Abstract
Background. There is little information on multiple human papillomavirus (HPV) infections and the potential for type competition in men, yet competition may impact the type-specific efficacy of HPV vaccination.
Methods. Among 2702 uncircumcised men in Kisumu, Kenya, who were seronegative for human immunodeficiency virus, the observed numbers of HPV types detected were compared with the expected number, which was simulated under the assumption of independent infections. To assess the potential for HPV type competition, adjusted odds ratios for pairwise combinations of prevalent HPV type infections were estimated using semi-Bayesian methods.
Results. Half of all men were HPV positive, of whom 57% had multiple HPV types. We observed men without HPV infection and with ≥4 HPV types more often than expected if infections were independent. No negative associations between individual HPV types were observed. HPV types 31, 39, 56, 58, and 59 were positively associated with both carcinogenic vaccine types HPV-16 and HPV-18 (2-sided P value <.05).
Conclusions. Men who were HPV infected were likely to test positive for >1 HPV type. Cross-sectional associations between individual HPV types were positive and did not appear to be type-specific. Thus, we did not identify HPV types that are candidates for potential HPV type competition in men.
Human papillomavirus (HPV) infection is the main cause of cervical cancer in women [1, 2] and is responsible for other genital cancers, including anal and penile carcinoma in men. Coinfection with multiple HPV types is common and observed in 20%–73% of HPV-infected males [3–11]. Multiple HPV-type infections have been associated with acquisition of other HPV types and increased HPV persistence in men [7] and cervical precancerous lesions in women [12–14].
There are currently 2 FDA-approved HPV vaccines that provide protection against HPV-16 and 18 [15–17] or HPV-16, 18, 6, and 11 [18–20]. Nearly 70% of cases of cervical cancer have been attributed to infection with oncogenic HPV types 16 and 18 [21, 22], and low-risk HPV types 6 and 11 are responsible for 90% of genital warts. HPV-type coinfections could affect the population-based impact of HPV vaccination in both young women and men due to potential HPV type competition and subsequent type replacement [23, 24], that is, an increase in the prevalence of nonvaccine HPV types in the population when vaccine-preventable HPV types are reduced or eliminated [25]. Type competition may result from some yet unknown biological mechanism, whereby infection with 1 HPV type inhibits the acquisition or persistence of other HPV types [7, 26]. In contrast, if infection with a specific HPV type facilitates the acquisition or persistence of other HPV types, it is possible that when 1 HPV type is prevented by vaccination, the other type might be reduced in the population.
If HPV types do compete, this will be reflected in the population as a low probability of coinfection with 2 specific HPV types. However, there are several reasons why, in addition to the possibility of type competition, that 2 HPV types may be less likely to occur together within a multiple infection. The prevalence of HPV types detected in a sample population are dependent on the HPV type distribution in the general population in that geographical area, as well as the HPV types circulating within individual networks of sexual contacts. In addition, observed positive associations between HPV types may be due to the common transmission route and risk factors for all HPV types, such as age [25, 27, 28], condom use [9], circumcision status [6, 11], and lifetime [9, 29, 30] or recent [28] number of sexual partners. Thus, any association between individual HPV types reflects a combination of exposure to HPV, the type distribution among sexual contacts, and host immunological and behavioral characteristics [7, 26, 31].
To date, there are no detailed studies of multiple HPV infections and type competition in an African population, where the HPV genotype distribution may differ from those in North America, Europe, or South America [32]. Given the recent approval of HPV prophylactic vaccination in the United States for young men [33], data on HPV coinfections in men and their female sexual partners are needed to assess the potential for future HPV type replacement. We previously reported the risk factors for HPV infection [3], prevalence by HPV stratified by penile site [34], and association between HPV infection and the risk of human immunodeficiency virus (HIV) acquisition [35] among men from Kisumu, Kenya, participating in a randomized controlled trial (RCT) of male circumcision [36]. In the present analysis, we investigated the associations between vaccine-preventable HPV types (16, 18, 6, and 11) and 41 other HPV types using semi-Bayesian methodology.
METHODS
Study Population and Design
From February 2002 to December 2006, an RCT was conducted in Kisumu, Kenya, to determine the effectiveness of male circumcision in reducing HIV incidence [36]. Male participants were recruited from sexually transmitted infection (STI) clinics, workplaces, and community organizations. To be included in the RCT, men were required to be 18–24 years of age, uncircumcised, HIV-seronegative, and sexually active and to have a hemoglobin level of ≥90 g/L.
An observational cohort study of the effect of circumcision on the natural history of penile HPV infection was nested in the RCT [3, 34, 35]. The present analysis is a cross-sectional study at the baseline visit of uncircumcised, HIV-seronegative men who were eligible for participation in the main RCT (n = 4489) and consented to HPV testing (n = 2705) [3]. In the present analysis, 3 men were excluded due to inconsistent records in the database. The final population for this analysis includes 2702 men who provided penile samples and information on sexual risk factors at baseline, of which 2228 were enrolled in the RCT. The study protocol was approved by the institutional review boards at all collaborating institutions.
Medical Examination and Sample Collection
After participants provided informed consent, trained male interviewers administered a standardized questionnaire on sociodemographic characteristics, medical conditions, and sexual behavior. A trained physician or clinical officer conducted a physical exam during which a genital examination was conducted to inspect for ulcers and warts. Blood was collected for herpes simplex virus type 2 (Kalon Biological) and syphilis testing (Becton Dickinson; rapid plasma reagin with Treponema pallidum hemagglutination assay confirmation). Urine samples were collected for polymerase chain reaction (PCR) detection of Neisseria gonorrhoeae and Chlamydia trachomatis (Roche Diagnostics).
Exfoliated penile cells were collected from 2 anatomical sites using 2 prewetted Dacron swabs: (1) shaft and external foreskin tissue (shaft specimen) and (2) glans, coronal sulcus, and inner foreskin tissue (glans specimen) [34]. The penile swabs were placed in separate 15-mL tubes containing 2-mL of Tris buffer 0.01 mol/L and processed on the day of collection. All samples were sent to the Department of Pathology at the Vrije Universiteit University Medical Center for laboratory detection of HPV DNA.
Detection of HPV DNA
Laboratory Detection
HPV DNA testing was performed on uncircumcised men at the baseline study visit. DNA was isolated from exfoliated penile cell samples [37, 38], and the presence of human DNA was evaluated by β-globin–specific PCR, followed by agarose gel electrophoresis. The presence of HPV DNA was assessed by GP5+/6+ PCR, followed by hybridization of PCR products using an enzyme immunoassay readout with 2 HPV oligoprobe cocktails that together detect 44 HPV types. Subsequent HPV genotyping was performed by reverse line blot hybridization (RLBH) of PCR products, as described elsewhere [37, 38]. HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 were classified as high-risk (carcinogenic) HPV (HR-HPV) types. Low-risk (LR-HPV) types included 6, 11, 26, 30, 32, 34, 40, 42, 43, 44, 53, 54 55, 57, 61, 64, 67, 69, 70, 71 (equivalent to CP8061), 72, 73, 81 (CP8304), 82 (IS39 and MM4 subtypes), 83 (MM7), 84 (MM8), 85 (cand85), 86, 89 (cand89 equivalent to CP6108), and JC9710. Types considered vaccine preventable were HPV-16, 18, 6, and 11, whereas all others were nonvaccine preventable HPV types. HPV types detected by PCR but not genotyped were designated as HPV-X, indicating a type, subtype, or variant undetectable with RLBH probes.
Definition of Multiple Infections
HPV DNA detection methods were carried out on the shaft and glans samples separately. Given that the aims of the present analyses relate to evaluating the potential for HPV type replacement following population-based HPV vaccination and not site-specific infection, we present pooled HPV results for the shaft and glans specimens. A single infection is defined as HPV DNA positivity to any 1 single HPV type in the glans, the shaft, or both sites. A multiple infection is defined as the detection of ≥2 different HPV types in either the glans or the shaft combined. For example, if a man was HPV-16 positive in the shaft and HPV-35 positive in the glans, or if a man was HPV-16 and HPV-35 positive in the glans and HPV negative in the shaft, the man was classified as having multiple infections. If HPV-X occurred alone, the infection was classified as a single infection, although it could represent >1 untyped HPV infection. If HPV-X was detected with additional typed infections, the infection was classified as a multiple HPV infection.
Statistical Analysis
Distribution of Number of HPV Genotypes
The observed number of men with 0, 1, 2, 3, 4, 5, and 6 or more concurrent HPV type infections was compared to the frequency that would be expected under the assumption that each HPV infection is independent of all others. Infection with each of the 45 possible HPV types was simulated for each man by random generation of a binary variable with the probability of infection equal to the observed prevalence of that type in the study population. Expected frequencies for each number of concurrent HPV infections were calculated as the average frequency over 1000 stochastic simulations of 2702 observations [26]. For HR-HPV simulations, the observed probabilities of the 14 HR-HPV types were used to simulate the expected number of infections with only HR-HPV types. As done elsewhere [3], all analyses included males who were HPV positive regardless of β-globin positivity and were conducted using SAS software, version 9.2 (SAS).
Correlates of Multiple HPV Infections
Univariate logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for potential correlates of multiple versus single HPV infections. Variables were identified a priori as potential correlates of multiple HPV infection based on the previous literature [3, 7, 9]. Multivariate logistic regression was used to estimate associations between each potential correlate and multiple HPV infections, simultaneously adjusting for all other potential correlates.
HPV Type Associations in Multiple Infections
We used hierarchical regression analysis to obtain semi-Bayesian estimates of the ORs [39] between the 4 vaccine-preventable HPV types and 41 other outcome HPV types, adjusting for the potential correlates of multiple HPV infections. Shrinkage methods, such as the one we used here, reliably reduce the overall error in the ensemble of estimates and enhance the precision of each estimate by incorporating prior information from the study data [40, 41]. HPV-16, 18, 6, and 11 type-specific prior means, μj, where j = 1–4, were estimated from the data, and their variability was propagated through hyperpriors. The μj are the average of the log OR between the individual vaccine type and all 41 other HPV types, adjusted for age, travel to Nairobi (in 6 months prior to baseline), bathing frequency, number of sexual partners (in the 6 months prior to baseline), consistent condom use (in the 6 months prior to baseline), and current N. gonorrhoeae and C. trachomatis infections. The prior variance for each μj, τ2, was set equal to 0.17 because we assumed that 95% of the log ORs should fall within a 5-fold range based on previous literature [24, 26, 31, 42, 43], which corresponds to a variance of [ln(5)/3.92]2 = 0.17.
Model estimates are reported as the exponentiated posterior medians and 95% credible intervals, analogous to adjusted odds ratios (AORs) and corresponding 95% CIs. Potential evidence of HPV type nonindependence is an AOR estimate <1.0: the odds of a nonvaccine-preventable type are lower in men with a vaccine-preventable HPV type compared with men without a vaccine-preventable type.
Sensitivity Analysis
The case of the bivalent HPV vaccine was also considered; analyses were conducted as outlined above, except HPV-16 and 18 were considered exposure types, and the 43 other types, including HPV-6 and 11, were considered outcome types. Further, we explored results obtained when setting τ2 to 0.35 or 1.38, reflecting a 10- and 100-fold prior 95% confidence limit. For comparison, maximum likelihood estimates of HPV type associations are also presented (Supplementary Table 1).
RESULTS
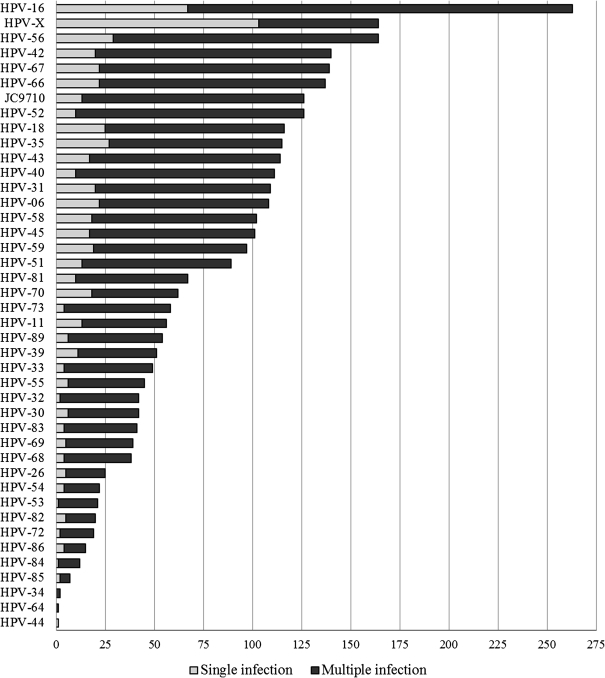
Among 2702 men, with a median age of 20 years (range, 17–28), 51% were HPV positive (n = 1379). A single HPV type was detected in 22% (n = 592) and multiple HPV types were detected in 29% (n = 787) of men. The 5 most prevalent types overall were HPV-16 (n = 263; 10%), HPV-X (n = 164; 6%), HPV-56 (n = 164; 6%), HPV-42 (n = 140; 5%), and HPV-67 (n = 139; 5%). The 5 most prevalent types within multiple infections were HPV-16 (n = 196; 25%), HPV-56 (n = 135; 17%), HPV-42 (n = 120; 15%), HPV-67 (n = 117; 15%), and HPV-52 (n = 116; 15%) (Figure 1). All HPV types were more likely to be detected as multiple infections rather than single infections, with the exception of HPV-X, which was less likely to be detected in the presence of other typed HPV infections (37% multiple infections, 63% single infections).
Figure 1.
Human papillomavirus (HPV) genotype distribution of single and multiple infections, in order of descending prevalence.
The number of HPV types detected within an individual ranged from 0 to 11 infections. The observed frequency of zero HPV infections and infections with 4, 5, and 6 or more HPV types were higher than expected under the assumption that infections are independent (2-sided P value <.01) (Table 1). Frequencies of 1 or 2 HPV types were less than expected (P < .01). The distribution of observed high-risk genotype infections was similar to infection with any HPV genotype.
Table 1.
Comparison of the Observed and Expected Number of Human Papillomavirus Types Detected
| Any HPV |
HR-HPV |
|||||
| No. of HPV Genotypes | Observed | Expected | P Valuea | Observed | Expected | P Valuea |
| 0 | 1323 | 832.9 | <.01 | 1749 | 1494.3 | <.01 |
| 1 | 592 | 1002.1 | <.01 | 571 | 909.4 | <.01 |
| 2 | 349 | 581.1 | <.01 | 230 | 251.5 | .15 |
| 3 | 194 | 214.6 | .15 | 103 | 41.6 | <.01 |
| 4 | 111 | 57.3 | <.01 | 34 | 4.8 | <.01 |
| 5 | 68 | 11.9 | <.01 | 11 | 0.4 | <.01 |
| ≥6 | 65 | 2.2 | <.01 | 4 | 0.0 | <.01 |
| Mean (SD) | 1.15 (1.60) | 1.15 (1.05) | 0.58 (0.96) | 0.58 (0.74) | ||
Abbreviation: HR-HPV, high-risk human papillomavirus.
Two-sided P-value calculation: For i = 0–6+ types, let Oi be the observed number of men with i infections and let Ei be the expected number of men with i infections (based on 1000 simulations) assuming independence. For simulated data sets j = 1–1000, let Oij be the number of men with i infections. Then for i = 0–6+ types, the 2-sided P value was calculated as proportion of simulated data sets where |Oij-Ei|>= |Oi-Ei|.
Multiple HPV infections were more common among men who were younger, bathed less often than daily, and had chlamydial infection. Men were less likely to have multiple versus single HPV type infections if they were older, or reported consistent condom use in the previous 6 months (Table 2). The strongest independent positive correlate of multiple HPV infections was bathing less often than daily (AOR, 2.1 [95% confidence interval, 1.0--4.4] vs daily bathing). In contrast, men who reported always using a condom in the previous 6 months had a 30% lower odds of multiple HPV infections (AOR, 0.7 [0.5–0.9] vs inconsistent users).
Table 2.
Correlates Multiple Human Papillomavirus (HPV) Infections Among 1379 Men With HPV Infection at Baselinea
| Single HPV | Multiple HPV | Unadjusted OR (95% CI) | Adjusted OR (95% CI)b | |
| Median (IQR) Age, years | 21 (19–22) | 20 (19–22) | 0.9 (.9–1.0) | 0.9 (.9–1.0) |
| Recent travel to Nairobic | ||||
| No | 480 (81.4) | 670 (85.5) | ref | ref |
| Yes | 110 (18.6) | 114 (14.5) | 0.7 (.6–1.0) | 0.7 (.5–1.0) |
| Bathing frequency | ||||
| Daily | 571 (97.6) | 751 (96.3) | ref | ref |
| Less than daily | 14 (2.4) | 29 (3.7) | 1.6 (.8–3.0) | 2.1 (1.0–4.4) |
| Recent no. of sexual partnersc | ||||
| 0–1 | 330 (56.2) | 417 (53.1) | ref | ref |
| ≥2 | 257 (43.8) | 369 (47.0) | 1.1 (.9–1.4) | 1.1 (.9–1.4) |
| Recent condom usec | ||||
| Not always | 410 (76.2) | 590 (82.9) | ref | ref |
| Always | 128 (23.8) | 122 (17.1) | 0.7 (.5–0.9) | 0.7 (.5–0.9) |
| Neisseriagonorrhoeae | ||||
| No | 565 (98.1) | 754 (96.7) | ref | ref |
| Yes | 11 (1.9) | 26 (3.3) | 1.8 (.9–3.6) | 1.9 (.9–4.2) |
| Chlamydiatrachomatis | ||||
| No | 551 (95.8) | 723 (92.7) | ref | ref |
| Yes | 24 (4.2) | 57 (7.3) | 1.8 (1.1–3.0) | 1.7 (1.0–2.8) |
Missing data: Travel to Nairobi in 6 months prior to baseline (n = 5), bathing frequency (n = 14), number of sexual partners in 6 months prior to baseline (n = 6), condom use in 6 months prior to baseline (men reporting no sex in last 6 months included in “always” category; (n = 129), N. gonorrhoeae (n = 23), C. trachomatis (n = 24).
Abbreviations: CI, confidence interval; HPV, human papillomavirus; IQR, interquartile range; ref, reference category for the odds ratio.
Data are numbers (%) unless otherwise specified.
Estimates are adjusted for all potential correlates.
In the previous 6 months.
In the main analysis using semi-Bayesian logistic regression, there were no negative associations between the 4 vaccine-preventable HPV types (HPV-16, 18, 6, and 11) and the other 41 HPV types (AORs >1.0; Table 3). Although a few negative associations were observed in the semi-Bayesian models with less precision and in the maximum likelihood models, all confidence intervals were wide and included the null value 1.0 (Supplementary Table 1). There was no clear pattern of differences in HPV type associations within or across HPV clades. HPV-16 was positively associated with 17 of the 41 nonvaccine HPV types (range of AORs, 1.7–3.6). Men with HPV-16 infection were 3.6 times as likely to also be infected with HPV-40 compared with men without HPV-16 infection (Table 3; AOR, 3.6 [2.4–5.2]). HPV-18 was positively associated with 33 nonvaccine types (range of AORs, 1.8–4.3) and had the strongest association with HPV-26 (AOR, 4.3 [2.1–7.7]). For the vaccine-preventable low-risk types 6 and 11, 12 nonvaccine HPV types were positively associated with HPV-6, and 14 HPV types were positively associated with HPV-11. HPV-52 was most strongly associated with HPV-6 (AOR, 3.4 [2.1–5.3]), and HPV-45 was most strongly associated with HPV-11 (AOR, 2.8 [1.5–4.9]). Results from models including only HPV-16 and HPV-18 as the vaccine-preventable types were almost identical to the 4 vaccine type models (Supplementary Table 2). Additional positive associations between low-risk types HPV-6 and HPV-11 with HPV-16 and HPV-18 were observed. When analyses were restricted to HPV-positive men, point estimates tended to be closer to the null, and HPV type associations appeared not to differ within or across HPV clades (Table 4). HPV-40 (AOR, 2.1 [1.4–2.9]) and HPV-26 (AOR, 3.4 [1.7–5.8] remained the HPV types most strongly associated with HPV-16 and HPV-18, respectively.
Table 3.
Estimated Odds Ratios Between the 4 Vaccine-Preventable Human Papillomavirus (HPV) Types and All Other Nonvaccine HPV Types Among All Men
| HPV-16, AOR (95% CI)a | HPV-18, AOR (95% CI)a | HPV-6, AOR (95% CI)a | HPV-11, AOR (95% CI)a | |
| Clade A3 | ||||
| HPV-61 | 2.2 (.9–4.5) | 2.7 (1.1–5.5) | 2.0 (.8–4.0) | 2.3 (.9–4.7) |
| HPV-72 | 2.7 (1.3–4.8) | 2.6 (1.2–4.9) | 1.8 (.8–3.4) | 2.7 (1.2–5.2) |
| HPV-81 | 1.7 (1.0–2.8) | 2.4 (1.2–4.0) | 2.8 (1.5–4.7) | 1.8 (.8–3.3) |
| HPV-83 | 1.8 (.9–3.0) | 2.8 (1.4–5.0) | 1.9 (.9–3.5) | 2.6 (1.2–4.8) |
| HPV-84 | 1.8 (.8–3.4) | 2.2 (1.0–4.3) | 1.8 (.8–3.5) | 2.1 (.9–4.3) |
| HPV-89 | 1.7 (1.0–2.9) | 2.5 (1.3–4.2) | 1.5 (.7–2.8) | 1.9 (.9–3.5) |
| Clade A5 | ||||
| HPV-26 | 1.7 (.8–3.1) | 4.3 (2.1–7.7) | 1.7 (.8–3.3) | 2.6 (1.1–5.1) |
| HPV-51 | 2.2 (1.4–3.4) | 1.8 (1.0–3.1) | 2.1 (1.1–3.6) | 1.8 (.9–3.2) |
| HPV-69 | 2.5 (1.4–4.2) | 2.8 (1.4–5.0) | 1.9 (.9–3.6) | 1.8 (.8–3.4) |
| HPV-82 | 1.5 (.7–2.7) | 2.3 (1.0–4.3) | 1.8 (.8–3.5) | 2.7 (1.2–5.2) |
| Clade A6 | ||||
| HPV-53 | 2.6 (1.3–4.6) | 2.3 (1.0–4.2) | 1.8 (.8–3.4) | 2.3 (1.0–4.5) |
| HPV-56 | 2.1 (1.4–3.0) | 1.9 (1.1–2.9) | 2.9 (1.8–4.3) | 2.4 (1.3–3.9) |
| HPV-66 | 2.0 (1.3–3.0) | 2.3 (1.3–3.5) | 1.8 (1.0–2.9) | 1.7 (.8–2.9) |
| Clade A7 | ||||
| HPV-39 | 2.1 (1.2–3.4) | 2.2 (1.1–3.9) | 2.0 (1.0–3.5) | 2.4 (1.1–4.4) |
| HPV-45 | 1.7 (1.1–2.6) | 2.9 (1.7–4.6) | 2.0 (1.1–3.3) | 2.8 (1.5–4.9) |
| HPV-59 | 2.3 (1.5–3.5) | 2.9 (1.7–4.6) | 1.7 (.9–2.9) | 2.4 (1.2–4.2) |
| HPV-68 | 1.3 (.7–2.2) | 2.1 (1.0–3.9) | 1.6 (.7–2.9) | 2.1 (.9–4.0) |
| HPV-70 | 1.6 (.9–2.6) | 1.7 (.9–3.0) | 1.3 (.6–2.4) | 2.0 (1.0–3.8) |
| Clade A9 | ||||
| HPV-31 | 2.9 (1.9–4.2) | 2.8 (1.6–4.4) | 1.6 (.8–2.7) | 1.8 (.9–3.2) |
| HPV-33 | 1.9 (1.0–3.1) | 2.6 (1.3–4.5) | 1.4 (.7–2.6) | 2.5 (1.1–4.6) |
| HPV-35 | 2.5 (1.6–3.7) | 1.9 (1.1–3.1) | 2.4 (1.4–3.9) | 2.0 (1.0–3.4) |
| HPV-52 | 1.7 (1.1–2.5) | 2.3 (1.3–3.6) | 3.4 (2.1–5.3) | 1.9 (1.0–3.3) |
| HPV-58 | 2.6 (1.7–3.9) | 2.3 (1.3–3.6) | 1.7 (.9–2.8) | 1.9 (.9–3.2) |
| Clade A10 | ||||
| HPV-44 | 2.1 (.9–4.2) | 2.7 (1.1–5.5) | 2.0 (.8–4.0) | 2.3 (.9–4.6) |
| HPV-55 | 1.5 (.8–2.6) | 2.0 (1.0–3.5) | 2.6 (1.3–4.5) | 2.6 (1.2–4.8) |
| Clade A11 | ||||
| HPV-34 | 2.0 (.9–4.0) | 3.1 (1.3–6.3) | 2.0 (.8–4.1) | 2.3 (.9–4.6) |
| HPV-64 | 2.1 (.9–4.2) | 2.7 (1.1–5.5) | 2.0 (.8–4.0) | 2.3 (.9–4.6) |
| HPV-73 | 2.2 (1.2–3.5) | 3.0 (1.6–5.1) | 2.0 (1.0–3.6) | 2.3 (1.1–4.2) |
| Other | ||||
| HPV-30 | 1.7 (.9–2.9) | 3.1 (1.5–5.3) | 1.7 (.8–3.1) | 2.3 (1.0–4.3) |
| HPV-32 | 1.7 (.9–2.9) | 2.3 (1.1–4.0) | 2.4 (1.2–4.3) | 2.3 (1.0–4.3) |
| HPV-40 | 3.6 (2.4–5.2) | 2.2 (1.3–3.5) | 2.0 (1.1–3.3) | 2.4 (1.2–4.2) |
| HPV-42 | 2.1 (1.4–3.0) | 2.3 (1.3–3.6) | 2.0 (1.2–3.2) | 2.6 (1.4–4.4) |
| HPV-43 | 1.7 (1.1–2.6) | 2.3 (1.3–3.7) | 3.0 (1.7–4.8) | 2.0 (1.0–3.6) |
| HPV-54 | 1.4 (.7–2.6) | 2.6 (1.2–4.7) | 1.8 (.8–3.4) | 2.0 (.9–3.9) |
| HPV-57 | 2.2 (.9–4.5) | 2.7 (1.1–5.5) | 2.0 (.8–4.0) | 2.3 (.9–4.7) |
| HPV-67 | 2.4 (1.6–3.4) | 1.8 (1.1–2.9) | 2.6 (1.5–4.1) | 2.0 (1.0–3.5) |
| HPV-71 | 2.2 (0.9–4.5) | 2.7 (1.1–5.5) | 2.0 (.8–4.0) | 2.3 (.9–4.7) |
| HPV-85 | 2.0 (.9–3.8) | 2.8 (1.2–5.6) | 2.1 (.9–4.3) | 2.2 (.9–4.5) |
| HPV-86 | 1.8 (.9–3.4) | 3.2 (1.5–6.1) | 1.7 (.7–3.2) | 2.4 (1.0–4.7) |
| HPV-JC9710 | 1.8 (1.1–2.6) | 2.8 (1.7–4.3) | 1.5 (.8–2.5) | 2.1 (1.1–3.7) |
| HPV-X | 1.2 (.8–1.8) | 1.7 (1.0–2.7) | 1.7 (1.0–2.7) | 1.3 (.6–2.3) |
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval.
ORs for pairwise associations are adjusted for age, travel to Nairobi (in previous 6 months), bathing frequency, number of sexual partners (in previous 6 months), consistent condom use (in previous 6 months), and current Neisseria gonorrhoeae and Chlamydia trachomatis infection.
Table 4.
Estimated Odds Ratios Between the 4 Vaccine-Preventable Human Papillomavirus (HPV) Types and All Other Nonvaccine HPV Types Among Men With at Least 1 HPV Infection
| HPV-16, AOR (95% CI)a | HPV-18, AOR (95% CI)a | HPV-6, AOR (95% CI)a | HPV-11, AOR (95% CI)a | |
| Clade A3 | ||||
| HPV-61 | 2.1 (.9–4.4) | 2.8 (1.1–5.8) | 2.1 (.8–4.2) | 2.3 (1.0–4.8) |
| HPV-72 | 2.1 (1.0–3.6) | 2.3 (1.1–4.3) | 1.6 (.7–3.1) | 2.4 (1.1–4.7) |
| HPV-81 | 1.1 (.7–1.8) | 1.7 (.9–2.8) | 2.0 (1.1–3.3) | 1.5 (.7–2.6) |
| HPV-83 | 1.2 (.7–2.0) | 2.1 (1.1–3.7) | 1.6 (.8–2.8) | 2.2 (1.1–4.0) |
| HPV-84 | 1.5 (.7–2.7) | 2.0 (.9–3.8) | 1.6 (.7–3.0) | 2.0 (.9–3.9) |
| HPV-89 | 1.2 (.7–1.8) | 1.8 (1.0–3.0) | 1.2 (.6–2.1) | 1.6 (.8–2.8) |
| Clade A5 | ||||
| HPV-26 | 1.3 (.7–2.2) | 3.4 (1.7–5.8) | 1.5 (.7–2.8) | 2.3 (1.1–4.4) |
| HPV-51 | 1.3 (.8–2.0) | 1.3 (.7–2.1) | 1.5 (.8–2.4) | 1.4 (.7–2.4) |
| HPV-69 | 1.8 (1.0–2.8) | 2.2 (1.1–3.7) | 1.6 (.8–2.8) | 1.6 (.7–2.9) |
| HPV-82 | 1.2 (.6–2.2) | 1.9 (.9–3.6) | 1.6 (.7–3.0) | 2.4 (1.1–4.6) |
| Clade A6 | ||||
| HPV-53 | 1.9 (1.0–3.4) | 1.9 (.9–3.5) | 1.6 (.7–3.0) | 2.1 (1.0–4.0) |
| HPV-56 | 1.2 (.8–1.6) | 1.2 (.7–1.8) | 1.8 (1.1–2.6) | 1.7 (.9–2.7) |
| HPV-66 | 1.1 (.8–1.6) | 1.5 (.9–2.2) | 1.2 (.7–1.8) | 1.2 (.6–2.1) |
| Clade A7 | ||||
| HPV-39 | 1.4 (.8–2.2) | 1.7 (.9–2.9) | 1.5 (.8–2.6) | 2.0 (1.0–3.6) |
| HPV-45 | 1.1 (.7–1.6) | 1.9 (1.1–3.0) | 1.4 (.8–2.2) | 2.1 (1.1–3.5) |
| HPV-59 | 1.4 (.9–2.1) | 2.0 (1.2–3.1) | 1.2 (.7–2.0) | 1.8 (.9–3.1) |
| HPV-68 | 0.9 (.5–1.6) | 1.7 (.8–2.9) | 1.3 (.6–2.3) | 1.8 (.8–3.2) |
| HPV-70 | 1.0 (.6–1.6) | 1.3 (.7–2.2) | 1.0 (.5–1.8) | 1.7 (.8–3.0) |
| Clade A9 | ||||
| HPV-31 | 1.7 (1.1–2.4) | 1.9 (1.1–3.0) | 1.1 (.6–1.9) | 1.4 (.7–2.4) |
| HPV-33 | 1.3 (.7–2.0) | 1.9 (1.0–3.2) | 1.2 (.6–2.1) | 2.0 (1.0–3.6) |
| HPV-35 | 1.4 (.9–2.1) | 1.3 (.8–2.1) | 1.6 (1.0–2.5) | 1.5 (.8–2.5) |
| HPV-52 | 1.0 (.6–1.4) | 1.5 (.9–2.3) | 2.1 (1.3–3.2) | 1.4 (.8–2.5) |
| HPV-58 | 1.5 (1.0–2.2) | 1.6 (.9–2.4) | 1.2 (.7–1.9) | 1.5 (.7–2.5) |
| Clade A10 | ||||
| HPV-44 | 2.0 (.8–4.1) | 2.7 (1.1–5.5) | 2.0 (.8–4.0) | 2.3 (.9–4.8) |
| HPV-55 | 1.1 (.6–1.8) | 1.5 (.8–2.7) | 2.0 (1.0–3.4) | 2.1 (1.0–3.9) |
| Clade A11 | ||||
| HPV-34 | 1.9 (.8–3.9) | 3.1 (1.3–6.3) | 1.9 (.8–3.8) | 2.3 (.9–4.7) |
| HPV-64 | 2.0 (.8–4.1) | 2.7 (1.1–5.5) | 2.0 (.8–4.0) | 2.3 (.9–4.8) |
| HPV-73 | 1.4 (.8–2.3) | 2.2 (1.2–3.6) | 1.6 (.8–2.6) | 1.9 (.9–3.4) |
| Other | ||||
| HPV-30 | 1.2 (.7–2.0) | 2.3 (1.2–3.9) | 1.4 (.7–2.4) | 1.9 (.9–3.5) |
| HPV-32 | 1.2 (.7–2.0) | 1.7 (.9–3.0) | 1.9 (.9–3.2) | 1.9 (.9–3.6) |
| HPV-40 | 2.1 (1.4–2.9) | 1.6 (.9–2.5) | 1.4 (.8–2.3) | 1.8 (1.0–3.1) |
| HPV-42 | 1.2 (.8–1.7) | 1.5 (.9–2.2) | 1.3 (.8–2.0) | 1.9 (1.0–3.0) |
| HPV-43 | 1.0 (.7–1.5) | 1.5 (.9–2.4) | 2.0 (1.2–3.0) | 1.5 (.8–2.6) |
| HPV-54 | 1.1 (.6–2.0) | 2.1 (1.0–3.9) | 1.5 (.7–2.9) | 1.8 (.8–3.4) |
| HPV-57 | 2.1 (.9–4.4) | 2.8 (1.1–5.8) | 2.1 (.8–4.2) | 2.3 (1.0–4.8) |
| HPV-67 | 1.3 (.9–1.9) | 1.2 (.7–1.9) | 1.7 (1.0–2.5) | 1.5 (.8–2.5) |
| HPV-71 | 2.1 (.9–4.4) | 2.8 (1.1–5.8) | 2.1 (.8–4.2) | 2.3 (1.0–4.8) |
| HPV-85 | 1.8 (.8–3.4) | 2.6 (1.2–5.2) | 2.0 (.9–4.0) | 2.1 (.9–4.2) |
| HPV-86 | 1.5 (.7–2.8) | 2.7 (1.3–5.2) | 1.5 (.7–2.9) | 2.2 (1.0–4.3) |
| HPV-JC9710 | 1.0 (.7–1.5) | 1.8 (1.1–2.7) | 1.0 (.6–1.6) | 1.6 (.8–2.6) |
| HPV-X | 0.7 (.4–.9) | 1.1 (.6–1.7) | 1.1 (.6–1.6) | 0.9 (.5–1.6) |
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval.
ORs for pairwise associations are adjusted for age, travel to Nairobi (in previous 6 months), bathing frequency, number of sexual partner (in previous 6 months), consistent condom use (in previous 6 months), and current Neisseria gonorrhoeae and Chlamydia trachomatis infection.
DISCUSSION
Half of all uncircumcised, HIV-negative men in this study from Kenya were infected with at least 1 HPV type at baseline. Nearly 30% of surveyed men had multiple penile HPV type infections, with HPV-16 and 18 accounting for >25% of all infections. If prevalent carcinogenic HPV-16 and 18 infections were prevented by mass vaccination, there is a theoretical possibility that this could result in other HR-HPV types filling the ecological niche of HPV-16 and/or 18 [44]. Because there are reported differences in genotype distributions across sexes and geographical regions [5, 32, 45], the potential for HPV type replacement could differ across study populations. The present study represents, to our knowledge, the first detailed investigation of multiple HPV infections and type associations within a cohort of young men from Africa. Using a cross-sectional study design, we did not find any negative associations between vaccine-preventable types HPV 16, 18, 6, and 11, and 41 nonvaccine-preventable HPV types.
All HPV genotypes were more likely to be detected as multiple rather than single-type infections. Further, all associations between HPV types were positive, suggesting that men who become infected are more likely to have >1 HPV infection, possibly due to host behavioral or immunological factors [7, 26, 31]. When analyses were restricted to HPV-infected men, associations between specific HPV types generally shifted closer toward 1.0, which suggests that specific HPV types are not associated with one another. We also observed a higher number of men than expected with ≥4 HPV type infections, suggesting that individual HPV infections were not independent from each other. This is likely due to the fact that men with ≥4 HPV infections reported less condom use in the last 6 months and more lifetime partners than did men with fewer HPV infections. These observations in men are consistent with results from studies among women [42, 46, 47]. Numerous prospective studies have found that women with HPV infection at baseline are more likely to acquire additional HPV types [7, 23, 26, 31, 43] and that acquisition of multiple HPV types occurs more often than expected [7, 24, 26]. Therefore, it is likely that the observed pattern of number and types of HPV infections reflect differences in HPV persistence or acquisition in men with multiple infections.
Positive associations between HPV types were observed yet appeared not to be type-specific. Our results among Kenyan men are consistent with a prospective study of American female college students that found no 2 HPV types are more likely to be acquired together than any other HPV types [26]. Most studies that have examined HPV type associations in women have reported positive or no associations between HPV types, regardless of the pairwise combinations, analytical methods, HPV genotyping methods, or study population [26, 46]. However, among female colposcopy clinic attendees in Italy, where the genotype distribution likely differs compared to the general population, coinfection with species A7 and A10 and with HPV-31 and 52 occurred less often than expected [48]. A large cross-sectional study of Danish women referred for testing based on clinical suspicion of infection found that HPV-51 was negatively associated with HPV-16 [42].
In this large sample of men with an HPV prevalence of 50%, we examined type-type associations, even for rarer HPV types, using semi-Bayesian methods that incorporated a shrinkage factor. We chose shrinkage methods because they allowed us to include all 4 vaccine types and numerous potential confounders in each model. In addition, these methods serve as an adjustment for multiple comparisons [49] and reduce the number of spurious associations as compared with maximum likelihood methods [50]. There is, however, a trade-off between precision and bias in methods that incorporate a shrinkage factor [40]. We therefore conducted sensitivity analyses to understand the effect of our statistical model choices and to compare our results with other studies that used maximum likelihood estimation [42, 46, 47]. Different methods to analyze our data resulted in wider confidence intervals and, in some cases, nonsignificant ORs <1.0. However, all methods resulted in the same conclusions regarding the lack of evidence of negative associations between prevalent HPV type infections in men. As in previous studies [42, 46–48], we made multiple comparisons across outcomes, which can increase the possibility of falsely concluding that certain HPV types are associated. By including all 4 vaccine types in each of our models, the number of comparisons was reduced compared with other pairwise analyses [24, 42, 43, 46, 47].
In the present study, we could only determine if the number and type of HPV infections were independent. The causes of nonindependence may be differences in host susceptibility, the distribution of HPV genotypes among female sexual partners, primer competition during PCR detection, or molecular interactions between HPV types that inhibit infection with other HPV types (type competition). Although we were able to control for several measured confounders, it is likely that there is residual confounding by other unmeasured behavioral risk factors, immunological differences between men [7, 26], and HPV-type exposure from female sexual partners. In populations where negative associations between HPV types are observed, molecular studies and prospective studies of couples are needed to address whether these associations are likely due to true biological type competition or competition for consensus primers.
Our study has additional limitations to consider when interpreting the findings. We detected 103 single HPV-X infections. If HPV-X represents multiple untyped infections, our reported prevalence of multiple infections and the number of HPV types detected are an underestimation. The likely impact of untyped HPV infections on the conclusions regarding HPV-type competition is minimal because inferences are mainly drawn from type-specific analyses. In addition, the prevalence and the type distribution of HPV has been shown to differ by circumcision status; thus, the results from this cohort of uncircumcised men may not be generalizable to circumcised populations [6, 11]. No participants received HPV prophylactic vaccination, so we could only observe differences between individuals who were naturally HPV uninfected and infected with HPV-16, 18, 6, and 11. Given the cross-sectional design, it is also not known whether infection with specific HPV types affects HPV acquisition or HPV persistence of additional HPV types.
With the recent approval of HPV vaccination in men [33], these data fill an important gap in our knowledge of the distribution and associations between HPV types in multiple infections among men. Future prospective studies in populations pre- and postvaccination are needed to observe the natural history of multiple infections and assess the long-term potential for HPV type replacement.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://www.oxfordjournals.org/our_journals/jid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgements.
We would like to thank the UNIM staff, the young men of Kisumu who volunteered to participate in this study, and the late Dr Jeckoniah O. Ndinya-Achola for his dedication to this study; Dr Stephen Cole for his help developing the analytical methods; and Dr Myron Cohen and Dr Steven Meshnick for their helpful contributions to this manuscript. The authors would also like to gratefully acknowledge the assistance provided by Dr Corette Parker, Norma Pugh, and Bonnie Knoke at RTI International in preparing the data for these analyses.
Financial support.
This work was supported by the National Cancer Institute, National Institutes of Health (NIH; grant R01 CA114773-04). The randomized controlled trial was supported by the Division of AIDS, National Institute of Allergy and Infectious Diseases, NIH (grant AI50440), and by the Canadian Institutes of Health Research (CIHR; grant HCT 44180). A. F. R. was supported by an NIH predoctoral training grant in infectious disease epidemiology (principal investigator Meshnick, grant T32 AI070114). S. M. was the recipient of a CIHR Investigator award.
Potential conflicts of interest.
C. P. receives partial salary support from the UNC-GSK Center of Excellence in Pharmacoepidemiology and has received honoraria for talks at GlaxoSmithKline. J. S. S. has received consultancy or research grants from GlaxoSmithKline and Merck Corporation over the past 5 years. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.Bosch FX, Lorincz A, Munoz N, Meijer C, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–65. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith JS, Backes DM, Hudgens MG, et al. Prevalence and risk factors of human papillomavirus infection by penile site in uncircumcised Kenyan men. Int J Cancer. 2010;126:572–7. doi: 10.1002/ijc.24770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lajous M, Mueller N, Cruz-Valdez A, et al. Determinants of prevalence, acquisition, and persistence of human papillomavirus in healthy Mexican military men. Cancer Epidemiol Biomarkers Prev. 2005;14:1710–6. doi: 10.1158/1055-9965.EPI-04-0926. [DOI] [PubMed] [Google Scholar]
- 5.Giuliano AR, Lazcano-Ponce E, Villa LL, et al. The human papillomavirus infection in men study: human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol Biomarkers Prev. 2008;17:2036–43. doi: 10.1158/1055-9965.EPI-08-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernandez BY, Wilkens LR, Zhu X, et al. Circumcision and human papillomavirus infection in men: a site-specific comparison. J Infect Dis. 2008;197:787–94. doi: 10.1086/528379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kjaer SK, Munk C, Winther JF, Jorgensen HO, Meijer CJ, van den Brule AJ. Acquisition and persistence of human papillomavirus infection in younger men: a prospective follow-up study among Danish soldiers. Cancer Epidemiol Biomarkers Prev. 2005;14:1528–33. doi: 10.1158/1055-9965.EPI-04-0754. [DOI] [PubMed] [Google Scholar]
- 8.Ng'ayo MO, Bukusi E, Rowhani-Rahbar A, et al. Epidemiology of human papillomavirus infection among fishermen along Lake Victoria shore in the Kisumu district, Kenya. Sex Transm Infect. 2008;84:62–6. doi: 10.1136/sti.2007.027508. [DOI] [PubMed] [Google Scholar]
- 9.Nielson CM, Harris RB, Flores R, et al. Multiple-type human papillomavirus infection in male anogenital sites: prevalence and associated factors. Cancer Epidemiol Biomarkers Prev. 2009;18:1077–83. doi: 10.1158/1055-9965.EPI-08-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin HR, Franceschi S, Vaccarella S, et al. Prevalence and determinants of genital infection with papillomavirus, in female and male university students in Busan, South Korea. J Infect Dis. 2004;190:468–76. doi: 10.1086/421279. [DOI] [PubMed] [Google Scholar]
- 11.Gray RH, Serwadda D, Kong X, et al. Male circumcision decreases acquisition and increases clearance of high-risk human papillomavirus in HIV-negative men: a randomized trial in Rakai, Uganda. J Infect Dis. 2010;201:1455–62. doi: 10.1086/652184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sasagawa T, Basha W, Yamazaki H, Inoue M. High-risk and multiple human papillomavirus infections associated with cervical abnormalities in Japanese women. Cancer Epidemiol Biomarkers Prev. 2001;10:45–52. [PubMed] [Google Scholar]
- 13.Fife KH, Cramer HM, Schroeder JM, Brown DR. Detection of multiple human papillomavirus types in the lower genital tract correlates with cervical dysplasia. J Med Virol. 2001;64:550–9. doi: 10.1002/jmv.1085. [DOI] [PubMed] [Google Scholar]
- 14.Trottier H, Mahmud S, Costa MC, et al. Human papillomavirus infections with multiple types and risk of cervical neoplasia. Cancer Epidemiol Biomarkers Prev. 2006;15:1274–80. doi: 10.1158/1055-9965.EPI-06-0129. [DOI] [PubMed] [Google Scholar]
- 15.Harper DM, Franco EL, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364:1757–65. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 16.Paavonen J, Naud P, Salmeron J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–14. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 17.Romanowski B, de Borba PC, Naud PS, et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet. 2009;374:1975–85. doi: 10.1016/S0140-6736(09)61567-1. [DOI] [PubMed] [Google Scholar]
- 18.Munoz N, Manalastas R, Jr, Pitisuttithum P, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24–45 years: a randomised, double-blind trial. Lancet. 2009;373:1949–57. doi: 10.1016/S0140-6736(09)60691-7. [DOI] [PubMed] [Google Scholar]
- 19.Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. New Engl J Med. 2011;364:401–11. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villa LL, Costa RL, Petta CA, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6:271–8. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 21.Smith JS, Lindsay L, Hoots B, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007;121:621–32. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 22.Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. New Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 23.Rousseau MC, Pereira JS, Prado JC, Villa LL, Rohan TE, Franco EL. Cervical coinfection with human papillomavirus (HPV) types as a predictor of acquisition and persistence of HPV infection. J Infect Dis. 2001;184:1508–17. doi: 10.1086/324579. [DOI] [PubMed] [Google Scholar]
- 24.Mendez F, Munoz N, Posso H, et al. Cervical coinfection with human papillomavirus (HPV) types and possible implications for the prevention of cervical cancer by HPV vaccines. J Infect Dis. 2005;192:1158–65. doi: 10.1086/444391. [DOI] [PubMed] [Google Scholar]
- 25.Rousseau MC, Villa LL, Costa MC, Abrahamowicz M, Rohan TE, Franco E. Occurrence of cervical infection with multiple human papillomavirus types is associated with age and cytologic abnormalities. Sex Transmi Dis. 2003;30:581–7. doi: 10.1097/00007435-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Thomas KK, Hughes JP, Kuypers JM, et al. Concurrent and sequential acquisition of different genital human papillomavirus types. J Infect Dis. 2000;182:1097–102. doi: 10.1086/315805. [DOI] [PubMed] [Google Scholar]
- 27.Mejlhede N, Bonde J, Fomsgaard A. High frequency of multiple HPV types in cervical specimens from Danish women. APMIS. 2009;117:108–14. doi: 10.1111/j.1600-0463.2008.00019.x. [DOI] [PubMed] [Google Scholar]
- 28.Rousseau MC, Abrahamowicz M, Villa LL, Costa MC, Rohan TE, Franco EL. Predictors of cervical coinfection with multiple human papillomavirus types. Cancer Epidemiol Biomarkers Prev. 2003;12:1029–37. [PubMed] [Google Scholar]
- 29.Oliveira LH, Rosa ML, Cavalcanti SM. Patterns of genotype distribution in multiple human papillomavirus infections. Clin Microbiol Infect. 2008;14:60–5. doi: 10.1111/j.1469-0691.2007.01887.x. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen A, Kjaer SK, Munk C, Iftner T. Type-specific HPV infection and multiple HPV types: prevalence and risk factor profile in nearly 12,000 younger and older Danish women. Sex Trans Dis. 2008;35:276–82. doi: 10.1097/OLQ.0b013e31815ac5c7. [DOI] [PubMed] [Google Scholar]
- 31.Plummer M, Schiffman M, Castle PE, Maucort-Boulch D, Wheeler CM. A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J Infect Dis. 2007;195:1582–9. doi: 10.1086/516784. [DOI] [PubMed] [Google Scholar]
- 32.Bruni L, Diaz M, Castellsague X, Ferrer E, Bosch FX, de Sanjose S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202:1789–99. doi: 10.1086/657321. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59:630–2. [PubMed] [Google Scholar]
- 34.Smith JS, Moses S, Hudgens MG, et al. Human papillomavirus detection by penile site in young men from Kenya. Sex Transm Dis. 2007;34:928–34. doi: 10.1097/OLQ.0b013e318065b8ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith JS, Moses S, Hudgens MG, et al. Increased risk of HIV acquisition among Kenyan men with human papillomavirus infection. J Infect Dis. 2010;201:1677–85. doi: 10.1086/652408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369:643–56. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 37.van den Brule AJ, Pol R, Fransen-Daalmeijer N, Schouls LM, Meijer CJ, Snijders PJ. GP5+/6+ PCR followed by reverse line blot analysis enables rapid and high-throughput identification of human papillomavirus genotypes. J Clin Microbiol. 2002;40:779–87. doi: 10.1128/JCM.40.3.779-787.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snijders PJ, van den Brule A, Jacobs MV, Pol RP, Meijer CJ. HPV DNA detection and typing in cervical scrapes by general primer GP5+/6+ PCR. In: Davy CE, Doorbar J, editors. Methods in molecular medicine: human papillomaviruses—methods and protocols. Totowa, NJ: Humana Press; 2005. pp. 101–14. [DOI] [PubMed] [Google Scholar]
- 39.Witte JS, Greenland S, Haile RW, Bird CL. Hierarchical regression analysis applied to a study of multiple dietary exposures and breast cancer. Epidemiology. 1994;5:612–21. doi: 10.1097/00001648-199411000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Greenland S. Principles of multilevel modelling. Int J Epidemiol. 2000;29:158–67. doi: 10.1093/ije/29.1.158. [DOI] [PubMed] [Google Scholar]
- 41.Greenland S, Poole C. Empirical-Bayes and semi-Bayes approaches to occupational and environmental hazard surveillance. Arch Environ Health. 1994;49:9–16. doi: 10.1080/00039896.1994.9934409. [DOI] [PubMed] [Google Scholar]
- 42.Mejlhede N, Pedersen BV, Frisch M, Fomsgaard A. Multiple human papilloma virus types in cervical infections: competition or synergy? APMIS. 2010;118:346–52. doi: 10.1111/j.1600-0463.2010.2602.x. [DOI] [PubMed] [Google Scholar]
- 43.Liaw KL, Hildesheim A, Burk RD, et al. A prospective study of human papillomavirus (HPV) type 16 DNA detection by polymerase chain reaction and its association with acquisition and persistence of other HPV types. J Infect Dis. 2001;183:8–15. doi: 10.1086/317638. [DOI] [PubMed] [Google Scholar]
- 44.Dillner J, Arbyn M, Dillner L. Translational mini-review series on vaccines: monitoring of human papillomavirus vaccination. Clin Exp Immunol. 2007;148:199–207. doi: 10.1111/j.1365-2249.2007.03384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR. Prevalence of HPV infection among men: a systematic review of the literature. J Infect Dis. 2006;194:1044–57. doi: 10.1086/507432. [DOI] [PubMed] [Google Scholar]
- 46.Chaturvedi AK, Myers L, Hammons AF, et al. Prevalence and clustering patterns of human papillomavirus genotypes in multiple infections. Cancer Epidemiol Biomarkers Prev. 2005;14:2439–45. doi: 10.1158/1055-9965.EPI-05-0465. [DOI] [PubMed] [Google Scholar]
- 47.Vaccarella S, Franceschi S, Snijders PJ, Herrero R, Meijer CJ, Plummer M. Concurrent infection with multiple human papillomavirus types: pooled analysis of the IARC HPV Prevalence Surveys. Cancer Epidemiol Biomarkers Prev. 2010;19:503–10. doi: 10.1158/1055-9965.EPI-09-0983. [DOI] [PubMed] [Google Scholar]
- 48.Spinillo A, Dal Bello B, Alberizzi P, et al. Clustering patterns of human papillomavirus genotypes in multiple infections. Virus Res. 2009;142:154–9. doi: 10.1016/j.virusres.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 49.Greenland S, Robins JM. Empirical-Bayes adjustments for multiple comparisons are sometimes useful. Epidemiology. 1991;2:244–51. doi: 10.1097/00001648-199107000-00002. [DOI] [PubMed] [Google Scholar]
- 50.Greenland S. A semi-Bayes approach to the analysis of correlated multiple associations, with an application to an occupational cancer-mortality study. Stat Med. 1992;11:219–30. doi: 10.1002/sim.4780110208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.