Abstract
The effect of Ocean Acidification (OA) on marine biota is quasi-predictable at best. While perturbation studies, in the form of incubations under elevated pCO2, reveal sensitivities and responses of individual species, one missing link in the OA story results from a chronic lack of pH data specific to a given species' natural habitat. Here, we present a compilation of continuous, high-resolution time series of upper ocean pH, collected using autonomous sensors, over a variety of ecosystems ranging from polar to tropical, open-ocean to coastal, kelp forest to coral reef. These observations reveal a continuum of month-long pH variability with standard deviations from 0.004 to 0.277 and ranges spanning 0.024 to 1.430 pH units. The nature of the observed variability was also highly site-dependent, with characteristic diel, semi-diurnal, and stochastic patterns of varying amplitudes. These biome-specific pH signatures disclose current levels of exposure to both high and low dissolved CO2, often demonstrating that resident organisms are already experiencing pH regimes that are not predicted until 2100. Our data provide a first step toward crystallizing the biophysical link between environmental history of pH exposure and physiological resilience of marine organisms to fluctuations in seawater CO2. Knowledge of this spatial and temporal variation in seawater chemistry allows us to improve the design of OA experiments: we can test organisms with a priori expectations of their tolerance guardrails, based on their natural range of exposure. Such hypothesis-testing will provide a deeper understanding of the effects of OA. Both intuitively simple to understand and powerfully informative, these and similar comparative time series can help guide management efforts to identify areas of marine habitat that can serve as refugia to acidification as well as areas that are particularly vulnerable to future ocean change.
Introduction
Since the publication of two reports in 2005–2006 [1], [2], the drive to forecast the effects of anthropogenic ocean acidification (OA) on marine ecosystems and their resident calcifying marine organisms has resulted in a growing body of research. Numerous laboratory studies testing the effects of altered seawater chemistry (low pH, altered pCO2, and undersaturation states - Ω - for calcium carbonate polymorphs) on biogenic calcification, growth, metabolism, and development have demonstrated a range of responses in marine organisms (for reviews see [3]–[8]). However, the emerging picture of biological consequences of OA – from data gathered largely from laboratory experiments – is not currently matched by equally available environmental data that describe present-day pH exposures or the natural variation in the carbonate system experienced by most marine organisms. Although researchers have documented variability in seawater carbonate chemistry on several occasions in different marine ecosystems (e.g., [9]–[15]), this variation has been under-appreciated in these early stages of OA research.
Recently, a deeper consideration of ecosystem-specific variation in seawater chemistry has emerged (e.g., [16]–[18]), one that is pertinent to the study of biological consequences of OA. Specifically, assessments of environmental heterogeneity present a nuanced complement to current laboratory experiments. The dynamics of specific natural carbonate chemistry on local scales provide critical context because outcomes of experiments on single species are used in meta-analyses to project the overall biological consequences of OA [7], [19], to forecast ecosystem-level outcomes [20], and ultimately to contribute to policy decisions [21] and the management of fisheries [22], [23]. As noted earlier [24], natural variability in pH is seldom considered when effects of ocean acidification are considered. Natural variability may occur at rates much higher than the rate at which carbon dioxide is decreasing ocean pH, about −0.0017 pH/year [25], [26]. This ambient fluctuation in pH may have a large impact on the development of resilience in marine populations, or it may combine with the steady effects of acidification to produce extreme events with large impacts [24]. In either case, understanding the environmental variability in ocean pH is essential.
Although data on the natural variation in the seawater CO2 system are emerging, nearly all high-resolution (e.g. hourly) time series are based on pCO2 sensors, with comparatively few pH time series found in the literature. From a research perspective, the absence of information regarding natural pH dynamics is a critical data gap for the biological and ecological arm of the multidisciplinary investigation of OA. Our ability to understand processes ranging from physiological tolerances to local adaptation is compromised. Specifically, laboratory experiments to test tolerances are often not designed to encompass the actual habitat exposure of the organisms under study, a critical design criterion in organismal physiology that also applies to global change biology [27]–[29]. It is noted that neither pH nor pCO2 alone provide the information sufficient to fully constrain the CO2 system, and while it is preferred to measure both, the preference for measuring one over the other is evaluated on a case-by-case basis and is often dictated by the equipment available.
In this light, data that reveal present-day pH dynamics in marine environments and therefore ground pH levels in CO2 perturbation experiments in an environmental context are valuable to the OA research community in two major ways. First, estimates of organismal resilience are greatly facilitated. Empiricists can contextualize lab experiments with actual environmental data, thereby improving them. Notably, the majority of manipulative laboratory experiments in OA research (including our own) have been parameterized using pCO2 levels as per the IPCC emission scenario predictions [30]. One consequence of this practice is that organisms are potentially tested outside of the current exposure across their biogeographic range, and tolerances are not bracketed appropriately. This situation may not be a lethal issue (i.e. negating all past observations in experiments where environmental context was not known); however, the lack of information about the ‘pH seascape’ may be translated through these organismal experiments in a manner that clouds the perspective of vulnerability of marine ecosystems. For example, recent data on the heterogeneity of pH in coastal waters of the Northeastern Pacific [31], [32] that are characterized by episodic upwelling has caused biologists to re-examine the physiological tolerances of organisms that live there. Specifically, resident calcifying marine invertebrates and algae are acclimatized to existing spatial and temporal heterogeneity [17], [18], and further, populations are likely adapted to local to regional differences in upwelling patterns [33].
Secondly, in addition to improving laboratory experiments, data regarding the nature of the pH seascape also facilitate hypothesis-generating science. Specifically, heterogeneity in the environment with regard to pH and pCO2 exposure may result in populations that are acclimatized to variable pH or extremes in pH. Although this process has been highlighted in thermal biology of marine invertebrates [34], such insight is not available with regard to gradients of seawater chemistry that occur on biogeographic scales. With that said, recent field studies have demonstrated that natural variation in seawater chemistry does influence organismal abundance and distribution [16], [35], [36]. With our newfound access to pH time series data, we can begin to explore the biophysical link between environmental seawater chemistry and resilience to baseline shifts in pH regimes, to identify at-risk populations as well as tolerant ones. Additionally, the use of sensors in the field can identify hidden patterns in the CO2 system, revealing areas that are refugia to acidification or carbonate undersaturation; such knowledge could enable protection, management, and remediation of critical marine habitats and populations in the future.
The recent development of sensors for in situ measurements of seawater pH [37], [38] has resulted in the ability to record pH more readily in the field in a manner that can support biological and ecological research. Since 2009, the Martz lab (SIO) has constructed 52 “SeaFET” pH sensors for 13 different collaborators (see http://martzlab.ucsd.edu) working in a broad range of settings. Using subsamples of data from many of these sensors, here we examine signatures of pH heterogeneity, presenting time series snapshots of sea-surface pH (upper 10 m) at 15 locations, spanning various overlapping habitat classifications including polar, temperate, tropical, open ocean, coastal, upwelling, estuarine, kelp forest, coral reef, pelagic, benthic, and extreme. Naturally, at many sites, multiple habitat classifications will apply. Characteristic patterns observed in the 30-day snapshots provide biome-specific pH signatures. This comparative dataset highlights the heterogeneity of present-day pH among marine ecosystems and underscores that contemporary marine organisms are currently exposed to different pH regimes in seawater that are not predicted until 2100.
Results
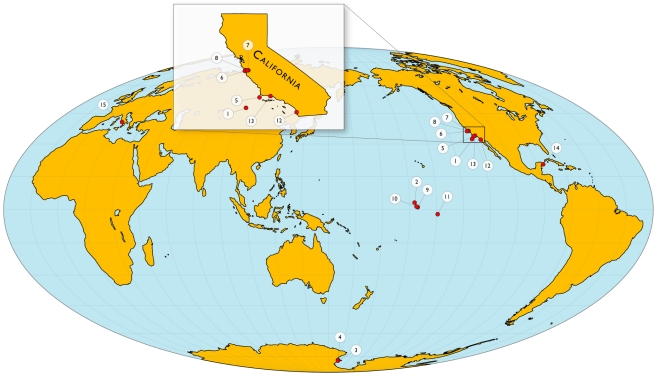
Overall, the patterns of pH recorded at each of the 15 deployment sites (shown in Figure 1, Table 1) were strikingly different. Figure 2 presents the temporal pattern of pH variation at each of these sites, and, for the sake of comparison, these are presented as 30-day time series “snapshots.” Note that all deployments generated >30 days of data except for sensors 3, 4, and 13, where the sensors were deliberately removed due to time constraints at the study sites. Though the patterns observed among the various marine ecosystems are driven by a variety of oceanographic forcing such as temperature, mixing, and biological activity, we do not provide a separate analysis of controlling factors on pH at each location. Each time series was accompanied by a different set of ancillary data, some rich with several co-located sensors, others devoid of co-located sensors. Given these differences in data collection across sites, here we focus on the comparative pH sensor data as a means to highlight observed pH variability and ecosystem-level differences between sites. For purposes of comparison, the metrics of variability presented here are pH minima, maxima, range, standard deviation, and rate of change (see Table 2). The rate presented in Table 2 and Figure 3 represents a mean instantaneous rate of change in pH hr−1, where a rate was calculated for each discrete time step as the absolute value of pH difference divided by the length of time between two adjacent data points.
Figure 1. Map of pH sensor (SeaFET) deployment locations.
See Table 1 for details regarding deployment locations.
Table 1. Summary of pH sensor deployment data shown in Figure 1.
| Category | Sensor-Site | Latitude | Longitude | DD1 | WD2 | to 3 | PI |
| Open Ocean(p) | 1-CCE1 | 33.5 N | 122.5 W | 2 | 4000 | 6-Mar-2011 | Send |
| Reef(b) | 2-Kingman offshore | 6.43961 N | 162.3949 W | 10 | 10 | 27-Apr-2010 | Smith |
| Polar(b) | 3-Cindercones | 77.8000 S | 166.6712 E | 15 | 16 | 12-Oct-2010 | Hofmann |
| Polar(b) | 4-Cape Evans | 77.6343 S | 166.4484 E | 15 | 16 | 2-Nov-2010 | Hofmann |
| Upwelling(p) | 5-Point Conception (CCE2) | 34.32 N | 120.80 W | 2 | 770 | 27-Mar-2011 | Send |
| Upwelling(p) | 6-Point Ano Nuevo (M1) | 36.8 N | 122 W | 2 | 800 | 15-Apr-2010 | Johnson |
| Tidal Estuary(p) | 7-Elkhorn Slough, CA (L1) | 36.8125 N | 121.7748 W | 1 | 8 | 15-Sep-2008 | Johnson |
| Near Shore(p) | 8-Monterey Bay L20 | 36.8135 N | 121.8290 W | 1 | 19 | 1-Aug-2010 | Johnson |
| Reef(b) | 9-Palmyra, fore reef | 5.86614 N | 162.1172 W | 10 | 10 | 20-Apr-2010 | Smith |
| Reef(b) | 10-Palmyra, reef terrace | 5.884 N | 162.1218 W | 5 | 5 | 19-Apr-2010 | Smith |
| Reef(b) | 11-Moorea, fringing reef | 17.4803 S | 149.7989 W | 10 | 11 | 10-Feb-2011 | Hofmann |
| Kelp(p) | 12-La Jolla | 32.80853 N | 117.2890 W | 7 | 20 | 28-Jul-2010 | Levin |
| Kelp(b) | 13-SBC Mohawk Reef | 34.3943 N | 119.73 W | 8 | 9 | 24-Jul-2010 | Hofmann |
| Extreme(b) | 14-Puerto Morelos | 20 N | 86.5 W | 5 | 5 | 27-Aug-2010 | Paytan |
| Extreme(b) | 15-Ischia (South zone) | 40.7303 N | 13.9636 E | 1 | 3 | 10-May-2010 | Micheli |
p = pelagic.
b = benthic.
1-Deployment Depth in meters.
2-Water Depth in meters.
3-Starting time of the 30-day window shown in Figure 1.
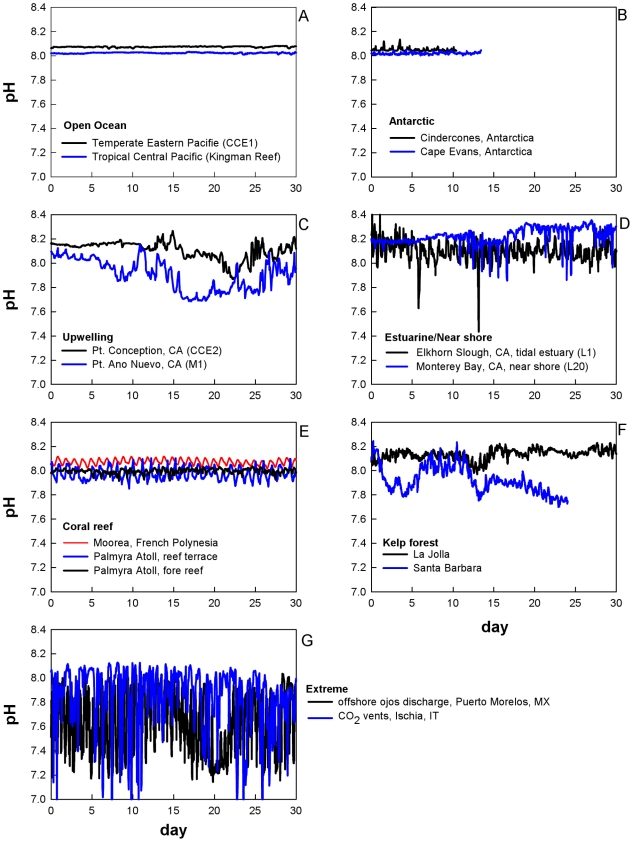
Figure 2. pH dynamics at 15 locations worldwide in 0–15 m water depth.
All panels are plotted on the same vertical range of pH (total hydrogen ion scale). The ordinate axis was arbitrarily selected to encompass a 30-day period during each sensor deployment representative of each site during the deployment season. See Table 1 for details regarding sensor deployment.
Table 2. Summary data for pH temporal profiles collected at 15 sensor locations.
| Site | Mean | Max | Min | SD | Range | Rate1 |
| CCE-1 | 8.074 | 8.082 | 8.059 | 0.004 | 0.024 | 0.001 |
| Kingman Reef | 8.023 | 8.034 | 8.009 | 0.004 | 0.025 | 0.001 |
| Cindercones | 8.050 | 8.134 | 8.039 | 0.013 | 0.096 | 0.006 |
| Cape Evans | 8.020 | 8.050 | 8.002 | 0.008 | 0.047 | 0.006 |
| Pt. Conception (CCE2) | 8.108 | 8.266 | 7.869 | 0.074 | 0.397 | 0.009 |
| Pt. Ano Nuevo | 7.905 | 8.152 | 7.685 | 0.126 | 0.467 | 0.013 |
| Elkhorn Slough (L1) | 8.101 | 8.427 | 7.435 | 0.082 | 0.992 | 0.043 |
| Monterey Bay (M1) | 8.222 | 8.356 | 7.857 | 0.070 | 0.499 | 0.025 |
| Palmyra, fore reef | 7.997 | 8.035 | 7.915 | 0.018 | 0.121 | 0.008 |
| Palmyra, reef terrace | 7.974 | 8.104 | 7.851 | 0.048 | 0.253 | 0.014 |
| Moorea, fringing reef | 8.072 | 8.118 | 8.017 | 0.022 | 0.101 | 0.006 |
| La Jolla | 8.134 | 8.229 | 7.970 | 0.043 | 0.259 | 0.021 |
| SBC Mohawk Reef | 7.922 | 8.244 | 7.700 | 0.111 | 0.544 | 0.028 |
| Puerto Morelos | 7.651 | 8.048 | 7.143 | 0.241 | 0.905 | 0.317 |
| Ischia (South zone) | 7.845 | 8.129 | 6.699 | 0.274 | 1.430 | 0.110 |
1-calculated as mean(abs(pHt2−pHt1)/(t2−t1)).
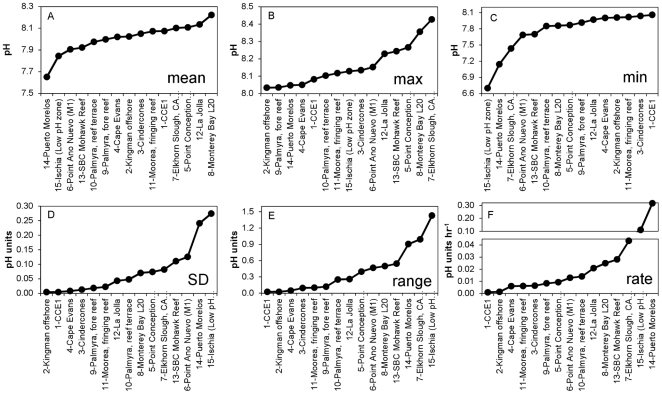
Figure 3. Metrics of short-term pH variability at 15 locations worldwide, ranked by ascending values.
Mean = geometric mean; Max = maximum value recorded; Min = minimum value recorded; SD = standard deviation; Range = Max - Min; Rate = mean of the absolute rate of change between adjacent data points.
In terms of general patterns amongst the comparative datasets, the open ocean sites (CCE1 and Kingman Reef) and the Antarctic sites (Cape Evans and Cindercones) displayed the least variation in pH over the 30-day deployment period. For example, pH range fluctuated between 0.024 to 0.096 at CCE1, Kingman Reef, Cape Evans, and Cindercones (Figure 2A, B and Table 2). In distinct contrast to the stability of the open ocean and Antarctic sites, sensors at the other five site classifications (upwelling, estuarine/near-shore, coral reef, kelp forest, and extreme) captured much greater variability (pH fluctuations ranging between 0.121 to 1.430) and may provide insight towards ecosystem-specific patterns. The sites in upwelling regions (Pt. Conception and Pt. Ano Nuevo, Figure 2C), the two locations in Monterey Bay, CA (Figure 2D), and the kelp forest sites (La Jolla and Santa Barbara Mohawk Reef, Figure 2F) all exhibited large fluctuations in pH conditions (pH changes>0.25). Additionally, at these 6 sites, pH oscillated in semi-diurnal patterns, the most apparent at the estuarine sites. The pH recorded in coral reef ecosystems exhibited a distinct diel pattern characterized by relatively consistent, moderate fluctuations (0.1<pH change<0.25; Figure 2E). At the Palmyra fore reef site, pH maxima occurred in the early evening (∼5:00 pm), and pH minima were recorded immediately pre-dawn (∼6:30 am). On a fringing reef site in Moorea, French Polynesia, a similar diel pattern was observed, with pH maxima occurring shortly after sunset (∼7:30 pm) and pH minima several hours after dawn (∼10:00 am). Finally, the greatest transitions in pH over time were observed at locations termed our “Extreme” sites - a CO2 venting site in Italy (site S2 in ref. [36]) and a submarine spring site in Mexico. For these sites, the patterns were extremely variable and lacked a detectable periodicity (Figure 2G).
The sites examined in this study do not comprehensively represent pH variability in coastal ecosystems, partly because we focused on surface epipelagic and shallow benthic pH variability. Many organisms that may be impacted by pH variability and ocean acidification reside at intermediate (>10 m) to abyssal depths. Notable regimes missing from Figure 2 include seasonally stratified open ocean locations that exhibit intense spring blooms; the equatorial upwelling zone; other temperate (and highly productive) Eastern Continental Boundary upwelling areas; subsurface oxygen minimum zones and seasonal dead zones; and a wide variety of unique estuarine, salt marsh, and tide pool environments. Spring bloom locations exhibit a marked increase in diel pCO2 variability during the peak bloom with a coincident drawdown similar in magnitude but opposite in sign to the upwelling signals shown in Figure 2 [39]. Equatorial upwelling locations undergo significant stochastic variability, as observed by pCO2 sensors in the TAO array (data viewable at http://www.pmel.noaa.gov/). Intertidal vegetated and tide pool habitats may exhibit major pH fluctuations due to macrophyte or animal respiratory cycles [15], while CO2 production in oxygen minimum zones can reduce pH to a limit of about 7.4 [40].
Due to local temperature differences, variable total alkalinity, and seasonal differences between deployment dates at each site, a comparison of average pH across the datasets would be somewhat misleading. However, some information can be gleaned from an examination of the averages: the overall binned average of all 15 mean values in Table 1 is 8.02±0.1. This pH value is generally in agreement with the global open ocean mean for 2010 of 8.07, a value generated by combining climatology data for temperature, salinity, phosphate, silicate [41]–[43], total alkalinity [44], and pCO2 [45] for the year 2000, corrected to 2010 using the average global rise of 1.5 µatm pCO2 yr−1. Rather than make a point-by-point comparison of the mean pH of each dataset, we focus instead on the differences in observed variability amongst the sites. For this analysis, summary statistics of the comparative datasets were ranked in order to examine the range of variability across all 15 sites (Fig. 3).
Discussion
Collected by 15 individual SeaFET sensors in seven types of marine habitats, data presented here highlight natural variability in seawater pH. Based on Figure 3, it is evident that regions of the ocean exhibit a continuum of pH variability. At sites in the open ocean (CCE-1), Antarctica, and Kingman reef (a coastal region in the permanently stratified open Pacific Ocean with very low residence times, and thus representative of the surrounding open ocean water), pH was very stable (SD<0.01 pH over 30 days). Elsewhere, pH was highly variable across a range of ecosystems where sensors were deployed. The salient conclusions from this comparative dataset are two-fold: (1) most non-open ocean sites are indeed characterized by natural variation in seawater chemistry that can now be revealed through continuous monitoring by autonomous instrumentation, and (2) in some cases, seawater in these sites reaches extremes in pH, sometimes daily, that are often considered to only occur in open ocean systems well into the future [46]. Admittedly, pH is only part of the story with regard to the biological impacts of OA on marine organisms. However, continuous long-term observations provided by sensors such as the SeaFET are a great first step in elucidating the biophysical link between natural variation and physiological capacity in resident marine organisms.
In the end, knowledge of spatial and temporal variation in seawater chemistry is a critical resource for biological research, for aquaculture, and for management efforts. From a biological perspective, the evolutionary history of the resident organisms will greatly influence the adaptation potential of organisms in marine populations. Thus, present-day natural variation will likely shape capacity for adaptation of resident organisms, influencing the resilience of critical marine ecosystems to future anthropogenic acidification. Below we discuss the comparative SeaFET-collected data and, where applicable, the biological consequences of the temporal heterogeneity that we found in each of the marine ecosystems where sensors were deployed.
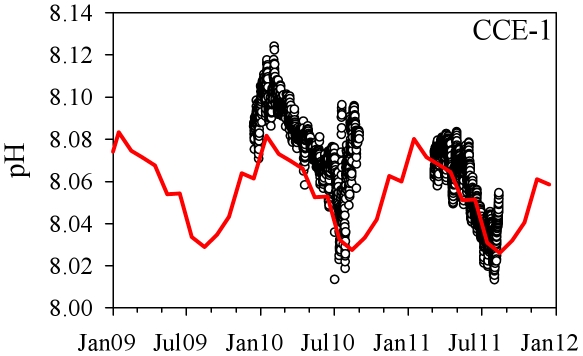
As the most stable area, the open ocean behaves in a predictable way and generally adheres to global models attempting to predict future CO2 conditions based on equilibration of the surface ocean with a given atmospheric pCO2 (e.g. [47]). This can be shown with longer-term pH records obtained with SeaFET sensors, which are available at the CCE-1 mooring (Fig. 4). The ambient pH values for this open ocean location can be predicted to better than ±0.02 from the CO2-corrected climatology mentioned above; pH has dropped by about 0.015 units since 2000. At CCE-1, the annual carbonate cycle followed the sea surface temperature cycle, and pH was driven mostly by changes in the temperature dependence of CO2 system thermodynamics (Figure 4). SeaFET observations at CCE-1 agree with the climatology to +0.017±0.014 pH units, with episodic excursions from the climatology but a general return to the climatological mean. Although the annual cycle in the open ocean is somewhat predictable, it is notable that even at these seemingly stable locations, climatology-based forecasts consistently underestimate natural variability. Our observations confirm an annual mean variability in pH at CCE-1 of nearly 0.1, suggest an inter-annual variability of ∼0.02 pH, and capture episodic changes that deviate from the climatology (Figure 4). Similar underestimates of CO2 variability were observed at nine other open ocean locations, where the Takahashi pCO2 climatology overlaps PMEL moorings with pCO2 sensors (not shown). Thus, on both a monthly (Fig. 2) and annual scale (Fig. 4), even the most stable open ocean sites see pH changes many times larger than the annual rate of acidification. This natural variability has prompted the suggestion that “an appropriate null hypothesis may be, until evidence is obtained to the contrary, that major biogeochemical processes in the oceans other than calcification will not be fundamentally different under future higher CO2/lower pH conditions” [24].
Figure 4. Comparison between sensor data (symbols) and the pH climatology (line) near CCE-1.
Similarly, the sensors deployed on the benthos in the Antarctic (Cindercones and Cape Evans, Figure 2B) recorded relatively stable pH conditions when compared to other sites in the study. Very few data exist for the Southern Ocean; however, open-water areas in this region experience a strong seasonal shift in seawater pH (∼0.3–0.5 units) between austral summer and winter [48], [49] due to a decline in photosynthesis during winter and a disequilibrium of air-sea CO2 exchange due to annual surface sea ice and deep water entrainment [50]. Given the timing of deployment of our sensor in McMurdo Sound (austral spring: October–November), the sensor did not capture the change in seawater chemistry that might have occurred in the austral winter [49]. In general, due to sea ice conditions, observations from the Southern Ocean are limited, with water chemistry data falling into two categories: (1) discrete sampling events during oceanographic cruises (e.g. US Joint Global Ocean Flux Study, http://www1.whoi.edu/) and (2) single-point measurements from locations under sea ice [49], [51], [52]. Biologically speaking, the Southern Ocean is a region expected to experience acidification and undersaturated conditions earlier in time than other parts of the ocean [47], and calcifying Antarctic organisms are thought to be quite vulnerable to anthropogenic OA given the already challenging saturation states that are characteristic of cold polar waters [53]–[56]. Short-term CO2 perturbation experiments have shown that Antarctic calcifying marine invertebrates are sensitive to decreased saturation states [51], [57], although the number of species-level studies and community-level studies are very limited. The Western Antarctic Peninsula and the sub-Antarctic islands will experience pronounced increases in temperature [54] and could consequently undergo more variation and/or undersaturation given the increased potential for biological activity. Importantly, depending on the patterns of seasonally-dependent saturation state that will be revealed with improved observations [58], Antarctic organisms may experience more variation than might be expected, a situation that will influence their resilience to future acidification.
Three other types of study sites – the coastal upwelling, kelp forest and estuarine/near-shore sites – all exhibited variability due to a combination of mixing, tidal excursions, biological activity, and variable residence time (Fig. 2). Although these sites are all united by fairly obvious heterogeneity in pH, organisms living in these areas encounter unique complexities in seawater chemistry that will influence their physiological response, resilience, and potential for adaptation.
Typically, estuarine environments have riverine input that naturally creates very low saturation states [59]–[61]. Seawater chemistry conditions in these areas often shift dramatically, challenging biogenic calcification by resident organisms. Additionally, these species must also tolerate abiotic factors that interact with pH, such as temperature [62]. Two sensors in the Monterey Bay region, L1 (at the mouth of Elkhorn Slough) and L20 (∼2 km seaward and north of L1), recorded rapid changes in pH. However, as opposed to riverine input, the low pH fluctuations observed here are likely due to isopycnal shoaling or low CO2 water that is pulsing up to the near shore on internal tides. These locations may also experience high river run-off in the rainy season, but such conditions were not reflected in the time series shown in Fig. 2.
Organisms living in upwelling regions may be acclimatized and adapted to extremes in seawater chemistry; here, deep CO2-enriched waters reach the surface and may shoal onto the benthos on the continental shelf [31], [32]. Data collected from our upwelling sites support the patterns found by cruise-based investigations; pH fluctuations were often sharp, and large transitions of up to ∼0.35 pH units occurred over the course of days (Fig. 2). Laboratory studies on calcifying marine invertebrates living in upwelling regions suggest that these organisms maintain function under such stochastic conditions. However, overall performance may be reduced, suggesting that these species are indeed threatened by future acidification [17], [18], [63].
For kelp forests, although there is less influence from riverine inputs, pH variation is quite dynamic at these sites in the coastal California region (Fig 2; [18]). Patterns here are likely driven by fluctuations in coastal upwelling, biological activity, currents, internal tides, seasonally shoaling isopleths, as well as the size of the kelp forest, which may influence residence times via reduced flow. Kelps may respond positively to increased availability of CO2 and HCO3 −, which may allow for reduced metabolic costs and increased productivity [64]. Increased kelp production may elevate pH within the forest during periods of photosynthesis, causing wider daily fluctuations in pH, though this is speculative at this time. As a result, kelp forests, particularly those of surface canopy forming species such as Macrocystis pyrifera, may contain a greater level of spatial heterogeneity in terms of the pH environment; vertical gradients in pH may form due to enhanced levels of photosynthesis at shallower depths. Such gradients may increase the risk of low pH exposure for benthic species while buffering those found within the surface canopy. Kelp forests provide habitat to a rich diversity of organisms from a wide range of calcifying and non-calcifying taxa [65]. As with organisms from the other coastal locations (estuarine and upwelling), the biota living within kelp forest environments are most likely acclimatized to this degree of natural variation. However, continued declines in oxygenation and shoaling of hypoxic boundaries observed in recent decades in the southern California bight [66], [67] are likely accompanied by a reduction in pH and saturation state. Thus, pH exposure regimes for the coastal California region's kelp forest biota may be changing over relatively short time scales. Over longer temporal scales as pH and carbonate saturation levels decrease, the relative abundances of these species may change, with community shifts favoring non-calcified species, as exemplified by long-term studies in intertidal communities by Wootton et al. [15].
For all the marine habitats described above, one very important consideration is that the extreme range of environmental variability does not necessarily translate to extreme resistance to future OA. Instead, such a range of variation may mean that the organisms resident in tidal, estuarine, and upwelling regions are already operating at the limits of their physiological tolerances (a la the classic tolerance windows of Fox – see [68]). Thus, future acidification, whether it be atmospheric or from other sources, may drive the physiology of these organisms closer to the edges of their tolerance windows. When environmental change is layered upon their present-day range of environmental exposures, they may thereby be pushed to the “guardrails” of their tolerance [20], [68].
In contrast to more stochastic changes in pH that were observed in some sites, our coral reef locations displayed a strikingly consistent pattern of diel fluctuations over the 30-day recording period. Similar short-term pH time series with lower daily resolution [69], [70] have reported regular diel pH fluctuation correlated to changes in total alkalinity and oxygen levels. These environmental patterns of pH suggest that reef organisms may be acclimatized to consistent but moderate changes in the carbonate system. Coral reefs have been at the center of research regarding the effects of OA on marine ecosystems [71]–[73]. Along with the calcification biology of the dominant scleractinian corals and coralline algae, the biodiversity on coral reefs includes many other calcifying species that will likely be affected [74]–[77]. Across the existing datasets in tropical reef ecosystems, the biological response of calcifying species to variation in seawater chemistry is complex (see [78]) –all corals or calcifying algal species will not respond similarly, in part because these calcifying reef-builders are photo-autotrophs (or mixotrophs), with algal symbionts that complicate the physiological response of the animal to changes in seawater chemistry.
Finally, the “Extreme” sites in our comparative dataset are of interest in that the low pH levels observed here represent a natural analogue to OA conditions in the future, demonstrating how the abundance and distribution of calcifying benthic organisms, as well as multi-species assemblages, can vary as a function of seawater chemistry [16], [35], [36], [79]. The variability in seawater pH was higher at both the groundwater springs off the coast of Mexico and the natural CO2 vents off the coast of Italy than at any of the other sensor locations. Offshore of Puerto Morelos, Mexico (and at other sites along the Mesoamerican Reef), natural low-saturation (Ω∼0.5, pH 6.70–7.30, due to non-ventilated, high CO2, high alkalinity groundwater) submarine springs have been discharging for millennia. Here, variability in pH is due to long-term respiration driving a low ratio of alkalinity to dissolved inorganic carbon in effluent ground water. These sites provide insight into potential long-term responses of coral backreef ecosystems to low saturation conditions [79]. Unlike Puerto Morelos, the variability of pH at volcanic CO2 vents at Ischia, Italy is almost purely abiotically derived, due entirely to CO2 venting and subsequent mixing. This site in the Mediterranean Sea hosts a benthic assemblage that reflects the impacts of OA on rocky reef communities [16], [36].
Overall, the ‘extreme’ systems provide an opportunity to examine how variability in pH and extreme events (sensu [80]) affects ecological processes. Knowledge of this biophysical link is essential for forecasting ecological responses to acidification in ecosystems with sharp fluctuations in pH, such as upwelling or estuarine environments. Despite reductions in species richness, several calcifying organisms are found in low pH conditions close to the vents [16] and the springs [79]. The persistence of calcifying organisms at these extreme sites, where mean pH values are comparable to those that have reduced organism performance in laboratory experiments (i.e., pHT 7.8; reviewed in [16]), suggest that long exposures to such variability in pH, versus a consistently low-pH environment, could play an important role in regulating organism performance. Variability in pH could potentially promote acclimatization or adaptation to acidification through repeated exposure to low pH conditions [24]; alternatively, transient exposures to high pH conditions could buffer the effects of acidification by relieving physiological stress. Thus, the ecological patterns coupled with the high fluctuations in pH at the extreme sites highlight the need to consider carbonate chemistry variability in experiments and models aimed at understanding the impacts of acidification.
Examination of Figure 3 indicates that no single simple statistical metric is sufficient to characterize the pH of an ecosystem. Not surprisingly, there is considerable overlap between panels D, E and F, confirming that sites with the greatest range and variance will exhibit the greatest instantaneous rates of change. While these simple metrics are easily understood and therefore helpful in a descriptive sense, the fact that observed pH may not exhibit a normal distribution should serve as a reminder that each system is unique and complex. Depending on the mechanism (e.g. mixing vs. biological) driving the observed change, the metric used to characterize the system will hold a different meaning. For example, during the periods compared here, the sensor in Monterey Bay at M1 (Fig. 2C) has a higher SD and range than the sensor located in the La Jolla Kelp forest (Fig. 2F), yet the average instantaneous rate of change in the La Jolla Kelp forest is higher than at M1 (Fig. 3D–F).
In summary, together, these pH time series create a compelling argument for the collection of more continuous data of this kind. Specifically, these data represent a critical step in understanding the consequences of ocean change: the linkage of present-day pH exposures to organismal tolerance and how this translates into ecological change in marine ecosystems [27], [81]. Long-term datasets exist, but many are in open-ocean locations (HOTS, BATS, ESTOC) and do not capture environmental variation in the coastal marine habitats that are of such critical ecological and economic value [20]. Additionally, they often do not capture changes in pH at physiologically relevant timescales since they are limited by ship-board sampling frequencies. The processes that combine to drive changes in seawater chemistry are complex; water chemistry at any location in the ocean is a result of air-sea exchange, land-water interactions, and the physical, chemical, and biological processes occurring in the water column and on the benthos. In the shallow coastal ecosystems measured here, the combination of background oceanography, resident biological processes, and residence time of the water are likely driving the daily variability or lack thereof in seawater pH. All of these processes can contribute to the mean, minimum, maximum, and diurnal or seasonal variability occurring within and among ecosystems or habitats. At this juncture, it is not clear what aspect of this variability is most biologically significant (e.g. minimum pH, maximum pH, hours spent below the yearly mean low pH); however, investigations in thermal biology have begun to tease apart the parameters that are relevant to organismal physiology [27].
As a final note, we do concede that, like pCO2, pH may not tell the whole story. It may in fact be saturation state and not pH that is the main driver of the mechanistic and physiological impact of OA, at least for calcifying organisms. While our comparative data set provides a unique look into natural pH variability, the overall picture of variability in the carbonate system will remain incomplete until we are able to fully characterize the CO2 system with additional sensors for dissolved inorganic carbon and alkalinity. There is also a need to monitor other hydrographic variables in addition to the carbonate system. Modern and paleo OA events are accompanied by shifts in temperature, stratification, and dissolved oxygen [82]. The impacts of OA can depend on values of interacting stressors such as temperature and oxygen, and pH may in turn alter tolerance to these stressors, with major consequences for organism function [83]. However, at present, the use of autonomous sensors can greatly improve our perspectives of how future acidification might influence species physiology, fitness, and interactions in marine ecosystems.
Materials and Methods
All necessary permits were obtained for the described field studies. Permits were issued for sensor deployments by: Moss Landing Harbor District (L01); Monterey Bay National Marine Sanctuary (L20 and M1); Délégation à la Recherche, Territorial Government of French Polynesia (Moorea Coral Reef LTER); US Fish and Wildlife (Palmyra Atoll National Wildlife Refuge). For all other sites in this study, no specific permits were required for sensor deployments.
The pH sensors used in this study are based on a modified version of the Honeywell DuraFET®, an ion sensitive field effect transistor (ISFET), with an integrated data logger and power supply [37]. We refer to these autonomous versions of the DuraFET as “SeaFET”. Data presented in Figure 2 were recorded by several different generations of SeaFET, with the most notable difference being that sensors 1, 5, 8, 12, and 14 were operated inside of a flow manifold that was flushed with a submersible pump (Sea-Bird SBE5) before each measurement while the other ten sensors were protected by a passively flushed copper guard.
The pH is reported on the total hydrogen ion concentration scale (see e.g., [84]). Calibrations for sensors 6–8 were carried out pre-deployment in the MBARI test tank, where tank pH was measured using the spectrophotometric method [85]. Vicarious calibration of sensors 3, 4, and 11–15 was accomplished by first deploying the sensor and then collecting one or more discrete samples within <1 m of the sensor, usually several days after deployment; sensors 2, 9, and 10 were calibrated in a common vessel prior to deployment with vicarious in situ calibration immediately following. Analyses of discrete samples used for vicarious calibration and subsequent calculation of pH at the deployment temperature were carried out as recommended by Dickson et al. [86]. Temperature dependence of the SeaFET sensor was accounted for as described in ref. [37]. Sensors 1 and 5 were calibrated to a point, within ∼1 week of mooring deployment, where pH was calculated from the pCO2 measured by the self-calibrating PMEL MapCO2 sensor co-located on the mooring and a total alkalinity value calculated from a region-specific conservative relationship between total alkalinity and salinity: AT = 2131+50.8*(Salinity−31.25) (equation provided by Simone Alin).
As discussed in ref. [37], DuraFET sensors operate with short term precision of ±0.0005 pH units and exhibit stability over weeks to months of ±0.005 pH. In situ validation of sensor stability was not incorporated into every sensor deployment presented here, but in several cases (e.g. Palmyra, CCE1 mooring) ancillary data were available that allowed us to confirm this stability of the sensors. Several factors combine to determine the overall uncertainty of the sensor pH value; the 1st-order error is due to the uncertainty in the pH of the solution in contact with the sensor at the time that a pH value is ascribed as a calibration point. Because the bottle samples were processed using high accuracy CO2 analysis methods, we attribute the majority pH uncertainty to sampling error due, for example, to small scale gradients at the deployment site when an in situ sample is collected for vicarious calibration. It is therefore likely that the uncertainty of pH is highest at the most dynamic sites because these locations exhibit the most intense gradients. Yet establishing uncertainty in sampling error requires a constant field presence to carry out a meaningful number of discrete samples over an appropriate period of time. Such validation is beyond the scope of the studies presented in this work. Based on past in situ validations, we (KSJ, TRM) have observed sampling errors ranging from ±0.0007 to ±0.015 pH units. We therefore report here that the worst-case uncertainty in pH is around ±0.015, and in many instances, we expect the uncertainty to be better than ±0.01 pH units. In our experience, biofouling can compromise data quality for the passively flushed SeaFET within ∼2 months of deployment in high fouling environments, and, in general, the pumped version remains stable for much longer periods. In every case shown in Figure 1, the 30-day window was selected early in the time series to avoid deleterious effects of biofouling. We are now archiving the quality-controlled time series presented in this work at http://martzlab.ucsd.edu/data.html. Longer time series from CCE1 and CCE2 can be viewed at http://mooring.ucsd.edu/projects/cce/cce_data.html.
As noted in Table 1, data were recorded in the upper 15 m of the ocean. However, the water depth at each location was highly variable with some of the deployment locations categorized as pelagic and others benthic (i.e., within 5 m of the bottom; Table 1).
Acknowledgments
The authors gratefully acknowledge support from various organizations that facilitated the deployment of sensors: the U.S. Santa Barbara Coastal Long Term Ecological Research (LTER) site (Director: Dan Reed), the U.S. Moorea Coral Reef LTER site (Directors: Russell Schmitt, Sally Holbrook, Peter Edmunds, Robert Carpenter), Centro de Investigación Científica de Yucatán (CICY), Cancun, Mexico (specifically Mario Rebolledo-Vieyra and Laura Hernandez); and the Stazione Zoologica “Anton Dohrn” (Naples, Italy) all supported the deployment of sensors.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: SeaFET development was supported through a grant to TRM by the U.S. National Science Foundation (NSF) grant OCE 0844394. Work by GEH was supported by NSF grants ANT 0944201 and OCE 1040960, and the collaboration between the lead authors (GEH and TRM) was supported by the University of California's multi-campus research program, Ocean Acidification: A Training and Research Consortium, (http://oceanacidification.msi.ucsb.edu). The David and Lucile Packard Foundation supported initial development of the Durafet sensor for oceanographic applications and deployments on the M1, L01 and L20 moorings. Additionally, the datasets presented here were supported by several lines of funding to the contributors of the data: NSF grant OCE 1040952 to AP; NSF grant OCE 0927445 to LAL and TRM; FM and KJK were supported by a Chambers Fellowship to FM, Stanford University setup funds (FM); funding for JES and NNP was provided by the Gordon and Betty Moore Foundation, the Nature Conservancy, the WWW Foundation, Scripps Institution of Oceanography development office and by generous donations from Scott and Karin Wilson and the Rhodes family; the CCE moorings were implemented and operated with support from National Oceanic and Atmospheric (NOAA) Climate Program Office, NOAA National Marine Fisheries Service, and the NOAA Ocean Acidification Program, with support from the California Current Ecosystem Long Term Ecological Research program (CCE LTER), and from University of California San Diego; and EBR was supported by a minigrant from the U.S. Moorea Coral Reef LTER. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kleypas JA, Feely RA, Fabry VJ, Langdon C, Sabine CL, et al. 2006. 88 Impacts of Ocean Acidification on Coral Reefs and Other Marine Calcifiers: A Guide for Future Research, report of a workshop held 18–20 April 2005, St. Petersberg, FL sponsored by NSF, NOAA, and U.S. Geological Survey.
- 2.Royal Society. Ocean acidification due to increasing atmospheric carbon dioxide. London: Royal Society; 2005. [Google Scholar]
- 3.Byrne M. Impact of ocean warming and ocean acidification on marine invertebrate life history stages: vulnerabilities and potential persistence in a changing ocean. Oceanogr Mar Biol. 2011;49:1–42. [Google Scholar]
- 4.Doney SC, Fabry VJ, Feely RA, Kleypas JA. Ocean Acidification: The Other CO2 Problem. Annu Rev Mar Sci. 2009;1:169–192. doi: 10.1146/annurev.marine.010908.163834. [DOI] [PubMed] [Google Scholar]
- 5.Fabry VJ, Seibel BA, Feely RA, Orr JC. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J Mar Sci. 2008;65:414–432. [Google Scholar]
- 6.Hofmann GE, Barry JP, Edmunds PJ, Gates RD, Hutchins DA, et al. The Effect of Ocean Acidification on Calcifying Organisms in Marine Ecosystems: An Organism-to-Ecosystem Perspective. Annu Rev Ecol Evol S. 2010;41:127–147. [Google Scholar]
- 7.Kroeker KJ, Kordas RL, Crim RN, Singh GG. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol Lett. 2010;13:1419–1434. doi: 10.1111/j.1461-0248.2010.01518.x. [DOI] [PubMed] [Google Scholar]
- 8.Gattuso JP, Hansson L, editors. Ocean Acidification. Oxford: Oxford University Press; 2011. [Google Scholar]
- 9.Bates NR, Hansell DA, Carlson CA. Distribution of CO2 species, estimates of net community production and air-sea CO2 exchange in the Ross Sea polyna. J Geophys Res. 1998;103:2883–2896. [Google Scholar]
- 10.Copin-Montégut C, Bégovic M, Merlivat L. Variability of the partial pressure of CO2 on diel to annual time scales in the Northwestern Mediterranean Sea. Mar Chem. 2004;85:169–189. [Google Scholar]
- 11.DeGrandpre MD, Hammar TR, Smith SP, Sayles FL. In situ measurements of seawater pCO2. Limnol Oceanogr. 1995;40:969–975. [Google Scholar]
- 12.Hales B, Takahashi T, Bandstra L. Atmospheric CO2 uptake by a coastal upwelling system. Global Biogeochem Cy. 2005;19 doi: 10.1029/2004GB002295. [Google Scholar]
- 13.Ohde S, van Woesik R. Carbon dioxide flux and metabolic processes of a coral reef, Okinawa. Bull Mar Sci. 1999;65:559–576. [Google Scholar]
- 14.van Geen A, Takesue RK, Goddard J, Takahashi T, Barth JA, et al. Carbon and nutrient dynamics during coastal upwelling off Cape Blanco, Oregon. Deep-Sea Res Pt II. 2000;47:975–1002. [Google Scholar]
- 15.Wootton JT, Pfister CA, Forester JD. Dynamic patterns and ecological impacts of declining ocean pH in a high-resolution multi-year dataset. P Natl Acad Sci USA. 2008;105:18848–18853. doi: 10.1073/pnas.0810079105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kroeker KJ, Micheli F, Gambi MC, Martz TR. Divergent ecosystem responses within a benthic marine community to ocean acidification. P Natl Acad Sci USA. 2011 doi: 10.1073/pnas.1107789108. doi/10.1073/pnas.1107789108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomsen J, Gutowska MA, Saphörster J, Heinemann A, Trübenbach K, et al. Calcifying invertebrates succeed in a naturally CO2-rich coastal habitat but are threatened by high levels of future acidification. Biogeosciences Discuss. 2010;7:5119–5156. [Google Scholar]
- 18.Yu PC, Matson PG, Martz TR, Hofmann GE. The ocean acidification seascape and its relationship to the performance of calcifying marine invertebrates: Laboratory experiments on the development of urchin larvae framed by environmentally-relevant pCO2/pH. J Exp Mar Biol Ecol. 2011a;400:288–295. [Google Scholar]
- 19.Hendriks IE, Duarte CM, Alvarez M. Vulnerability of marine biodiversity to ocean acidification: A meta-analysis. Estuar Coast Shelf S. 2010;86:157–164. [Google Scholar]
- 20.Turley C, Eby M, Ridgwell AJ, Schmidt DN, Findlay HS, et al. The societal challenge of ocean acidification. Mar Pollut Bull. 2010;60:787–792. doi: 10.1016/j.marpolbul.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Kelly RP, Foley MM, Fisher WS, Feely RA, Halpern BS, et al. Mitigating Local Causes of Ocean Acidification with Existing Laws. Science. 2011;332:1036–1037. doi: 10.1126/science.1203815. [DOI] [PubMed] [Google Scholar]
- 22.Cooley SR, Lucey N, Kite-Powell H, Doney SC. Nutrition and income from molluscs today imply vulnerability to ocean acidification tomorrow. Fish and Fisheries. 2011 doi: 10.1111/j.1467-2979.2011.00424.x. [Google Scholar]
- 23.Le Quesne WJF, Pinnegar JK. The potential impacts of ocean acidification: scaling from physiology to fisheries. Fish Fish. 2011 doi: 10.1111/j.1467-2979.2011.00423.x. [Google Scholar]
- 24.Joint I, Doney SC, Karl DM. Will ocean acidification affect marine microbes? The ISME Journal. 2011;5:1–7. doi: 10.1038/ismej.2010.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byrne RH, Mecking S, Feely RA, Liu X. Direct observations of basin-wide acidification of the North Pacific Ocean. Geophys Res Lett. 2010;37:L02601. doi: 02610.01029/02009GL040999. [Google Scholar]
- 26.Dore JE, Lukas R, Sadler DW, Church MJ, Karl DM. Physical and biogeochemical modulation of ocean acidification in the central North Pacific. P Natl Acad Sci USA. 2009;106:12235–12240. doi: 10.1073/pnas.0906044106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helmuth B, Broitman BR, Yamane L, Gilman SE, Mach K, et al. Organismal climatology: analyzing environmental variability at scales relevant to physiological stress. J Exp Biol. 2010;213:995–1003. doi: 10.1242/jeb.038463. [DOI] [PubMed] [Google Scholar]
- 28.Pörtner HO. Ecosystem effects of ocean acidification in times of ocean warming: a physiologist's view. Mar Ecol-Prog Ser. 2008b;373:203–217. [Google Scholar]
- 29.Somero GN. The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‚ ‘winners’ and ‚‘losers’. J Exp Biol. 2010;213:912–920. doi: 10.1242/jeb.037473. [DOI] [PubMed] [Google Scholar]
- 30.Meehl GA, Stocker TF, Collins WD, Friedlingstein P, Gaye AT, et al. The Physical Science Basis. Contribution of the Working Group in the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, editors. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- 31.Fassbender AJ, Sabine CL, Feely RA, Langdon C, Mordy CW. Inorganic carbon dynamics during northern California coastal upwelling. Cont Shelf Res. 2011;31:1180–1192. [Google Scholar]
- 32.Feely RA, Sabine CL, Hernandez-Ayon JM, Ianson D, Hales B. Evidence for Upwelling of Corrosive “Acidified” Water onto the Continental Shelf. Science. 2008:1155676. doi: 10.1126/science.1155676. [DOI] [PubMed] [Google Scholar]
- 33.Hauri C, Gruber N, Plattner G-K, Alin S, Feely RA, et al. Ocean Acidification in the California Current System. Oceanography. 2009;22:60–71. [Google Scholar]
- 34.Sanford E, Kelly MW. Local Adaptation in Marine Invertebrates. Annu Rev Mar Sci. 2011;3:509–535. doi: 10.1146/annurev-marine-120709-142756. [DOI] [PubMed] [Google Scholar]
- 35.Fabricius KE, Langdon C, Uthicke S, Humphrey C, Noonan S, et al. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nature Clim Change. 2011;1:165–169. [Google Scholar]
- 36.Hall-Spencer JM, Rodolfo-Metalpa R, Martin S, Ransome E, Fine M, et al. Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature. 2008 doi: 10.1038/nature07051. doi: 10.1038/nature07050. [DOI] [PubMed] [Google Scholar]
- 37.Martz TR, Connery JG, Johnson KS. Testing the Honeywell Durafet® for seawater pH applications. Limnol Oceanogr Methods. 2010;8:172–184. [Google Scholar]
- 38.Seidel MP, DeGrandpre MD, Dickson AG. A sensor for in situ indicator-based measurements of seawater pH. Mar Chem. 2008;109:18–28. [Google Scholar]
- 39.Martz TR, DeGrandpre MD, Strutton PG, McGillis WR, Drennan WM. Sea surface pCO2 and carbon export during the Labrador Sea spring-summer bloom: An in situ mass balance approach. J Geophys Res. 2009 doi: 10.1029/2008JC005060. [Google Scholar]
- 40.Culberson C, Pytkowicz R. Oxygen-total carbon dioxide correlation in the Eastern Pacific Ocean. J Oceanogr. 1970;26:95–100. [Google Scholar]
- 41.Antonov JI, Seidov D, Boyer TP, Locarnini RA, Mishonov AV, et al. World Ocean Atlas 2009, Volume 2: Salinity. 2010. 184 S. Levitus, Ed. NOAA Atlas NESDIS 69, U.S. Government Printing Office, Washington, D.C.
- 42.Garcia HE, Locarnini RA, Boyer TP, Antonov JI. World Ocean Atlas 2009, Volume 4: Nutrients (phosphate, nitrate, silicate). 2010. 398 S. Levitus, Ed. NOAA Atlas NESDIS 71, U.S. Government Printing Office, Washington, D.C.
- 43.Locarnini RA, Mishonov AV, Antonov JI, Boyer TP, Garcia HE. World Ocean Atlas 2009, Volume 1: Temperature. 2010. 184 S. Levitus, Ed. NOAA Atlas NESDIS 68, U.S. Government Printing Office, Washington, D.C.
- 44.Lee K, Tong LT, Millero FJ, Sabine CL, Dickson AG, et al. Global relationship of total alkalinity and temperature in surface waters of the world's oceans. Geophys Res Lett. 2006;33:L19605/19601–L19605/19605. [Google Scholar]
- 45.Takahashi T, Sutherland SC, Wanninkhof R, Sweeney C, Feely RA, et al. Climatological mean and decadal change in surface ocean pCO2, and net sea-air CO2 flux over the global oceans. Deep-Sea Res Pt II. 2009;56:554–577. [Google Scholar]
- 46.Caldeira K, Wickett ME. OceanographyAnthropogenic carbon and ocean pH. Nature. 2003;425:365. doi: 10.1038/425365a. [DOI] [PubMed] [Google Scholar]
- 47.Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature. 2005;437:681. doi: 10.1038/nature04095. [DOI] [PubMed] [Google Scholar]
- 48.McNeil BI, Matear RJ. Southern Ocean acidification: A tipping point at 450-ppm atmospheric CO2. P Natl Acad Sci USA. 2008;105:18860–18864. doi: 10.1073/pnas.0806318105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McNeil BI, Sweeney C, Gibson JAE. Natural seasonal variability of aragonite saturation state within two Antarctic coastal ocean sites. Antarct Sci. 2011 doi: 10.1017/S0954102011000204. [Google Scholar]
- 50.McNeil BI, Tagliabue A, Sweeney C. A multi-decadal delay in the onset of corrosive ‘acidified’ waters in the Ross Sea of Antarctica due to strong air-sea CO2 disequilibrium. Geophys Res Lett. 2010;37:L19607. doi: 19610.11029/12010GL044597. [Google Scholar]
- 51.Cummings V, Hewitt J, Van Rooyen A, Currie K, Beard S, et al. Ocean Acidification at High Latitudes: Potential Effects on Functioning of the Antarctic Bivalve Laternula elliptica. PLoS ONE. 2011;6:e16069. doi: 10.1371/journal.pone.0016069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Littlepage JL. Oceanographic investigations in McMurdo Sound, Antarctic. Antarct Res Ser. 1965;5:1–37. [Google Scholar]
- 53.Andersson AJ, Mackenzie FT, Bates NR. Life on the margin: implications of ocean acidification on Mg-calcite, high latitude and cold-water marine calcifiers. Mar Ecol-Prog Ser. 2008;373:265–273. [Google Scholar]
- 54.Fabry VJ, McClintock JB, Mathis JT, Grebmeier JM. Ocean Acidification at High Latitudes: The Bellwether. Oceanography. 2009;22:160–171. [Google Scholar]
- 55.McClintock JB, Amsler MO, Angus RA, Challener RC, Schram JB, et al. The Mg-Calcite Composition of Antarctic Echinoderms: Important Implications for Predicting the Impacts of Ocean Acidification. J Geol. 2011;119:457–466. [Google Scholar]
- 56.Sewell MA, Hofmann GE. Antarctic echinoids and climate change: a major impact on the brooding forms. Glob Change Biol. 2011;17:734–744. [Google Scholar]
- 57.Kawaguchi S, Kurihara H, King R, Hale L, Berli T, et al. Will krill fare well under Southern Ocean acidification? Biol Lett. 2011;7:288–291. doi: 10.1098/rsbl.2010.0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rintoul SR, Sparrow MD, Meredith MP, Wadley V, Speer K, et al. The Southern Ocean Observing System: Initial Science and Implementation Strategy. 2011. Scientific Committee on Antarctic Research and Scientific Committee on Oceanic Research.
- 59.Miller AW, Reynolds AC, Sobrino C, Riedel GF. Shellfish Face Uncertain Future in High CO2 World: Influence of Acidification on Oyster Larvae Calcification and Growth in Estuaries. PLoS ONE. 2009;4:e5661. doi: 10.1371/journal.pone.0005661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salisbury J, Green M, Hunt C, Campbell J. Coastal Acidification by Rivers: A Threat to Shellfish? EOS. 2008;89:513–514. [Google Scholar]
- 61.Waldbusser G, Voigt E, Bergschneider H, Green M, Newell R. Biocalcification in the Eastern Oyster (Crassostrea virginica) in Relation to Long-term Trends in Chesapeake Bay pH. Estuar Coast. 2011;34:221–231. [Google Scholar]
- 62.Parker LM, Ross PM, O'Connor WA, Borysko L, Raftos DA, et al. Adult exposure influences offspring response to ocean acidification in oysters. Glob Change Biol. 2011 doi: 10.1111/j.1365-2486.2011.02520.x. [Google Scholar]
- 63.Gaylord B, Hill TM, Sanford E, Lenz EA, Jacobs LA, et al. Functional impacts of ocean acidification in an ecologically critical foundation species. J Exp Biol. 2011;214:2586–2594. doi: 10.1242/jeb.055939. [DOI] [PubMed] [Google Scholar]
- 64.Hepburn CD, Pritchard DW, Cornwall CE, McLeod RJ, Beardall J, et al. Diversity of carbon use strategies in a kelp forest community: implications for a high CO2 ocean. Glob Change Biol. 2011;17:2488–2497. [Google Scholar]
- 65.Foster MS, Schiel DR. The ecology of giant kelp forests in California: a community profile. US Fish Wildl Serv Biol Rep. 1985;85:1–152. [Google Scholar]
- 66.Bograd SJ, Castro CG, Di Lorenzen E, Palacios DM, Bailey H, et al. Oxygen declines and the shoaling of the hypoxic boundary in the California Current. Geophys Res Lett. 2008;35:L12607. doi: 12610.11029/12008GL034185. [Google Scholar]
- 67.McClatchie S, Goericke R, Auad G, Cosgrove R, Vetter R. Oxygen in the Southern California Bight: multidecadal trends and implications for demersal fisheries. Geophys Res Lett. 2010 doi: 10.1029/2010GL044497. [Google Scholar]
- 68.Hofmann GE, Todgham AE. Living in the Now: Physiological Mechanisms to Tolerate a Rapidly Changing Environment. Annu Rev Physiol. 2010;72:127–145. doi: 10.1146/annurev-physiol-021909-135900. [DOI] [PubMed] [Google Scholar]
- 69.Yates KK, Dufore C, Smiley N, Jackson C, Halley RB. Diurnal variation of oxygen and carbonate system parameters in Tampa Bay and Florida Bay. Mar Chem. 2007;104:110–124. [Google Scholar]
- 70.Yates KK, Halley RB. CO3 2− concentration and pCO2 thresholds for calcification and dissolution on the Molokai reef flat, Hawaii. Biogeosciences. 2006;3:357–369. [Google Scholar]
- 71.Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, et al. Coral Reefs Under Rapid Climate Change and Ocean Acidification. Science. 2007;318:1737–1742. doi: 10.1126/science.1152509. [DOI] [PubMed] [Google Scholar]
- 72.Kleypas JA, Buddemeier RW, Archer D, Gattuso J-P, Langdon C, et al. Geochemical Consequences of Increased Atmospheric Carbon Dioxide on Coral Reefs. Science. 1999;284:118–120. doi: 10.1126/science.284.5411.118. [DOI] [PubMed] [Google Scholar]
- 73.Veron JEN, Hoegh-Guldberg O, Lenton TM, Lough JM, Obura DO, et al. The coral reef crisis: The critical importance of <350ppm CO2. Mar Pollut Bull. 2009;58:1428–1436. doi: 10.1016/j.marpolbul.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 74.Anthony KRN, Kline DI, Diaz-Pulido G, Dove S, Hoegh-Guldberg O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc Natl Acad Sci USA. 2008;105:17442–17446. doi: 10.1073/pnas.0804478105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kleypas JA, Yates KK. Coral reefs and ocean acidification. Oceanography. 2009;22:108–117. [Google Scholar]
- 76.Price NN, Hamilton SL, Smith JE. Species-specific consequences of ocean acidification for the calcareous tropical green algae Halimeda. Mar Ecol-Prog Ser. 2011;440:67–78. [Google Scholar]
- 77.Przeslawski R, Ahyong S, Byrne M, Worheide G, Hutchings P. Beyond corals and fish: the effects of climate change on noncoral benthic invertebrates of tropical reefs. Glob Change Biol. 2008;14:2773–2795. [Google Scholar]
- 78.Jury CP, Whitehead RF, Szmant AM. Effects of variations in carbonate chemistry on the calcification rates of Madracis auretenra ( = Madracis mirabilis sensu Wells, 1973): bicarbonate concentrations best predict calcification rates. Glob Change Biol. 2010;16:1632–1644. [Google Scholar]
- 79.Crook ED, Potts D, Rebolledo-Vieyra M, Hernandez L, Paytan A. Calcifying coral abundance near low pH springs: implications of future ocean acidification. Coral Reefs. 2011 In Press, DOI: 10.1007/s00338-011-0839-y. [Google Scholar]
- 80.Gaines SD, Denny MW. The largest, smallest, highest, lowest, longest, and shortest – Extremes in Ecology. Ecology. 1993;74:1677–1692. [Google Scholar]
- 81.Helmuth B. From cells to coastlines: how can we use physiology to forecast the impacts of climate change? J Exp Biol. 2009;212:753–760. doi: 10.1242/jeb.023861. [DOI] [PubMed] [Google Scholar]
- 82.Gattuso JP, Bijma J, Gehlen M, Riebesell U, Turley C. Ocean acidification: knowns, unknowns and perspectives. In: Gattuso JP, Hansson L, editors. Ocean Acidification. Oxford: Oxford University Press; 2011. [Google Scholar]
- 83.Pörtner HO, Langenbuch M, Michaelidis B. Synergistic effects of temperature extremes, hypoxia, and increases in CO2 on marine animals: From Earth history to global change. J Geophys Res. 2005;110:C09S10. doi: 10.1029/2004JC002561. [Google Scholar]
- 84.Marion GM, Millero FJ, Camões MF, Spitzer P, Feistel R, et al. pH of seawater. Mar Chem. 2011;126:89–96. [Google Scholar]
- 85.Clayton TD, Byrne RH. Spectrophotometric seawater pH measurements: total hydrogen ion concentration scale calibration of m-cresol purple and at-sea results. Deep-Sea Res, Pt I. 1993;40:2115–2129. [Google Scholar]
- 86.Dickson AG, Sabine CL, Christian JR. 2007. Guide to best practices for ocean CO2 measurements, PICES Special Publication 3 IOCCP Report No. 8. 191.