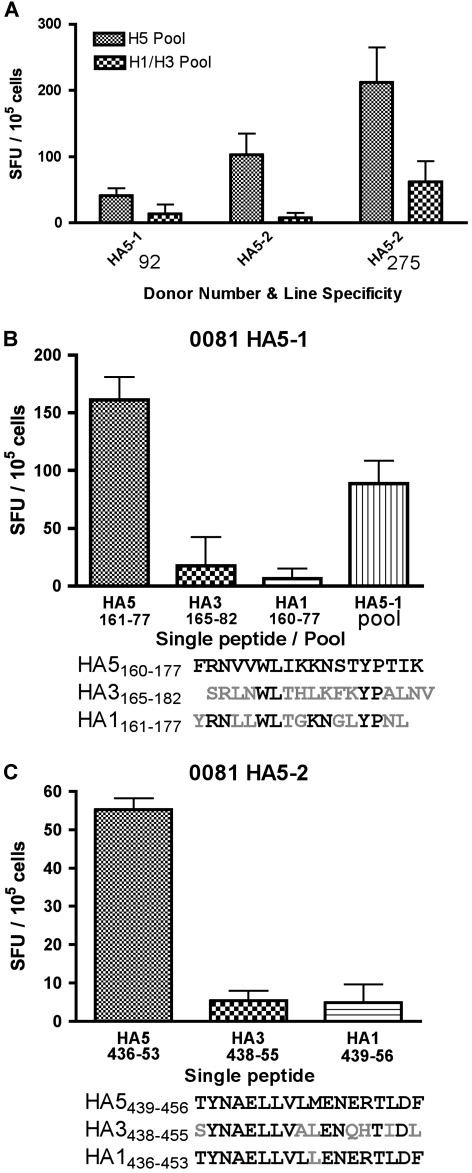
For further analysis of the H5-specific responders, STLs and cultured ELISPOT experiments were performed. Tables 2 and 3 show the results of direct ex vivo and cultured ELISPOT from a number of different donors who were positive for H5 responses at initial screening, and PBMCs were then expanded as STL. Figure 2A shows 3 examples of HA STLs that show higher responses to H5N1 HA pools than to H1N1 or H3N2 HA pools. When similar experiments were done with other lines, 4 donors had H5-specific STL after expansion in vitro (Table 2). Ten donors had responses to internal pools that were either H3N2 peptide pool specific or cross-reactive with both H5N1 and H3N2 peptide pools (Table 3).
Table 2.
H5 Hemagglutinin (HA) Responders and Generation of HA5-Specific Short-Term T-Cell Lines (STLs)
| Ex Vivo Responses |
STL |
||||
| Donora and Stimulation Poolb | H5N1c | H3N2 | H1N1 | Single Peptide Identityd | CD4/CD8 Cell Typee |
| Donor 0081 | |||||
| HA5-1 | + | − | − | HA5160–77 | ND |
| HA5-2 | + | − | − | HA5439–56, HA5344–64 | CD8, CD4 |
| Donor 0092 | |||||
| HA5-1 | ++ | − | − | CD8 | |
| HA5-2 | ++ | − | − | CD8 | |
| Donor 0148 | |||||
| HA5-2 | ++ | − | − | HA5344–64 | CD4 |
| Donor 0275 | |||||
| HA5-2 | + | − | − | CD4 | |
Abbreviation: ND, no data.
Donor number assigned during collection.
Peptide pool used to stimulate the STL or peripheral blood mononuclear cells (PBMCs), as shown in Table 1.
Ex vivo enzyme-linked immunospot response on fresh PBMCs; ++ indicates strong response (>100 spot-forming units [SFU]/106 PBMCs); +, medium response (40–99 SFU/106 PBMCs); −, no response (<40 SFU/106 cells).
Single peptide defined by incubation of STLs with single peptides.
Lymphocyte subset CD4/CD8 defined by tumor necrosis factor α or interferon-γ secretion in intracellular cytokine staining (ICS) after restimulation.
Table 3.
Internal Peptide-Specific Short-Term T-Cell Lines (STLs)
| Ex Vivob,c | Expanded STL Specificity |
||||
| Donor andPoola | H3N2 | H5N1 | H3N2 | Single Peptide Identifiedd | CD4/CD8e |
| Donor 0018 | |||||
| NP-1 | ++ | +++ | +++ | NP199–216 | CD8 |
| Donor 0053 | |||||
| NP-1 | ++ | ND | +++ | CD4 | |
| Donor 0080 | |||||
| NP-1 | ++ | ++ | ++ | CD8/CD4 | |
| NS1/2 | ++ | ++ | ++ | CD4 | |
| Donor 0081 | |||||
| PB2-2 | ++ | ND | +++ | PB2427–44, PB2637–55 | ND |
| NS1/2 | ++ | ND | ++ | NS1163–85 | CD4 |
| Donor 0092 | |||||
| PB1-1 | ++ | ND | + | CD4 | |
| NP-1 | ++ | + | ++ | ND | |
| PA-1 | ++ | ND | + | CD4 | |
| Donor 0109 | |||||
| NP-1 | + | ND | + | ND | |
| Donor 0130 | |||||
| NP-2 | + | ND | ++ | ND | |
| Donor 0141 | |||||
| NP-1 | ++ | + | ++ | ND | |
| NP-2 | ++ | + | ++ | CD4 | |
| Donor 0142 | |||||
| M1/2 | ++ | + | ++ | CD4 | |
| NP-1 | ++ | + | ++ | CD8 | |
| Donor 0275 | |||||
| M1/2 | ++ | + | ++ | CD4 | |
| NP-2 | ++ | + | ++ | CD4 | |
| Donor 0492 | |||||
| NP-2 | ++ | ND | ++ | CD8/CD4 | |
Abbreviation: ND, no data.
Peptide pool as defined in Table 1.
Ex vivo enzyme-linked immunospot (ELISPOT) response on fresh peripheral blood mononuclear cells (PBMCs); ++ indicates strong response (>100 spot-forming units [SFU]/106 PBMCs); +, medium response (40–99 SFU/106 PBMCs); −, no response (<40 SFU/106 cells).
Derived STL. Rested ELISPOT response: −. no response, +, ≤50 SFU/105 cells; ++, 50–100 SFU/105 cells; +++ > 100 SFU/105 cells.
Single peptide identified by stimulation in ELISPOT.
CD4/CD8 defined by tumor necrosis factor α or interferon-γ intracellular cytokine staining (ICS) after restimulation.
Figure 2.
Hemagglutinin (HA)–specific short-term T-cell lines show specificity for H5 pools, but not H3 or H1 pools, and are specific to single H5 HA peptides. A, Peripheral blood mononuclear cells (PBMCs) were expanded in vitro using HA5-1 or HA5-2 peptide pools and interleukin (IL) 2. Ten days later, cells were rested overnight in IL-2–free media and then tested in an enzyme-linked immunospot spot (ELISPOT) assay using H5 pools, HA5-1 or HA5-2, depending on the specificity of the line, or a mixture of H3/H1 pools from the same corresponding region of the HA protein. B, C, STLs were restimulated with antigen pulsed autologous B cells. After 10-day stimulation and 30-hour rest, cells were tested in their response to specific peptides and the release of interferon-γ was measured by ELISPOT assay. A, B, C, Results shown are spot-forming units (SFU) per 105 cells of duplicate or triplicate wells (± standard deviations).
To determine the T-cell recognition of the single peptides containing potential epitopes from H5 HA pools in individuals with H5N1-specific T-cell responses, STLs were generated and tested by cultured ELISPOT assays using single peptides. The STL grown from donor 0081 for the HA5-1 pool was able to recognize only the peptide with the amino acid sequence 160–177 of HA5160–77 FRNVVWLIKKNSTYPTIK and not the equivalent peptides from H1 or H3 (Figure 2B). Another line grown from this donor using the HA5-2 pool was able to respond only to the peptide HA5439–56; TYNAELLVLMENERTLDF from the H5 strain of virus but not to the equivalent H1 and H3 peptides (Figure 2C). A similar response to HA5439–56 was also found with a second donor (data not shown). A second line generated from donor 0081 using pool HA5-2 was specific to peptide HA5344–64 KKRGLFGAIAGFIEGGWQGMV (Table 2). Notably, HA5344–64 was CD4-restricted and HA5439–56 was CD8-restricted, so the H5 HA specificity was not limited to only CD8 T cells.
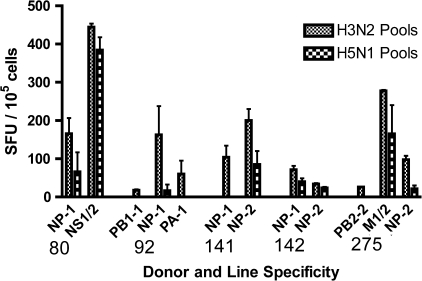
To investigate whether this cohort had cross-reactive responses against peptides from the internal proteins of influenza, STLs were generated to internal peptide pools that had been found to be positive in initial ELISPOT screening. These lines were tested for reactivity against seasonal (H3) peptides and also H5 peptides. STLs from 5 donors were able to recognize internal peptide pools from both H3N2 and H5N1 (Figure 3 and Table 3). We found 1 CD4 clone that was able to respond to peptide NS1163–85 GHTIEDVKNAIGVLIGGL, a peptide derived from an internal H3N2 NS1 protein that has not been documented elsewhere. We also identified several previously unknown individual peptides containing potential T-cell epitopes in H3N2 internal proteins that were cross-reactive with equivalent H5N1 peptides: PA163–80 RIKTRLFTIRQEMASRGL, PB2427–44 RLNTMHQLLRHFQKDAKV, PB2637–55 TVNVRGSGMRILVRGNSPV, NP199–216 RGINDRNFWRGENGRRTR, and PA406–21 KACELTDSIWIELDEI, noted in Table 3.
Figure 3.
Short-term T-cell lines derived from H3N2 internal peptide pools show cross-reactive responses to both H3N2 and H5N1 peptide pools. Short-term lines were rested overnight in H10 media and then tested in a cultured enzyme-linked immunospot spot assay against peptide pools from internal genes of H3N2 and H5N1 peptide pools. Line specificity is shown on the axis, and duplicate values with background subtracted are shown ± standard deviations. Abbreviations: NP, nucleoprotein; NS, non-structural protein; PB1 and 2, polymerase basic protein 1 and 2; PA, polymerase acidic protein; M, matrix protein; SFU, spot-forming units.
DISCUSSION
We used ELISPOT assays to analyze T-cell responses to influenza in members of a community exposed to H5N1 and found that 24 of 747 (3.2%) had specific responses to H5 HA peptides but little or no response to equivalent H3 or H1 HA peptides. H5-specific responses were further confirmed by cultured ELISPOT assays and by growing STLs and clones. Although H5 HA-specific CD4 T-cell responses can be generated in unexposed healthy individuals by in vitro expansion from PBMCs [26], this is the first study to detect H5 HA-specific T-cell responses directly ex vivo in a cohort at high risk of H5N1 exposure. In contrast, we did not detect any ex vivo H5 HA-specific T-cell responses in 271 unexposed healthy controls.
Almost 5% of participants (37 of 747; 4.9%) had horse erythrocyte HAI antibody titers ≥1:40, and 4 of them had both an H5N1-positive antibody titer and H5-specific T-cell responses. The poor correlation between the antibody and T-cell measurements may be a result of different kinetics of persistence after virus exposure. H5N1 antibodies have been shown to persist after severe infection [27] but decline after mild or asymptomatic infection [5]. H5-specific T-cell responses are seen only in a small proportion of confirmed cases, possibly because T-cell responses are short-lived, as seen with seasonal influenza [28], and it is not known how long specific H5 T-cell responses may persist. Both HAI and T-cell assays may not identify all infections, because there have been studies in which only 2 of 5 virologically confirmed H5N1 cases have antibodies detectable by HAI [12], and H5N1-specific T cells are not detected in all persons convalescing after confirmed H5N1 infection (HHT Trang, A. Fox, T. Dong, LQ Mai, VTK Lien, T. Powell, TN Duong, NTT Yen, PQ Thai, NT Hien, P Horby, authors' unpublished data). Therefore, measured rates of prevalence could be underestimated whichever assay (antibody or T cell) is used. Multiple time points and samples would answer questions of persistence [5, 27], and further studies are needed to explore this issue. Not many donors make both T-cell and antibody responses, and this may be due to underlying issues of immune repertoire between different donors or for the H5N1 cohort differences in clinical interventions [29]. Because the measured proportion of H5 HAI–positive results was greater in patients who had recovered from H5N1 infection than in the community cohort, HAI correlates to some extent with rate of infection or exposure. T-cell or antibody responses have been shown to persist for up to 6 months in vaccine studies [30, 31] but to decline after early time points. Most studies use only early time points [less than one month] after infection [15, 16]. Therefore, this study was undertaken during the influenza season to obtain samples during or shortly after influenza exposure [25].
The detection of subclinical H5N1 infection has several important implications. First, it can provide a more accurate assessment of the probability of animal-to-human transmission and of the severity of human H5N1 infection. Second, persons with subclinical or asymptomatic H5N1 infection will not be hospitalized and may be at risk of coinfection with another seasonal virus, which could reassort with H5N1 [32]. Third, identifying groups with different severities of H5N1 infection can contribute to our understanding of the pathogenesis of severe H5N1 influenza and factors that may influence susceptibility to severe disease [6].
Most donors have cross-reactive H5N1 T-cell responses to peptides from internal influenza genes that have been shown elsewhere to respond to infected target cells [19, 20]. There could be an important role for cross-reactive T-cell responses in protection against H5N1. Further characterization of these recognized peptides will help in the design of universal influenza vaccines that target the less variable internal genes of the virus.
H5 HA-specific T cells are most likely to have been generated as a result of prior infection with, or exposure to, a low level of H5N1 virus. CD8 T cells are stimulated more readily by virus infection rather than inactivated vaccines [31, 33]. A low level of infection may have occurred because upper human respiratory tract lacks the α2,3-galactose sialic acid receptors that H5N1 viruses preferentially bind [34] so that the H5N1 virus is unable to replicate to a high titer.
In conclusion, we report evidence of possible subclinical H5N1 infection demonstrated with T-cell ELISPOT assays. These responses were not detectable by horse erythrocyte HAI. We consider that detection of H5N1-specific T-cell responses may be a useful adjunct to serology to identify the prevalence of infection with H5N1. Further research is needed with different cohorts in different geographical areas to determine whether this is universally applicable. Characterization and comparison of T-cell responses between asymptomatic responders and patients who have recovered from H5N1 infection may provide insights into the immune responses associated with severity of infection. Identification of cross-reactive epitopes in asymptomatic individuals may provide useful information for universal influenza vaccine design.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://www.oxfordjournals.org/our_journals/jid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments.
We acknowledge help and assistance from many coworkers in Vietnam and blood donors in Oxford and Vietnam. We thank Craig Waugh for cell sorting, Tim Rostron for HLA typing, and Alastair Waugh and Rebecca Horsfall for EBV production.
Financial support.
This work was supported by the UK Medical Research Council (grant G0600520 to T. D.) and the Wellcome Trust UK (grants 081613/Z/06/Z and 077078/Z/05/Z).
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions.
T.J.P., A.F., Y.P., L.T.Q.M.. V.T.K.L., N.L.K.H., and L.W. performed experiments; T.D., PH, A.F., N.T.H., S.L.R.J., N.T.T.Y., P.Q.T., T.N.D., B.A.A., C.P.S., J.J.F., A.J.M., and L.L.Y.L. designed the study; T.J.P., A.F., V.T.K.L., N.L.K.H., L.W., and T.D. analyzed data; T.J.P., T.D., A.F., and P.H. wrote the article.
References
- 1.Beigel JH, Farrar J, Han AM, et al. Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005;353:1374–85. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- 2.Gambotto A, Barratt-Boyes SM, de Jong MD, Neumann G, Kawaoka Y. Human infection with highly pathogenic H5N1 influenza virus. Lancet. 2008;371:1464–75. doi: 10.1016/S0140-6736(08)60627-3. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization. Confirmed human cases of avian influenza A(H5N1). Avian Influenza Weekly Update 302. 14 October 2011. http://www.who.int/influenza/human_animal_interface/H5N1_cumulative_table_archives/en/index.html. Accessed 8 April 2011.
- 4.Salomon R, Webster RG. The influenza virus enigma. Cell. 2009;136:402–10. doi: 10.1016/j.cell.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchy P, Vong S, Chu S, et al. Kinetics of neutralizing antibodies in patients naturally infected by H5N1 virus. PLoS One. 2010;5:e10864. doi: 10.1371/journal.pone.0010864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vong S, Ly S, Van Kerkhove MD, et al. Risk factors associated with subclinical human infection with avian influenza A (H5N1) virus–Cambodia, 2006. J Infect Dis. 2009;199:1744–52. doi: 10.1086/599208. [DOI] [PubMed] [Google Scholar]
- 7.Schultsz C, Nguyen VD, Hai le T, et al. Prevalence of antibodies against avian influenza A (H5N1) virus among cullers and poultry workers in Ho Chi Minh City, 2005. PLoS One. 2009;4:e7948. doi: 10.1371/journal.pone.0007948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katz JM, Lim W, Bridges CB, et al. Antibody response in individuals infected with avian influenza A (H5N1) viruses and detection of anti-H5 antibody among household and social contacts. J Infect Dis. 1999;180:1763–70. doi: 10.1086/315137. [DOI] [PubMed] [Google Scholar]
- 9.Ceyhan M, Yildirim I, Ferraris O, et al. Serosurveillance study on transmission of H5N1 virus during a 2006 avian influenza epidemic. Epidemiol Infect. 2010;138:1274–80. doi: 10.1017/S095026880999166X. [DOI] [PubMed] [Google Scholar]
- 10.Barr IG, McCauley J, Cox N, et al. Epidemiological, antigenic and genetic characteristics of seasonal influenza A(H1N1), A(H3N2) and B influenza viruses: basis for the WHO recommendation on the composition of influenza vaccines for use in the 2009–2010 Northern Hemisphere season. Vaccine. 2009;28:1156–67. doi: 10.1016/j.vaccine.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 11.Nicholson KG, Wood JM, Zambon M. Influenza. Lancet. 2003;362:1733–45. doi: 10.1016/S0140-6736(03)14854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowe T, Abernathy RA, Hu-Primmer J, et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol. 1999;37:937–43. doi: 10.1128/jcm.37.4.937-943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Temperton NJ, Hoschler K, Major D, et al. A sensitive retroviral pseudotype assay for influenza H5N1-neutralizing antibodies. Influenza Other Respi Viruses. 2007;1:105–12. doi: 10.1111/j.1750-2659.2007.00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia JM, Pepin S, Lagarde N, et al. Heterosubtype neutralizing responses to influenza A (H5N1) viruses are mediated by antibodies to virus haemagglutinin. PLoS One. 2009;4:e7918. doi: 10.1371/journal.pone.0007918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McElhaney JE, Xie D, Hager WD, et al. T cell responses are better correlates of vaccine protection in the elderly. J Immunol. 2006;176:6333–9. doi: 10.4049/jimmunol.176.10.6333. [DOI] [PubMed] [Google Scholar]
- 16.Hammitt LL, Bartlett JP, Li S, et al. Kinetics of viral shedding and immune responses in adults following administration of cold-adapted influenza vaccine. Vaccine. 2009;27:7359–66. doi: 10.1016/j.vaccine.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 17.Zinckgraf JW, Sposato M, Zielinski V, Powell D, Treanor JJ, von Hofe E. Identification of HLA class II H5N1 hemagglutinin epitopes following subvirion influenza A (H5N1) vaccination. Vaccine. 2009;27:5393–401. doi: 10.1016/j.vaccine.2009.06.081. [DOI] [PubMed] [Google Scholar]
- 18.Jameson J, Cruz J, Terajima M, Ennis FA. Human CD8+ and CD4+ T lymphocyte memory to influenza A viruses of swine and avian species. J Immunol. 1999;162:7578–83. [PubMed] [Google Scholar]
- 19.Lee LY, Ha DL, Simmons C, et al. Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J Clin Invest. 2008;118:3478–90. doi: 10.1172/JCI32460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreijtz JH, de Mutsert G, van Baalen CA, Fouchier RA, Osterhaus AD, Rimmelzwaan GF. Cross-recognition of avian H5N1 influenza virus by human cytotoxic T-lymphocyte populations directed to human influenza A virus. J Virol. 2008;82:5161–6. doi: 10.1128/JVI.02694-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babon JA, Cruz J, Orphin L, et al. Genome-wide screening of human T-cell epitopes in influenza A virus reveals a broad spectrum of CD4+ T-cell responses to internal proteins, hemagglutinins, and neuraminidases. Hum Immunol. 2009;70:711–21. doi: 10.1016/j.humimm.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vuola JM, Keating S, Webster DP, et al. Differential immunogenicity of various heterologous prime-boost vaccine regimens using DNA and viral vectors in healthy volunteers. J Immunol. 2005;174:449–55. doi: 10.4049/jimmunol.174.1.449. [DOI] [PubMed] [Google Scholar]
- 23.Todryk SM, Pathan AA, Keating S, et al. The relationship between human effector and memory T cells measured by ex vivo and cultured ELISPOT following recent and distal priming. Immunology. 2009;128:83–91. doi: 10.1111/j.1365-2567.2009.03073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong T, Stewart-Jones G, Chen N, et al. HIV-specific cytotoxic T cells from long-term survivors select a unique T cell receptor. J Exp Med. 2004;200:1547–7. doi: 10.1084/jem.20032044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen HT, Dharan NJ, Le MT, et al. National influenza surveillance in Vietnam, 2006–2007. Vaccine. 2009;28:398–402. doi: 10.1016/j.vaccine.2009.09.139. [DOI] [PubMed] [Google Scholar]
- 26.Yang J, Gebe JA, Huston L, et al. H5N1 strain-specific hemagglutinin CD4+ T cell epitopes restricted by HLA DR4. Vaccine. 2009;27:3862–9. doi: 10.1016/j.vaccine.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitphati R, Pooruk P, Lerdsamran H, et al. Kinetics and longevity of antibody response to influenza A H5N1 virus infection in humans. Clin Vaccine Immunol. 2009;16:978–81. doi: 10.1128/CVI.00062-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMichael AJ, Gotch FM, Dongworth DW, Clark A, Potter CW. Declining T-cell immunity to influenza, 1977–82. Lancet. 1983;2:762–4. doi: 10.1016/s0140-6736(83)92297-3. [DOI] [PubMed] [Google Scholar]
- 29.de Jong MD, Simmons CP, Thanh TT, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12:1203–7. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crowe BA, Bruhl P, Gerencer M, et al. Evaluation of the cellular immune responses induced by a non-adjuvanted inactivated whole virus A/H5N1/VN/1203 pandemic influenza vaccine in humans. Vaccine. 2011;29:166–73. doi: 10.1016/j.vaccine.2010.10.065. [DOI] [PubMed] [Google Scholar]
- 31.Mbawuike IN, Piedra PA, Cate TR, Couch RB. Cytotoxic T lymphocyte responses of infants after natural infection or immunization with live cold-recombinant or inactivated influenza A virus vaccine. J Med Virol. 1996;50:105–11. doi: 10.1002/(SICI)1096-9071(199610)50:2<105::AID-JMV1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 32.Octaviani CP, Ozawa M, Yamada S, Goto H, Kawaoka Y. High level of genetic compatibility between swine-origin H1N1 and highly pathogenic avian H5N1 influenza viruses. J Virol. 2010;84:10918–22. doi: 10.1128/JVI.01140-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webster RG, Askonas BA. Cross-protection and cross-reactive cytotoxic T cells induced by influenza virus vaccines in mice. Eur J Immunol. 1980;10:396–401. doi: 10.1002/eji.1830100515. [DOI] [PubMed] [Google Scholar]
- 34.Shinya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y. Avian flu: influenza virus receptors in the human airway. Nature. 2006;440:435–6. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.