Abstract
(See the editorial commentary by Tossonian and Conway, on pages 10–12.)
Background. The benefits of antiretroviral therapy during early human immunodeficiency virus type 1 (HIV-1) infection remain unproved.
Methods. A5217 study team randomized patients within 6 months of HIV-1 seroconversion to receive either 36 weeks of antiretrovirals (immediate treatment [IT]) or no treatment (deferred treatment [DT]). Patients were to start or restart antiretroviral therapy if they met predefined criteria. The primary end point was a composite of requiring treatment or retreatment and the log10 HIV-1 RNA level at week 72 (both groups) and 36 (DT group).
Results. At the June 2009 Data Safety Monitoring Board (DSMB) review, 130 of 150 targeted participants had enrolled. Efficacy analysis included 79 individuals randomized ≥72 weeks previously. For the primary end point, the IT group at week 72 had a better outcome than the DT group at week 72 (P = .005) and the DT group at week 36 (P = .002). The differences were primarily due to the higher rate of progression to needing treatment in the DT group (50%) versus the IT (10%) group. The DSMB recommended stopping the study because further follow-up was unlikely to change these findings.
Conclusions. Progression to meeting criteria for antiretroviral initiation in the DT group occurred more frequently than anticipated, limiting the ability to evaluate virologic set point. Antiretrovirals during early HIV-1 infection modestly delayed the need for subsequent treatment.
Clinical Trials Registration. NCT00090779.
The role of antiretroviral therapy (ART) during acute and early human immunodeficiency virus type 1 (HIV-1) infection has been an area of investigation for several years; however, the benefits of such treatment remain unproved, and best practice for clinical management of this unique stage of infection remains unknown [1]. The initiation of ART shortly after HIV-1 infection has been shown to preserve HIV-1–specific immune responses that could improve virologic control on discontinuation of treatment [2–4]. However, prospective observational studies have evaluated the effects of early ART followed by treatment cessation on subsequent virologic control with conflicting results [5–15], and few randomized clinical trials have been performed in the setting of acute and early HIV-1 infection [16–20]. We hypothesized that 36 weeks of ART administered to participants within 6 months of acquiring HIV-1 infection would lower the virologic set point after treatment discontinuation.
The AIDS Clinical Trials Group (ACTG) Setpoint Study (A5217) was a multicenter, randomized clinical trial during which individuals with recent but not acute HIV-1 infection were randomized to begin immediate treatment (IT) with a 36-week course of ART and then discontinue treatment, or to defer treatment (DT) until prespecified criteria for initiation of therapy were met. During a scheduled interim review, the Data and Safety Monitoring Board (DSMB) of the National Institute of Allergy and Infectious Diseases (NIAID) noted a higher than expected rate of disease progression and subsequent initiation of ART in the DT group, limiting the ability to compare the actual virologic set point between the 2 groups. As a result of these findings in June 2009, the DSMB stated that further enrollment and follow-up as designed could not be justified and recommended study discontinuation. We report here on the data collected through the time of the DSMB recommendations.
METHODS
Study Participants
The study enrolled men and nonpregnant women who were ≥18 years of age, were HIV-1 infected within the last 6 months but beyond the acute phase of infection (with a positive HIV-1 Western blot), and had no prior ART, acceptable laboratory parameters, and an HIV-1 RNA level ≥500 copies/mL. Recent infection was defined as a nonreactive detuned HIV-1 antibody test consistent with infection of <6 months duration at the time of screening, or documented seroconversion (ie, a documented negative HIV-1 enzyme immunoassay [EIA] or a negative or indeterminate Western blot within 6 months before the study). We initially used the Vironostika HIV-1 detuned EIA, with recent infection defined as a standardized optical density measurement ≤0.75 [21]. In 2008, the Vironostika assay was no longer available, and the detuned Ora-Quick rapid test was used to confirm recent infection in the absence of documented seroconversion [22–24]. All detuned assays were performed at either the University of North Carolina at Chapel Hill or the Blood Systems Research Institute in San Francisco. We excluded participants who met immunologic or clinical criteria for treatment at entry [25], including the occurrence of Centers for Disease Control and Prevention (CDC) category B or C diagnoses, CD4+ T-cell count <350 cells/mm3, or CD4+ T-cell percentage of <14%. Baseline genotypic resistance testing was performed on all participants, with therapy adjusted as necessary when data became available. Individuals with baseline resistance to >1 component of the initial study regimen were excluded. The institutional review board at each participating site approved the study protocol, and written informed consent was obtained from all participants.
Study Design
ACTG 5217 was a 96-week, multicenter, randomized, open-label study that began in February 2005 and was performed at 25 sites in the United States and 2 in Peru. One hundred fifty recently HIV-1–infected adults were to be randomized 1:1 to the IT group versus the DT group. Participants in the IT group received 36 weeks of ART followed by treatment discontinuation. Participants in the DT group were followed up off treatment throughout the study; however, individuals in either group who met prespecified criteria for treatment initiation or reinitiation were advised to begin ART.
Screening evaluations included confirmation of recent HIV-1 infection as defined above, genotypic resistance testing, CD4+ T-cell count, and HIV-1 RNA level. Baseline and on-study evaluations occurred at weeks 0, 1, 2, and 4 and every 4 weeks thereafter for the duration of the study and included CD4+ T-cell count and HIV-1 RNA level, the latter measured using the Roche Amplicor Monitor assay, version 1.5, at a laboratory certified by Division of AIDS Virology Quality Assurance program.
Study Treatment
The study provided fixed-dose combination emtricitabine-tenofovir DF (one 200/300 mg tablet, once daily) and lopinavir-ritonavir (200/50 mg tablets, 2 tablets twice daily or 4 tablets once daily) for the first 36 weeks for individuals in the IT group and for the remaining duration of the study for individuals in either group who had met eligibility for initiation of ART. However, study participants were allowed to receive any alternative provider-prescribed potent ART regimen.
Criteria for Initiating ART
Participants in either arm who met protocol-specified criteria for treatment initiation or reinitiation were advised but not required to begin treatment. The prespecified criteria for initiating ART were designed to be consistent with treatment guidelines for chronic infection at the time [25] and included (1) CD4+ T-cell count <350 cells/mm3 at 2 consecutive determinations ≥4 weeks apart, ≥12 weeks into the study or ≥12 weeks after treatment discontinuation; (2) confirmed CD4+ T-cell count <200 cells/mm3 or CD4+ T-cell percentage <14% at any time during the study; (3) confirmed HIV-1 RNA level >750 000 copies/mL ≥4 weeks into the study or >200 000 copies/mL ≥12 weeks into the study; or (4) CDC category B or C diagnosis. The time requirements were to accommodate the fluctuations in CD4+ T-cell counts and HIV-1 RNA levels characteristically seen with recent seroconversion.
Statistical Analysis
The primary end point was constructed as a composite measure that consisted of the average viral load at weeks 72 and 76 for study participants who continued to week 72 off treatment and an assigned viral load rank for those who met criteria for initiating ART before these study visits and thus could not contribute an off-treatment HIV-1 RNA assessment. Such individuals were considered to have experienced a poor outcome and were assigned an HIV-1 RNA rank. The assigned rank was either the last observed rank carried forward or the worst rank, according to an analysis plan that was designed to be, if anything, biased against finding a treatment effect; details of the algorithm for assigning ranks are provided in the Supplementary appendix. Differences in the primary end point between the 2 treatment groups at week 72 were then assessed using the Wilcoxon rank sum 1-sided test, use of which reflects the focus of interest in only the 1-sided alternative hypothesis that immediate short-term therapy is superior to delay in starting therapy. However, at 72 weeks, participants in the IT group would have been off therapy for 36 weeks, whereas those in the DT group would have been off therapy for 72 weeks. To provide additional insight about any benefits of treatment, a difference in virologic set point observed at 72 weeks would trigger an additional analysis: comparison of the end point at week 72 in the IT group (36 weeks after ART was interrupted) with the end point at week 36 in the DT group (detailed further in [26]). The secondary end point, time to meeting eligibility for initiating or reinitiating ART, was assessed via Kaplan-Meier plot and log-rank test.
Power calculations were based on a 1-sided, 2-sample t test at α = .05, adjusted for the use of methods based on ranked data. The planned sample size of 75 individuals randomized to each group provided >90% power to detect a plasma HIV-1 RNA improvement of 0.6 log10 copies/mL in the IT group compared with the DT group. This provided power of ≥80% for the combined 2-step test, using Bonferroni inequality.
Study Monitoring
The initial monitoring plan included 2 interim safety reviews conducted by the NIAID Therapeutics DSMB. However, given slower than expected accrual, annual reviews of efficacy and futility analyses started in the summer of 2008. Subsequently, the DSMB performed an annual review of safety and efficacy data on 25 June 2009 and recommended premature discontinuation of the study.
RESULTS
Baseline Characteristics
We had enrolled 130 eligible of 150 targeted participants at the time of the June 2009 DSMB review. Baseline characteristics were well balanced between the groups (Table 1).
Table 1.
Baseline Characteristics
| All Eligible Subjects |
Subjects Included in Primary Analysis |
|||||
| Characteristic | IT Group (n = 66) | DT Group (n = 64) | Total (n = 130) | IT Group (n = 39) | DT Group (n = 40) | Total (n = 79) |
| Sex | ||||||
| Male | 58 (88) | 59 (92) | 117 (90) | 34 (87) | 40 (100) | 74 (94) |
| Female | 8 (12) | 5 (8) | 13 (10) | 5 (13) | 0 (0) | 5 (6) |
| Age, median (IQR), y | 34 (25–40) | 33 (27–42) | 33 (26–42) | 37 (23–42) | 36.0 (28.5–42.5) | 36 (26–42) |
| Race | ||||||
| White | 49 (74) | 56 (88) | 105 (81) | 31 (79) | 38 (95) | 69 (87) |
| Black/African American | 10 (15) | 3 (5) | 13 (10) | 6 (15) | 2 (5) | 8 (10) |
| Other/unknown | 7 (11) | 5 (8) | 12 (9) | 2 (5) | 0 (0) | 2 (3) |
| Ethnicity | ||||||
| Hispanic or Latino | 14 (21) | 9 (14) | 23 (18) | 7 (18) | 1 (3) | 8 (10) |
| Not Hispanic or Latino | 52 (79) | 55 (86) | 107 (82) | 32 (82) | 39 (98) | 71 (90) |
| Transmission risk | ||||||
| MSM | 52 (79) | 55 (86) | 107 (82) | 31 (79) | 36 (90) | 67 (85) |
| Heterosexual | 10 (15) | 7 (11) | 17 (13) | 8 (21) | 3 (8) | 11 (14) |
| IVDU | 1 (2) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) |
| MSM and IVDU | 3 (5) | 1 (2) | 4 (3) | 0 (0) | 1 (3) | 1 (1) |
| Unknown | 0 (0) | 1 (2) | 1 (1) | 0 (0) | 0 (0) | 0 (0) |
| CD4+ T-cell count, median (IQR), cells/mm3a | 514 (415–671) | 557 (441–721) | 540 (435–697) | 488 (402, 671) | 552 (435–704) | 534 (415–697) |
| CD4+ T-cell count, cells/mm3 | ||||||
| 201–350 | 4 (6) | 2 (3) | 6 (5) | 3 (8) | 2 (5) | 5 (6) |
| 351–500 | 26 (39) | 24 (38) | 50 (38) | 17 (44) | 14 (35) | 31 (39) |
| >500 | 36 (55) | 38 (59) | 74 (57) | 19 (49) | 24 (60) | 43 (54) |
| HIV-1 RNA, median (IQR), log10 copies/mLa | 4.4 (3.9–4.8) | 4.4 (4.0–4.7) | 4.4 (4.0–4.7) | 4.4 (3.9–4.8) | 4.4 (4.1–4.9) | 4.4 (4.0–4.9) |
| HIV-1 RNA, copies/mL | ||||||
| <10 000 | 17 (26) | 16 (25) | 33 (25) | 10 (26) | 8 (20) | 18 (23) |
| ≥10 000 | 49 (74) | 48 (75) | 97 (75) | 29 (74) | 32 (80) | 61 (77) |
Except where otherwise indicated, data represent No. (%) of subjects. P > .05 for all comparisons between treatment groups.
Abbreviations: DT, deferred treatment; HIV-1, human immunodeficiency virus, type 1; IQR, interquartile range; IT, immediate treatment; IVDU, intravenous drug use; MSM, men who have sex with men.
Baseline CD4+ T-cell counts and baseline HIV-1 RNA levels were the values at study entry.
Study Status
At the time of the June 2009 DSMB review, 52 (40%) of 130 participants were still in the study, 52 (40%) had completed the protocol, 24 (18.5%) had left the study before week 96, and 2 in the DT group had died. One death was a suicide 14 weeks into the study, and the other occurred 8 weeks into the study and was of unknown cause. Reasons for going off study prematurely are shown in Table 2. Forty-five of 66 (68%) IT participants had completed 36 weeks of ART, 13 of 66 (20%) were in the midst of treatment when the study was stopped, 4 (6%) discontinued ART prematurely, and 4 (6%) discontinued the study before week 36. Fifty-five of 66 (83%) treated participants chose the study-provided ART regimen, and 88% of all participants randomized to the IT group achieved complete virologic suppression by week 24. One participant randomized to the IT group initiated study medications and was promptly discontinued and excluded from the efficacy analysis after review of the baseline pol sequence analysis showed multidrug resistance.
Table 2.
Summary of Study Status at the Time of Data and Safety Monitoring Board Recommendations
| Treatment Group, No. (%) |
|||
| Status | Immediate (n = 66) | Deferred (n = 64) | Total (n = 130) |
| In study | 29 (44) | 23 (36) | 52 (40) |
| Died | 0 (0) | 2 (3) | 2 (2) |
| Completed protocol | 26 (39) | 26 (41) | 52 (40) |
| Premature discontinuationa | 11 (17) | 13 (20) | 24 (18) |
| Subject refused further participation | 4 (36) | 2 (15) | 6 (25) |
| Nonadherence to study requirements | 0 (0) | 1 (8) | 1 (4) |
| Subject relocated, no remote follow-up planned | 2 (18) | 8 (62) | 10 (42) |
| Subject could not be contacted | 3 (27) | 2 (15) | 5 (21) |
| Investigator/clinician decision | 1 (9) | 0 (0) | 1 (4) |
| Other (pregnancy) | 1 (9) | 0 (0) | 1 (4) |
Four subjects (all in the deferred treatment group) met criteria for initiation of antiretroviral therapy before prematurely leaving the study.
Eligibility for Initiation or Reinitiation of ART
When all 130 participants were included, regardless of length of time on protocol, 7 of 66 (11%) in the IT group and 23 of 64 (36%) in the DT group met eligibility for initiation/reinitiation of ART, with 13 (20%) of those in the DT group meeting criteria within the first 36 weeks. The majority of participants who met criteria for treatment initiation met immunologic criteria (6 in IT group, 14 in DT group), and a few met virologic criteria (5 in DT group). Five individuals met eligibility owing to the occurrence of a CDC category B or C event (4 in the DT group, 1 in the IT group) (Table 3). A total of 5 individuals, all in the DT group, progressed to AIDS—1 because of persistent herpes simplex infection, 1 because of CD4+ T-cell count <200 cells/mm3, and 3 because of CD4+ T-cell percentage <14%.
Table 3.
Summary of Eligibility Criteria Met for Initiation of Antiretroviral Therapy (ART)
| Treatment Group, No. |
||
| Criteria | Immediate (n = 66) | Deferred (n = 64) |
| Total meeting ART criteria | 7 (11%) | 23 (36%) |
| CD4+ T-cell percentage <14% and HIV-1 RNA >200 000 copies/mLa | 0 | 1 |
| CD4+ T-cell count <350 cells/mm3 (2 consecutive visits) | 6 | 10 |
| CD4+ T-cell percentage <14% | 0 | 2 |
| CD4+ T-cell count <200 cells/mm3 | 0 | 1 |
| HIV-1 RNA >750 000 copies/mL (2 consecutive visits) | 0 | 1 |
| HIV-1 RNA >200 000 copies/mL (2 consecutive visits) | 0 | 4 |
| CDC category B or C disease | 1 | 4 |
| Herpes simplex for >1 month | 0 | 1 |
| Oral hairy leukoplakia | 0 | 1 |
| Fatigue | 1 | 0 |
| Idiopathic thrombocytopenic purpura | 0 | 2 |
Abbreviations: CDC, Centers for Disease Control and Prevention; HIV-1, human immunodeficiency virus, type 1.
Only the first criterion that study participants met was counted in the summary of eligibility for ART initiation. One individual met 2 eligibility criteria simultaneously. Laboratory values meeting eligibility criteria were confirmed over 2 consecutive visits.
Primary Efficacy Analysis
Efficacy analysis was limited to 79 participants (39 and 40 from the IT and DT groups, respectively) who had been randomized ≥72 weeks before the DSMB review. By week 72, 50% of the 40 DT participants versus 10% of the 39 IT participants had met criteria for initiation/reinitiation of ART. At week 36, 27.5% of the 40 DT participants had met criteria for starting ART.
For the primary end point, the IT group at week 72 had a better outcome than the DT group at 72 weeks (P = .005; 1-sided Wilcoxon test) or 36 weeks (P = .002; 1-sided Wilcoxon test). The outcome was the same when the analysis was based on available data for all enrolled participants (ie, including an additional 50 participants) instead of being restricted only to 79 who were randomized ≥72 weeks before the DSMB recommendations. Thus, superiority was demonstrated for the IT group. Because of the higher-than-expected number of individuals meeting criteria for initiating ART, the primary analysis was highly influenced by the higher rate of progression in the DT group. Because off-treatment HIV-1 RNA levels were unobserved for all participants who met criteria for initiating ART, we were unable to make conclusions regarding the actual virologic set point.
Time to Meeting Eligibility Criteria for Initiating or Reinitiating ART
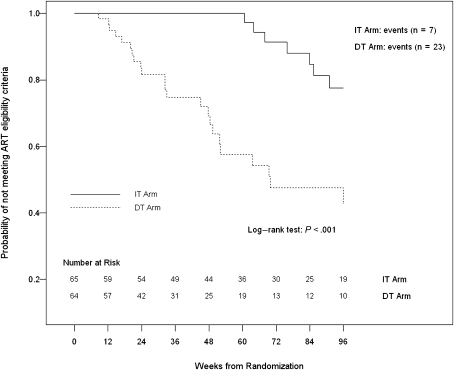
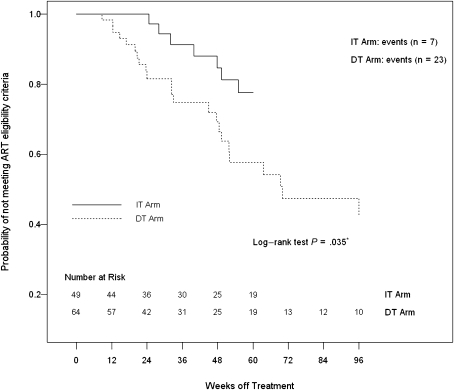
A secondary end point was time to meeting eligibility criteria for initiating or reinitiating ART, which was significantly shorter in the DT group than in the IT group using data up to 76 and 96 weeks (P < .001 for both, by log-rank test). Figure 1 shows the time to meeting eligibility criteria for starting ART over the 96 weeks of the study. In a different analysis that compared the first 36 weeks of the study for participants in the DT group and the period from weeks 36 to 72 for participants in the IT group, the time to meeting criteria for initiating ART remained shorter in the DT group than in the IT group (Figure 2). Using the time when ART was interrupted (week 36) as the time origin for the IT group and week 0 as the time origin for the DT group, the curves remain significantly different (P = .035; log-rank test), but the analysis includes only those in the IT group who continued ART through week 36 (n = 49), compared with all in the DT group (n = 64), and therefore it is not a randomized comparison. Treated participants experienced an additional delay (∼16 weeks) beyond the 36 weeks of treatment before failures began to occur.
Figure 1.
Time to meeting eligibility criteria for initiation or reinitiation of antiretroviral therapy (ART) for the immediate treatment (IT) and deferred treatment (DT) groups over the 96 weeks of the study; times were significantly longer in the IT group (P < .001; log–rank test).
Figure 2.
Time to meeting eligibility criteria for initiation or reinitiation of antiretroviral therapy (ART) for the immediate treatment (IT) and deferred treatment (DT) groups. The time origin for the IT group was the time when ART was interrupted (week 36), and the time origin for the DT group was week 0. The curves remain significantly different (P = .035 by log–rank test), but the analysis includes only those in the IT group who continued ART through week 36 (n = 49), compared with all in the DT group (n = 64), and therefore it is not a truly randomized comparison.
Proportional hazard models adjusted for treatment group were used to evaluate whether any of the baseline characteristics predicted time to meeting criteria for initiating ART. Baseline CD4+ T-cell count <540 cells/mm3 and baseline viral load ≥4.4 log10 copies/mL were associated with shorter time to meeting criteria for starting ART (hazard ratio, 4.49 [P = .0003] and 3.99 [P = .0007], respectively). No significant interaction effect was found between treatment group and baseline CD4+ T-cell count or baseline HIV-1 RNA value.
Observed HIV-1 RNA Values
Among participants who contributed an observed week 72 and/or week 76 HIV-1 RNA value (26 of 39 [67%] in the IT group and 11 of 40 [27.5%] in the DT group), the mean HIV-1 RNA levels at weeks 72 and 76 were similar between the 2 treatment groups (3.99 log10 copies/mL in the IT group and 4.15 log10 copies/mL in the DT group). Among participants (26 in the IT group and 22 in the DT group) who contributed an observed week 72 and/or week 76 HIV-1 RNA value (IT group) or an observed week 36 and/or week 40 HIV-1 RNA value (DT group), the point estimate for the mean HIV-1 RNA level at week 36 in the DT group was higher than that measured at week 72 in the IT group (4.37 log10 copies/mL vs 3.99 log10 copies/mL), but this finding must be interpreted with caution, given that the confidence intervals overlap and the remaining individuals were a selected subgroup of the original participants. The mean log10 copies/mL change in HIV-1 RNA from baseline was −0.29 at 72 weeks in the IT group and 0.07 at 36 weeks in the DT group (Table 4).
Table 4.
Observed log10 HIV-1 RNA Values
| HIV-1 RNA Values | Immediate Therapy | Deferred Therapy |
| HIV-1 RNA, mean (SE), log10 copies/mL | ||
| Week 0 | 4.36 (0.10) (n = 39) | 4.50 (0.09) (n = 40) |
| Week 36 | … | 4.37 (0.09) (n = 22) |
| Week 72 | 3.99 (0.13) (n = 26) | 4.15 (0.13) (n = 11) |
| Change in HIV-1 RNA from weeks 0 to 36, mean (SE), log10 copies/mL | NA | 0.07 (0.09) |
| Change in HIV-1 RNA from weeks 0 to 72, mean (SE), log10 copies/mL | −0.29 (0.16) | 0.01 (0.17) |
Abbreviations: HIV-1, human immunodeficiency virus, type 1; NA, not applicable; SE, standard error.
DISCUSSION
This is the largest randomized controlled trial designed to assess whether ART initiated during early but not acute HIV-1 infection is associated with better virologic outcomes than deferred ART. The study demonstrated a better outcome in the IT group, as measured by the composite end point at week 72 as well as at week 36 for the DT group versus week 72 for the IT group. Owing to the higher-than-anticipated rates of progression and, consequently, initiation of therapy, the difference in virologic set points between the 2 groups could not be statistically evaluated. In addition, participants in the IT group took significantly longer to meet the criteria for starting ART. Treated participants appear to have been protected not only while on treatment but also for a brief period of time thereafter. Thus, a limited period of ART during early HIV-1 infection delayed the need for subsequent initiation of long-term ART.
The best practice for the clinical management of primary HIV-1 infection remains unknown. Although prospective observational data from the HIV-CAUSAL Collaboration demonstrated the lowest mortality rate (6/1000 person-years) in seroconverters who started ART, compared with the overall mortality rate of 10/1000 person-years among individuals with established infection who started ART [27], no randomized clinical trials to date provide definitive recommendations for ART use in this patient population [16–20]. Efforts to evaluate the potential effect of ART on virologic set point in recently infected individuals have been limited, in part because of the challenges involved in identifying recently infected persons [5–15]. A study comparing 58 individuals who received ART during primary HIV-1 infection with 116 who remained untreated found no differences in virologic set point 12 months after withdrawal of effective ART [6]. Among the very few randomized clinical trials evaluating the impact of ART on virologic set point in recently infected persons, no persistent significant differences have been observed in HIV-1 RNA levels [16, 17, 20]. Like our study, a recently completed randomized controlled trial of temporary treatment versus no treatment during acute HIV-1 infection in the Netherlands observed a delay in the need for long-term ART in the immediate treatment group, although the delay appeared to be longer in the Dutch study [20]. However, whether such a delay in treatment yields durable or substantial clinical benefits remains unknown. A substudy of A5217 is underway to address whether immediate versus deferred treatment during primary infection results in improvements in markers of inflammation and immune activation, which may provide further insight into the potential benefits of treating primary infection.
Perhaps the most compelling finding of the A5217 study is that the time between the diagnosis of early infection and the need for initiation of ART was shorter than anticipated in the DT group. Investigators from the CASCADE study evaluated 14 387 individuals with well-estimated dates of HIV seroconversion to predict the proportion of participants with CD4+ counts below certain thresholds at various years after seroconversion [28]. Their model, which incorporated data from a median 4.4 years of follow-up since seroconversion while AIDS free and ART naive, predicted that 27% of individuals would develop a CD4+ count <350 cells/mm3 within 2 years after seroconversion. Our observation that half of the participants in the DT group who were included in the primary analysis met criteria for treatment initiation by week 72 would be consistent with a more rapid progression to this threshold. The slower rate of disease progression observed in the CASCADE cohort study may be related to a treatment selection bias resulting from exclusion of those persons from the analysis who received early treatment because of a greater perceived risk for disease progression. In addition, some observational cohort studies have found evidence of an increase in HIV virulence over time, as evidenced by higher postseroconversion HIV-1 RNA levels, lower postseroconversion CD4+ T cells, and/or higher viral replicative capacity [29–33], although these observations are not supported by findings of other studies [34, 35]. Although the median baseline CD4+ T-cell count in the current study is not inconsistent with this hypothesis, the relatively small number of participants is insufficient to support or refute the possibility of changing virulence in the epidemic.
Recently revised treatment guidelines recommend treatment initiation at higher CD4+ T-cell count thresholds [36, 37]. These guidelines consider emerging data highlighting the consequences of untreated HIV-1 infection and the potential role of ART in preventing serious non-AIDS conditions, which may result from the persistent immune activation and systemic inflammation associated with uncontrolled viral replication [27, 38–41]. The results of our current study may be of interest to clinicians and patients struggling with the decision of whether to initiate ART during recent HIV infection. Our results suggest that if immediate therapy is not begun, progression to meeting standard criteria for ART initiation may occur more rapidly than expected, especially with changing treatment paradigms.
Limitations of the study include the methods currently available for classifying study participants as having recent HIV infection. Detuned assays, used to confirm early infection for 78% of the participants, are limited in precision [42, 43]. The potential errors in disease stage classification were thought to favor exclusion of those with recent infection rather than inappropriate inclusion of participants with advanced HIV. We are unable to exclude, however, the possibility of this latter type of misclassification contributing to the more rapid rate of progression. Another possible contribution to a selection bias favoring rapid progression for this cohort may result from oversampling of individuals with symptomatic seroconversion syndromes (55% overall), who are more likely to seek medical attention and may experience more rapid disease progression [44, 45]. Finally, it is not possible to extrapolate the observed study results to individuals who present with acute HIV infection (HIV-1 RNA positive, HIV-1 antibody negative), because our study enrolled only participants with recent but not acute infection.
In conclusion, this randomized, controlled trial of immediate versus deferred ART in the setting of recent HIV-1 infection demonstrated a treatment effect in favor of immediate treatment. A limited period of ART during early HIV-1 infection modestly delayed the need for subsequent initiation of long-term ART. The study was unable to answer the initial question regarding virologic set point, and durable clinical benefits of this strategy remain unproved. However, the higher than anticipated rate of disease progression among untreated individuals, which prevented us from drawing conclusions regarding the virologic set point, is a compelling finding of this study and contributes to the growing body of evidence favoring earlier treatment.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://www.oxfordjournals.org/our_journals/jid/). Supplementary materials consist of data provided by the authors that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the authors.
Notes
Acknowledgments.
We would like to thank all the members of the ACTG 5217 team, including Roula Qaqish, James Rooney, David Currin, Ana Martinez, Rick Hecht, Donna Mildvan, and Charles Rinaldo. We are thankful to Abbott Laboratories and Gilead Sciences for providing study medications. We would like to express our special appreciation to Mark Byroads for his excellent work as the study data manager. We would also like to thank and acknowledge Karen Messer and Florin Vaida for their assistance with study design and analysis plan during the protocol development and early implementation phase.
We gratefully acknowledge the hard work of the study staff at all the ACTG and Acute Infection and Early Disease Research Program (AIEDRP) sites who screened and enrolled patients for this trial: M. Graham Ray and Beverly Putnam, University of Colorado Hospital Clinical Research Site (CRS) (Clinical Trial Unit [CTU] grant U01 AI69450, Colorado Clinical and Translational Sciences Institute [CCTSI] grant UL1 RR025780, AIEDRP grant P01 AI55356, M01 RR00051); Aaron Diamond AIDS Research Center, The Rockefeller University Hospital (AIEDRP grants P01 AI041534 and AI047033); Michael F. Para, MD, and Kathy J. Watson, RN, Ohio State University (CTU grant AI069474); Joanne Santangelo, NP, and Dee Dee Pacheco, University of California, San Diego (CTU grant AI69432, AIEDRP grant AI43638, P01 grant AI74621, California HIV Research Program grant RN07-SD-702); Pamela Poethke, RN, Miriam Hospital (CTU grant AI69472); Janine Maenza, MD, and Claire Stevens, PA-C, University of Washington AIDS Clinical Trials Unit (CTU grant AI 069434, Clinical Research Center [CRC] grant UL1-RR-025014), University of Washington Primary Infection Clinic (CTU grant AI27664); Ge-Youl Kim, RN, BSN, and Michael K. Klebert, PhD, RN, ANP-BC, Washington University (CTU grant AI 069495); Pablo Tebas, MD, and Joseph Quinn, RN, BSN, University of Pennsylvania (CTU grant U01-AI-096497-05 and Center for AIDS Research [CFAR] grant P30-AI-045008-12); Margaret A. Fischl, MD, and Hector Bolivar, MD, University of Miami AIDS Clinical Research Unit (CTU grant AI069477); Karen Coleman and Baiba Berzins, Northwestern University CRS (CTU grant AI 069471); Timothy Lane, MD, and Kim Epperson RN, BSN, Moses H. Cone Memorial Hospital (CTU grant A106943-01); Mario Guerrero and Sadia Shaik, Harbor-UCLA Medical Center (CTU grant AI 069424, CRC grant RR00425); Javier R. Lama, MD, MPH, Investigaciones Medicas en Salud (INMENSA) CTU grant U01AI069438); Donna Pittard, BSN, and Susan Pedersen, BS, BSN, UNC AIDS Clinical Trials Unit (CTU grant 5-U01 AI069423, Center for AIDS Research [CFAR] grant AI050410, CTSA grant UL 1RR025747); Carol Greisberger, RN, BSN, and Mary Adams, RN, BSN, MPh, University of Rochester CRS (CTU grant U01AI069511-02 [as of 12 February 2008], CRC grant 5-MO1 RR00044); Allan S. Tenorio, MD, and Beverly E. Sha, MD, Rush University Medical Center (CTU grant U01 AI069471); C. Bradley Hare, MD, and Deborah Zeitschel, RN, MSN, UCSF AIDS CTU/CRS (CTU grant 5UO1 AI069502); Susan Aileen Olender, MD, Chrisa Hunnewell, ANP, and Madeline Torres, RN, BSN, Columbia University (CTU grant AI27665); Eric Rosenberg, MD, Amy Sbrolla, RN, and Teri Flynn, ANP, Massachusetts General Hospital, Boston (CTU grant AI069472), Brigham and Women’s Hospital (CTU grant AI69472), Indiana University (CTU grant AI25859), Beth Israel Medical Center (CTU grant AI46370); Charles E. Davis, Jr, MD, and William Blattner, MD, University of Maryland IHV Baltimore Treatment CRS (CTU grant U01- AI069447); Juan V. Guanira, MD, MPH, Asociacion Civil Impacta Salud y Educacion—Miraflores (CTU grant U01AI069438); Christine Hurley, RN, and Roberto Corales, DO-AIDS Care (CTU grant U01AI069511-02 [as of 12 February 2008], CRC grant 5-MO1 RR00044); Molly Eaton, MD, and Ericka Patrick, RN, Ponce de Leon Center (CTU grant 5UO1 AI069418, CFAR grant P30 AI050409, Atlanta Clinical and Translational Science Award grant UL1 RR025009).
Finally, we would like to thank all the patients who participated in this study while grappling with a recent diagnosis of HIV infection.
Financial support.
This work was supported by the National Institute of Allergy and Infectious Diseases (U01AI068636), the National Institute of Mental Health, and the National Institute of Dental and Craniofacial Research. The protocol was originally developed as Study AIN503 within the Acute Infection and Early Disease Research Program (U01AI043638). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health. The project was also supported by a grant (AI68634) from the National Institute of Allergy and Infectious Diseases to the Statistical and Data Analysis Center and from individual site grants listed in the Acknowledgments.
Potential conflicts of interest.
S. F. is a consultant for Gen-Probe, Abbott Molecular and Roche Molecular Systems. C. B. H. has received consulting fees and honoraria from Abbott Laboratories and consulting and speaker’s fees and honoraria from Gilead Sciences. M. M. has received consulting and speaker’s fees from Gilead Sciences. K. T. T. has received research grants from GlaxoSmithKline, Merck, and Tibotec and consulting fees from Merck. R. C. is a DSMB member and has received honoraria from Takeda. E. S. D. has received research grants from Abbott, Merck, Pfizer, Gilead, and ViiV and consulting fees from Gilead, ViiV, Merck, and Bristol-Myers Squibb. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Bell SK, Little SJ, Rosenberg ES. Clinical management of acute HIV infection: best practice remains unknown. J Infect Dis. 2010;202(Suppl 2):S278–88. doi: 10.1086/655655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altfeld M, Rosenberg ES, Shankarappa R, et al. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J Exp Med. 2001;193:169–80. doi: 10.1084/jem.193.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binley JM, Trkola A, Ketas T, et al. The effect of highly active antiretroviral therapy on binding and neutralizing antibody responses to human immunodeficiency virus type 1 infection. J Infect Dis. 2000;182:945–9. doi: 10.1086/315774. [DOI] [PubMed] [Google Scholar]
- 4.Markowitz M, Vesanen M, Tenner-Racz K, et al. The effect of commencing combination antiretroviral therapy soon after human immunodeficiency virus type 1 infection on viral replication and antiviral immune responses. J Infect Dis. 1999;179:527–37. doi: 10.1086/314628. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg ES, Altfeld M, Poon SH, et al. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407:523–6. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 6.Desquilbet L, Goujard C, Rouzioux C, et al. Does transient HAART during primary HIV-1 infection lower the virological set-point? AIDS. 2004;18:2361–9. [PubMed] [Google Scholar]
- 7.Kaufmann DE, Lichterfeld M, Altfeld M, et al. Limited durability of viral control following treated acute HIV infection. PLoS Med. 2004;1:e36. doi: 10.1371/journal.pmed.0010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Streeck H, Jessen H, Alter G, et al. Immunological and virological impact of highly active antiretroviral therapy initiated during acute HIV-1 infection. J Infect Dis. 2006;194:734–9. doi: 10.1086/503811. [DOI] [PubMed] [Google Scholar]
- 9.Hecht FM, Wang L, Collier A, et al. A multicenter observational study of the potential benefits of initiating combination antiretroviral therapy during acute HIV infection. J Infect Dis. 2006;194:725–33. doi: 10.1086/506616. [DOI] [PubMed] [Google Scholar]
- 10.Lampe FC, Porter K, Kaldor J, Law M, Kinloch-de Loes S, Phillips AN. Effect of transient antiretroviral treatment during acute HIV infection: comparison of the Quest trial results with CASCADE natural history study. Antivir Ther. 2007;12:189–93. [PubMed] [Google Scholar]
- 11.Fidler S, Fox J, Touloumi G, et al. Slower CD4 cell decline following cessation of a 3 month course of HAART in primary HIV infection: findings from an observational cohort. AIDS. 2007;21:1283–91. doi: 10.1097/QAD.0b013e3280b07b5b. [DOI] [PubMed] [Google Scholar]
- 12.Steingrover R, Pogany K, Fernandez Garcia E, et al. HIV-1 viral rebound dynamics after a single treatment interruption depends on time of initiation of highly active antiretroviral therapy. AIDS. 2008;22:1583–8. doi: 10.1097/QAD.0b013e328305bd77. [DOI] [PubMed] [Google Scholar]
- 13.Pantazis N, Touloumi G, Vanhems P, Gill J, Bucher HC, Porter K. The effect of antiretroviral treatment of different durations in primary HIV infection. AIDS. 2008;22:2441–50. doi: 10.1097/QAD.0b013e328319ea4e. [DOI] [PubMed] [Google Scholar]
- 14.Volberding P, Demeter L, Bosch RJ, et al. Antiretroviral therapy in acute and recent HIV infection: a prospective multicenter stratified trial of intentionally interrupted treatment. AIDS. 2009;23:1987–95. doi: 10.1097/QAD.0b013e32832eb285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koegl C, Wolf E, Hanhoff N, et al. Treatment during primary HIV infection does not lower viral set point but improves CD4 lymphocytes in an observational cohort. Eur J Med Res. 2009;14:277–83. doi: 10.1186/2047-783X-14-7-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinloch-De Loes S, Hirschel BJ, Hoen B, et al. A controlled trial of zidovudine in primary human immunodeficiency virus infection. N Engl J Med. 1995;333:408–13. doi: 10.1056/NEJM199508173330702. [DOI] [PubMed] [Google Scholar]
- 17.Niu MT, Bethel J, Holodniy M, Standiford HC, Schnittman SM. Zidovudine treatment in patients with primary (acute) human immunodeficiency virus type 1 infection: a randomized, double-blind, placebo-controlled trial. DATRI 002 Study Group. Division of AIDS Treatment Research Initiative. J Infect Dis. 1998;178:80–91. doi: 10.1086/515612. [DOI] [PubMed] [Google Scholar]
- 18.Dybul M, Hidalgo B, Chun TW, et al. Pilot study of the effects of intermittent interleukin-2 on human immunodeficiency virus (HIV)-specific immune responses in patients treated during recently acquired HIV infection. J Infect Dis. 2002;185:61–8. doi: 10.1086/338123. [DOI] [PubMed] [Google Scholar]
- 19.Zala C, Salomon H, Ochoa C, et al. Higher rate of toxicity with no increased efficacy when hydroxyurea is added to a regimen of stavudine plus didanosine and nevirapine in primary HIV infection. J Acquir Immune Defic Syndr. 2002;29:368–73. doi: 10.1097/00126334-200204010-00007. [DOI] [PubMed] [Google Scholar]
- 20.Grijsen M, Steingrover R, Wit F, et al. Programs and abstracts of the 18th Conference on Retroviruses and Opportunistic Infections (Boston) Alexandria, VA: CROI; 2011. An RCT comparing no treatment with 24 or 60 weeks of temporary ART during primary HIV infection [abstract 161] [Google Scholar]
- 21.Kothe D, Byers RH, Caudill SP, et al. Performance characteristics of a new less sensitive HIV-1 enzyme immunoassay for use in estimating HIV seroincidence. J Acquir Immune Defic Syndr. 2003;33:625–34. doi: 10.1097/00126334-200308150-00012. [DOI] [PubMed] [Google Scholar]
- 22.Fiscus SA, Cachafeiro A, Kshatriya R, et al. Program and abstracts of the 16th Conference on Retroviruses and Opportunistic Infections (Montreal) Alexandria, VA: CROI; 2009. Comparison of 6 HIV incidence assays with the less sensitive Vironostika HIV antibody assay for screening subjects for A5217 [abstract V122] [Google Scholar]
- 23.Kshatriya R, Cachafeiro AA, Kerr RJ, Nelson JA, Fiscus SA. Comparison of two rapid human immunodeficiency virus (HIV) assays, Determine HIV-1/2 and OraQuick Advance Rapid HIV-1/2, for detection of recent HIV seroconversion. J Clin Microbiol. 2008;46:3482–3. doi: 10.1128/JCM.00665-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soroka SD, Granade TC, Candal D, Parekh BS. Modification of rapid human immunodeficiency virus (HIV) antibody assay protocols for detecting recent HIV seroconversion. Clin Diagn Lab Immunol. 2005;12:918–21. doi: 10.1128/CDLI.12.8.918-921.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panel on Clinical Practices for Treatment of HIV Infection. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Washington, DC: Department of Health and Human Services; 2004:1–115. [Google Scholar]
- 26.Messer K, Vaida F, Hogan C. Robust analysis of biomarker data with informative missingness using a two-stage hypothesis test in an HIV treatment interruption trial: AIEDRP AIN503/ACTG A5217. Contemp Clin Trials. 2006;27:506–17. doi: 10.1016/j.cct.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Ray M, Logan R, Sterne JA, et al. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS. 2010;24:123–37. doi: 10.1097/QAD.0b013e3283324283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lodi S, Phillips A, Touloumi G, et al. Programs and abstracts of the 17th Conference on Retroviruses and Opportunistic Infections (San Francisco) Alexandria, VA: CROI; 2010. Proportion of individuals likely to need treatment for CD4 thresholds <200, <350, and <500 cells/mm3 [abstract 525] [Google Scholar]
- 29.Dorrucci M, Rezza G, Porter K, Phillips A. Temporal trends in postseroconversion CD4 cell count and HIV load: the Concerted Action on Seroconversion to AIDS and Death in Europe Collaboration, 1985–2002. J Infect Dis. 2007;195:525–34. doi: 10.1086/510911. [DOI] [PubMed] [Google Scholar]
- 30.Gras L, Jurriaans S, Bakker M, et al. Viral load levels measured at set-point have risen over the last decade of the HIV epidemic in the Netherlands. PLoS One. 2009;4:e7365. doi: 10.1371/journal.pone.0007365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crum-Cianflone N, Eberly L, Zhang Y, et al. Is HIV becoming more virulent? Initial CD4 cell counts among HIV seroconverters during the course of the HIV epidemic: 1985–2007. Clin Infect Dis. 2009;48:1285–92. doi: 10.1086/597777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dorrucci M, Phillips AN, Longo B, Rezza G. Changes over time in post-seroconversion CD4 cell counts in the Italian HIV-Seroconversion Study: 1985–2002. AIDS. 2005;19:331–5. [PubMed] [Google Scholar]
- 33.Gali Y, Berkhout B, Vanham G, Bakker M, Back NK, Arien KK. Survey of the temporal changes in HIV-1 replicative fitness in the Amsterdam Cohort. Virology. 2007;364:140–6. doi: 10.1016/j.virol.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 34.Herbeck JT, Gottlieb GS, Li X, et al. Lack of evidence for changing virulence of HIV-1 in North America. PLoS One. 2008;3:e1525. doi: 10.1371/journal.pone.0001525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Troude P, Chaix ML, Tran L, et al. No evidence of a change in HIV-1 virulence since 1996 in France. AIDS. 2009;23:1261–7. doi: 10.1097/QAD.0b013e32832b51ef. [DOI] [PubMed] [Google Scholar]
- 36.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Washington, DC: Department of Health and Human Services; 2009:1–161. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed 21 November 2010. [Google Scholar]
- 37.Thompson MA, Aberg JA, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA. 2010;304:321–33. doi: 10.1001/jama.2010.1004. [DOI] [PubMed] [Google Scholar]
- 38.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–26. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 40.Jonsson Funk M, Fusco J, Cole S, et al. Programs and abstracts of the 18th International AIDS Conference (Vienna, Austria) Geneva: International AIDS Society; 2010. HAART initiation and clinical outcomes: insights from the CASCADE cohort of HIV-1 seroconverters on “When to Start” [abstract THLBB201] [Google Scholar]
- 41.Sterne JA, May M, Costagliola D, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–63. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen MS, Gay CL, Busch MP, Hecht FM. The detection of acute HIV infection. J Infect Dis. 2010;202(Suppl 2):S270–7. doi: 10.1086/655651. [DOI] [PubMed] [Google Scholar]
- 43.Busch MP, Pilcher CD, Mastro TD, et al. Beyond detuning: 10 years of progress and new challenges in the development and application of assays for HIV incidence estimation. AIDS. 2010;24:2763–71. doi: 10.1097/QAD.0b013e32833f1142. [DOI] [PubMed] [Google Scholar]
- 44.Lavreys L, Baeten JM, Chohan V, et al. Higher set point plasma viral load and more-severe acute HIV type 1 (HIV-1) illness predict mortality among high-risk HIV-1-infected African women. Clin Infect Dis. 2006;42:1333–9. doi: 10.1086/503258. [DOI] [PubMed] [Google Scholar]
- 45.Vanhems P, Lecomte C, Fabry J. Primary HIV-1 infection: diagnosis and prognostic impact. AIDS Patient Care STDS. 1998;12:751–8. doi: 10.1089/apc.1998.12.751. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.