Abstract
Background. Semen is the main carrier of sexually transmitted viruses, including human immunodeficiency virus type 1 (HIV-1). However, semen is not just a mere passive transporter of virions but also plays an active role in HIV-1 transmission through cytokines and other biological factors.
Methods. To study the relationship between viruses and the chemokine-cytokine network in the male genital tract, we measured the concentrations of 21 cytokines/chemokines and the loads of HIV-1 and of 6 herpesviruses in seminal and blood plasma from HIV-1–infected and HIV-uninfected men.
Results. We found that (1) semen is enriched in cytokines and chemokines that play key roles in HIV-1 infection or transmission; (2) HIV-1 infection changes the chemokine-cytokine network in semen, further enriching it in cytokines that modulate its replication; (3) HIV-1 infection is associated with Epstein-Barr virus (EBV) and cytomegalovirus (CMV) compartmentalized seminal reactivation; (4) CMV and EBV concomitant seminal shedding is associated with higher HIV-1 loads in blood and seminal plasma; and (5) CMV seminal reactivation increases the seminal levels of the CCR5 ligands RANTES and eotaxin, and of the CXCR3 ligand monokine induced by gamma interferon (MIG).
Conclusions. HIV-1 infection results in an aberrant production of cytokines and reactivation of EBV and CMV that further changes the seminal cytokine network. The altered seminal milieu in HIV-1 infection may be a determinant of HIV-1 sexual transmission.
Semen is a composite biological fluid in which spermatozoa and other cells are immersed in plasma containing soluble factors essential for fertilization in particular cytokines [1]. Previous studies have shown that in comparison with other body fluids, semen is enriched in several cytokines, in particular interleukin (IL) 7 and transforming growth factor (TGF)-β, suggesting that the male genital tract has unique immunological features [2–5]. It is not clear whether the cytokines in semen reflect innate and adaptive responses only or whether they have precise reproductive functions as well [6]. Whichever their role is, cytokines create in semen an environment that alters the replication and transmission of pathogens. Indeed, several sexually transmitted viruses, in particular human immunodeficiency virus (HIV) and its simian counterpart, simian immunodeficiency virus (SIV), exploit various physical or biological properties of semen or alter the genital cytokine milieu for their own benefit [7–9]. For example, in rhesus macaques vaginal exposure to SIV enhances the vaginal expression of MIP-3α, which is necessary to establish SIV genital infection [9]. HIV-1 is often accompanied by infections by other pathogens (HIV copathogens), in particular herpesviruses, which are often also shed in semen [10]. Copathogens can further change the seminal cytokine milieu, thus affecting their replication as well as HIV-1 replication. This complex and yet not understood relationship between viruses may play a critical role in HIV transmission and pathogenesis [11].
In this study, we addressed the relationship between the cytokine milieu and coinfecting viruses in the male genital tract of HIV-1–infected individuals by comparing the levels of 21 cytokines and chemokines as well as the loads of HIV-1 and 6 coinfecting herpesviruses in seminal and blood plasma samples from HIV-1–infected and HIV-uninfected men. We found that semen is particularly enriched in cytokines that are known to play a role in HIV-1 infection and replication (including IL-7, macrophage inflammatory protein (MIP)-3α, IL-8, RANTES, and MIP-1β) [9, 12, 13] and that HIV-1 infection is associated with a dramatic change of the semen cytokine spectrum. Also, we found that HIV-1 infection is frequently associated with the reactivation of Epstein-Barr virus (EBV) and cytomegalovirus (CMV), especially in semen, where they modulate the cytokine spectrum by increasing the levels of the CCR5 ligands RANTES and eotaxin and of the CXCR3 ligand monokine induced by gamma interferon (MIG). The aberrant production of cytokines and the preferential reactivation of EBV and CMV in semen of HIV-1–infected individuals may be an important determinant in HIV-1 infectivity and evolution in the male genital tract.
METHODS
Study Population
The protocol was approved by the institutional review boards of the National Institutes of Health and the All India Institute of Medical Sciences (AIIMS). Study participants were recruited in AIIMS between September 2008 and November 2009. The presence of genital ulcers and/or genital discharge as well as VDRL reactivity were identified as exclusion criteria for enrollment. After 2–5 days of sexual abstinence, semen was obtained by masturbation, collected into a sterile container, and processed within 1 hour of ejaculation. Semen was centrifuged at 800 g for 10 minutes, and the resulting supernatant (plasma) was stored at −80°C.
Eighty-four therapy-naive HIV-infected patients were enrolled; 1 patient was excluded from further analysis because he was infected with HIV-2. Owing to the sociocultural stigma of HIV/AIDS in India, a few enrolled patients missed follow-up visits. Thus, HIV-1 blood plasma load was available from 74 individuals; paired HIV-1 blood plasma load and CD4+ T-cell counts were available from 70 of these. Seminal plasma was collected from 50 HIV-1–infected individuals, and paired blood and seminal plasma samples from 43 of the 50. Thirty-three HIV-uninfected individuals, among healthy volunteers referred to the AIIMS fertility clinic, were enrolled as HIV-uninfected controls. Blood and semen were collected from 27 and 28 individuals respectively; paired blood and semen samples were available from 22 HIV-uninfected controls.
Real-Time Polymerase Chain Reaction
Nucleic acid extraction, real-time polymerase chain reaction, and the primers and probes used for amplifications are described in the Supplemental Methods. HIV-1 load was measured using a set of primers previously validated for load quantification of different HIV-1 group M subtypes, including subtype C [14].
Quantification of Cytokines and Chemokines
We measured levels of the cytokines and chemokines using a multiplex-bead-array assay described in the Supplemental Methods.
Statistical Analyses
Unless otherwise specified, we used medians (25th–75th percentiles, interquartile range [IQR]) to summarize continuous variables. We compared continuous variables between groups using the Mann–Whitney test and assessed linear association of continuous variables using the nonparametric Spearman rank correlation coefficient (ρ). The Kruskall–Wallis test with Dunn multiple comparison test was used to test for differences among >2 independent groups. We compared categorical variables using Fisher`s exact and χ2 tests and performed 2-way hierarchical cluster analysis on log-transformed cytokine levels using Ward’s method.
RESULTS
HIV-1 and Herpesvirus Loads in Blood and Semen
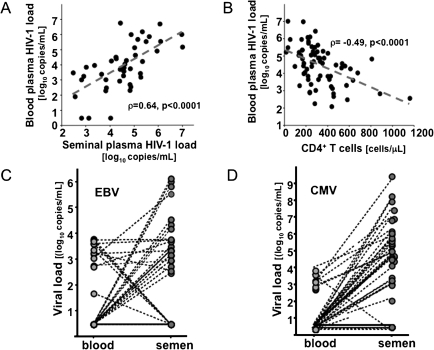
Sociodemographic characteristics of the enrolled patients are shown in Table1. HIV-1 RNA load was quantified in semen and blood plasmas from therapy-naive HIV-1–infected individuals. The median CD4+ T-cell count in this cohort was 281 cells/mm3 (IQR, 185–430). HIV-1 RNA was detected in blood plasma from all the enrolled patients (100%) with a median load of 4.58 log10 copies/mL (IQR, 3.6–5.2). A significant negative correlation was found between the CD4+ T-cell count and the HIV-1 plasma load (ρ = −0.49; P < .0001). In seminal plasma, HIV-1 RNA was detected in 94% of the patients with a median load of 3.89 log10 copies/mL (IQR, 3–5). There was a significant positive correlation between the HIV-1 loads in blood plasma and seminal plasma (ρ = 0.64; P < .0001) and a significant negative correlation between the CD4+ T-cell count and the seminal HIV-1 RNA load (ρ = −0.43; P = .002) (Figure 1A and B).
Table 1.
Sociodemographic Characteristics of the Enrolled HIV-1–Infected and HIV-Uninfected Individuals
| No. (%) |
|||
| Characteristic | HIV-1–Infected (n = 83)a | HIV-Uninfected (n = 33) | P |
| Age, years | >.1 | ||
| ≤30 | 23 (27) | 14 (42) | |
| >30 | 60 (73) | 19 (58) | |
| Marital status | <.01 | ||
| Unmarried | 19 (23) | 0 (0) | |
| Married or long-term consensual relationship | 64 (77) | 33 (100) | |
| Employment status | <.05 | ||
| Unemployed | 9 (11) | 0 (0) | |
| Self-employed | 44 (53) | 14 (42) | |
| Full-time wage earner | 30 (36) | 19 (58) | |
| Mode of HIV-1 acquisition | |||
| Heterosexual intercourse | 70 (84.3) | NA | |
| Injection drug use or transfusion | 5 (6.0) | NA | |
| Males who have sex with malesb | 6 (7.2) | NA | |
| Unknown | 2 (2.5) | NA | |
Abbreviations: HIV-1, human immunodeficiency virus type 1; NA, not applicable.
Eighty-four patients were initially enrolled, but 1 was immediately excluded because he was infected by HIV-2.
None of the HIV-uninfected controls reported any history of homosexual sex. However, the difference in the proportion of males who have sex with males between HIV-1–infected and HIV-uninfected individuals was not statistically significant (P > .1).
Figure 1.
Human immunodeficiency virus type 1 (HIV-1), cytomegalovirus (CMV), and Epstein-Barr virus (EBV) loads in blood and seminal plasma. A, Correlation between the HIV-1 load in blood and seminal plasma. B, Correlation between HIV-1 blood plasma load and the CD4+ T-cell count. C, D, For each HIV-1–infected individual, a dotted line connects the EBV (C) and CMV (D) loads in blood and seminal plasma. Note that there is a significant positive correlation between the blood and seminal HIV-1 loads and a significant negative correlation between the HIV-1 blood plasma load and the CD4+ T-cell count. Note also that high CMV and EBV seminal plasma loads are often found in patients in whom blood plasma HIV-1 is undetectable.
Seminal and blood plasmas were tested for 6 herpesviruses known to be found in semen (herpes simplex virus [HSV]–2, EBV, CMV, human herpesvirus [HHV] 6, HHV-7, and HHV-8), and the results are summarized in Table 2. HSV-2 DNA was found in seminal plasma of 8% of HIV-1–infected individuals (median load, 2.1 log10 copies/mL [IQR, 1.9–2.3]) but in none of their blood plasma samples. No HSV-2 DNA was detected in blood or semen samples from HIV-uninfected individuals.
Table 2.
The Frequency of Detection and the Median Load of Herpesviruses in Blood and Semen Plasma Samples From HIV-1–Infected and HIV-Uninfected Men
| HIV-Uninfecteda |
HIV-1–Infecteda |
Pb |
||||
| Herpesvirus | Blood Plasma | Semen Plasma | Blood Plasma | Semen Plasma | Blood | Semen |
| HSV-2 | 0 | 0 | 0 | 4/50 (8); 2.1 (1.9–2.3) | NS | NS |
| EBV | 0 | 1/28 (3.5); 5.5 | 21/74 (28); 3.2 (2.7–3.6) | 28/50 (56); 3.5 (3.1–4.7) | <.001 | <.001 |
| CMV | 0 | 1/28 (3.5); 3.2 | 12/74 (16); 3.1 (2.9–3.4) | 35/50 (70); 5.3 (4.4–6.1) | <.001 | <.001 |
| HHV-6 | 0 | 3/28 (6); 3.7 (3.7–4.5) | 0 | 1/50 (2); 3.3 | NS | NS |
| HHV-7 | 0 | 3/28 (6); 3 (2.2–3.1) | 0 | 6/50 (12); 2.7 (2.3–3.1) | NS | NS |
| HHV-8 | 0 | 0 | 0 | 3/50 (6); 3.3 (3.1–3.5) | NS | NS |
Abbreviations: CMV, cytomegalovirus; EBV, Epstein-Barr virus; HHV, human herpesvirus; HIV, human immunodeficiency virus; HSV, herpes simplex virus; NS, not significant.
Data are presented as frequency of DNA detection (%); median DNA viral load (interquartile range), expressed as log10 copies/mL.
Significance of the difference in frequency of DNA detection between HIV-uninfected and HIV-1–infected individuals.
Among the HIV-1–infected individuals, HHV-6, HHV-7, and HHV-8 DNA were detected in 2%, 12%, and 6% of the seminal plasma samples, respectively (median loads, 3.3 [1 patient], 2.7 [IQR, 2.3–3.1], and 3.3 [IQR, 3.1–3.5] log10 copies/mL, respectively). Among HIV-uninfected individuals, HHV-6, HHV-7, and HHV-8 DNA were detected in 6%, 6%, and 0% of the seminal plasma samples, respectively (median loads, 3.79 [IQR, 3.7–4.5] and 3 [IQR, 2.2–3.1] log10 copies/mL, respectively). There was no significant difference in seminal loads of these viruses between HIV-1–infected and HIV-uninfected individuals. None of these herpesviruses was detected in blood plasma from either HIV-1–infected or HIV-uninfected men.
On the contrary, the presence of EBV DNA was strongly associated with HIV-1 infection (P < .001): it was found in 28% (median load, 3.2 log10 copies/mL [IQR, 2.7–3.6]) and 56% (median load, 3.5 log10 copies/mL [IQR, 3.1–4.7]) of blood and seminal plasma samples from HIV-1–infected individuals, respectively. Among HIV-uninfected individuals, EBV DNA was found in none of the blood plasma samples and in only 1 (3.5%; 5.52 log10 copies/mL) of the semen samples. Moreover, the frequency of EBV DNA in HIV-1–infected individuals was significantly higher in seminal than in blood plasma (P < .001). Among the HIV-1–infected individuals with detectable EBV DNA, 54% had EBV DNA in seminal plasma but not in blood plasma, 27% had EBV DNA in both, and the remaining 19% had EBV DNA in blood but not seminal plasma (P < .01) (Figure 1C).
As with EBV DNA, the presence of CMV DNA was strongly associated with HIV-1 infection: CMV DNA was found in blood plasma of 16% (median load, 3.12 log10 copies/mL [IQR, 2.9–3.4]) and seminal plasma of 70% (median load, 5.3 log10 copies/mL [IQR, 4.4–6.1]) (P < .01) of HIV-1–infected patients. In contrast, in HIV-uninfected individuals CMV DNA was not detected in any of the blood plasma samples and in only 3.5% (1 patient; 3.27 log10 copies/mL) of the seminal plasma samples. Moreover, among the HIV-1–infected patients with detectable CMV DNA, 77.4% had CMV DNA in seminal plasma but not in blood plasma, 19.4% had CMV DNA in both semen and blood plasma, and the remaining 3.2% had CMV DNA in blood plasma but not in seminal plasma (P < .01) (Figure 1D).
In summary, none of the tested herpesviruses was detected in the blood plasma of the 27 HIV-uninfected individuals. In contrast, either EBV or CMV was found in the blood plasma of 37% of the HIV-1–infected individuals (P < .001). In 18% of these, both EBV and CMV DNA were detected, in 57% only EBV DNA was detected, and in the remaining 25% only CMV DNA was detected. No significant difference was found in the blood plasma levels of EBV or CMV DNA between the patients with only one of the viruses and those with both of these viruses.
In the seminal plasma of HIV-uninfected individuals, neither HSV-2 nor HHV-8 DNA was found; EBV or CMV DNA was found in 3.5% and HHV-6 or HHV-7 DNA in 6% of the semen samples from these individuals. On the contrary, HSV-2, EBV, CMV, HHV-6, HHV-7, or HHV-8 DNA was found in seminal plasma samples from 92% of the HIV-1–infected individuals. However, although all these herpesviruses were detected in semen of HIV-1–infected individuals, only the frequency of EBV and CMV was significantly higher than in HIV-uninfected individuals (P < .01) (Table 2). Among the HIV-1–infected patients, CMV and EBV DNA were found in 39% and 24% respectively, and EBV and CMV were concomitantly shed in semen in 37%. No significant difference was found in the semen loads of EBV or CMV between the patients with detectable DNA for only one of these viruses and patients with both.
In conclusion, HIV-1 infection was significantly associated with increased frequency of CMV and EBV DNA in both blood and seminal plasma samples. However, in the majority of the patients CMV and EBV were shed into semen even in the absence of blood plasma viremia, indicating that HIV-1 infection is often associated with a compartmentalized reactivation of CMV and EBV in the male genital tract.
Relation Between Herpesvirus Reactivation, HIV-1 Loads, and CD4+ T-cell Counts
Because HIV-1 infection was associated with increased frequency and load of CMV and EBV DNA but not of other herpesviruses, we investigated whether these 2 parameters correlate with CD4+ T-cell counts and HIV-1 RNA loads. Among HIV-1–infected patients, the presence of CMV DNA was associated with a lower median CD4+ T-cell count than in patients in whom no CMV DNA was detected: this difference was statistically significant for blood (178.5 [IQR, 163–296] vs 288.5 cells/mm3 [IQR, 220–439]; P = .03) but not for seminal plasma (265 [IQR, 180–397] vs 403 cells/mm3 [IQR, 219–611]; P = .08). No significant difference was found in the CD4+ T-cell counts between HIV-1–infected patients with and those without EBV DNA in blood or seminal plasma.
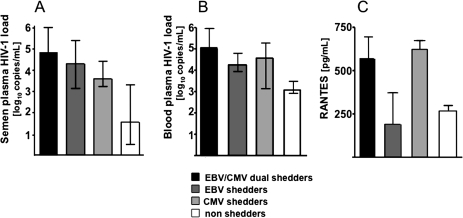
On the basis of the pattern of seminal detection of EBV and CMV DNA, we divided the HIV-1–infected patients into 4 subgroups: (1) EBV-CMV dual shedders (17/50; 34%), (2) EBV shedders (11/50; 22%), (3) CMV shedders (18/50; 36%), and (4) nonshedders (neither EBV nor CMV). The HIV-1 seminal loads were significantly different among these 4 groups (P = .01): in particular, dual shedders had a significantly higher HIV-1 seminal load than nonshedders (P < .05). No significant differences in the HIV-1 seminal loads were found in pairwise comparisons among these 4 groups (Figure 2A). A similar pattern was found in the levels of blood plasma HIV-1 for these 4 groups of patients (P = .008) (Figure 2B). Thus, in HIV-1–infected individuals, the concomitant shedding of EBV and CMV in semen is associated with HIV-1 RNA loads in both blood and seminal plasma that are higher than the loads in HIV-1–infected individuals shedding neither of these 2 herpesviruses.
Figure 2.
Cytomegalovirus (CMV) and Epstein-Barr virus (EBV) seminal shedding is associated with higher human immunodeficiency virus type 1 (HIV-1) loads and higher levels of seminal RANTES. A, B, HIV-1 loads in seminal (A) and blood plasma (B). C, Levels of seminal RANTES. Presented are medians and interquartile ranges. On the basis of the pattern of seminal EBV and CMV DNA, the HIV-1–infected patients were divided into 4 subgroups: (1) EBV-CMV dual shedders, (2) EBV shedders, (3) CMV shedders, and (4) nonshedders (neither EBV nor CMV). Note that the concomitant seminal shedding of EBV and CMV is associated with higher levels of HIV-1 seminal shedding, whereas CMV shedding is associated with higher levels of seminal RANTES.
Cytokines and Chemokines in the Blood and Semen of HIV-1–Infected and HIV-Uninfected Individuals
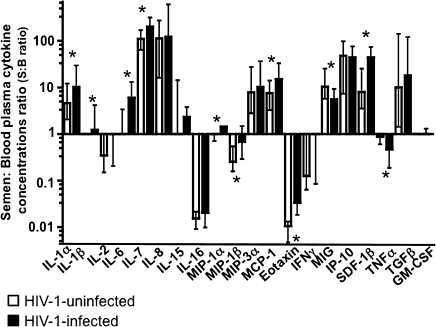
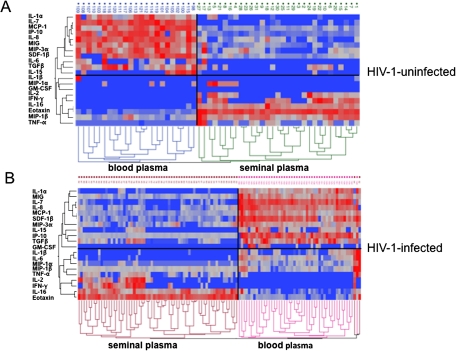
To evaluate the immunological profile of the blood and seminal plasma of HIV-1–infected and HIV-uninfected individuals, we measured the concentrations of 21 cytokines. We found that the cytokine spectra in blood and semen of HIV-uninfected men are profoundly different, because seminal plasma was enriched in IL-1α, IL-7, IL-8, MIP-3α, monocyte chemotactic protein (MCP) 1, MIG, interferon gamma-induced protein (IP) 10, stromal cell-derived factor (SDF) 1β, and TGF-β (P < .0001), whereas blood plasma was enriched in IL-2, IL-16, MIP-1β, and eotaxin (P < .0001). (RANTES was excluded from the analysis because the nonspecific spontaneous degranulation of this chemokine from platelets upon venipuncture renders its measurement not representative of its specific release [15]). Figure 3 displays the compartmentalization of cytokines by paired sample ratios of their seminal plasma to blood concentrations. The profound difference in the blood and semen cytokine profiles is also evident from the 2-way hierarchical cluster analysis of cytokine concentrations in blood and semen (Figure 4). In a comparison of the relative cytokine levels, 2 distinct cytokine profiles in blood and seminal plasma samples were identified in both HIV-uninfected and HIV-1–infected individuals.
Figure 3.
Cytokine spectra are different in blood and seminal plasma. Median and interquartile ranges of cytokine concentration ratios in seminal plasma and blood plasma samples (S:B) are shown for human immunodeficiency virus (HIV)–uninfected (white bars) and HIV-1–infected individuals (black bars). Asterisks denote a significant differences between the S:B ratios in HIV-uninfected versus HIV-1–infected individuals; ratios >1 or <1 indicate enrichment of a cytokine in semen or blood plasma, respectively. Note that HIV-1 infection significantly changes the compartmentalization of 11 of the 20 cytokines measured in blood and seminal plasma. Abbreviations: GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; IP, interferon gamma-induced protein; MCP, monocyte chemotactic protein; MIG, monokine induced by gamma interferon; MIP, macrophage inflammatory protein; SDF, stromal cell-derived factor; TGF, transforming growth factor; TNF, tumor necrosis factor.
Figure 4.
Two-way hierarchical cluster analysis of cytokine concentrations in blood and semen. A, Cluster analysis for human immunodeficiency virus (HIV)–uninfected individuals. B, Cluster analysis for HIV-1–infected individuals. The analysis was performed using Ward`s method on log-transformed blood and semen cytokine concentrations. Cytokine levels are expressed as color scales (blue represents low levels; red, high levels). Dendrograms under color scales classify 2 clusters according to cytokine profiles in different individuals. Dendrograms on left side reflect proximities of cytokines. Comparisons of relative cytokine levels and cytokine profiles in samples of blood and seminal plasma disclose 2 distinct cytokine profiles in both HIV-uninfected and HIV-1–infected individuals. Abbreviations: GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; IP, interferon gamma-induced protein; MCP, monocyte chemotactic protein; MIG, monokine induced by gamma interferon; MIP, macrophage inflammatory protein; SDF, stromal cell-derived factor; TGF, transforming growth factor; TNF, tumor necrosis factor.
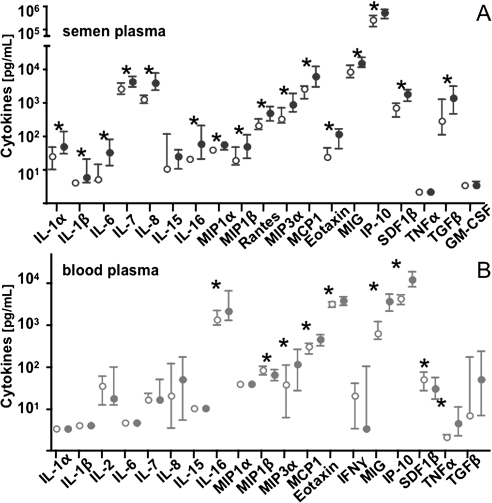
However, HIV-1 infection resulted in aberrant production of the cytokines in both seminal and blood plasma samples compared with those in HIV-uninfected individuals. In seminal plasma of HIV-1–infected individuals, there was a significant up-regulation of 16 of the 21 measured cytokines: IL-1α, IL-1β, IL-6, IL-7, IL-8, IL-16, MIP-1α, MIP-1β, MIP-3α, RANTES, MCP-1, eotaxin, MIG, IP-10, SDF-1β, and TGF-β (P < .05) (Figure 5A). In blood plasma, HIV-1 infection was associated with an altered production of 9 of the 20 analyzed cytokines: whereas MIP-1β and SDF-1β were significantly down-regulated, we found a significant up-regulation of IL-16, MIP-3α, MCP-1, eotaxin, MIG, IP-10, and tumor necrosis factor (TNF) α (P < .05) (Figure 5B).
Figure 5.
Differential effect of human immunodeficiency virus type 1 (HIV-1) infection on cytokines in blood and seminal plasma. A, Levels of cytokines in seminal plasma of HIV-uninfected (open circles) and HIV-1–infected individuals (filled circles). B, Levels of cytokines in blood plasma of HIV-uninfected (open circles) and HIV-1–infected individuals (filled circles). Values presented are medians and interquartile ranges. Asterisks denote a significant difference between median levels of a cytokine in HIV-uninfected and HIV-1–infected individuals. Interleukin (IL) 2 and interferon (IFN) γ were not detected in any sample of seminal plasma, and no granulocyte-macrophage colony-stimulating factor (GM-CSF) was found in blood plasma. The levels of RANTES in blood plasma are not presented because the aspecific spontaneous degranulation of this chemokine from platelets on venipuncture renders its measurement not representative of its specific release. Note that HIV-1 infection differently affects the production of each cytokine in blood and seminal plasma. Abbreviations: IP, interferon gamma-induced protein; MCP, monocyte chemotactic protein; MIG, monokine induced by gamma interferon; MIP, macrophage inflammatory protein; SDF, stromal cell-derived factor; TGF, transforming growth factor; TNF, tumor necrosis factor.
HIV-1 infection was also associated with an increased compartmentalization of most of the cytokines. In samples from HIV-1–infected patients, the median ratios of seminal plasma to blood plasma concentrations for 11 cytokines were significantly different from those in HIV-uninfected individuals: IL-1α (10.2 vs 4.6), IL-1β (1.2 vs 1), IL-6 (6 vs 1), IL-7 (204 vs 110), MIP-1α (1.4 vs 1), MCP-1 (14.9 vs 7.5), SDF-1β (44.5 vs 7.9), MIG (5 vs 10.3), TNF-α (0.46 vs 0.87), MIP-1β (0.68 vs 0.25), and eotaxin (0.03 vs 0.01) (P < .05) (Figure 3). In summary, HIV-1 infection has a differential effect on cytokines in blood and seminal plasma, resulting in profound changes in the compartmentalization of the majority of the evaluated cytokines.
Effect of Seminal Shedding of Herpesviruses on the Cytokine Spectra of HIV-1–Infected Individuals
Next, we evaluated the effects of CMV and EBV seminal shedding on cytokine concentrations in semen of HIV-1–infected individuals. Among the 47 HIV-1–infected individuals whose seminal cytokine levels were measured, 34 (72%) were CMV shedders. In the seminal plasma of these individuals, we found higher levels of RANTES, eotaxin, and MIG than in HIV-1–infected individuals not shedding CMV in semen (2.2-fold, 1.8-fold, and 1.2-fold, respectively; P < .05). Analysis of cytokines in seminal plasma of HIV-1–infected patients revealed no significant differences between 25 patients (53%) who were shedding EBV into seminal plasma and 22 patients without EBV DNA seminal shedding (P > .1).
Finally, the seminal levels of RANTES were significantly different (P < .03) among the 4 subgroups: 571 pg/mL (IQR, 325–751) for EBV-CMV dual shedders , 192 pg/mL (IQR, 147–649) for EBV shedders, 622 pg/mL (IQR, 460–767) for CMV shedders, and 267 pg/mL (IQR, 234–292) for nonshedders. Although the post hoc analysis did not reach statistical significance for the pairwise comparisons of the median RANTES seminal levels in these 4 groups, these data indicate that in semen of HIV-1–infected individuals CMV shedding, independent of EBV shedding, is associated with higher levels of RANTES (Figure 2C). No significant differences were found among the 4 subgroups in the seminal levels of eotaxin and MIG. In summary, we found that CMV seminal shedding in HIV-1–infected individuals is associated with changes in the seminal cytokine spectrum.
DISCUSSION
Seminal cytokines, which play an important role in HIV sexual transmission, both affect and are affected by the replication of HIV-1 and other copathogens, in particular herpesviruses. A deep understanding of immunological and virological features of semen and their changes with HIV-1 infection is of paramount importance for our knowledge of the biology of HIV-1 sexual transmission [2, 6, 16].
Our data show that semen and blood are 2 separate immunological compartments, in which concentrations of cytokines and loads of coinfecting herpesviruses are profoundly different. We found that semen is enriched in IL-1α, IL-7, IL-8, MIP-3α, MCP-1, MIG, IP-10, SDF-1β, and TGF-β, whereas blood is enriched in IL-2, IL-16, MIP-1β, and eotaxin. With HIV infection, the levels of blood and semen cytokines are significantly altered, thus affecting the compartmentalization of the semen and blood cytokine networks. HIV-1 infection changes the seminal cytokine spectrum by up-regulating 16 of the 21 measured cytokines (IL-1α, IL-1β, IL-6, IL-7, IL-8, IL-16, MIP-1α, MIP-1β, MIP-3α, RANTES, MCP-1, eotaxin, MIG, IP-10, SDF-1β, and TGF-β). Concurrently, in blood MIP-1β and SDF-1β are down-regulated, and IL-16, MIP-3α, MCP-1, eotaxin, MIG, IP-10, and TNF-α up-regulated. As a result of these changes, HIV-1 infection emphasizes the blood-semen compartmentalization of some cytokines (IL-1α, IL-1β, IL-6, IL-7, MCP-1, SDF-1β, and TNF-α), whereas it reduces that of others (MIG, eotaxin, and MIP-1β).
Because the cytokine network in semen is different from that in blood and the increase in the levels of seminal cytokines is not always accompanied by an up-regulation of the same cytokines in the blood, the up-regulated seminal cytokines seem to be produced locally in the male genital tract. Thus, the changes in cytokine levels that occur with HIV-1 infection may reflect a profound dysregulation of the functional state of immune cells resident in the male genital tract.
In particular, the increase in proinflammatory cytokines IL-1α, IL-6, IL-8, MIP-1β, MIP-3α, MCP-1, and RANTES could reflect the expansion and activation of resident T cells, whereas the increase in TGF-β, naturally enriched in semen to facilitate the induction of immune tolerance to seminal antigens, could result from the aberrant accumulation of regulatory T cells, as described elsewhere for lymphoid tissues [17, 18]. Furthermore, remarkably high levels of IL-7 (200 times higher than in blood) may play a crucial role in the persistence and survival of memory T cells, thus enriching both the pool of HIV-1 targets and the pool of effector cells crucial to containing HIV-1 replication in this compartment. Seminal CCR5-binding cytokines, up-regulated as the result of HIV-1 infection may initially reduce replication of CCR5-tropic HIV-1 variants but select for more cytopathic ones [19]. At the same time, the increased level of CXCR4 ligands may suppress CXCR4-tropic HIV-1 variants that are negatively selected during HIV-1 sexual transmission, thus representing one of the “gatekeepers” for this HIV-1 variant [20]. In general, the complex seminal cytokine network may finely regulate the replication and the transmission of different HIV variants.
The seminal cytokine network already altered by HIV-1 infection is further altered by herpesviruses reactivated in the male genital tract. Our data indicate that such reactivation may occur as a local phenomenon, because ∼75% of the patients had CMV seminal shedding and 54% of the patients had EBV seminal shedding in the absence of blood plasma viremia. Moreover, the median load of CMV in seminal plasma was >2 log10 copies/mL higher than in blood plasma. These findings are consistent with a previous report on CMV seminal shedding in HIV-1–infected therapy-naive individuals [21]. Our work indicates that the source of the EBV and CMV seminal shedding is not a systemic reactivation of the infection accompanied by a “spillover” of virus from the blood but rather their compartmentalized reactivation in the male genital tract, further indicating that these 2 compartments are immunologically different.
The reactivation of herpesviruses in the genital tract is associated with an increase of HIV-1 seminal load. Future experiments should reveal whether the high HIV-1 load and its immunological consequences promote the reactivation of herpesviruses or vice versa. Of course, a possibility remains that other sexual pathogens not evaluated in our study may contribute to the modification of the cytokine network and promote replication of both herpesviruses and HIV-1. Nevertheless, the association of dual EBV and CMV seminal shedding with a lower CD4+ T-cell count may indicate that the HIV-1–mediated loss of immune control over CMV and EBV infections causes a reactivation that could remain initially localized and become systemic only afterward [22, 23]. Herpesviral genital reactivation is also accompanied by changes in seminal cytokine spectrum. Indeed, we found that the seminal shedding of CMV was associated with an increased seminal concentration of the 2 CCR5 ligands RANTES and eotaxin, as well as with the increase of the CXCR3 ligand MIG.
The net effect on HIV-1 replication of a complex cytokine mixture is difficult to predict on the basis of the effects of individual cytokines, especially because these effects are often ambivalent. For example, RANTES, up-regulated by CMV, will block infection by CCR5-utilizing HIV-1 variants but promote activation and expansion of CCR5-expressing T cells, potentially enhancing HIV-1 replication. It is conceivable that the seminal cytokine spectra altered by local infection with HIV-1, CMV, EBV, and probably other viruses may favor selection of particular HIV-1 variants. The occurrence of such selection was demonstrated in pig-tailed macaques: in animals coinfected with HHV-6, which up-regulates RANTES, SIV acquired resistance to RANTES [19]. Seminal cytokines may also play a role in the selection of recently described transmitter/founder HIV-1 variants. Selection of such variants may be a multistage process starting in the male genital tract, in which the local inflammation, herpesviral reactivation, and cytokine levels may play an important role [5, 24].
Finally, coinfecting viruses and cytokines present in semen may alter not only HIV-1 replication and evolution in the male genital tract but also the probability of HIV-1 transmission in the female genital tract. In fact, cytokines and high loads of seminal pathogens may alter the immunological landscape of the female genital tract when delivered with the ejaculate, modifying the recruitment and activation status of immune cells therein [18]. Moreover, seminal cytokines together with viruses shed in semen may determine the efficiency of HIV male-to-female and male-to-male HIV transmission and thus should be considered as another target in strategies aimed at preventing HIV-1 transmission. Such strategies require a deeper understanding of the complex relationship between the cytokine milieu of semen, HIV-1, and coinfecting viruses. Our data represent one of the first steps toward this goal.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://www.oxfordjournals.org/our_journals/jid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the authors.
Notes
Acknowledgments.
The contribution of A. M. to this study is a part of her PhD Program, All India Institute of Medical Sciences, New Delhi, India. The contribution of A. I. to this study is a part of his PhD Program, University of Milano, Italy. The contribution of E. S. to this study is a part of her PhD Program, Vita-Salute San Raffaele University, Milano, Italy.
Financial support.
This work was supported in part by the Intramural Research Program of the National Institute of Child Health and Human Development (NICHD) and the NIH Intramural-to-India (I-to-I) Program. The work of S. S. was supported by the Extramural Research Grant from Indian Council of Medical Research, New Delhi.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Owen DH, Katz DF. A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J Androl. 2005;26:459–69. doi: 10.2164/jandrol.04104. [DOI] [PubMed] [Google Scholar]
- 2.Politch JA, Tucker L, Bowman FP, Anderson DJ. Concentrations and significance of cytokines and other immunologic factors in semen of healthy fertile men. Hum Reprod. 2007;22:2928–35. doi: 10.1093/humrep/dem281. [DOI] [PubMed] [Google Scholar]
- 3.Robertson SA, Guerin LR, Bromfield JJ, Branson KM, Ahlstrom AC, Care AS. Seminal fluid drives expansion of the CD4+CD25+ T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biol Reprod. 2009;80:1036–45. doi: 10.1095/biolreprod.108.074658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson SA, Ingman WV, O'Leary S, Sharkey DJ, Tremellen KP. Transforming growth factor beta–a mediator of immune deviation in seminal plasma. J Reprod Immunol. 2002;57:109–28. doi: 10.1016/s0165-0378(02)00015-3. [DOI] [PubMed] [Google Scholar]
- 5.Anderson JA, Ping LH, Dibben O, et al. HIV-1 populations in semen arise through multiple mechanisms. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001053. e1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabatte J, Lenicov FR, Cabrini M, et al. The role of semen in sexual transmission of HIV: beyond a carrier for virus particles. Microbes Infect. 2011;13:977–82. doi: 10.1016/j.micinf.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Munch J, Rucker E, Standker L, et al. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell. 2007;131:1059–71. doi: 10.1016/j.cell.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Boukari H, Brichacek B, Stratton P, et al. Movements of HIV-virions in human cervical mucus. Biomacromolecules. 2009;10:2482–8. doi: 10.1021/bm900344q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Q, Estes JD, Schlievert PM, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–8. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dejucq N, Jegou B. Viruses in the mammalian male genital tract and their effects on the reproductive system. Microbiol Mol Biol Rev. 2001;65:208–31. doi: 10.1128/MMBR.65.2.208-231.2001. first and second pages, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lisco A, Vanpouille C, Margolis L. War and peace between microbes: HIV-1 interactions with coinfecting viruses. Cell Host Microbe. 2009;6:403–8. doi: 10.1016/j.chom.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Sereti I, Dunham RM, Spritzler J, et al. IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood. 2009;113:6304–14. doi: 10.1182/blood-2008-10-186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–23. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- 14.Ptak RG, Gallay PA, Jochmans D, et al. Inhibition of human immunodeficiency virus type 1 replication in human cells by Debio-025, a novel cyclophilin binding agent. Antimicrob Agents Chemother. 2008;52:1302–17. doi: 10.1128/AAC.01324-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malnati MS, Tambussi G, Clerici E, et al. Increased plasma levels of the C-C chemokine RANTES in patients with primary HIV-1 infection. J Biol Regul Homeost Agents. 1997;11:40–2. [PubMed] [Google Scholar]
- 16.Baeten JM, Kahle E, Lingappa JR, et al. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med. 2011;3:77ra29. doi: 10.1126/scitranslmed.3001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersson J, Boasso A, Nilsson J, et al. The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J Immunol. 2005;174:3143–7. doi: 10.4049/jimmunol.174.6.3143. [DOI] [PubMed] [Google Scholar]
- 18.Robertson SA, Guerin LR, Moldenhauer LM, Hayball JD. Activating T regulatory cells for tolerance in early pregnancy—the contribution of seminal fluid. J Reprod Immunol. 2009;83:109–16. doi: 10.1016/j.jri.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Biancotto A, Grivel JC, Lisco A, et al. Evolution of SIV toward RANTES resistance in macaques rapidly progressing to AIDS upon coinfection with HHV-6A. Retrovirology. 2009;6:61. doi: 10.1186/1742-4690-6-S2-I3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Margolis L, Shattock R. Selective transmission of CCR5-utilizing HIV-1: the “gatekeeper” problem resolved? Nat Rev Microbiol. 2006;4:312–7. doi: 10.1038/nrmicro1387. [DOI] [PubMed] [Google Scholar]
- 21.Sheth PM, Danesh A, Sheung A, et al. Disproportionately high semen shedding of HIV is associated with compartmentalized cytomegalovirus reactivation. J Infect Dis. 2006;193:45–8. doi: 10.1086/498576. [DOI] [PubMed] [Google Scholar]
- 22.Kaur A, Rosenzweig M, Johnson RP. Immunological memory and acquired immunodeficiency syndrome pathogenesis. Philos Trans R Soc Lond B Biol Sci. 2000;355:381–90. doi: 10.1098/rstb.2000.0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunt PW, Martin JN, Sinclair E, et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis. 2011;203:1474–83. doi: 10.1093/infdis/jir060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keele BF. Identifying and characterizing recently transmitted viruses. Curr Opin HIV AIDS. 2010;5:327–34. doi: 10.1097/COH.0b013e32833a0b9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.