Abstract
We recently discovered the antisense protein of human T-cell leukemia virus (HTLV) type 2 (APH-2), whose messenger RNA is encoded by the antisense strand of the HTLV-2 genome. We quantified proviral load, level of tax, and APH-2 in a series of blood samples obtained from a cohort of HTLV-2 carriers. We determined whether APH-2 promotes cell proliferation. APH-2 was detectable in most samples tested and was correlated with proviral load. APH-2 levels were not correlated with lymphocyte count in vivo, consistent with the inability of APH-2 to promote cell proliferation in vitro. APH-2 does not promote cell proliferation and does not cause lymphocytosis.
Human T-cell leukemia virus type 1 (HTLV-1) and type 2 (HTLV-2) infect T lymphocytes in vivo [1]. Their 5′ long terminal repeat (LTR) serves as a viral promoter to encode, among others, the Tax transactivator protein, whereas another promoter located in the 3′-LTR is used to encode the antisense HTLV-1 bZip factor (HBZ) protein or the antisense protein of HTLV-2 (APH-2), respectively [2, 3].
HTLV-2 infection is associated with lymphocytosis [4], rarely with cases of myelopathies that may resemble HTLV-1 Associated Myelopathy/Tropical Spastic Paraparesis (HAM/TSP), and with rare cases of lymphoproliferative disease in patients coinfected with human immunodeficiency virus. The discovery of HBZ opened new avenues of research. Surprisingly, HBZ mRNA and HBZ protein may have distinct functions. Indeed, HBZ promotes proliferation of T-cell lines, whereas HBZ downregulates Tax-mediated viral transcription [5]. In line with these observations, tax mRNA is not detected in most leukemic Adult T cell Leukemia/Lymphoma (ATLL) cases, whereas HBZ is always expressed [5, 6]. Furthermore, HBZ expression is correlated with proviral load (PVL) and HAM/TSP severity [7]. Even if a recent report demonstrated that HBZ promotes cell transformation when expressed in transgenic animals [8], the model of ATLL development suggests that Tax stimulates the initiation of transformation, whereas HBZ is involved in the maintenance of the transformed stage and in cell proliferation [9].
We have discovered APH-2, which represents the HTLV-2 counterpart of HBZ and which represses Tax-2–mediated transcription [3], suggesting that APH-2 might function like HBZ [3]. Consistent with this, lymphocytosis is commonly observed in HTLV-2 carriers [4].
We quantified APH-2 and tax expression in addition to HTLV-2 PVL in a series of samples obtained from HTLV-2 asymptomatic carriers. APH-2 was expressed in most samples tested. APH-2 and tax levels are correlated with PVL. However, neither APH-2 nor tax is correlated with lymphocyte count. Finally, in constrast to HBZ, APH-2 is not capable of promoting lymphocytosis.
Methods
HTLV-2 carrier blood samples were obtained from human subjects enrolled in the HTLV Outcomes Study cohort, which followed for >15 years the 151 HTLV-1 subjects, 387 HTLV-2 subjects, and 799 uninfected controls [10]. From the total of 387 HTLV-2 subjects, we selected 3 groups of 20 based on PVL measurements at the baseline visit of the cohort: 20 subjects with the lowest (10−6 to 10−4.7 copies/cell), 20 subjects surrounding the median (10−4.5 to 10−3.19 copies/cell) and 20 subjects with the highest PVLs (10−2.8 to 10−1.6 copies/cell), as described in a previously published longitudinal study [11]. Adequate remaining specimens and complete current measurements were available from 51 of these 60 subjects. Subjects included 36 women and 15 men (age range, 37–85 years); 18 were Hispanic, 12 were black, and 18 were white (data not available for 3 subjects). In total, 33 samples belonged to HTLV-2 subtype A and 5 belonged to HTLV-2 subtype B, while data were unavailable for the remaining samples. None of these 51 samples originated from patients presenting a clinical manifestation. The 336 samples that were not used had also a detectable PVL. Frozen peripheral blood mononuclear cells (5×106 PBMCs) were obtained from visits 7 or 8, approximately 15 years after the baseline visit. All subjects gave written informed consent for future research use of their biologic specimens, and the protocol was approved by the Committee on Human Research of the University of California, San Francisco, consistent with the Declaration of Helsinki.
High-molecular-weight DNA as well as total RNA was extracted from the same cryopreserved PBMC samples, thus allowing us to perform an accurate measurement of HTLV-2 PVL and of both APH-2 and tax transcripts. Because these PBMCs were never placed in culture, the level of tax and APH-2 transcripts therefore represents the in vivo situation. HTLV-2 infected C19 cells, uninfected Jurkat cells, or Kit225 cells were grown in Roswell Park Memorial Institute (RPMI) 1640 medium, supplemented with 10% fetal bovine serum (FBS) and antibiotics, and maintained at 37°C in 5% CO2.
High-molecular-weight DNA was extracted, and HTLV-2 PVL was measured in duplicate using a previously described real-time polymerase chain reaction (PCR) technique [12]. RNA was extracted from the same ex vivo samples using TRIzol reagent (Invitrogen) and kept at −80°C until use in real-time PCR experiments. Complementary DNA (cDNA) was obtained from 500 ng of RNA using High Capacity RNA-to-cDNA Master Mix (Applied Biosystems). RNA samples were incubated for 5 minutes at 25°C, 30 minutes at 42°C, and 5 minutes at 85°C in a GeneAmp PCR System 9700 thermocycler (Applied Biosystems). The cDNA samples were diluted (1/10), and 2.5 μL of each sample was tested using the FastStart Universal SYBR Green Master (Roche) containing ROX (Carboxy-X-rhodamine) as an internal reference. The cDNA samples were amplified with APH-2 primers (5′-CCCCAAGACTATTTTAGGAGATTGC-3′ (sense) and 5′-CCGATCCCGACCCCAGAG-3′) (antisense) or tax primers (5′-GACAGAGCCTCCTATATGG-3′ (sense) and 5′-GGTATTGGAGAGGAGAGC-3′) (antisense) [13]. The 18S primers (5′-GTGGAGCGATTTGTCTGGTT-3′ and 5′-CGCTGAGCCAGTCAGTGTAG-3′) were used for normalization. Samples were incubated for 10 minutes at 95°C; then 40 cycles were performed using a StepOne Plus thermocycler (Applied Biosystems) (10 seconds at 95°C and 30 seconds at 60°C), and the melt curve was performed between 60°C and 95°C. APH-2 and Tax-2 primers’ efficacy was determined on a series of C19 cDNA dilutions (0.5 pg to 500 ng). As a control for DNA contamination, quantitative PCR was directly performed without performing the reverse transcriptase (RT) step on RNA obtained from 2 randomly chosen HTLV-2 carriers. RNA extracted from uninfected Jurkat cells was also included in each run. As an interassay reproducibility control, a known concentration of HTLV-2 C19 cDNA samples was used in duplicate for each experiment. The data were then analyzed with StepOne software (version 2.1; Applied Biosystems).
Total RNA was extracted from stable transfectants as described above. One microgram of DNase I-treated total RNA was used to synthesize cDNA using reverse-transcriptase SuperScript III and random primers (Invitrogen). To quantify the level of APH-2 and β-actin transcripts, real-time PCR (see above) was performed using APH-2 primers (see above) and human β-actin primers: 5′-AGGCCAACCGCGAGAAGATG-3′ (sense) and 5′-CCAGAGGCGTACAGGGATAG-3′ (antisense). Relative expression level of APH-2 was calculated by the delta cycle threshold (ΔCt) method.
An MTT assay was performed. Kit225 cells stably transfected with pME18Sneo/APH-2, pME18Sneo/sHBZ or control empty vector were maintained in RPMI medium containing 10% FBS and antibiotics, supplemented with recombinant interleukin 2 (rIL-2; 85 U/mL) and G418 (600 μg/mL). Thirty days after transfection, stable transfectants were washed 3 times with RPMI containing 10% FBS and antibiotics and cultured in this same medium in the absence of rIL-2 and G418. Forty-eight hours later, cells were counted and resuspended in medium supplemented with rIL-2 (2.5 U/mL). Twenty thousand cells were seeded per well in a 96-well plate, and viability was determined by measuring 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide dye absorbance at the indicated time points.
The Shapiro-Wilk test was used to test the data for normality. The variables HTLV-II PVL, APH-2, and tax showed skewed distributions and were log10 transformed to approximate normal distributions. The assay limit of detection (data not shown) was recoded as the log10 of 10−6 for PVL and as a 10−9 for APH-2 and tax. We used linear regression (PROC CORR) with calculation of coefficients of determination (R2) to explore the hypothesis of linear correlations between APH-2 and tax versus other variables of interest. The strength of relationship between covariates was also assessed using general linear models (PROC GLM), with similar results. For comparisons between groups, we used t tests with equal variance (except tax high vs tax intermediate, which used the Satterthwaite method). The contingency tables comparing APH-2 versus tax detection within the 3 PVL groups were analyzed using Fisher exact test due to low cell counts. All statistical analyses were performed using SAS software, version 9.1.3 (SAS Institute).
Results
PVL values were determined for each sample obtained at visit 7 or 8 and compared to those measured at baseline more than 10 years before. Those results demonstrated that PVL from visit 7 or 8 was strongly correlated with PVL measured at baseline, confirming, as previously reported [11], that HTLV-2 PVL does not increase with time (Figure 1A).
Figure 1.
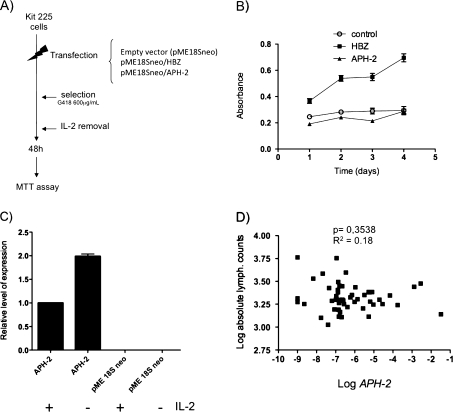
APH-2 and tax messenger RNA (mRNA) levels vary according to proviral load (PVL). A, PVL is stable over time among human T-cell leukemia virus type 2 (HTLV-2)–infected individuals. Each dot represents the log PVL measured at baseline as a function of the log PVL measured at visit 7or 8 for a given HTLV-2 carrier. Coefficients of determination (R2) and P values were derived from linear regression. B, C, Log PVL as a function of (B) log APH-2 or (C) log tax mRNA expression. Each dot represents 1 sample. The vertical dotted lines represent the limit of detection of the assays. Coefficients of determination (R2) and P values were derived from linear regression. D, Median log APH (top) or median log tax (bottom) values according to PVL divided into 3 groups. The t tests were used to derive P values between groups. E, Number of HTLV-2 carriers with detectable or undetectable APH-2 and tax mRNA, according to PVL divided into 3 groups. Fisher exact tests were used to derive P values for each contingency table.
We quantified APH-2 and tax on the same samples and determined whether their levels were similar (Figure 1B–1E). APH-2 was expressed in 94% of the samples independent of HTLV-2 subtype, and its level was significantly correlated (P = .001) with PVL (Figure 1B). Expression of tax was not detected in the samples with the lowest PVL but was also correlated with PVL across all samples (P = .0002) (Figure 1C). Consistent with our previous data showing that APH-2 suppresses Tax-mediated transactivation on the 5′-LTR [3], APH-2 was more likely than tax to be detected in samples with low and intermediate PVL (Figure 1D and 1E). The level of APH-2 expression differed significantly between low and high PVL groups (P = .0473). The level of tax expression also differed significantly between the low and high PVL groups, as well as between the intermediate and high PVL groups (P < .0001 and P = .0074, respectively) (Figure 1D). Both APH-2 and tax were expressed in samples with high PVL (17 of 17 vs 16 of 17, respectively), suggesting that either some cells might express both transcripts of the high PVL group represents heterogeneous populations of cells that express either tax or APH-2 (Figure 1D and 1E). In addition, APH-2 was more frequently detected than tax in the low and intermediate PVL groups (P = .046 and P < .0001, respectively) (Figure 1E).
Because APH-2 was expressed in most samples tested (Figure 1B and 1D) and because HBZ promotes T-cell proliferation and HTLV-2 carriers develop lymphocytosis, we then asked whether APH-2 could also stimulate cell proliferation. Kit225 CD4+ T cells were transfected with HBZ, APH-2, or an empty vector and grown in the presence of neomycin (G418) to select for transfected cells (Figure 2A). Interleukin 2 (IL-2) was then removed from the cell culture medium for 48 hours. Cell proliferation was then assessed in the presence of a low concentration of recombinant exogenous IL-2 (2.5 U/mL). As previously observed, under those conditions, HBZ-positive cells proliferated, whereas APH-2 cells behaved as control cells, suggesting that APH-2 is not involved in cell proliferation in vitro (Figure 2B). As a control, APH-2 expression was assessed and normalized to β-actin (Figure 2C). This confirmed that suboptimal levels of IL-2 have no effect on APH-2 expression. These results demonstrate that although APH-2 is widely expressed in vivo, its function is not to promote cell proliferation. Consistent with those results, we did not find any correlation between APH-2 levels and the number of lymphocytes in vivo (Figure 2D).
Figure 2.
APH-2 expression does not promote lymphocyte proliferation. A, Kit225 lymphocytes were transfected with APH-2, HBZ, or the backbone plasmid. After neomycin selection, cell viability was tested by removing interleukin 2 (IL-2) from the culture medium for 48 h. Recombinant IL-2 (2.5 U/mL) was later added. Cell viability was then assessed 1, 2, 3, and 4 days later. B, Functional analyses of APH-2 or HBZ genes on proliferation of T cells. C, Determination of APH-2 expression by quantitative real-time polymerase chain reaction (RT-PCR). Relative expression level of APH2 was calculated by the ΔCt method. Values were first normalized to β-actin expression and then compared to the normalized expression of APH-2 in IL-2 (85 U/mL) conditions, which was considered as 1. B, C, Data represent the mean value of 2 independent experiments. D, APH-2 messenger RNA (mRNA) expression does not correlate with lymphocyte counts in human T-cell leukemia virus type 2 (HTLV-2) carriers. Each dot represents the log of the absolute lymphocyte count as a function of the log APH-2 mRNA value obtained for a given HTLV-2 carrier. Coefficients of determination (R2) and P values were derived from linear regression.
Discussion
The discovery of HBZ and its effects on cell proliferation and transformation solved a number of questions that have remained unanswered for several years. We previously demonstrated that HTLV-2 cell lines also express an antisense transcript that we named APH-2 [3]. As is the case with HBZ, APH-2 expression is correlated with 5′-LTR downregulation. Because persons with HTLV-2 develop lymphocytosis, this prompted us to evaluate APH-2 expression in vivo and APH-2 properties in vitro.
Our results demonstrate that APH-2 is expressed in vivo in most HTLV-2 carriers and is correlated with PVL, a situation that is reminiscent of HBZ [6, 7]. Contrary to previous reports on HTLV-1 [7], HTLV-2 tax levels were also correlated with PVL. These results were quite unexpected because, in the HTLV-1 situation, (1) HBZ and tax are generally mutually exclusive and (2) only HBZ levels are correlated with PVL [6]. Our data demonstrate that APH-2, in contrast with HBZ, cannot promote cell proliferation.
It is established that Tax-1 and Tax-2 present a number of functional dissimilarities. However, recent reports demonstrated that, even if Tax plays a major role in HTLV-1 pathogenesis, HBZ is a key player in the late steps of disease development [8]. The lack of APH-2 effect on lymphocyte proliferation might also explain the differences in leukemogenesis that are observed between HTLV-1– and HTLV-2–infected individuals [14].
Notes
Acknowledgments.
We acknowledge Bariza Blanquier for her help with the settings of the quantitative PCR experiments. We thank the different members of the Mahieux laboratory for their helpful suggestions.
Financial support.
R. M. is supported by Ecole Normale Supérieure de Lyon, E. D. by the Ministère de la Recherche, and E. L. M. by the National Heart Lung and Blood Institute (NHLBI) (grant K24-HL-75036). We acknowledge the financial support from l’Association de Recherche sur le Cancer, from l’Institut National du Cancer, from INSERM, and from l’Ecole Normale Supérieure de Lyon to our group. Samples were obtained from the HTLV outcomes study funded by NHLBI grant 2R01-HL-62235.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Journo C, Douceron E, Mahieux R. HTLV gene regulation: because size matters, transcription is not enough. Future Microbiol. 2009;4:425–40. doi: 10.2217/fmb.09.13. [DOI] [PubMed] [Google Scholar]
- 2.Gaudray G, Gachon F, Basbous J, Biard-Piechaczyk M, Devaux C, Mesnard JM. The complementary strand of the human T-cell leukemia virus type 1 RNA genome encodes a bZIP transcription factor that down-regulates viral transcription. J Virol. 2002;76:12813–22. doi: 10.1128/JVI.76.24.12813-12822.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halin M, Douceron E, Clerc I, et al. Human T-cell leukemia virus type 2 produces a spliced antisense transcript encoding a protein that lacks a classic bZIP domain but still inhibits Tax2-mediated transcription. Blood. 2009;114:2427–38. doi: 10.1182/blood-2008-09-179879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartman MT, Kaidarova Z, Hirschkorn D, et al. Long-term increases in lymphocytes and platelets in human T-lymphotropic virus type II infection. Blood. 2008;112:3995–4002. doi: 10.1182/blood-2008-05-155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satou Y, Yasunaga J, Yoshida M, Matsuoka M. HTLV-I basic leucine zipper factor gene mRNA supports proliferation of adult T cell leukemia cells. Proc Natl Acad Sci U S A. 2006;103:720–5. doi: 10.1073/pnas.0507631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saito M, Matsuzaki T, Satou Y, et al. In visvo expression of the HBZ gene of HTLV-1 correlates with proviral load, inflammatory markers and disease severity in HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP) Retrovirology. 2009;6:19. doi: 10.1186/1742-4690-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Usui T, Yanagihara K, Tsukasaki K, et al. Characteristic expression of HTLV-1 basic zipper factor (HBZ) transcripts in HTLV-1 provirus-positive cells. Retrovirology. 2008;5:34. doi: 10.1186/1742-4690-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satou Y, Yasunaga J, Zhao T, et al. HTLV-1 bZIP factor induces T-cell lymphoma and systemic inflammation in vivo. PLoS Pathog. 2011;7:e1001274. doi: 10.1371/journal.ppat.1001274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuoka M, Green PL. The HBZ gene, a key player in HTLV-1 pathogenesis. Retrovirology. 2009;6:71. doi: 10.1186/1742-4690-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy EL, Glynn SA, Fridey J, et al. Increased prevalence of infectious diseases and other adverse outcomes in human T lymphotropic virus types I- and II-infected blood donors. Retrovirus Epidemiology Donor Study (REDS) Study Group. J Infect Dis. 1997;176:1468–75. doi: 10.1086/514143. [DOI] [PubMed] [Google Scholar]
- 11.Kwaan N, Lee TH, Chafets DM, et al. Long-term variations in human T lymphotropic virus (HTLV)-I and HTLV-II proviral loads and association with clinical data. J Infect Dis. 2006;194:1557–64. doi: 10.1086/508899. [DOI] [PubMed] [Google Scholar]
- 12.Lee TH, Chafets DM, Busch MP, Murphy EL. Quantitation of HTLV-I and II proviral load using real-time quantitative PCR with SYBR Green chemistry. J Clin Virol. 2004;31:275–82. doi: 10.1016/j.jcv.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Besson G, Kazanji M. One-step, multiplex, real-time PCR assay with molecular beacon probes for simultaneous detection, differentiation, and quantification of human T-cell leukemia virus types 1, 2, and 3. J Clin Microbiol. 2009;47:1129–35. doi: 10.1128/JCM.02006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roucoux DF, Murphy EL. The epidemiology and disease outcomes of human T-lymphotropic virus type II. AIDS Rev. 2004;6:144–54. [PubMed] [Google Scholar]