Abstract
Tetrahydrobiopterin (BH4) deficiency is a genetic disorder associated with a variety of metabolic syndromes such as phenylketonuria (PKU). In this article, the signaling pathway by which BH4 deficiency inactivates mTORC1 leading to the activation of the autophagic pathway was studied utilizing BH4-deficient Spr-/- mice generated by the knockout of the gene encoding sepiapterin reductase (SR) catalyzing BH4 synthesis. We found that mTORC1 signaling was inactivated and autophagic pathway was activated in tissues from Spr-/- mice. This study demonstrates that tyrosine deficiency causes mTORC1 inactivation and subsequent activation of autophagic pathway in Spr-/- mice. Therapeutic tyrosine diet completely rescued dwarfism and mTORC1 inhibition but inactivated autophagic pathway in Spr-/- mice. Tyrosine-dependent inactivation of mTORC1 was further supported by mTORC1 inactivation in Pahenu2 mouse model lacking phenylalanine hydroxylase (Pah). NIH3T3 cells grown under the condition of tyrosine restriction exhibited autophagy induction. However, mTORC1 activation by RhebQ64L, a positive regulator of mTORC1, inactivated autophagic pathway in NIH3T3 cells under tyrosine-deficient conditions. In addition, this study first documents mTORC1 inactivation and autophagy induction in PKU patients with BH4 deficiency.
Key words: tetrahydrobiopterin, autophagy, mTORC1, tyrosine, phenylalanine, phenylketonuria, Akt, AMPK
Introduction
Tetrahydrobiopterin (BH4) is an obligatory cofactor for aromatic amino acid hydroxylases including phenylalanine hydroxylase (PAH), tyrosine hydroxylase (TH) and tryptophan hydroxylase (TPH) as well as three forms of nitric oxide synthases (NOS).1,2 BH4 is synthesized through de novo biosynthesis and the salvage pathway. BH4 is synthesized from GTP through sequential enzymatic reactions mediated by GTP cyclohydrolase I (GTPCH), 6-pyruvoyl-tetrahydropterin synthase (PTPS) and sepiapterin reductase (SR). Through the salvage or regeneration pathway, BH4 is regenerated from preexisting dihydropterins by dihydrofolate reductase (DHFR) and dihydropteridine reductase (DHPR).1 Mutations in enzymes associated with BH4 homeostasis cause BH4 deficiency, a metabolic disorder associated with symptoms of phenylketonuria (PKU), especially BH4-responsive PKU as well as neurodegenerative diseases such as Parkinson disease.3–6 Untreated cases of BH4-responsive PKU have been reported to be generally accompanied by a significant reduction in height and weight growth and microcephaly.7–9 Since BH4 is required for the biochemical reactions catalyzed by TH or TPH,4,5,10 BH4 deficiency in cerebrospinal fluid has been shown to cause profound neurological impairments associated with deficit of neurotransmitters dopamine or serotonin. BH4 deficiency also creates a situation whereby NOS is impaired and generates superoxide.1,2,11 Thus, BH4 deficiency is also responsible for the oxidative stress associated with many neurological features.
mTORC1 is an evolutionarily conserved Ser/Thr protein kinase complex that is a central controller of cell proliferation, cell size and cell cycle.12,13 mTORC1 activity is believed to upregulate anabolic cellular processes such as protein synthesis and ribosome biogenesis, but to downregulate catabolic processes such as autophagy.13–17 Regulation of mTORC1 activity is mediated by growth factors, energy status and amino acid signaling. Insulin and other growth factors activate mTORC1 through the activation of phosphatidylinositol-3-kinase (PI3K) and its downstream signaling molecule Akt. Energy status can also regulate mTORC1 activity through AMP-activated protein kinase (AMPK), which is activated by a high ratio of AMP to ATP. Activated AMPK phosphorylates and activates TSC2, a negative regulator of mTORC1, leading to the downregulation of mTORC1 activity.13,18 Amino acids are important activators of mTORC1 but the mechanism by which amino acids signal to mTORC1 activation is largely unknown. Amino acid-dependent control of mTORC1 seems to be mediated by class III phosphatidylinositol-3-kinase (PI3K class III, also known as Vps34).19–21 Although the signal pathways between Vps34 and mTORC1 remain to be clarified, there are studies indicating that the amino acid signal is propagated through the Vps34/PDK1/PRAS40 pathway. Based on the loss of leucine effect in Pdk−/− mice, Sanchez Canedo et al.22 have proposed the ample requirement of PDK1 in amino acid-dependent activation of mTORC1. PDK1 could stimulate mTORC1 through the activation of AGC kinases, Akt, SGK and PKC leading to the inactivation of proline-rich Akt substrate 40 (PRAS40), an inhibitor of substrate binding to mTORC1.23–25 Recently, Ras-related small GTP binding proteins, Rag GTPases, have emerged as important intermediate partners through which amino acid signaling regulates mTORC1 function.26,27 Work by Sancak et al.28 has proposed that the colocalization of Rag GTPases with mTORC1 at the lysosomal membrane is important for mTORC1 activation by amino acid signaling.
Amino acid signaling is also known to be important for the activation of autophagic pathway. Under amino acid starvation conditions, mTORC1 directly binds to the ULK1-mATG13-FIP200 complex, a signaling complex that mediates autophagic vesicle formation.29,30 Amino acid starvation induces autophagy (macroautophagy) in order to provide an internal source of nutrition that can support cellular energy production or biosynthesis through the degradation process.31–33 In this study, we established that mTORC1 inactivation by BH4 deficiency activates autophagic pathway in cultured cells and experimental mice. Inactivation of mTORC1 and subsequent induction of autophagy by BH4 deficiency was confirmed in human patients with BH4-responsive PKU.
Results
mTORC1 inactivation and subsequent induction of autophagy in BH4-deficient Spr−/− mice.
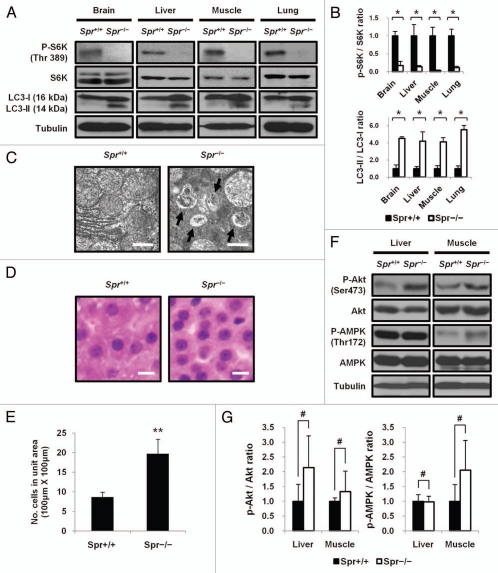
The biochemical mechanism by which BH4 deficiency induces autophagy was investigated in BH4-deficient Spr−/− mice generated through the knockout of Spr gene encoding SR that catalyzes the final step of BH4 synthesis.5 Autophagy was assessed by detecting the conversion of LC3-I into LC3-II, a hallmark of autophagy, in tissues of brain, liver, muscle and lung from BH4-deficient Spr−/− mice. Levels of LC3-II were markedly increased in these tissues from Spr−/− mice compared with those from Spr+/+ mice. Since autophagy is primarily regulated by mTORC1 signaling, mTORC1 activity was evaluated by monitoring its ability to phosphorylate S6K. Cellular levels of S6K in tissues of brain, liver, muscle or lung were the same as those in Spr+/+ mice. However, the phosphorylation state of S6K at Thr389 was inhibited in Spr−/− mice (Fig. 1A and B). Electron micrographs, indicating the formation of autophagic vesicles in liver cells from Spr−/− mice, confirmed the induction of the autophagy in Spr−/− mice (Fig. 1C). We then evaluated the role of mTORC1 signaling in controlling cell size in livers of Spr−/− mice in which mTORC1 activity was suppressed. Liver cells from Spr−/− mice were considerably reduced in size compared with those from Spr+/+ mice (Fig. 1D and E). To test whether mTORC1 activity in BH4-deficient Spr−/− mice was modulated by Akt or AMPK, each of which has emerged as an important upstream regulator of mTORC1, levels of phosphoylated Akt or AMPK in tissues from Spr+/+ or Spr−/− mice were measured. Levels of Akt and AMPK in tissues from Spr−/− mice did not differ from those in Spr+/+ mice. If mTORC1 is to be inactivated in Spr−/− mice by its positive regulator Akt, activation phosphorylation of Akt should be inhibited in Spr−/− mice. However, our data indicate that Akt phosphorylation is activated in Spr−/− mice. If mTORC1 is to be inactivated by its negative regulator AMPK, the level of phosphorylated AMPK should be elevated in Spr−/− mice. But we found that the levels of phosphorylated AMPK were not substantially enhanced in Spr−/− mice and thereby cannot account for the inhibition of mTORC1 in Spr−/− mice (Fig. 1F and G).
Figure 1.
Autophagy induction in BH4-deficient Spr−/− mice. (A) Inactivation of mTORC1 and induction of autophagy in Spr−/− mice. Tissue homogenates from 25-d-old Spr+/+ or Spr−/− mice fed a normal diet were analyzed by protein gel blotting to examine the phosphorylation status of S6K and the conversion of LC3-I into LC3-II. (B) Relative band intensities in protein gel blot in (A) were quantified by using ImageJ software and the ratio of phosphorylated S6K (p-S6K) to S6K or LC3-II to LC3-I was presented. The ratio of band intensities of p-S6K/S6K in each tissue from Spr+/+ mice was individually set to 1.0 (upper). The ratio of band intensities of LC-II/LC3-I in tissues from Spr+/+ mice was set to 1.0 (bottom). Values were means ± SD (n = 3 experiments). Statistical significance was determined by Student's t-test, *p < 0.05, compared with Spr+/+ mice. (C) Representative electron micrographs show the formation of autophagic vesicles in liver cells from 25-d-old Spr−/− mouse. Scale bars represent 2 µm. (D) Representative example of liver cells from Spr+/+ or Spr−/− mice indicating reduced cell size in Spr−/− mice. Liver sections from 25-d-old Spr+/+ (left) or Spr−/− (right) mice were stained with hematoxylin and eosine (H&E). Scale bars represent 20 µm. (E) Average number of cells in 10 randomly selected areas (100 µm × 100 µm) was presented. Statistical significance was determined by Student's t-test. Values represent means ± SD **p < 0.01, compared with Spr+/+ mice. (F) Dispensable role of BH4 deficiency on the activity of Akt or AMPK. Levels of Akt, AMPK, and their phosphorylated forms in livers and muscles from 25-d-old Spr−/− mice fed a normal diet were compared with those in Spr+/+ mice. The activation of Akt or AMPK was evaluated by examining the phosphorylation status of Akt at Ser473 or AMPK at Thr172 by protein gel blotting. (G) Relative band intensities in protein gel blot in (F) were quantified and ratios of p-Akt/Akt and p-AMPK/AMPK in the liver or muscle from Spr+/+ or Spr−/− mice were determined. The ratio of band intensities of p-Akt/Akt or p-AMPK/AMPK in tissues from Spr+/+ mice was respectively set to 1.0. Values represent means ± SD (n = 3 experiments). #, 0.1 < p < 1.0.
Tyrosine paucity induces autophagy in BH4-deficient Spr−/− mice.
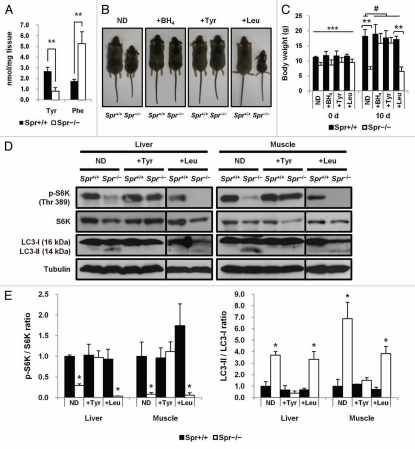
Since BH4 function is required for PAH, which converts phenylalanine into tyrosine, and amino acid availability is critical for the control of mTORC1 activation, it was necessary to determine whether tyrosine deficiency in BH4-deficient mice interferes with mTORC1 activity, resulting in the subsequent induction of autophagy. First, we attempted to show that BH4 deficiency leads to mTORC1 inhibition due to tyrosine deficiency in Spr−/− mice. Concentrations of phenylalanine and tyrosine in livers from Spr−/− mice were compared with those from control Spr+/+ mice. In accordance with the previous report in reference 5, phenylalanine levels in livers from Spr−/− mice were much higher than levels found in control Spr+/+ mice, while levels of tyrosine were fairly reduced in livers from Spr−/− mice (Fig. 2A). A causative correlation between tyrosine deficiency and growth retardation in Spr−/− mice was also investigated. We examined whether dietary tyrosine supplementation can rescue growth retardation in Spr−/− mice. For tyrosine replacement therapy, newborn Spr−/− mice were pretreated with BH4 by daily oral administration of a ‘high dose of BH4’ as indicated in Materials and Methods. All 35-d-old Spr−/− mice fed a normal diet displayed conspicuous growth retardation. Rescue of Spr−/− mice with BH4 supplementation was also performed as a positive control experiment as previously described in reference 5. Considerable weight gain was observed in Spr−/− mice that received tyrosine replacement therapy. The average body weight of Spr−/− mice fed a therapeutic tyrosine diet was not statistically different from that of Spr+/+ mice fed a normal diet. Although leucine is an important amino acid affecting mTORC1 signaling,34 no body weight gain was observed in Spr−/− mice fed a leucine-rich diet. Our data reveal that Spr−/− mice are rescued from growth retardation specifically by tyrosine supplementation (Fig. 2B and C). Knowing that mTORC1 is inactivated in Spr−/− mice with dwarfism, we examined whether mTORC1 activity is restored in rescued Spr−/− mice after tyrosine replacement therapy. Activities of mTORC1 in tissues of liver and muscle from Spr−/− mice were restored almost completely by tyrosine replacement therapy. Together with the activation of mTORC1, levels of LC3-II in tissues from Spr−/− mice were diminished by tyrosine replacement therapy. In order to ascertain that mTORC1 activity in Spr−/− mice is specifically restored by tyrosine supplementation, we compared the effect of leucine supplementation with that of tyrosine supplementation in Spr−/− mice. Neither the level of phosphorylated S6K nor lipidated LC3-II was affected by leucine supplementation in Spr−/− mice (Fig. 2D and E).
Figure 2.
Tyrosine deficiency causes autophagy induction following mTORC1 inactivation in Spr−/− mice. (A) Concentrations of liver phenylalanine and tyrosine in 25-d-old Spr+/+ or Spr−/− mice (n = 5 for each genotype). Data represent means ± SD, **p < 0.01, compared with Spr+/+ mice. (B) Weight gain of 35-d-old experimental mice. Newborn Spr−/− mice were pretreated with ‘high dose of BH4’ as described in Materials and Methods. For a control experiment, Spr−/− mice were fed a normal diet (ND) with or without BH4 solution (+BH4) for an additional 10 d. For replacement therapy, therapeutic tyrosine diet (+Tyr) or leucine diet (+Leu) was prepared as described in Materials and Methods. Spr−/− mice were fed a tyrosine or leucine supplemented diet for 10 d under ad libitum conditions. (C) Effects of tyrosine replacement therapy on weight gain of Spr−/− mice (n = 5 for each genotype per experimental group). Each experimental mouse was weighed before and after replacement therapy for 10 d. Data represent means ± SD, **p < 0.01. ***p < 0.001, compared with body weight of Spr+/+ mice fed a normal diet, one-way analysis of variance (ANOVA), #, 0.1 < p < 1.0. (D) Dietary tyrosine supplementation increases mTORC1 activity with subsequent inactivation of autophagic pathway in Spr−/− mice. Tissue homogenates from 35-d-old Spr+/+ or Spr−/− mice fed a normal diet, therapeutic tyrosine or leucine diet were analyzed by protein gel blotting to examine the phosphorylation of S6K and the conversion of LC3-I into LC3-II. (E) Band intensities of protein gel blot in (D) were quantified and ratios of p-S6K/S6K and LC3-II/LC3-I were determined (n = 3 experiments). The ratio of band intensities of p-S6K/S6K or LC3-II/LC3-I in liver or muscle tissue from Spr+/+ mice fed a normal diet was respectively set to 1.0. Values represent means ± SD, *p < 0.05.
Activation of autophagic pathway in Pahenu2 mice.
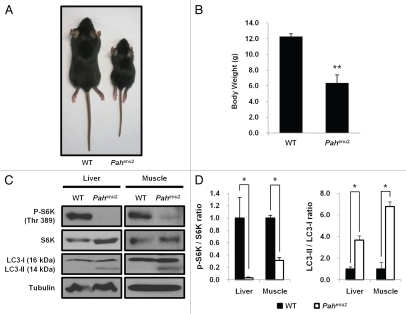
A genetic mouse model of PKU (Pahenu2) developed in the C57BL/6 background strain was employed to ascertain the induction of autophagy as well as mTORC1 inhibition by tyrosine deficiency. The Pahenu2 mice have a mutation in the gene for PAH in exon 7.35 In Pahenu2 mouse, PAH activities are minimal in the liver resulting in increased phenylalanine concentration but decreased tyrosine concentration in the brain.36,37 In the current study, Pahenu2 mice fed a normal diet exhibited significant growth retardation compared with the wild-type control (Fig. 3A and B). Activities of mTORC1 in tissues of liver or muscle were severely downregulated; however, the autophagic pathway was activated as assessed by the conversion of LC3-I into LC3-II in Pahenu2 mice with impaired PAH activity (Fig. 3C and D).
Figure 3.
Inactivation of mTORC1 and autophagy induction in Pahenu2 mice. (A and B) The growth of 25-d-old Pahenu2 mice was compared with that of 25-d-old wild-type mice (n = 6). Data represent means ± SD **p < 0.01, compared with the control group. (C) Inactivation of mTORC1 and induction of autophagy in Pahenu2 mice. Liver and muscle homogenates from 25-d-old wild-type or Pahenu2 mice fed a normal diet were analyzed by protein gel blotting to examine the phosphorylation of S6K and the conversion of LC3-I into LC3-II. (D) Band intensities of protein gel blot in (C) were quantified and ratios of p-S6K/S6K and LC3-II/LC3-I were presented. Values represent means ± SD (n = 3 experiments). *p < 0.05, compared with wild-type mice.
Dispensable role of phenylalanine excess on mTORC1 inactivation and autophagy induction in NIH3T3 cells.
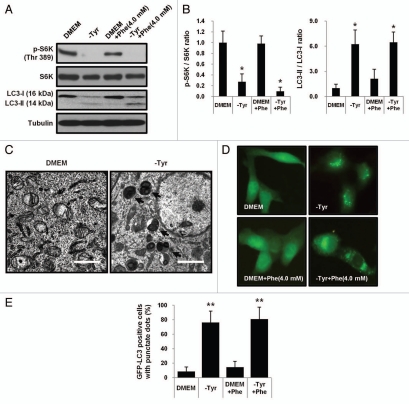
BH4 deficiency creates a metabolic situation characterized by an excess of phenylalanine and tyrosine paucity. The effect of tyrosine or phenylalanine availability on autophagy induction and mTORC1 function was investigated in NIH3T3 mouse fibroblasts. Cells were grown in media containing an excess amount of phenylalanine, or in media lacking tyrosine. Data in Figure 4A and B demonstrate autophagy induction as well as mTORC1 inactivation in NIH3T3 cells grown under tyrosine restriction. Phosphorylation of S6K was inhibited but the conversion of LC3-I into LC3-II was increased in NIH3T3 cells grown under tyrosine limitation. The result showing the absence of an effect of phenylalanine excess on autophagy induction in NIH3T3 cells grown in media containing 4.0 mM phenylalanine (normal growth medium contain 0.4 mM phenylalanine) also supports our notion that mTORC1 inactivation followed by autophagy induction in BH4 deficient Spr−/− mice is mediated by tyrosine deficiency, but not by phenylalanine excess. Additionally, ultrastructural analysis shows the accumulation of autophagic vesicles in NIH3T3 cells grown under tyrosine deficiency for 24 h (Fig. 4C). The important role of tyrosine availability in the activation of the autophagic pathway was further stressed by the accumulation of autophagic vesicles in NIH3T3-GFP-LC3 cells stably expressing GFP-LC3. Ectopically-expressed GFP-LC3 accumulated in autophagic vesicles was imaged by fluorescent microscopy and quantified (Fig. 4D and E).
Figure 4.
Induction of autophagy by tyrosine deficiency, but not by phenylalanine excess in NIH 3T3 cells. (A) NIH 3T3 cells were grown for 24 h in DMEM, tyrosine-restricted media (-Tyr), DMEM containing an excess amount of phenylalanine (4.0 mM) or tyrosine-restricted media containing 4.0 mM phenylalanine. Cells were examined for the phosphorylation of S6K and the conversion of LC3-I into LC3-II. (B) Band intensities of protein gel blot were quantified and the ratio of p-S6K/S6K or LC3-II/LC3-I was shown. The ratio of band intensities of p-S6K/S6K or LC-II/LC3-I in cells grown in complete DMEM was respectively set to 1.0. Values represent means ± SD (n = 3 experiments). *p < 0.05. (C) Electron micrographs demonstrate the induction of autophagy in NIH 3T3 cells grown in tyrosine-deprived DMEM for 24 h. Scale bars represent 2 µm. (D and E) NIH 3T3-GFP-LC3 cells stably expressing GFP-LC3 were incubated in DMEM, tyrosine-restricted media, DMEM containing excess amount of phenylalanine (4.0 mM) or tyrosine-restricted media containing 4.0 mM phenylalanine for 24 h. Accumulation of GFP-LC3 was visualized by fluorescent microscope and quantified. Combined results from three independent experiments were shown as means ± SD of the percentage of GFP-LC3-positive cells with punctate dots. **p < 0.01, compared with cells incubated in complete DMEM.
Inactivation of autophagic pathway by constitutively active Rheb in NIH3T3 cells grown under tyrosine deficiency.
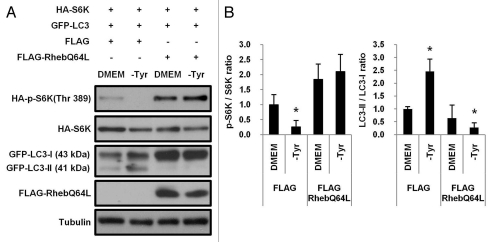
Since mTORC1 is an important negative regulator of autophagy,14,38 we examined whether autophagic pathway induced by tyrosine deficiency could be inactivated by mTORC1 activation. NIH3T3 cells were cotransfected with FLAG-RhebQ64L, a constitutively active mutant of Rheb, GFP-LC3 and HA-S6K. At 16 h after transfection, culture media were replaced with fresh DMEM or tyrosine-deficient media (-Tyr). Cells were then further incubated for 24 h. Ectopic expression of RhebQ64L strongly induced the phosphorylation of HA-S6K and suppressed the conversion of GFP-LC3-I into GFP-LC3-II in cotransfected NIH3T3 cells growing under tyrosine-deficient conditions (Fig. 5A and B).
Figure 5.
Inactivation of autophagic pathway by constitutively active Rheb in NIH 3T3 cells grown under tyrosine deficiency. (A) NIH 3T3 cells (1 × 106) grown in complete media were transiently cotransfected with Flag-RhebQ64L (0.8 µg), GFP-LC3 (0.1 µg) and HA-S6K (0.1 µg). At 16 h after transfection, culture media were replaced with fresh DMEM or tyrosine-deficient media (-Tyr) and incubated for additional 24 h. The phosphorylation of ectopically expressed S6K (HA-S6K) and conversion of GFP-LC3-I into GFP-LC3-II were examined by protein gel blotting. (B) Band intensities were quantified and the ratio of p-S6K/S6K or LC3-II/LC3-I was shown. (n = 3 experiments). Values represent means ± SD, *p < 0.05.
Activation of autophagic pathway in BH4-responsive PKU patients.
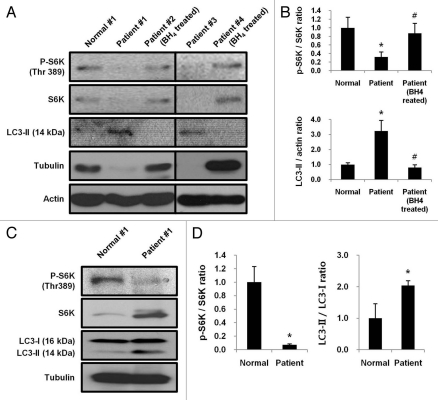
We tested the hypothesis that mTORC1-dependent autophagic pathway is activated in patients with BH4-deficiency. The phosphorylation of S6K in lymphocytes from untreated patients (Patient #1 and #3) was severely suppressed while levels of lipidated LC3-II were elevated. However, mTORC1 activities were recovered along with the inactivation of autophagic pathway in BH4-responsive PKU patients (Patient #2 and #4) who received regular BH4 treatment (400 mg/day) (Fig. 6A and B). Since lymphocytes from two untreated BH4-responsive PKU patients (Patient #1 and #3) revealed diminished levels of tubulin along with insufficient levels of S6K, we used actin as a loading control instead of tubulin to measure the phosphorylation of S6K in lymphocytes. An independent experiment was performed to test whether the reduced phosphorylation of S6K merely represents insufficient levels of S6K in Patients #1 and #3. A larger amount of lysate from Patient #1 (250 µg protein) than the amount of lymphocyte lysate protein from a healthy control (Normal #1, 50 µg protein) was subjected to protein gel blotting to determine the level of phosphorylated S6K using tubulin as a loading control. Although the lymphocytes lysate from Patient #1 contained much larger amount of S6K than Normal #1, the level of phosphorylated S6K was much lower in lymphocytes from Patient #1 than Normal #1, indicating a marked inactivation of mTORC1 in BH4-responsive PKU patients (Fig. 6C and D). Therefore, our data conclude that activities of mTORC1 were severely suppressed stimulating the induction of autophagy in Patients #1 and #3 affected by BH4 deficiency.
Figure 6.
Downregulation of mTORC1 activates autophagic pathway in BH4-responsive PKU patients. (A) Levels of phosphorylated S6K and LC3-II in lymphocytes from BH4-responsive PKU patients with BH4 deficiency left untreated (Patient #1 and #3) or treated with 400 mg/day BH4 (Patient #2 and #4) were examined by protein gel blotting. Patient #1, #2 and #4: PTPS deficiency, Patient #3: DHPR deficiency. (B) Band intensities were quantified and ratios of p-S6K/S6K and LC3-II/actin were determined. Values represent means ± SD (n = 3 experiments). *p < 0.05. #, 0.1 < p < 1.0. (C) Levels of S6K and phosphorylated S6K in 50 µg of lymphocyte lysate protein from a healthy child (Normal #1) were compared with those in 250 µg of lymphocyte lysate protein from BH4-responsive PKU patient (Patient #1). (D) Band intensities were quantified and the ratio of p-S6K/S6K or LC3-II/LC3-I in PKU patient was compared with that in normal control. Values represent means ± SD (n = 3 experiments). *p < 0.05.
Discussion
BH4 is present in most mammalian tissues and cells. Impaired BH4 homeostasis affects hydroxylations of aromatic amino acids and influences various neurological functions. Here, we first report the induction of mTORC1-dependent autophagy in BH4 deficiency.
The crosstalk between BH4 function and mTORC1-dependent autophagy was sought in BH4-deficient Spr−/− mice. We found that mTORC1 inactivation and subsequent activation of autophagy in Spr−/− mice are attributable to tyrosine deficiency. Amino acid starvation not only inactivates mTORC1 signaling but also induces autophagy to provide an internal source of nutrition that supports cellular energy production and biosynthesis through the degradation of self-cellular proteins.32,39 Enhanced levels of lipidated LC3-II were observed in tissues from Spr−/− mice (Fig. 1A). In addition, electron micrographs indicate autophagic vesicle formation in livers of Spr−/− mice (Fig. 1B). We evaluated the effect of mTORC1 inhibition on cell size in BH4-deficient Spr−/− mice since mTORC1 signaling functions as a positive regulator of cell growth and size.40 A significant reduction in cell size in the liver from Spr−/− mice strongly supports the functional link between mTORC1 inactivation and BH4 deficiency (Fig. 1D and E). The mechanism involved in mTORC1 inactivation by BH4 deficiency has not yet been proposed. Studies have demonstrated that PI3K/Akt pathway is a predominant upstream signal pathway that regulates mTORC1 activation.15 However, it is unlikely that BH4 deficiency inactivates mTORC1 signaling through the inactivation of PI3K/Akt pathway since PI3K/Akt pathway was not at least inhibited in BH4-deficient Spr−/− mice as assessed by the phosphorylation of Akt. Neither AMPK appear to participate in the inactivation of mTORC1 by BH4 deficiency in this mouse model because AMPK activity assessed by the phosphorylation of AMPK was not enhanced enough to account for the inhibition of mTORC1 in tissues from Spr−/− mice. Thus, these observations predict that mTORC1 inactivation in Spr−/− mice is not mediated by the modulation of Akt or AMPK (Fig. 1F and G).
The data in Figure 2 imply that mTORC1 inactivation and subsequent autophagy induction in BH4-deficient Spr−/− mice are mediated by the limited availability of tyrosine. Discernible growth retardation of Spr−/− mice with dwarfism has been shown to be rescued by tyrosine supplementation (Fig. 2B and C). Tyrosine replacement therapy restored mTORC1 activity along with the weight gain in Spr−/− mice. The important role of tyrosine availability on autophagic pathway, which is negatively regulated by mTORC1 signaling, was illustrated by results showing the restoration of mTORC1 activity after tyrosine replacement therapy (Fig. 2D and E). How mTORC1 signaling is wired to pathways that determine the size of animals and organs are the least known aspect of mTORC1 signaling. However, there are several lines of evidence that support the role of mTORC1 in regulating the whole-body size of animals and organs. Treatment of experimental mice with rapamycin, a known mTORC1 inhibitor, has been shown to reduce heart size and body mass.41,42 Although leucine deprivation in growth media has been known to be highly inhibitory to mTORC1 activity in cultured mammalian cells,34 supplementation with a high dose of leucine affects neither mTORC1 activity nor the weight gain in Spr−/− mice (Fig. 2B–E). The inactivation of autophagic pathway by tyrosine supplementation (Fig. 2D and E) indicates that tyrosine deficiency induces signals that activate the autophagic pathway in BH4-deficient Spr−/− mice, suggesting an interplay between BH4 deficiency and autophagy induction.
Our notion that tyrosine deficiency activates autophagic pathway by inactivating mTORC1 signaling in BH4-deficient Spr−/− mice was also examined in a genetic mouse model of PKU (Pahenu2). The inactivation of mTORC1 and growth retardation in Pahenu2 mice lacking the ability to convert phenylalanine into tyrosine strongly support that the induction of autophagy by the inactivation of mTORC1 in Spr−/− mice is mediated by tyrosine paucity (Fig. 3). However, a high level of phenylalanine interferes with neither mTORC1 function nor autophagic pathway in Spr−/− mice because the phosphorylation of S6K and the conversion of cytoplasmic LC3-I into lipidated LC3-II in NIH3T3 cells grown in media containing high concentration of phenylalanine was virtually the same as in cells grown in normal complete media (Fig. 4). The important role of mTORC1 inactivation in the activation of the autophagic pathway by tyrosine paucity was evidenced in NIH3T3 cells overexpressing constitutively active mutant of Rheb, RhebQ64L. The autophagic pathway was inhibited when mTORC1 activity was enhanced by RhebQ64L in NIH3T3 cells under tyrosine-deficient conditions (Fig. 5).
BH4 has been known to exert proliferative activities in cultured murine cells.43–45 Signaling through mTORC1 has been regarded as a central regulator of cell growth by controlling the protein synthetic machinery in mammalian cells.12,15 Decreased concentration of BH4 in the cerebral spinal fluid has been documented in many neurological diseases such as Parkinson and Alzheimer disease.46–48 Recent studies have disclosed that mTORC1 activities are modified in these neurodegenerative disorders.49 The present study confirms that tyrosine paucity resulting from BH4 deficiency causes mTORC1 inactivation followed by the induction of autophagy in experimental mice and reports that the mTORC1-dependent autophagic pathway is activated in BH4-responsive PKU patients. There are two types of BH4-responsive PKU patients; those with reduced binding affinity to BH4 due to mutations in PAH gene and those with defects in BH4 metabolism.50 The plasma concentration of phenylalanine is elevated in both types of BH4-responsive PKU patients.3,51 In Figure 6, we explored a hypothesis that the failure to convert phenylalanine to tyrosine and low birth weight in patients with BH4 deficiency7–9 might correlate with the inactivation of mTORC1 signaling and activation of autophagic pathway. Lymphocytes from BH4-responsive PKU patients with a high level of plasma phenylalanine (>400 µmol/L) contained insufficient amounts of S6K and phosphorylated S6K. Interestingly, diminished levels of tubulin were also observed in lymphocytes from BH4-responsive PKU patients. Since the inactivation of mTORC1 signaling pathway also promotes the subsequent activation of the autophagic pathway in PKU patients with BH4-responsiveness, it is likely that cells in patients with BH4 deficiency degrade the principal component of cytoskeleton such as tubulin. Janelidze et al.52 have previously shown that the level of S6K protein in brain tissue from rats was decreased along with the suppression of mTORC1 activity during focal ischemia. Decreased levels of tubulin were also reported in mouse embryonic fibroblast cells after incubation in media deprived of growth factors and amino acids.53 The phosphorylation of S6K by mTORC1 activation and LC3 conversion by autophagy induction in patients with BH4-deficient PKU were measured using actin as a loading control (Fig. 6A). We also noticed that mTORC1 activity was restored and the autophagic pathway was inactivated in BH4-responsive PKU patients treated with BH4. An independent experiment was performed to assess the possibility that the decreased levels of phosphorylated S6K in BH4-responsive PKU patients are attributable to the diminished S6K substrate levels. The result in Figure 6C and D reveals that both the level of S6K substrate and the activity of mTORC1 to phosphorylate S6K were seriously reduced in BH4-responsive PKU patients.
There have been studies emphasizing the potential role of tyrosine deficiency in the causation of mental retardation in maternal PKU.54–57 Low concentrations of tyrosine in both the fetus and pregnant mother have been implicated as an important factor in fetal damage in maternal PKU.56,57 In fact, treatment of pregnant PKU patients with tyrosine has been reported to exhibit some alleviation of the symptoms.55 Our study demonstrating the activation of autophagic pathway by mTORC1 inactivation in PKU-affected patients with BH4 deficiency may provide some additional insight into therapeutics that could be used to improve the symptoms of diseases associated with BH4 deficiency.
Materials and Methods
Materials and growth media.
BH4-2HCl (11.212-5) was obtained from Schircks Laboratories. L-DOPA (D9628), 5-hydroxytryptophan (H9772), carbidopa (C1335), ascorbic acid (A4544), N-acetyl-L-cysteine (A8199), L-tyrosine (T3754), L-leucine (L8912), L-phenylalanine (P5482), L-glutamine (G8540) and anti-α-tubulin antibody (T5168) were purchased from Sigma. Anti-phospho-S6K (Thr389) (#9234), Anti-phospho-Akt (Ser473) (#9271), anti-Akt (#9272), anti-phospho-AMPK (Thr172) (#2535) and anti-AMPK (#2632) antibodies were purchased from Cell Signaling Technology. Anti-S6K (sc-8414) and anti-actin (sc-1616-R) antibodies were obtained from Santa Cruz Biotechnology. Anti-LC3 antibody (NB100-2220) was from Novus Biological. Customized DMEM (LM001-95) deprived of tyrosine, phenylalanine and glutamine was supplied by WelGENE Inc. To prepare tyrosine-restricted medium, phenylalanine (0.4 mM) and glutamine (4 mM) along with 10% dialyzed FBS (WelGENE Inc., S001-01) were added to Tyr/Phe/Gln-deprived DMEM.
Experimental mice and replacement therapy.
All animal experiments were compliant with the guidelines of Institutional Animal Care and Use Committee (IACUC) at Korea Advanced Institute of Science and Technology (KAIST). Spr−/− mouse line on a mixed C57BL6/sv129 hybrid background was generated as previously described in reference 5. Both Spr+/+ and Spr−/− mice from the same mother were raised in the same cage and weaned at 20 d after birth. For analyses of mTORC1 activity and autophagy induction, Spr+/+ or Spr−/− mice were fed a normal diet for an additional 5 d after weaning and then sacrificed to prepare tissue samples for protein gel blotting. For tyrosine replacement therapy test, newborn Spr−/− mice were pretreated with a ‘high dose of BH4’ solution containing BH4 (122 µg/g body weight/day), L-DOPA (13.5 µg/g body weight/day), 5-hydroxytryptophan (9.5 µg/g body weight/day), carbidopa (2.5 µg/g body weight/day), ascorbic acid (100 µg/g body weight/day) and N-acetyl-L-cysteine (50 µg/g body weight/day) by oral administration as described by Elzaouk et al.58 for 25 d. Spr+/+ or Spr−/− mice were weaned at 20 d of age and fed a normal diet ad libitum for 5 d to acclimate them to normal mouse chow. After the acclimatization period, 25-d-old mice were kept in separate cages for tyrosine replacement therapy test. Mice were fed either a normal or therapeutic tyrosine diet in which 5.6% tyrosine (w/w) was added to the normal diet59 for an additional 10 d under ad libitum conditions. For leucine supplementation, 25-d-old Spr−/− mice pretreated with ‘high dose of BH4’ solution were fed a leucine supplemented diet in which 3.6% leucine (w/w) was added to the normal diet for 10 d under ad libitum conditions.
PAH-deficient C57BL6-Pahenu2 (Pahenu2) mice were kindly provided by Dr. Beat Thöny (University of Zürich). Pahenu2 mice were also raised and weaned at 20 d after birth. For analyses of mTORC1 activity and autophagy induction, wild-type or mutant Pahenu2 mice were fed a normal diet for an additional 5 d after weaning and then sacrificed to prepare tissue samples for protein gel blotting.
Determination of phenylalanine and tyrosine levels.
Concentrations of free phenylalanine and tyrosine in livers from Spr−/− mice were compared with those from Spr+/+ mice by reversed phase high-performance liquid chromatography (RP-HPLC) using the Pico-Tag method. Phenylisothiocyanate-derivatized free amino acids were analyzed on a Pico-Tag amino acid analysis column with monitoring of the elutes at 254 nm. The phenylalanine and tyrosine were identified and quantified by comparing their elution points and peak areas, respectively, with those of standards.
Electron microscopy.
Fragmented tissues from experimental mice or cell preparations from cultured NIH3T3 cells were fixed using Karnovsky's fixative solution (2% paraformaldehyde, 2% glutaraldehyde, 0.5% calcium chloride in cacodylate buffer, pH 7.2), washed with cacodylate buffer, dehydrated through a graded ethanol series and embedded in resin. Blocks were sectioned on a Reichert Jung Ultracut S (Leica, Germany), mounted on grids and stained with uranyl acetate and lead citrate. Images were analyzed using a Zeiss EM 902 A electron microscope.
Establishment of NIH3T3 cell line stably expressing GFP-LC3.
293T cells were cotransfected with retroviral pRe-vExTO2 expression vector containing cDNA encoding N-terminal GFP fusion of LC3 (GFP-LC3) and pGP expression vector for MuLV Gag-Pol (Takara, 6160) together with the pVpack-VSV-G expression vector encoding VSV-G protein (Stratagene, 217567). Viral particles were collected 48 h after transfection, filtered, and used to infect NIH3T3 cells. Transduced NIH3T3-GFP-LC3 cells were selected in DMEM containing 3 µg/ml puromycin (Amresco, J593) to establish stable subclones.
RhebQ64L mutant construct.
cDNA encoding mouse Rheb was cloned into EcoRI and BamHI sites of expression vector pFlag-CMV2 (Sigma, E7033) to produce pFlag-Rheb vector using PCR primers 5′-CGG AAT TCA ATG CCT CAG TCC AA-3′ and 5′-CGG GAT CCT CAC ATC ACC GAG C-3′. The mutant construct containing Q64L substitution was generated by using two-step PCR-based targeted mutagenesis. RhebQ64L was cloned into EcoRI and BamHI sites of expression vector pFlag-CMV2 to produce pFlag-RhebQ64L vector. Specific primers used to amplify the substituted fragment were 5′-AGA CAC AGC GGG GCT GGA TGA AT-3′ and 5′-ATT CAT CCA GCC CCG CTG TGT CT-3′.
Patients with BH4-responsive PKU.
BH4-responsive PKU affected children whose initial phenylalanine levels were higher than 400 µmol/L were selected from the Department of Pediatrics (Soonchunhyang University Hospital, South Korea). This study was approved by Institutional Review Board of each respective institution. Parents of all patients and controls gave their informed written consent for this study. PKU affected patients were given a single oral dose of BH4 (20 mg/kg body weight) and blood phenylalanine levels were determined 24 h after BH4 loading. Patients whose blood phenylalanine levels decreased more than 30% in 24 h after the BH4 loading test were diagnosed as BH4-responsive PKU. Two subjects, untreated 1- and 9-y-old female BH4-responsive PKU patients, were chosen for this study. Analysis of their genotypes disclosed that the 1-y-old patient was 6-pyruvoyl-tetrahydropterin synthase (PTPS)-deficient and the 9-y-old patient was dihydropteridine reducase (DHPR)-deficient. A blood sample was also drawn from other BH4-responsive PKU patients, an 11-y-old female and a 16-y-old male who received regular BH4 replacement therapy (400 mg/day) immediately after their birth. Medical records disclosed that both patients were BH4-deficient due to PTPS deficiency. Human mononuclear lymphocytes (1 × 105) from the normal or BH4-responsive PKU patients were isolated using Ficoll-paque (GE HealthCare, 17-1440-02) density gradient centrifugation as described in the manufacturer's instructions. Lymphocyte lysates were analyzed for the phosphorylation of S6K as a measure for mTORC1 activity and levels of LC3-II as a measure for autophagy induction by protein gel blotting.
Acknowledgements
We thank S.P. Oh for the Spr−/− mouse strain and B. Thöny for Pahenu2 mice. Authors are grateful to J.S. Choi (Korea Basic Science Institute) for amino acid analysis and H. Joe for editorial assistance. This study was supported in part by grants from the Korea Science and Engineering Foundation (C.O.J.), the Korea Research Foundation (C.O.J.) and the National Institute of Health, R01 CA101035 (G.G.M.).
Abbreviation
- BH4
tetrahydrobiopterin
- mTORC1
mammalian target of rapamycin complex 1
- PI3K
phosphatidylinositol-3-kinase
- S6K
ribosomal protein S6 kinase
- LC3
microtubule-associated protein 1 light chain 3
- PKU
phenylketonuria
- TH
tyrosine hydroxylase
- TPH
tryptophan hydroxylase
- NOS
nitric oxide synthase
- GTPCH
GTP cyclohydrolase I
- PTPS
6-pyruvoyl-tetrahydropterin synthase
- SPR
sepiapterin reductase
- DHFR
dihydrofolate reductase
- DHPR
dihydropteridine reductase
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Thöny B, Auerbach G, Blau N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J. 2000;347:1–16. doi: 10.1042/0264-6021:3470001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foxton RH, Land JM, Heales SJ. Tetrahydrobiopterin availability in Parkinson's and Alzheimer's disease; potential pathogenic mechanisms. Neurochem Res. 2007;32:751–756. doi: 10.1007/s11064-006-9201-0. [DOI] [PubMed] [Google Scholar]
- 3.Thöny B, Blau N. Mutations in the GTP cyclohydrolase I and 6-pyruvoyl-tetrahydropterin synthase genes. Hum Mutat. 1997;10:11–20. doi: 10.1002/(SICI)1098-1004(1997)10:1<11::AID-HUMU2> 3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 4.Takazawa C, Fujimoto K, Homma D, Sumi-Ichinose C, Nomura T, Ichinose H, et al. A brain-specific decrease of the tyrosine hydroxylase protein in sepiapterin reductase-null mice-as a mouse model for Parkinson's disease. Biochem Biophys Res Commun. 2008;367:787–792. doi: 10.1016/j.bbrc.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 5.Yang S, Lee YJ, Kim JM, Park S, Peris J, Laipis P, et al. A murine model for human sepiapterin-reductase deficiency. Am J Hum Genet. 2006;78:575–587. doi: 10.1086/501372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blau N, Heizmann CW, Sperl W, Korenke GC, Hoffmann GF, Smooker PM, et al. Atypical (mild) forms of dihydropteridine reductase deficiency: neurochemical evaluation and mutation detection. Pediatr Res. 1992;32:726–730. doi: 10.1203/00006450-199212000-00021. [DOI] [PubMed] [Google Scholar]
- 7.Ponzone A, Spada M, Ferraris S, Dianzani I, de Sanctis L. Dihydropteridine reductase deficiency in man: from biology to treatment. Med Res Rev. 2004;24:127–150. doi: 10.1002/med.10055. [DOI] [PubMed] [Google Scholar]
- 8.Takanashi J, Kanazawa M, Kohno Y. Central tegmental tract involvement in an infant with 6-pyruvoyltetrahydropterin synthetase deficiency. AJNR Am J Neuroradiol. 2006;27:584–585. [PMC free article] [PubMed] [Google Scholar]
- 9.Chien YH, Chiang SC, Huang A, Lin JM, Chiu YN, Chou SP, et al. Treatment and outcome of Taiwanese patients with 6-pyruvoyltetrahydropterin synthase gene mutations. J Inherit Metab Dis. 2001;24:815–823. doi: 10.1023/A:1013984022994. [DOI] [PubMed] [Google Scholar]
- 10.Lovenberg W, Levine RA, Robinson DS, Ebert M, Williams AC, Calne DB. Hydroxylase cofactor activity in cerebrospinal fluid of normal subjects and patients with Parkinson's disease. Science. 1979;204:624–626. doi: 10.1126/science.432666. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt TS, Alp NJ. Mechanisms for the role of tetrahydrobiopterin in endothelial function and vascular disease. Clin Sci (Lond) 2007;113:47–63. doi: 10.1042/CS20070108. [DOI] [PubMed] [Google Scholar]
- 12.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Noda T, Ohsumi Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J Biol Chem. 1998;273:3963–3966. doi: 10.1074/jbc.273.7.3963. [DOI] [PubMed] [Google Scholar]
- 15.Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Blommaart EF, Luiken JJ, Blommaart PJ, van Woerkom GM, Meijer AJ. Phosphorylation of ribosomal protein S6 is inhibitory for autophagy in isolated rat hepatocytes. J Biol Chem. 1995;270:2320–2326. doi: 10.1074/jbc.270.5.2320. [DOI] [PubMed] [Google Scholar]
- 17.Shigemitsu K, Tsujishita Y, Hara K, Nanahoshi M, Avruch J, Yonezawa K. Regulation of translational effectors by amino acid and mammalian target of rapamycin signaling pathways. Possible involvement of autophagy in cultured hepatoma cells. J Biol Chem. 1999;274:1058–1065. doi: 10.1074/jbc.274.2.1058. [DOI] [PubMed] [Google Scholar]
- 18.Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol. 2005;15:702–713. doi: 10.1016/j.cub.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 19.Nobukuni T, Joaquin M, Roccio M, Dann SG, Kim SY, Gulati P, et al. Amino acids mediate mTOR/raptor signaling through activation of class 3-phosphatidylinositol-3OH-kinase. Proc Natl Acad Sci USA. 2005;102:14238–14243. doi: 10.1073/pnas.0506925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nobukuni T, Kozma SC, Thomas G. hvps34, an ancient player, enters a growing game: mTOR Complex1/S6K1 signaling. Curr Opin Cell Biol. 2007;19:135–141. doi: 10.1016/j.ceb.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 21.Gulati P, Gaspers LD, Dann SG, Joaquin M, Nobukuni T, Natt F, et al. Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab. 2008;7:456–465. doi: 10.1016/j.cmet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez Canedo C, Demeulder B, Ginion A, Bayascas JR, Balligand JL, Alessi DR, et al. Activation of the cardiac mTOR/p70(S6K) pathway by leucine requires PDK1 and correlates with PRAS40 phosphorylation. Am J Physiol Endocrinol Metab. 2010;298:761–769. doi: 10.1152/ajpendo. 00421.2009. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Harris TE, Roth RA, Lawrence JC., Jr PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. J Biol Chem. 2007;282:20036–20044. doi: 10.1074/jbc.M702376200. [DOI] [PubMed] [Google Scholar]
- 24.Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9:316–323. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 25.Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, et al. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol. 2008;10:935–945. doi: 10.1038/ncb1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell. 2009;20:1981–1991. doi: 10.1091/mbc. E08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, et al. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Codogno P, Meijer AJ. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ. 2005;12:1509–1518. doi: 10.1038/sj.cdd.4401751. [DOI] [PubMed] [Google Scholar]
- 33.Dröge W. Autophagy and aging—importance of amino acid levels. Mech Ageing Dev. 2004;125:161–168. doi: 10.1016/j.mad.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Hara K, Yonezawa K, Weng QP, Kozlowski MT, Belham C, Avruch J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem. 1998;273:14484–14494. doi: 10.1074/jbc.273.23.14484. [DOI] [PubMed] [Google Scholar]
- 35.Ding Z, Harding CO, Rebuffat A, Elzaouk L, Wolff JA, Thony B. Correction of murine PKU following AAV-mediated intramuscular expression of a complete phenylalanine hydroxylating system. Mol Ther. 2008;16:673–681. doi: 10.1038/mt.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zagreda L, Goodman J, Druin DP, McDonald D, Diamond A. Cognitive deficits in a genetic mouse model of the most common biochemical cause of human mental retardation. J Neurosci. 1999;19:6175–6182. doi: 10.1523/JNEUROSCI.19-14-06175.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith CB, Kang J. Cerebral protein synthesis in a genetic mouse model of phenylketonuria. Proc Natl Acad Sci USA. 2000;97:11014–11019. doi: 10.1073/pnas.97.20.11014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors and stress. Mol Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Proud CG. Amino acids and mTOR signalling in anabolic function. Biochem Soc Trans. 2007;35:1187–1190. doi: 10.1042/BST0351187. [DOI] [PubMed] [Google Scholar]
- 40.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rovira J, Marcelo Arellano E, Burke JT, Brault Y, Moya-Rull D, Banon-Maneus E, et al. Effect of mTOR inhibitor on body weight: from an experimental rat model to human transplant patients. Transpl Int. 2008;21:992–998. doi: 10.1111/j.1432-2277.2008.00710.x. [DOI] [PubMed] [Google Scholar]
- 42.Shioi T, McMullen JR, Kang PM, Douglas PS, Obata T, Franke TF, et al. Akt/protein kinase B promotes organ growth in transgenic mice. Mol Cell Biol. 2002;22:2799–2809. doi: 10.1128/MCB.22.8.2799-809.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanaka K, Kaufman S, Milstien S. Tetrahydrobiopterin, the cofactor for aromatic amino acid hydroxylases, is synthesized by and regulates proliferation of erythroid cells. Proc Natl Acad Sci USA. 1989;86:5864–5867. doi: 10.1073/pnas.86.15.5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kerler F, Ziegler I, Schmid C, Bacher A. Synthesis of tetrahydrobiopterin in Friend erythroleukemia cells and its modulator effect on cell proliferation. Exp Cell Res. 1990;189:151–156. doi: 10.1016/0014-4827(90)90229-4. [DOI] [PubMed] [Google Scholar]
- 45.Anastasiadis PZ, Bezin L, Imerman BA, Kuhn DM, Louie MC, Levine RA. Tetrahydrobiopterin as a mediator of PC12 cell proliferation induced by EGF and NGF. Eur J Neurosci. 1997;9:1831–1837. doi: 10.1111/j.1460-9568.1997.tb00749.x. [DOI] [PubMed] [Google Scholar]
- 46.Choi HJ, Lee SY, Cho Y, No H, Kim SW, Hwang O. Tetrahydrobiopterin causes mitochondrial dysfunction in dopaminergic cells: implications for Parkinson's disease. Neurochem Int. 2006;48:255–262. doi: 10.1016/j.neuint.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 47.Curtius HC, Niederwieser A, Levine R, Muldner H. Therapeutic efficacy of tetrahydrobiopterin in Parkinson's disease. Adv Neurol. 1984;40:463–466. [PubMed] [Google Scholar]
- 48.Barford PA, Blair JA, Eggar C, Hamon C, Morar C, Whitburn SB. Tetrahydrobiopterin metabolism in the temporal lobe of patients dying with senile dementia of Alzheimer type. J Neurol Neurosurg Psychiatry. 1984;47:736–738. doi: 10.1136/jnnp.47.7.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swiech L, Perycz M, Malik A, Jaworski J. Role of mTOR in physiology and pathology of the nervous system. Biochim Biophys Acta. 2008;1784:116–132. doi: 10.1016/j.bbapap.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 50.Blau N, Erlandsen H. The metabolic and molecular bases of tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency. Mol Genet Metab. 2004;82:101–111. doi: 10.1016/j.ymgme.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 51.Spaapen LJ, Rubio-Gozalbo ME. Tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency, state of the art. Mol Genet Metab. 2003;78:93–99. doi: 10.1016/S1096-7192(02)00229-9. [DOI] [PubMed] [Google Scholar]
- 52.Janelidze S, Hu BR, Siesjo P, Siesjo BK. Alterations of Akt1 (PKBalpha) and p70(S6K) in transient focal ischemia. Neurobiol Dis. 2001;8:147–154. doi: 10.1006/nbdi.2000.0325. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi Y, Coppola D, Matsushita N, Cualing HD, Sun M, Sato Y, et al. Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis. Nat Cell Biol. 2007;9:1142–1151. doi: 10.1038/ncb1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bessman SP. Genetic failure of fetal amino acid “justification”: a common basis for many forms of metabolic, nutritional and “nonspecific” mental retardation. J Pediatr. 1972;81:834–842. doi: 10.1016/S0022-3476(72)80117-3. [DOI] [PubMed] [Google Scholar]
- 55.Bessman SP. Historical perspective: tyrosine and maternal phenylketonuria, welcome news. Am J Clin Nutr. 1998;67:357–358. doi: 10.1093/ajcn/67.3.357. [DOI] [PubMed] [Google Scholar]
- 56.van Spronsen FJ, van Rijn M, Bekhof J, Koch R, Smit PG. Phenylketonuria: tyrosine supplementation in phenylalanine-restricted diets. Am J Clin Nutr. 2001;73:153–157. doi: 10.1093/ajcn/73.2.153. [DOI] [PubMed] [Google Scholar]
- 57.Rohr FJ, Lobbregt D, Levy HL. Tyrosine supplementation in the treatment of maternal phenylketonuria. Am J Clin Nutr. 1998;67:473–476. doi: 10.1093/ajcn/67.3.473. [DOI] [PubMed] [Google Scholar]
- 58.Elzaouk L, Leimbacher W, Turri M, Ledermann B, Burki K, Blau N, et al. Dwarfism and low insulin-like growth factor-1 due to dopamine depletion in Pts−/− mice rescued by feeding neurotransmitter precursors and H4-biopterin. J Biol Chem. 2003;278:28303–28311. doi: 10.1074/jbc.M303986200. [DOI] [PubMed] [Google Scholar]
- 59.Matalon R, Michals-Matalon K, Bhatia G, Grechanina E, Novikov P, McDonald JD, et al. Large neutral amino acids in the treatment of phenylketonuria (PKU) J Inherit Metab Dis. 2006;29:732–738. doi: 10.1007/s10545-006-0395-8. [DOI] [PubMed] [Google Scholar]