Abstract
Mortality from adult bacterial meningitis exceeds 50% in sub-Saharan Africa. We postulated that—particularly in individuals infected with human immunodeficiency virus (HIV)—herpes simplex virus, varicella zoster virus, Epstein-Barr virus (EBV), and cytomegalovirus (CMV) in the cerebrospinal fluid (CSF) contribute to poor outcome. CSF from 149 Malawian adults with bacterial meningitis and 39 controls were analyzed using polymerase chain reaction. EBV was detected in 79 of 149 bacterial meningitis patients. Mortality (54%) was associated with higher CSF EBV load when adjusted for HIV (P = .01). CMV was detected in 11 of 115 HIV-infected patients, 8 of whom died. The mechanisms by which EBV and CMV contribute to poor outcome require further investigation.
Bacterial meningitis in adults in Malawi, as in many sub-Saharan African countries outside the “meningitis belt,” is associated with a mortality exceeding 50%, even following effective antibiotic therapy [1, 2]. One potentially important factor in bacterial meningitis previously not investigated is the role of coinfection of the brain with herpesviruses, particularly in individuals who are infected with human immunodeficiency virus (HIV). Herpesviruses infect >90% of the world’s population and persist indefinitely in a latent form [3]. Both primary infection and reactivation can cause neurological disease, including meningitis and encephalitis. Reactivation may occur at any time but is particularly common during concurrent illness or immunosuppression [4]. In Malawi >80% of adult bacterial meningitis occurs in HIV-infected patients who frequently have advanced immunosuppression [1]. We hypothesized that in the context of this immunodeficiency, the inflammatory process associated with bacterial meningitis triggers reactivation of herpesviruses in the central nervous system (CNS). Herpesvirus reactivation may alter the host inflammatory response [5], which is known to be the predominant cause of cerebral injury in meningitis [6], and thus contribute to poor outcome. The aim of this study was to describe the prevalence of herpes simplex virus types 1 and 2 (HSV1&2), varicella zoster virus (VZV), Epstein-Barr virus (EBV), and cytomegalovirus (CMV) in the cerebrospinal fluid (CSF) of adults with bacterial meningitis in Malawi, and to determine whether the presence of these viruses in the CSF is associated with increased mortality.
Methods
Queen Elizabeth Hospital in Blantyre, Malawi, is a teaching hospital offering free services to a population of approximately 1 million. From 2006 until 2009, 3 prospective adult (age ≥16 years) meningitis studies were conducted at the hospital; a randomized controlled trial of adjunctive oral glycerol in bacterial meningitis (n = 265) [7] and descriptive studies of suspected meningitis of any cause (n = 573) [8] and suspected bacterial meningitis (n = 161) (unpublished). CSF samples were stored at the time of diagnostic lumbar puncture. Blood samples were also obtained from participants in the randomized trial. Patients or their legal guardians gave written informed consent before recruitment. Each study was approved by the University of Malawi, College of Medicine Research Ethics Committee (P.04/05/363, P.05/06/471, P.09/08/700) and complied with all institutional guidelines. Patients were included in this study if they had an available CSF sample, documented HIV status, and bacterial meningitis defined as a CSF white cell count ≥100 cells/mm3 with polymorphs >50%, or a positive CSF Gram stain, bacterial culture, or Streptococcus pneumoniae polymerase chain reaction (PCR) test [9]. Defining bacterial meningitis using the above CSF white cell criteria identifies patients with the same clinical characteristics and outcomes as patients with microbiologically proven bacterial meningitis in this setting [1]. Controls were patients from one of the descriptive studies who did not have meningitis based on a CSF white cell count <5 cells/mm3 and negative CSF Gram stain, cryptococcal antigen test, bacterial culture, and mycobacterial culture.
Samples were stored at −70°C until DNA extraction from 200 μL using a DNA Mini Kit (Qiagen). Five microliters of extract was used for all assays except the CMV assay, which is optimized for use with 10 μL [10]. Real-time PCR was performed with an ABI7300 machine using TaqMan Master Mix (Applied Biosystems). Primers and probes targeting conserved regions of the DNA polymerase gene were used for HSV1&2 (forward-GACAGCGAATTCGAGATGCTG, reverse-ATGTTGTACCCGGTCACGAACT, HSV1 probe; 5′-FAM-CATGACCCTTGTGAAACA-MGB-3′-BHQ1, HSV2 probe; 5′-VIC-TGACCTTCGTCAAGCAG-MGB-3′-BHQ1) and VZV (forward-GCGCGGTAGTAACAGAGAATTTC, reverse-ACGTGCATGGACCGGTTAAT, probe; 5′-FAM-ACCATGTCATCGTTTCAA-MGB-3′-BHQ1). For VZV a selection of samples were also confirmed with a commercial VZV assay (Qiagen). The EBV assay targeted the BNRF1 gene, and the CMV assay targeted the UL123/UL55 genes [10, 11]. PCR conditions were activation of UNG 50oC for 2 minutes, activation of Taq 95oC for 15 minutes, amplification for 45 cycles at 95oC for 15 seconds, and 60oC for 1 minute. Each assay had a lower limit of detection of 1000 copies/mL assessed as the level at which the PCR reaction always gave a positive result. Positive controls consisted of extracted whole virus suspension (National Institute of Biological Standards and Controls, United Kingdom). A positive control for the virus being tested and a negative control were tested in each run. Clinical samples were analyzed in triplicate. Virus detection in ≥2 of 3 wells was considered positive. If virus was detected in 1 of 3 wells, the test was repeated and considered positive if it again yielded ≥1 of 3 wells positive. Samples positive for EBV were analyzed by quantitative PCR to determine EBV load using a plasmid-derived standard. The standard was quantified by ultraviolet spectrophotometry, serially diluted to 103–109 copies/mL, and optimized to 92% efficiency. EBV load was also quantified in matched whole blood samples where available.
Statistical analysis was performed using Stata software, version 10, with a level of significance of P < .05. Medians were compared using Wilcoxon rank-sum test. Associations were examined using Fisher exact test, stratified Cochran-Mantel-Haenszel tests, and logistic regression. Correlations were tested using Spearman rank correlation.
Results
CSF samples from 188 patients were analyzed. In total, 149 (of whom 115 were HIV positive) had bacterial meningitis and 39 (of whom 24 were HIV positive) had no meningitis (Table 1). CD4 testing was not routinely available in Malawi during the studies; however, where documented, there was no difference between samples with bacterial meningitis (n = 45) and those without meningitis (n = 23) (median 188 cells/mm3 vs 157 cells/mm3, respectively; P = .43). For patients with bacterial meningitis the CSF white cell count was lower in HIV-positive patients than in HIV-negative patients (median 365 cells/mm3 vs 960 cells/mm3; P = .009).
Table 1.
Patient Characteristics and Rates of Detection of HSV 1&2, VZV, EBV, and CMV
| No meningitis |
Bacterial meningitis |
|||
| HIV negative (n = 15) | HIV positive (n = 24) | HIV negative (n = 34) | HIV positive (n = 115) | |
| Male, no. (%) | 7 (47%) | 11 (46%) | 22 (65%) | 42 (37%) |
| Age, y | 27 (16–77) | 35 (21–50) | 27 (16–79) | 32 (16–78) |
| CD4 count,a cells/mm3 | … | 188 (25–553) | … | 157 (1–457) |
| Mortality (%)b | NA | NA | 12/34 (35%) | 64/108 (59%) |
| CSF white cell count, cells/mm3 | 0 | 0 | 960 (30–6160) | 365 (10–72 080) |
| CSF PCR results (%) | ||||
| HSV1 | 1/15 (7%) | 1/24 (4%) | 0/32 (0%) | 1/97 (1%) |
| HSV2 | 0/15 (0%) | 0/24 (0%) | 0/32 (0%) | 0/97 (0%) |
| VZV | 0/15 (0%) | 0/17 (0%) | 0/28 (0%) | 0/76 (0%) |
| EBV | 0/15 (0%) | 6/24 (25%) | 9/34 (26%) | 70/115 (61%) |
| CMV | 0/15 (0%) | 0/24 (0%) | 0/34 (0%) | 11/115 (10%) |
| EBV load in CSF, copies/mL | … | 1202 (182–2281) | 1102 (365–7269) | 6849 (511–223 323) |
| EBV load in blood, copies/mL | NA | NA | 2874 (1146–201 905) | 31 010 (751–35 658 200) |
Values are median (range) unless otherwise stated.
Abbreviations: CSF, cerebrospinal fluid; CMV, cytomegalovirus; EBV, Epstein-Barr virus; HIV, human immunodeficiency virus; HSV 1 & 2, herpes simplex virus types 1 and 2; NA, not available; PCR, polymerase chain reaction; VZV, varicella zoster virus.
CD4 count available for 23 patients without meningitis and 45 patients with meningitis.
Outcome data not available for 7 patients with meningitis.
Nine of the 90 positive PCR results were based on repeat testing following an initial result of 1 of 3 wells being positive (2 HSV1, 1 CMV, 6 EBV). Reclassifying these results as negative did not change the results of the statistical analyses.
HSV1 was detected in 2 of 39 patients without meningitis and 1 of 129 patients with bacterial meningitis (Table 1). HSV2 and VZV were not detected in any patients.
CMV was detected in 11 of 115 (10%) HIV-positive patients with bacterial meningitis (median CD4 count, 121 cells/mm3 [range, 1–251 cells/mm3]; n = 7) but was not detected in any HIV-negative patients with bacterial meningitis or 39 patients without meningitis.
EBV was found in the CSF in 79 of 149 (53%) patients with bacterial meningitis and was strongly associated with HIV seropositivity (odds ratio 5.2 [95% confidence interval, 2.2–11.9]; P < .001). In HIV-positive patients with bacterial meningitis, there was no correlation between CSF EBV load and CSF white cell count (ρ = −0.01, P = .94), CSF lymphocyte count (ρ = −0.02, P = .86) or CSF red cell count (ρ = −0.01, P = .98). For those bacterial meningitis patients with CD4 count data available there was no difference in the CD4 count between those with (n = 33) and without (n = 12) EBV in the CSF (median, 180 cells/mm3 vs 141 cells/mm3, respectively; P = .78) and CSF EBV load did not correlate with the CD4 count (ρ = 0.01, P = .97).
In HIV-positive patients without meningitis, EBV was present in 6 of 24 (25%) patients, which was significantly less than in those with bacterial meningitis (P = .002). All had relatively low EBV loads (range, 182–2281 copies/mL). EBV was not detected in any of the 15 HIV-negative patients without meningitis.
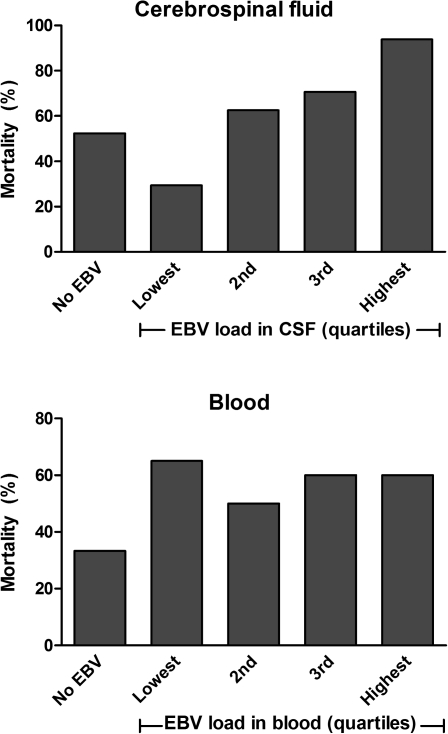
In-hospital mortality from bacterial meningitis was higher in HIV-positive (59% [64 of 108]) than HIV-negative (35% [12 of 34]) individuals (P = .016). Outcome data were unavailable for 7 HIV-positive patients. The presence of EBV in the CSF of HIV-positive patients was not associated with outcome (P = .32); however, when outcome was analyzed according to CSF EBV load, adjusting for HIV status, there was a significant association with mortality (P = .012) (Figure 1). When this analysis was repeated excluding those who had no EBV in the CSF (n = 75), the association remained (P = .018). Similarly, the association remained significant when other factors previously shown to be associated with poor outcome (age ≥32 years, Glasgow Coma Score <12, hemoglobin <10 g/dL) [1] were included in the model (P = .003).
Figure 1.
Mortality from bacterial meningitis in adults infected with human immunodeficiency virus (HIV) according to Epstein-Barr virus (EBV) load in cerebrospinal fluid (CSF) (n = 108) and blood (n = 83). In total, 52 patients had no EBV in the CSF, and 3 patients had no EBV in the blood. CSF EBV load quartile ranges are 511–2186 copies/mL, 2187–6602 copies/mL, 7096–19 958 copies/mL, and 19 958–223 323 copies/mL. Blood EBV load quartile ranges are 751–9013 copies/mL, 9645–30 768 copies/mL, 31 251–136 953 copies/mL, and 183 681–35 658 200 copies/mL.
EBV load in the blood was measured in 107 bacterial meningitis patients and detected in 80 of 83 (96%) HIV-positive patients compared with 14 of 24 (58%) HIV-negative patients (P < .001). Unlike the CSF, blood EBV load was not associated with outcome, adjusting for HIV status (P = .37) (Figure 1). Blood EBV load was higher than the corresponding CSF load for all but 6 individuals, 3 of whom had no detectable EBV in the blood. Of these 6 patients CSF white cell counts ranged from 120 to 5920 cells/mm3, blood white cell counts ranged from 4.5 to 22.2 cells/L, and 3 patients died.
In total, 8 of 10 patients with CMV died (outcome data were unavailable for 1 patient). All 8 patients who died had dual infection with EBV, whereas the 2 survivors did not. Although the presence of CMV did not increase the risk of death (P = .19), EBV/CMV dual infection was associated with death (P = .02).
Discussion
The cerebral injury that occurs in bacterial meningitis is largely due to a host-mediated inflammatory response [6]. Our data suggest that this process may be amplified by EBV reactivation and possibly CMV either in the brain or periphery. In contrast, we show that HSV1&2 and VZV are not commonly reactivated in adult bacterial meningitis in Malawi.
We found a very high prevalence of EBV in the context of bacterial meningitis, particularly in HIV-positive patients. EBV in the CSF of HIV-positive patients is associated with CNS lymphoma, although studies have detected EBV in other CNS diseases [12, 13]. Replicating EBV arises from B lymphocytes in the brain or peripheral circulation [14]. Given that we found virus in patients with no white cells in the CSF, and that replicative-cycle EBV messenger RNA has been detected in CSF from patients with other CNS infections [13], it is likely that some EBV was replicating rather than latent. Alternatively, the inflammatory process may allow latently infected lymphocytes into the CSF, leading to detection of latent virus. Immunosuppressed patients have increased levels of EBV in the blood due to a greater number of infected B lymphocytes [3]. If reactivation occurs in the circulation, then the disrupted blood-CSF barrier in meningitis may allow free virus into the CSF. Although most patients had higher EBV loads in the blood than in the CSF, the finding of higher CSF loads in some patients suggests that EBV can arise within the CNS.
Our data show that increasing CSF EBV load is associated with an increased risk of death from bacterial meningitis. EBV may simply be a marker of immune impairment, although this immune impairment appears to be poorly reflected in the CD4 count given the lack of correlation between CD4 count and EBV load. Alternatively, an immune threshold may exist at which EBV becomes detectable; however, our data show is the EBV load that is associated with outcome rather than just the presence of virus. We speculate that EBV plays a causative role. EBV causes neurological damage by direct infection of brain cells or immune-mediated damage [14, 15]. EBV can also induce cytokines, which may allow further viral reactivation and impaired clearance of other infections [5]. The presence of EBV in 25% of HIV-positive patients with normal CSF does show that EBV can occur in the CSF without causing inflammation; however, all of these patients had low EBV loads.
CMV was detected in 10% of HIV-positive patients with bacterial meningitis. The absence of CMV in other patients indicates that detection is associated with inflammation in the CSF in patients with underlying immunosuppression. CMV neurological disease usually occurs at CD4 counts <50 cells/mm3; however, in our study CMV-positive patients had a median CD4 count of 121 cells/mm3. A previous study showed that detection of CMV in the CSF of HIV-positive individuals was almost always associated with autopsy findings of CMV neurological disease, even when other neurological diseases were likely to be the primary illness [4]. Our study showed a significant association between dual EBV/CMV infection and mortality, suggesting that dual herpesvirus infections are associated with greater CSF inflammation and worse outcome [5, 13].
Our study supports the hypothesis that bacterial meningitis can trigger reactivation of herpesviruses, especially in HIV-positive patients. This could be further supported by demonstrating that the virus is replicating and not latent. We have shown that CSF EBV load and dual EBV/CMV infection are associated with increased mortality from bacterial meningitis and propose that this reflects a causative role resulting from the effects of EBV and CMV on the inflammatory process responsible for poor outcome.
Notes
Acknowledgments.
The authors thank Sarah White for her assistance with the statistical analysis.
Financial support.
This work was supported by grants from the Meningitis Research Foundation (0905.0) and the Wellcome Trust (084679/Z/08/Z), United Kingdom.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Scarborough M, Gordon SB, Whitty CJ, et al. Corticosteroids for bacterial meningitis in adults in sub-Saharan Africa. N Engl J Med. 2007;357:2441–50. doi: 10.1056/NEJMoa065711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hakim JG, Gangaidzo IT, Heyderman RS, et al. Impact of HIV infection on meningitis in Harare, Zimbabwe: a prospective study of 406 predominantly adult patients. AIDS. 2000;14:1401–7. doi: 10.1097/00002030-200007070-00013. [DOI] [PubMed] [Google Scholar]
- 3.Babcock GJ, Decker LL, Freeman RB, Thorley-Lawson DA. Epstein-Barr virus–infected resting memory B cells, not proliferating lymphoblasts, accumulate in the peripheral blood of immunosuppressed patients. J Exp Med. 1999;190:567–76. doi: 10.1084/jem.190.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cinque P, Vago L, Dahl H, et al. Polymerase chain reaction on cerebrospinal fluid for diagnosis of virus-associated opportunistic diseases of the central nervous system in HIV-infected patients. AIDS. 1996;10:951–8. doi: 10.1097/00002030-199610090-00004. [DOI] [PubMed] [Google Scholar]
- 5.Tang YW, Espy MJ, Persing DH, Smith TF. Molecular evidence and clinical significance of herpesvirus coinfection in the central nervous system. J Clin Microbiol. 1997;35:2869–72. doi: 10.1128/jcm.35.11.2869-2872.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheld WM, Koedel U, Nathan B, Pfister HW. Pathophysiology of bacterial meningitis: mechanism(s) of neuronal injury. J Infect Dis. 2002;186(Suppl 2):S225–33. doi: 10.1086/344939. [DOI] [PubMed] [Google Scholar]
- 7.Ajdukiewicz KM, Cartwright KE, Scarborough M, Mwambene JB, et al. Glycerol adjuvant therapy in adults with bacterial meningitis in a high HIV seroprevalence setting in Malawi: a double-blind, randomised controlled trial. Lancet Infect Dis. 2011;11:293–300. doi: 10.1016/S1473-3099(10)70317-0. [Epub 2011 Feb 18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen DB, Zijlstra EE, Mukaka M, Reiss M, et al. Diagnosis of cryptococcal and tuberculous meningitis in a resource-limited African setting. Trop Med Int Health. 2010;15:910–17. doi: 10.1111/j.1365-3156.2010.02565.x. [DOI] [PubMed] [Google Scholar]
- 9.Corless CE, Guiver M, Borrow R, Edwards-Jones V, et al. Simultaneous detection of Neisseria meningitidis, Haemophilus influenzae, and Streptococcus pneumoniae in suspected cases of meningitis and septicemia using real-time PCR. J Clin Microbiol. 2001;39:1553–8. doi: 10.1128/JCM.39.4.1553-1558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boeckh M, Huang M, Ferrenberg J, Stevens-Ayers T, et al. Optimization of quantitative detection of cytomegalovirus DNA in plasma by real-time PCR. J Clin Microbiol. 2004;42:1142–8. doi: 10.1128/JCM.42.3.1142-1148.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niesters HG, van Esser J, Fries E, Wolthers KC, et al. Development of a real-time quantitative assay for detection of Epstein-Barr virus. J Clin Microbiol. 2000;38:712–5. doi: 10.1128/jcm.38.2.712-715.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rojanawiwat A, Miura T, Thaisri H, Pathipvanich P, et al. Frequent detection of Epstein-Barr virus and cytomegalovirus but not JC virus DNA in cerebrospinal fluid samples from human immunodeficiency virus-infected patients in northern Thailand. J Clin Microbiol. 2005;43:3484–6. doi: 10.1128/JCM.43.7.3484-3486.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinberg A, Bloch K, Li S, Tang YW, Palmer M, Tyler KL. Dual infections of the central nervous system with Epstein-Barr virus. J Infect Dis. 2005;191:234–7. doi: 10.1086/426402. [DOI] [PubMed] [Google Scholar]
- 14.Menet A, Speth C, Larcher C, et al. Epstein-Barr virus infection of human astrocyte cell lines. J Virol. 1999;73:7722–33. doi: 10.1128/jvi.73.9.7722-7733.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volpi A. Epstein-Barr virus and human herpesvirus type 8 infections of the central nervous system. Herpes. 2004;11(Suppl 2):120A–7A. [PubMed] [Google Scholar]