Abstract
Our recent microarray analysis of infected human alveolar macrophages (AMs) found serine protease inhibitor 9 (PI-9) to be the most prominently expressed of a cluster of apoptosis-associated genes induced by virulent Mycobacterium tuberculosis. In the current study, we show that induction of PI-9 occurs within hours of infection with M. tuberculosis H37Rv and is maintained through 7 days of infection in both AMs and blood monocytes. Inhibition of PI-9 by small inhibitory RNA decreased M. tuberculosis–induced expression of the antiapoptotic molecule Bcl-2 and resulted in a corresponding increase in production of caspase 3, a terminal effector molecule of apoptosis. Further, PI-9 small inhibitory RNA mediated a significant reduction in the subsequent survival of M. tuberculosis within AMs. Thus PI-9 induction within human mononuclear phagocytes by virulent M. tuberculosis serves to protect these primary targets of infection from elimination by apoptosis and thereby promotes intracellular survival of the organism.
Alveolar macrophages (AMs) provide the first line of host defense against Mycobacterium tuberculosis after inhalation of airborne infectious droplets. Although human AMs are well equipped to eliminate the great majority of inhaled microbial pathogens, they remain permissive to intracellular growth of phagocytosed M. tuberculosis. The mechanisms by which M. tuberculosis subverts host immunity to survive within this intracellular environment remain incompletely understood. However, since Malloy et al observed that apoptosis of infected phagocytes results in killing of intracellular mycobacteria [1], the ability of virulent M. tuberculosis to inhibit apoptotic processes has been a subject of considerable interest in studies of the pathogenesis of tuberculosis [2–8].
The receptors of molecules associated with extrinsic apoptotic pathway are coupled to intracellular “death domains” that activate procaspase 8, which itself promotes further downstream activation of caspase cascades. In contrast, intrinsic pathways of apoptosis are initiated by release from the mitochondria of destructive molecules such as cytochrome c, Smac/Diablo, and apaf. Once released into the cytoplasm, the apoptosome triggers downstream caspase activation. The intrinsic apoptotic pathway is largely regulated by molecules of the Bcl-2 family via their effects on mitochondrial membrane permeability. More specifically, the ratio of antiapoptotic family members Bcl-2 and Bcl-xL to the proapoptotic member Bax appears to direct the cell toward either continued containment or release of mitochondrial contents and therefore toward cell survival or apoptosis, respectively [9]. Both pathways mediate activation of caspase 3, and this common end effector induces DNA fragmentation as the ultimate outcome of both intrinsic and extrinsic apoptosis [10].
Using a genome-wide microarray analysis of human AMs, we recently identified several novel apoptosis-associated genes as being significantly up-regulated 24 hours after infection by virulent M. tuberculosis strain H37Rv but not by its avirulent derivative, M. tuberculosis H37Ra [11]. The most prominent of these was SE.RPINB9, which encodes serine protease inhibitor 9 (PI-9). PI-9 has been previously identified as the sole natural endogenous inhibitor of granzyme B (GrB), a cytotoxic molecule produced by CD8 T cells and natural killer cells. Overexpression of PI-9 protects target cells from GrB-mediated apoptosis [12], which may be relevant to the capacity of intracellular M. tuberculosis to resist being killed by cytotoxic lymphocytes. However, because of the critical role of early intracellular survival in establishment of infection, the current study evaluated the capacity of PI-9 expressed by human phagocytes to protect against apoptosis. We hypothesized that M. tuberculosis–induced PI-9 serves to inhibit apoptotic pathways of host phagocytes. To investigate this possibility, we first evaluated whether intracellular infection with M. tuberculosis results in sustained induction of PI-9 by host phagocytes. We subsequently assessed the impact of specific inhibition of PI-9 on M. tuberculosis–induced expression of Bcl-2, Bax, tumor necrosis factor receptor, and Fas ligand (L), and on production of caspase 3. We then quantified viable M. tuberculosis within infected human AMs transfected with PI-9–specific small inhibitory RNA (siRNA) to define the effects of PI-9–mediated modulation of phagocyte apoptosis on intracellular survival of the organism. Our findings indicate that H37Rv-induced PI-9 promotes the viability of intracellular M. tuberculosis in the context of inhibition of the intrinsic apoptotic pathway via up-regulation of Bcl-2.
MATERIAL AND METHODS
Human Subjects
Healthy nonsmokers aged 20–45 underwent blood drawing and bronchoscopy with bronchoalveolar lavage, using procedures described elsewhere [13]. All protocols were approved by the institutional review boards of University Hospitals Case Medical Center and the Louis Stokes Cleveland Department of Veterans’ Affairs Medical Center.
Preparation of Human Phagocyte Populations
Bronchoalveolar lavage cells and peripheral blood mononuclear cells were isolated and AMs and monocytes (MNs) prepared by polystyrene adherence, as described elsewhere [14]. Adherent cell populations were >99% viable and 90%–95% pure by microscopy. For some experiments, MNs were isolated from peripheral blood mononuclear cells via negative selection using immmunomagnetic sorting (Human Monocyte Isolation Kit II, Miltenyi Biotech). The purity of sorted MNs was >98% based on CD14 staining.
Infection and Stimulation With M. tuberculosis
Mycobacterium tuberculosis strain H37Rv (ATCC) was cultured and prepared for infection as described elsewhere [15]. Cell monolayers were incubated with the organism for 90 minutes in 30% autologous serum using multiplicities of infection (MOIs) of 1:1 to 50:1. In some experiments, phagocytes were stimulated with M. tuberculosis H37Rv lysate, a French press preparation of irradiated late log-phase organisms (provided by Colorado State University under National Institutes of Health contract NOI-AI-75320).
Detection and Analysis of Gene Expression
Primers and probes for PI-9 and Bcl2 were designed using Applied Biosystems (ABI) Primer Express software, as follows: PI-9: forward, AACCCTTCGCACAACGTGTT; reverse, CTTTGCCCCTAGGAGAACCAT; and probe, 6FAM-TGTTCTCCTGTGAGCATCTCCTCTGCC-TAMRA; Bcl2: forward, AGAACCTTGTGTGACAAATGAGAAC; reverse, TACCCATTAGACATATCCAGCTTGAA; and probe, 6FAM-AGACATCAGCATGGCTCAAAGTGCAGCT-TAMRA. Primer and probes sequences reported elsewhere were used for Mcl-1 [16], Bcl-w [6], tumor necrosis factor (TNF) α, ribosomal 18S (R18), and M. tuberculosis 16 ribosomal RNA (rRNA) [17]. Fas and Bax primer/probes were purchased from ABI.
After total RNA extraction and reverse transcription [18], quantitative real-time polymerase chain reaction of complementary DNA was performed using the ABI 7700 detection system. Expression of messenger RNA (mRNA) copies was normalized to R18 copy numbers in the same sample and reported as fold induction within M. tuberculosis–stimulated samples relative to unstimulated control samples.
Inhibition of PI-9 Expression by siRNA
Target-specific PI-9 and nontargeting (control) siRNA constructs were purchased from Dharmacon. MNs obtained via negative immunoselection were nucleofected using a human MN nucleofector kit (Amaxa). For AMs, which tolerate nucleofection poorly, PI-9–specific and control siRNAs were introduced by passive transfection according to Dharmacon Accell protocols.
Enzyme-Linked Immunosorbent Assay for Intracellular PI-9
Cell lysates were assessed for PI-9 by enzyme-linked immunosorbent assay (ELISA) using polyclonal (coating) and conjugated monoclonal (detecting) anti–PI-9 antibodies (Sanquin Foundation) [19].
Western Blot Analysis for Caspase 3
After preparation in Laemmli sample buffer, electrophoretic separation, and transfer to nitrocellulose membranes, cell lysates were evaluated by overnight incubation with mouse antihuman caspase 3 (BD Bioscience) in Odyssey Blocking buffer, followed by a 1-hour incubation with IRDye 800 CW conjugated goat antimouse immunoglobulin G (LI-COR Biosciences). Blots were scanned using an Odyssey infrared image scanner, and results were quantified using Odyssey 3.0 software (LI-COR).
Statistical Analysis
Comparisons of multiple measures assessed using cells from the same groups of subjects were evaluated with paired t tests. Correlation between parameters was assessed by linear regression analysis. Differences were considered significant at P < .05.
RESULTS
Differing Kinetics of PI-9 Induction in AMs and MNs Infected With M. tuberculosis
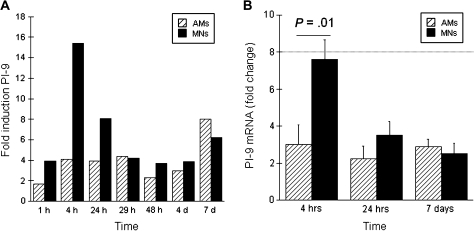
PI-9 was identified in a recent study of total gene expression in virulent M. tuberculosis–infected human AMs [11]. To clarify the kinetics of M. tuberculosis–induced PI-9 expression within human mononuclear phagocytes, we evaluated freshly isolated AMs and autologous blood MNs throughout a 7-day time course after infection with H37Rv using real-time polymerase chain reaction. Figure 1A indicates results of a representative kinetic experiment. PI-9 expression is represented as fold induction of PI-9 mRNA within M. tuberculosis–infected cells, as compared to that in uninfected cells. As illustrated, early induction of PI-9 (after 4 hours of infection) was greater in MNs than in AMs, but in both cell types expression was sustained throughout the 7-day course of the experiment.
Figure 1.
Differing kinetics of Mycobacterium tuberculosis–induced serine protease inhibitor 9 (PI-9) expression in alveolar macrophages (AMs) and blood monocytes (MNs). A, Representative study of the kinetics of PI-9 expression as assessed from 1 hour through 7 days after infection of AMs (striped bars) and MNs (solid bars) with M. tuberculosis strain H37Rv. Expression of PI-9 mRNA is represented as fold induction (PI-9 messenger RNA [mRNA] within M. tuberculosis infected cells as determined by real-time polymerase chain reaction divided by that of uninfected cells collected at the same time point). As illustrated, MNs displayed a greater early peak of PI-9 mRNA than did AMs (at 4 hours after M. tuberculosis infection), but PI-9 expression persisted in both cell types throughout the 7 day course of the experiment. B, Mean data from studies of 16 subjects confirmed that early differences in PI-9 expression resolved rapidly. As illustrated, H37Rv induced a 7.6-fold increase in PI-9 mRNA in MNs (solid bars) at 4 hours, compared to a 3.2 fold increase in AMs (striped bars). By 24 hours, however, PI-9 expression in MNs had declined significantly, so that induction of PI-9 was similar within M. tuberculosis–infected AMs and MNs. PI-9 expression continued through day 7 in both cell types, as illustrated.
The differing early kinetics of M. tuberculosis–induced PI-9 mRNA expression in H37Rv-infected MNs and AMs were confirmed in multiple experiments (Figure 1B). After 4 hours of culture, mean PI-9 expression in M. tuberculosis–infected AMs was 3.2 (standard error of mean [SEM] ±1.06)–fold greater than in cells incubated in medium alone, and similar levels of PI-9 induction by M. tuberculosis were sustained through a 7-day course of infection. In contrast, in MNs M. tuberculosis induced a 7.6 (SEM ±1.06)–fold increase in PI-9 expression at 4 hours, which was significantly greater than that observed in AMs at this time point (P < .01). PI- 9 expression in M. tuberculosis–infected MNs decreased significantly by 24 hours, however, and remained similar to that observed in AMs through day 7.
PI-9 Induction by M. tuberculosis Lystate
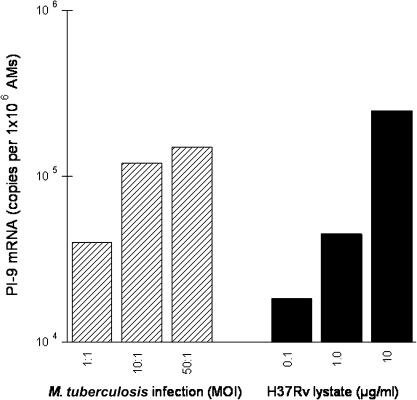
We next compared PI-9 mRNA induction in response to infection with intact M. tuberculosis H37Rv with that after incubation with H37Rv lysates. Results from one such experiment are displayed in Figure 2. As illustrated, both infection of AMs with M. tuberculosis H37Rv using increasing MOIs (1:1 to 50:1) and exposure of AMs to increasing concentrations of M. tuberculosis H37Rv lysate (0.1–10 μg/mL) induced dose-dependent increases in PI-9 mRNA.
Figure 2.
Intact organisms are not required for induction of phagocyte serine protease inhibitor 9 (PI-9) messenger RNA (mRNA) by Mycobacterium tuberculosis. Alveolar macrophage (AM) monolayers were infected with M. tuberculosis strain H37Rv at several multiplicities of infection (MOIs) (1:1 to 50:1; bacteria/cell) or exposed to H37Rv lysate, a French press preparation of irradiated organisms (1–10 μg/mL). Cells were harvested at 4 hours, and expression of mRNA for PI-9 was assessed. As illustrated, both intact H37Rv (striped bars) and M. tuberculosis cell lysates (solid bars) induce PI-9 in a dose-dependent fashion, indicating that viable organisms are not essential for M. tuberculosis–induced expression of PI-9.
Next, we confirmed differential induction of PI-9 by lysates of virulent M. tuberculosis H37Rv as opposed to avirulent M. tuberculosis H37Ra, as reported elsewhere for whole M. tuberculosis organisms [11]. In 5 experiments M. tuberculosis H37Rv lysate induced significantly greater expression of PI-9 mRNA than did M. tuberculosis H37Ra lysate (P < .001; not shown).
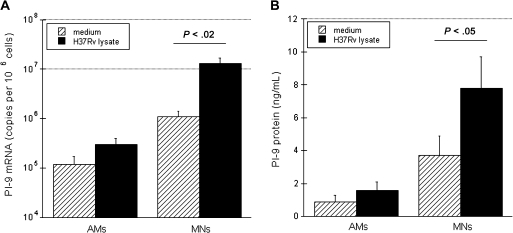
To confirm that induction of PI-9 mRNA by M. tuberculosis resulted in production of PI-9 protein, we then evaluated the effects of H37Rv lysate (1 μg/mL) on PI-9 mRNA and protein levels in both AMs and autologous MNs. As above, induction of PI-9 mRNA at 4 hours was particularly notable in MNs (>10-fold as compared with unstimulated cells [P < .02]) (Figure 3A). This corresponded to immunoreactivity of PI-9 in cell lysates of MNs and AMs at 24 hours as assessed by ELISA (Figure 3B). As illustrated, the observed increase in PI-9 mRNA was associated with significant increases in intracellular PI-9 protein in MNs (P < .05).
Figure 3.
Induction of serine protease inhibitor 9 (PI-9) messenger RNA (mRNA) and protein by Mycobacterium tuberculosis H37Rv lysate in alveolar macrophages (AMs) and blood monocytes (MNs). Induction of PI-9 mRNA and protein in AMs and MNs exposed to M. tuberculosis H37Rv lysate (1 μg/mL) was assessed. A, Induction of PI-9 mRNA in AMs and MNs after 4 hours of incubation with 1 μg/mL H37Rv lysate (solid bars) or medium alone (striped bars). B, Concentrations of PI-9 protein (as measured by enzyme-linked immunosorbent assay) in cell lysates of AMs and MNs after 24 hours of culture with H37Rv lysate or medium. Differences in the induction of MN PI-9 mRNA and PI-9 protein were both significant, as indicated.
The rapid expression of PI-9 in MNs indicated the suitability of this cell population for use in subsequent functional studies of the effects of inhibition of PI-9 using siRNA on other apoptosis-associated molecules. In addition, the demonstration that lysates of M. tuberculosis H37Rv were as effective as viable organisms in inducing PI-9 added to the utility of this model. Specifically, cells that have undergone transfection with siRNA via nucleofection are not fully tolerant to subsequent phagocytosis of intact M. tuberculosis but can readily be treated with bacterial lysates.
Inhibition of M. tuberculosis–Induced PI-9 mRNA and Its Effects on Bcl-2 Expression and Apoptosis
To evaluate the role of PI-9 in modulating phagocyte apoptosis after M. tuberculosis exposure, we assessed the impact of PI-9–specific siRNA on M. tuberculosis–induced expression of components of both the extrinsic and intrinsic apoptotic pathways, as well as on production of caspase 3. These initial mechanistic studies were performed using MNs rather than AMs because of the difficulty in confirming the efficiency of nucleofection in AMs (using flow cytometry–based assessment of uptake of green fluorescent protein–labeled vectors), which is due to the high autofluorescence of this population. The greater early production of PI-9 within MNs also made these cells a more robust model for demonstrating the efficacy of specific siRNA in inhibiting its production. For the purpose of nucleofection studies, MNs were obtained by negative isolation by immunomagnetic cell sorting, and nucleofection was performed with PI-9 and control siRNA constructs. In preliminary experiments the transfection efficiency of MNs (using green fluorescent protein–labeled constructs) was determined to be 50%–80% (not shown).
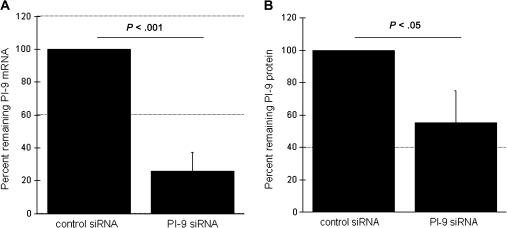
As illustrated in Figure 4, PI-9 siRNA successfully inhibited induction of PI-9 at both mRNA and protein levels. Expression of PI-9 mRNA in MNs transfected with PI-9 siRNA decreased by an average of 74% as compared with MNs transfected with control siRNA (P < .001) (Figure 4A). Likewise, M. tuberculosis–induced PI-9 protein (as assessed by ELISA of cell lysates) was reduced by 46% in MNs transfected with PI-9 siRNA, as compared with those transfected with control siRNA (P < .05) (Figure 4B).
Figure 4.
Induction of serine protease inhibitor 9 (PI-9) by Mycobacterium tuberculosis is effectively inhibited by specific small inhibitory RNA (siRNA). Induction of both PI-9 messenger RNA (mRNA) and protein PI-9 in response to H37Rv lysate (1 μg/mL) were assessed in blood monocytes (MNs) nucleofected with PI-9–specific siRNA. A, PI-9–specific siRNA significantly reduced M. tuberculosis–induced expression of PI-9 mRNA after 4 hours of stimulation, compared with that observed in cells transfected with control siRNA. B, Similarly, MNs transfected with PI-9–specific siRNA produced significantly less PI-9 protein in response to H37Rv lysate than did MNs transfected with control siRNA, as determined by enzyme-linked immunosorbent assay of cell lysates collected after 24 hours of stimulation. For both figures, results are displayed as the percentage of PI-9 mRNA or protein observed in MNs transfected with siRNA specific for PI-9, as compared with that observed in control siRNA-treated cells obtained from the same individual. Data represent means and standard deviations of values from studies performed on MNs from 8 subjects.
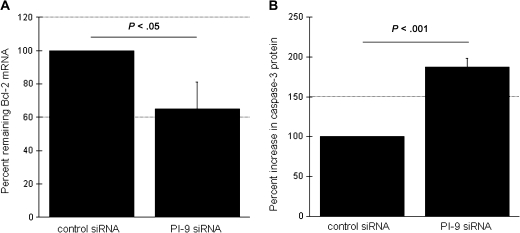
The impact of PI-9 on apoptosis-associated genes was then assessed by comparing their expression in MNs transfected with PI-9–specific or control siRNA in response to H37Rv lysate. This assessment included quantification of mRNA for antiapoptotic genes Bcl-2, Mcl-1, and Bcl-w and proapoptotic genes Bax, TNF-α, and Fas. Expression of caspase 3, the common end effector of both apoptotic pathways was determined as well. In MNs transfected with PI-9 siRNA, M. tuberculosis–induced Bcl-2 expression was reduced to 65% of that observed in MNs transfected with control siRNA (P < .05) (Figure 5A). In contrast, M. tuberculosis–induced expression of mRNA for Bax, Bcl-w, Mcl-1, TNF-α, and Fas was not significantly affected by transfection with PI-9 siRNA (data not shown). Interference with H37Rv-induced PI-9 expression therefore decreases the expression of the antiapoptotic molecule Bcl-2 but has no impact on expression of other genes of the intrinsic pathway or TNF-α and Fas of the extrinsic apoptotic pathway.
Figure 5.
Blood monocytes (MNs) transfected with serine protease inhibitor 9 (PI-9) small inhibitory RNA (siRNA) display decreased expression of Bcl-2 messenger RNA (mRNA) and increased levels of caspase 3 protein in response to stimulation with lysates of Mycobacterium tuberculosis H37Rv. A, Compared with transfection with control siRNA, transfection with PI-9 specific siRNA significantly decreased M. tuberculosis–induced expression of the antiapoptotic molecule Bcl-2 after 4 hours of incubation with H37Rv lysate. mRNA is again expressed as the percentage observed in MNs treated with PI-9 siRNA, compared the percentage in those treated with control siRNA. B, Consistent with this, MNs transfected with PI-9 specific siRNA demonstrated significantly increased production of MN caspase 3 protein (as quantified by Western blot analysis after 24 hours of incubation with H37Rv lysate), which is a common end effector of intrinsic and extrinsic apoptosis.
To confirm that PI-9 induction serves to prevent apoptosis of M. tuberculosis–stimulated MNs, we then examined the impact of PI-9 inhibition on production of caspase 3, the final effector molecule in both intrinsic and extrinsic apoptotic pathways. Caspase 3 was quantified by Western blot analysis of cell lysates of MNs treated with PI-9 siRNA or control siRNA and stimulated with H37Rv lysates. Caspase 3 protein was increased by a median of 89.7% in cells treated with PI-9 siRNA, as compared with those treated with control siRNA (P < .001) (Figure 5B). Thus, specific inhibition of M. tuberculosis–induced PI-9 results in increased phagocyte apoptosis, confirming the antiapoptotic role of PI-9 in M. tuberculosis infection.
Inhibition of M. tuberculosis–Induced PI-9 Expression and the Organism’s Viability Within Human Alveolar Macrophages
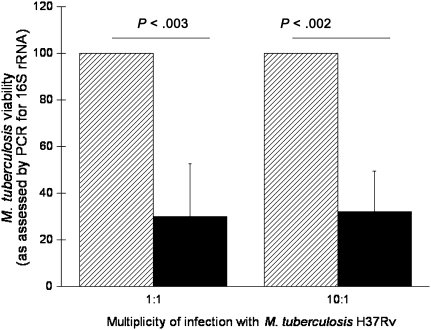
To confirm the impact of PI-9 expression to the intracellular survival of M. tuberculosis after initial exposure to the organism in the lung, AMs from 4 subjects were prepared. Alveolar macrophages were pretreated with control siRNA or PI-9–specific siRNA by passive transfection in duplicates. After 48 hours, AMs were infected with M. tuberculosis H37Rv at an MOI of either 1:1 or 10:1. The viability of intracellular M. tuberculosis was quantified by assessment of expression of M. tuberculosis 16S rRNA, which we found displayed strong correlation with direct measurement of intracellular colony-forming units (P < .001; not shown). M. tuberculosis 16S rRNA expression was assessed 7 days after infection of AMs treated with control and PI-9–specific siRNA. As illustrated in Figure 6, M. tuberculosis 16S rRNA within AMs treated with PI-9–specific siRNA was reduced to only 30.0% (± 22.6%) of that observed in AMs treated with control siRNA (100%) after infection using an MOI of 1:1 (P < .003). Likewise, 16S rRNA of M. tuberculosis within PI-9 siRNA-treated AMs after 10:1 infection was reduced to 32.0% (± 17.6%) as compared with that observed within AMs treated with control siRNA (P < .002). These data indicate that M. tuberculosis–induced PI-9 expression serves to facilitate the organism’s intracellular survival within AMs.
Figure 6.
Specific inhibition of serine protease inhibitor 9 (PI-9) results in decreased viability of Mycobacterium tuberculosis within infected alveolar macrophages (AMs). Compared with transfection with control small inhibitory RNA (siRNA), transfection with PI-9–specific siRNA led to significant decreases in viable intracellular M. tuberculosis within infected AMs, as determined by expression of M. tuberculosis 16S ribosomal RNA (rRNA). 16s rRNA expression within AMs treated with PI-9 siRNA (solid bars) is represented as the percentage of that observed within AMs treated with control siRNA (striped bars). The reduction in M. tuberculosis viability after PI-9 inhibition was statistically significant within AMs infected with the organism at multiplicities of infection of both 1:1 and 10:1. Results represent means and standard deviations of values from studies performed on AMs obtained from 4 subjects. Abbreviation: PCR, polymerase chain reaction.
DISCUSSION
The ability of virulent M. tuberculosis to survive within the intracellular environment of host mononuclear phagocytes has long been a major focus of tuberculosis research. Although the capacity of the organism to inhibit normal phagosome maturation and acidification was the first identified mechanism of facilitating intracellular survival described for M. tuberculosis [20], more recent studies demonstrated that virulent M. tuberculosis inhibits phagocyte apoptosis as an additional means of promoting its own survival [4, 5, 7]. Using a genome-wide microarray approach, we recently identified a novel antiapoptotic molecule, PI-9, as being significantly induced in human alveolar macrophages 24 hours after infection by M. tuberculosis H37Rv [11]. In the current study, we demonstrate induction of PI-9 in AMs and MNs within 4 hours of infection with M. tuberculosis H37Rv. Early expression of PI-9 was induced in response to lysates of H37Rv in a manner similar to that induced by the intact organism. Inhibition of M. tuberculosis–induced PI-9 expression in cells transfected with specific siRNA resulted in decreased Bcl-2 mRNA, increased production of caspase 3, and decreased viability of the intracellular organism. These findings confirm a role for PI-9 among host genes manipulated by virulent M. tuberculosis in order to maintain its intracellular “niche” and indicate a previously undescribed interaction between PI-9 and the intrinsic apoptotic pathway in response to M. tuberculosis infection.
Earlier studies of PI-9 have primarily focused on the interaction of this protein with GrB and its induction by viral pathogens. PI-9 was first identified by a directed search for eukaryotic serine protease inhibitors (serpins) with homology to the viral product serpin cytokine response modifier A. The unusual reactive center of the PI-9 molecule suggested specificity for interaction with GrB, a product of cytotoxic T cells, because this is the only known serine protease that cleaves after acidic residues [21]. PI-9 binds GrB and prevents this component of cytotoxic lymphocyte granules from inciting target cell DNA degradation and resulting apoptosis. PI-9 is strongly expressed within cytotoxic CD8+ T cells and natural killer cells, suggesting that it normally serves to protect these cells from self-induced apoptosis resulting from misdirected granule release. The recent observation of PI-9 expression in human blood MNs and dendritic cells, as well as the augmentation of expression of PI-9 at immune privileged sites (eg, placenta, testis, eyes) [19], further suggests a protective role for this molecule against the effects of degranulating cytotoxic cells that release GrB. Previous studies of Jurkat cells transfected with PI-9–expressing vectors found that these cells were protected from apoptosis mediated by GrB. In contrast, PI-9 expression had no impact on either the extrinsic apoptotic pathway (in response to stimulation with anti-Fas antibodies) or the intrinsic apoptotic pathway (as induced by staurosporine) in this experimental system [12]. In addition to its antiapoptotic profile, PI-9 binds caspase 1 (interleukin [IL] 1–converting enzyme) [22], whereas IL-1β has itself been shown to induce PI-9 mRNA [23], suggesting the involvement of PI-9 in autoregulatory limitation of IL-1–mediated inflammation. In a study of acutely ill children, PI-9 was induced in blood MNs during infection with Epstein–Barr virus, but not with bacterial pathogens [24], suggesting that its up-regulation may be more specifically initiated by intracellular pathogens. Our recent microarray study was the first to document expression of PI-9 in human mononuclear phagocytes in response to infection with virulent M. tuberculosis [11].
Although expression of PI-9 by virulent M. tuberculosis–infected mononuclear phagocytes may suggest a means for the organism to evade the lytic effects of cytotoxic lymphocytes (through resistance to GrB), the effect of PI-9 expression in these host cells and, in particular, its role in intracellular infections in the absence of cytotoxic lymphocytes have not been investigated until recently. However, the very early induction of PI-9 observed here in both AMs and MNs suggested the possibility that this response could contribute to initial resistance of the organism to the antimicrobial defenses of mononuclear phagocytes themselves.
Various studies have indicated the ability of M. tuberculosis to inhibit phagocyte apoptotic pathways as a means to further its own intracellular survival. A previous limited microarray assessment focused on known apoptosis-associated genes indicated differential induction of macrophage apoptotic pathways by virulent H37Rv as compared to avirulent M. tuberculosis strain H37Ra [7]. These differences seemed to be based on increased resistance of the virulent organism to the effects of M. tuberculosis–induced TNF-α and thus implied a role for subversion of the extrinsic apoptotic pathway in survival of the H37Rv. Findings of other studies have suggested that M. tuberculosis–induced modulation of intrinsic apoptotic pathways contributes to viability of intracellular bacilli as well. Induction of Mcl-1, an antiapoptotic member of the Bcl-2 family, was found to be associated with survival of H37Rv infection in THP-1 cells and MN–derived macrophages [6]. Another antiapoptotic member of the Bcl-2 family, the nuclear factor–κb–dependent molecule bfl-1/A1, was more strongly induced by H37Rv than by H37Ra in a THP-1 cell line infection model. Inhibition of blf-1/A1 with specific siRNA in this system resulted in increased apoptosis of M. tuberculosis–infected cells as well as enhancement of phagosome-lysosome fusion [25]. A murine model of infection further supports the role of regulation of the intrinsic apoptotic pathway in response to M. tuberculosis, as survival of the pathogen is associated with an increase in the ratio of the expression of antiapoptotic Bcl-2 to that of the proapoptotic Bcl-2 family member Bax [26]. In addition, infection with M. tuberculosis at high MOIs has been reported to induce apoptosis by a distinct mechanism that is both TNF-α and caspase independent and that is followed by rapid progression to cell necrosis [27].
Our current findings further illustrate the complexity of the capacity of M. tuberculosis to inhibit apoptosis of host mononuclear phagocytes. Inhibition of PI-9 with specific siRNA results in decreased Bcl-2, but not Bax, indicating that M. tuberculosis–induced PI-9 serves to increase the Bcl-2/Bax ratio (as has shown elsewhere to correlate with the intracellular survival of the bacilli) [26]. This observation also indicates a previously unknown role for PI-9 in inhibiting GrB-independent apoptosis and stands in contrast to a prior report that PI-9 did not impact staurosporine-dependent activation of the intrinsic apoptotic cascade [12]. Given that the inciting factors in intrinsically mediated apoptosis are poorly understood, this discrepancy could be attributable to differences in the manner in which M. tuberculosis and staurosporine initiate this pathway. Alternatively, these stimuli may have differing effects in the distinct cell types studied (primary mononuclear phagocytes vs the Jurkat T-cell leukemia cell line). The continued expression of PI-9 in 7-day cultured AMs also implies that H37Rv-infected AMs may be enabled to survive the encounter with GrB-secreting cytotoxic lymphocytes on subsequent recruitment of these cells to the lung.
In summary, induction of mononuclear phagocyte PI-9 in response to virulent M. tuberculosis inhibits the intrinsic apoptotic pathway and thereby promotes survival of both host cells and the intracellular pathogen. These findings suggest that, in combination with its other effects of inhibiting inflammation and providing resistance to GrB-mediated cytotoxicity, induction of PI-9 within host phagocytes may play a multidimensional role in the pathogenesis of human M. tuberculosis infection.
Notes
Financial support.
This study was supported by the National Institutes of Health (NIH) (grants HL 51636 and AI-95383); the Center for AIDS Research (grant AI-36219); and the US Department of Veteran’s Affairs Merit Review program (grants to Z.T. and R.F.S.). Research bronchoscopy procedures were performed in the Dahms Clinical Research Unit of the Case Western Reserve University Clinical and Translational Science Collaborative at University Hospitals of Cleveland-Case Medical Center (NIH UL1 RR024989) from the National Center for Research Resources.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Molloy A, Laochumroonvorapong P, Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guerin. J Exp Med. 1994;180:1499–509. doi: 10.1084/jem.180.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fratazzi C, Arbeit RD, Carini C, et al. Macrophage apoptosis in mycobacterial infections. J Leukoc Biol. 1999;66:763–4. doi: 10.1002/jlb.66.5.763. [DOI] [PubMed] [Google Scholar]
- 3.Keane J, Balcewicz-Sablinska MK, Remold HG, et al. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect Immun. 1997;65:298–304. doi: 10.1128/iai.65.1.298-304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keane J, Remold HG, Kornfeld H. Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J Immunol. 2000;164:2016–20. doi: 10.4049/jimmunol.164.4.2016. [DOI] [PubMed] [Google Scholar]
- 5.Rios-Barrera VA, Campos-Pena V, Aguilar-Leon D, et al. Macrophage and T lymphocyte apoptosis during experimental pulmonary tuberculosis: their relationship to mycobacterial virulence. Eur J Immunol. 2006;36:345–53. doi: 10.1002/eji.200535202. [DOI] [PubMed] [Google Scholar]
- 6.Sly LM, Hingley-Wilson SM, Reiner NE, McMaster WR. Survival of Mycobacterium tuberculosis in host macrophages involves resistance to apoptosis dependent upon induction of antiapoptotic Bcl-2 family member Mcl-1. J Immunol. 2003;170:430–7. doi: 10.4049/jimmunol.170.1.430. [DOI] [PubMed] [Google Scholar]
- 7.Spira A, Carroll JD, Liu G, et al. Apoptosis genes in human alveolar macrophages infected with virulent or attenuated Mycobacterium tuberculosis: a pivotal role for tumor necrosis factor. Am J Respir Cell Mol Biol. 2003;29:545–51. doi: 10.1165/rcmb.2002-0310OC. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Jiang R, Takayama H, Tanaka Y. Survival of virulent Mycobacterium tuberculosis involves preventing apoptosis induced by Bcl-2 upregulation and release resulting from necrosis in J774 macrophages. Microbiol Immunol. 2005;49:845–52. doi: 10.1111/j.1348-0421.2005.tb03673.x. [DOI] [PubMed] [Google Scholar]
- 9.Wesche DE, Lomas-Neira JL, Perl M, Chung CS, Ayala A. Leukocyte apoptosis and its significance in sepsis and shock. J Leukoc Biol. 2005;78:325–37. doi: 10.1189/jlb.0105017. [DOI] [PubMed] [Google Scholar]
- 10.Logue SE, Martin SJ. Caspase activation cascades in apoptosis. Biochem Soc Trans. 2008;36:1–9. doi: 10.1042/BST0360001. [DOI] [PubMed] [Google Scholar]
- 11.Silver RF, Walrath J, Lee H, et al. Human alveolar macrophage gene responses to Mycobacterium tuberculosis strains H37Ra and H37Rv. Am J Respir Cell Mol Biol. 2009;40:491–504. doi: 10.1165/rcmb.2008-0219OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bird CH, Sutton VR, Sun J, et al. Selective regulation of apoptosis: the cytotoxic lymphocyte serpin proteinase inhibitor 9 protects against granzyme B-mediated apoptosis without perturbing the Fas cell death pathway. Mol Cell Biol. 1998;18:6387–98. doi: 10.1128/mcb.18.11.6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walrath J, Zukowski L, Krywiak A, Silver RF. Resident Th1-like effector memory cells in pulmonary recall responses to Mycobacterium tuberculosis. Am J Respir Cell Mol Biol. 2005;33:48–55. doi: 10.1165/rcmb.2005-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toossi Z, Hirsch CS, Hamilton BD, Knuth CK, Friedlander MA, Rich EA. Decreased production of TGF-beta 1 by human alveolar macrophages compared with blood monocytes. J Immunol. 1996;156:3461–8. [PubMed] [Google Scholar]
- 15.Silver RF, Li Q, Boom WH, Ellner JJ. Lymphocyte-dependent inhibition of growth of virulent Mycobacterium tuberculosis H37Rv within human monocytes: requirement for CD4+ T cells in purified protein derivative-positive, but not in purified protein derivative-negative subjects. J Immunol. 1998;160:2408–17. [PubMed] [Google Scholar]
- 16.Young JL, Sukhova GK, Foster D, Kisiel W, Libby P, Schonbeck U. The serpin proteinase inhibitor 9 is an endogenous inhibitor of interleukin 1beta-converting enzyme (caspase-1) activity in human vascular smooth muscle cells. J Exp Med. 2000;191:1535–44. doi: 10.1084/jem.191.9.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilkinson RJ, Desjardin LE, Islam N, et al. An increase in expression of a Mycobacterium tuberculosis mycolyl transferase gene (fbpB) occurs early after infection of human monocytes. Mol Microbiol. 2001;39:813–21. doi: 10.1046/j.1365-2958.2001.02280.x. [DOI] [PubMed] [Google Scholar]
- 18.Islam N, Kanost AR, Teixeira L, et al. Role of cellular activation and tumor necrosis factor-alpha in the early expression of Mycobacterium tuberculosis 85B mRNA in human alveolar macrophages. J Infect Dis. 2004;190:341–51. doi: 10.1086/421522. [DOI] [PubMed] [Google Scholar]
- 19.Bladergroen BA, Strik MC, Bovenschen N, et al. The granzyme B inhibitor, protease inhibitor 9, is mainly expressed by dendritic cells and at immune-privileged sites. J Immunol. 2001;166:3218–25. doi: 10.4049/jimmunol.166.5.3218. [DOI] [PubMed] [Google Scholar]
- 20.Rook GA, Hernandez-Pando R. The pathogenesis of tuberculosis. Annu Rev Microbiol. 1996;50:259–84. doi: 10.1146/annurev.micro.50.1.259. [DOI] [PubMed] [Google Scholar]
- 21.Sun J, Bird CH, Sutton V, et al. A cytosolic granzyme B inhibitor related to the viral apoptotic regulator cytokine response modifier A is present in cytotoxic lymphocytes. J Biol Chem. 1996;271:27802–9. doi: 10.1074/jbc.271.44.27802. [DOI] [PubMed] [Google Scholar]
- 22.Annand RR, Dahlen JR, Sprecher CA, et al. Caspase-1 (interleukin-1beta-converting enzyme) is inhibited by the human serpin analogue proteinase inhibitor 9. Biochem J. 1999;342:655–65. [PMC free article] [PubMed] [Google Scholar]
- 23.Kannan-Thulasiraman P, Shapiro DJ. Modulators of inflammation use nuclear factor-kappa B and activator protein-1 sites to induce the caspase-1 and granzyme B inhibitor, proteinase inhibitor 9. J Biol Chem. 2002;277:41230–9. doi: 10.1074/jbc.M200379200. [DOI] [PubMed] [Google Scholar]
- 24.Classen CF, Bird PI, Debatin KM. Modulation of the granzyme B inhibitor proteinase inhibitor 9 (PI-9) by activation of lymphocytes and monocytes in vitro and by Epstein–Barr virus and bacterial infection. Clin Exp Immunol. 2006;143:534–42. doi: 10.1111/j.1365-2249.2006.03006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhiman R, Kathania M, Raje M, Majumdar S. Inhibition of bfl-1/A1 by siRNA inhibits mycobacterial growth in THP-1 cells by enhancing phagosomal acidification. Biochim Biophys Acta. 2008;1780:733–42. doi: 10.1016/j.bbagen.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Mogga SJ, Mustafa T, Sviland L, Nilsen R. Increased Bcl-2 and reduced Bax expression in infected macrophages in slowly progressive primary murine Mycobacterium tuberculosis infection. Scand J Immunol. 2002;56:383–91. doi: 10.1046/j.1365-3083.2002.01140.x. [DOI] [PubMed] [Google Scholar]
- 27.Lee J, Remold HG, Ieong MH, Kornfeld H. Macrophage apoptosis in response to high intracellular burden of Mycobacterium tuberculosis is mediated by a novel caspase-independent pathway. J Immunol. 2006;176:4267–74. doi: 10.4049/jimmunol.176.7.4267. [DOI] [PubMed] [Google Scholar]