Abstract
T helper 17 (TH17) cells have well-described roles in autoimmune disease. Recent evidence suggests that this effector T cell subset is also involved in tumour immunology and may be a target for cancer therapy. In this Review, we summarize recent findings regarding the nature and relevance of TH17 cells in mouse models of cancer and human disease. We describe the interplay between TH17 cells and other immune cells in the tumour microenvironment, and we assess both the potential antitumorigenic and pro-tumorigenic activities of TH17 cells and their associated cytokines. Understanding the nature of TH17 cell responses in the tumour microenvironment will be important for the design of more efficacious cancer immunotherapies.
Although the link between inflammation and cancer has been noted for more than a century, investigators have only recently started to address the cellular, molecular and genetic causal relationships between these two events. Compelling evidence has shown that inflammation orchestrates the microenvironment around tumours, contributing to the proliferation, migration and survival of cancer cells that can result in tumour invasion, migration and metastasis. However, inflammatory reactions in the tumour microenvironment are an important component of the tumour-associated immune response. Inflammatory cells and molecules may have crucial roles in initiating and maintaining protective antitumour immunity. The specific nature of the inflammatory response and the tissue context may determine the beneficial versus the detrimental effects of inflammation on tumour pathology.
T helper 17 (TH17) cells are an important inflammatory component and have been shown to promote inflammation in a number of autoimmune diseases1–8. Research on these cells is rapidly evolving, and recent reviews have extensively covered basic TH17 cell biology, TH17 cell lineage development and the relevance of TH17 cells in autoimmune diseases 1–4,9,10. In this Review, we summarize recent reports of TH17 cells in patients with cancer and in mouse tumour models. We examine the phenotype, recruitment, generation and function of tumour-associated TH17 cells, focusing on their production of cytokines and on their interplay with other immune cells in the tumour microenvironment. Finally, we discuss the clinical relevance of TH17 cells in tumour immunology and highlight their therapeutic potential.
TH17 cells
Interleukin-17 (IL-17; originally termed CTLA8, also known as IL-17A) belongs to a family of six members (IL-17A, IL-17B, IL-17C, IL-17D, IL-17E and IL-17F) and has been of great interest recently owing to the discovery that the production of IL-17 characterizes a subset of CD4+ helper T cells (TH17 cells). The development of TH17 cells is distinct from the development of TH1, TH2 and regulatory T (TReg) cells and is characterized by unique transcription factors and cytokine requirements (FIG. 1). For further information on TH17 lineage development, we refer the reader to some recent reviews1–4,9.
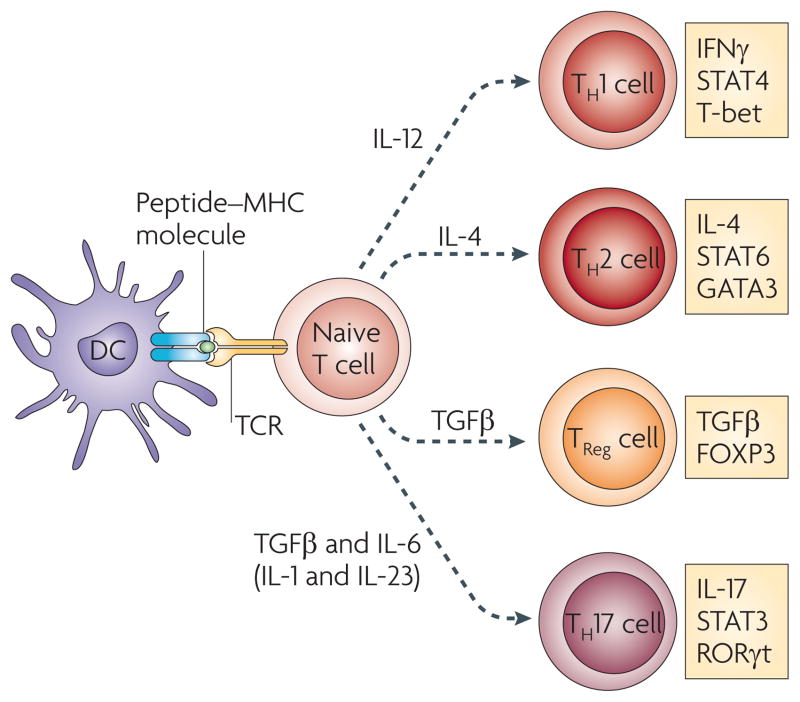
Figure 1. Differentiation of helper T cell subsets.
Following activation by antigen-presenting cells such as dendritic cells (DCs), naive CD4+ T cells can be polarized into different effector T cell subsets — T helper 1 (TH1), TH2, TH17 and regulatory T (TReg) cells — depending on the local cytokine environment. The differentiation of each of these effector T cell subsets is controlled by distinct sets of transcription factors. In the presence of interleukin-6 (IL-6) and transforming growth factor-β (TGFβ), naive T cells can differentiate into TH17 cells, which are characterized by expression of the transcription factors retinoic acid receptor-related orphan receptor-γt (RORγt) and signal transducer and activator of transcription 3 (STAT3). Furthermore, IL-1 and IL-23 can promote and/or stabilize TH17 cell differentiation and expansion. FOXP3, forkhead box P3; GATA3, GATA-binding protein 3; IFNγ, interferon-γ; TCR, T cell receptor.
Tumour-associated TH17 cells
Tissue distribution
It is well appreciated that TH17 cells contribute to autoimmunity1–4. However, one of the main physiological roles of TH17 cells is to promote host defence against infectious agents, including certain bacteria, fungi, viruses and protozoa, and TH17 cells are thought to be particularly important in maintaining barrier immunity at mucosal surfaces, such as the gut and lungs, as well as in the skin11,12. Consistent with this, TH17 cells are highly prevalent in the mucosal tissues of healthy individuals11,12.
More recently, TH17 cells have been investigated in patients with diverse cancer types, including ovarian cancer and prostate cancer, and this list continues to grow (TABLE 1). A caveat of these studies is that most have examined TH17 cells in peripheral blood, rather than in the tumour itself. However, it is generally agreed that although TH17 cells may remain a minor population in the peripheral blood of patients with cancer, they can be more prevalent at the tumour site itself. Support for this has come from extensive study of the tissue distribution of TH17 cells in patients with ovarian cancer. In these patients, the prevalence of TH17 cells in tumour-draining lymph nodes and peripheral blood is similar to that found in the peripheral blood of healthy donors; however, higher proportions of TH17 cells are found in tumours than at these other sites. This suggests that TH17 cells may be induced in and/or recruited to the tumour microenvironment13,14. In addition to these studies from human cancers, TH17 cells are also found in mouse tumour models. Consistent with the findings from human tumours, TH17 cells are more prevalent in the mouse tumour tissue itself, although TH17 cells are generally present at lower frequencies than other T cell subsets13. In summary, although not the predominant T cell subset within the tumour, TH17 cells are present in the tumour microenvironment.
Table 1.
TH17 cells in human carcinomas: immunological, clinical and pathological associations
| Human cancer type | Presence of TH17 cells | Effects associated with the presence of TH17 cells | Refs |
|---|---|---|---|
| B cell (non-Hodgkin) cancer | Limited TH17 cells in the tumour | Tumours inhibited TH17 cell formation and promoted TReg cells through IL-2 | 94 |
| Breast cancer | Fewer TH17 cells in blood in HER2+ than HER2− patients | Reverse relationship between TH17 and TReg cells; trastuzumab* decreased TReg cells and increased TH17 cells | 95 |
| Colon cancer | TH17 cells detected in the tumours | Polyfunctional TH17 cells present | 14 |
| Gastric cancer | TH17 cells detected in blood and IL17mRNA in the tumour | Blood TH17 cells and IL-17 increased in advanced cancer | 96 |
| Hepatocellular cancer | Increased TH17 cells in the tumour | Polyfunctional TH17 cells; TH17 cells correlate with angiogenesis in viral hepatitis associated carcinoma | 14,97 |
| Melanoma | Increased TH17 cells in the tumour | Polyfunctional TH17 cells. IFNα2 increased TReg cells, but not TH17; tremelimumab‡ increased TH17 cells | 14,67,98 |
| Myeloma | Bone marrow | Polyfunctional TH17 cells; DCs induce TH17 cells | 99 |
| Ovarian cancer | Increased TH17 cells in the tumour | Polyfunctional TH17 cells negatively correlated with TReg cells; tumour IL-17 levels positively predict survival | 13,14,28,100 |
| Pancreatic cancer | Increased TH17 cells in the tumour | Polyfunctional TH17 cells in the tumour | 14 |
| Prostate cancer | Increased TH17 cells in the tumour | TH17 cells were higher in those responsive to immunotherapy than in non-responders and negatively correlated with stages | 66,101 |
| Renal cell cancer | Increased TH17 cells in the tumour | Polyfunctional TH17 cells in the tumour | 14,102 |
| Small cell lung cancer | Increase in number of peripheral TH17 cells | Higher levels of peripheral TH17 cells are observed in patients with limited-stage disease and in long-term survivors | 103 |
DC, dendritic cell; HER2, human epidermal growth factor receptor 2; IFN, interferon; IL, interleukin; TH17, T helper 17; TReg cell, regulatory T cell.
Herceptin; Genentech/Roche.
Pfizer/Medarex.
Expression of tissue-homing molecules
Human primary tumour-infiltrating TH17 cells isolated from colon carcinomas, hepatocellular carcinomas, melanoma, ovarian carcinoma, pancreatic cancers and renal cell carcinomas express high levels of CXC-chemokine receptor 4 (CXCR4), CC-chemokine receptor 6 (CCR6), several CD49 integrins and the C-type lectin receptor CD161 (also known as KLRB1), but do not express CD62L or CCR7 (REF. 14). This suggests that these TH17 cells do not home to lymphoid tissues, but some of the above homing molecules might be involved in TH17 cell migration to, and retention within, inflammatory tissues and tumours14–17. For example, high levels of CXC-chemokine ligand 12 (CXCL12) (the ligand for CXCR4)18,19 and CCL20 (the ligand for CCR6)20 are found in human tumour micro-environments. Therefore the expression of CXCR4 and CCR6 could facilitate TH17 cell trafficking to tumours. However, these molecules are not exclusively expressed by tumour-infiltrating TH17 cells; high levels of CCR6 and CD161 are expressed by TH17 cells isolated from healthy donors21, as well as by non-TH17 cells from tumours and other inflamed tissues22. Therefore, to date, there are no known specific chemokine receptors or homing molecules associated with TH17 cells in the tumour environment. As such, there are no specific markers for identifying and isolating these TH17 cells for functional experiments.
Activation markers
Conventional effector T cells often express HLA-DR, CD25 and granzyme B, but tumour-infiltrating TH17 cells have been shown to express negligible levels of these molecules14. This suggests that these TH17 cells might not be conventional effector T cells and might not mediate effector functions through the granzyme B pathway. In addition, TH17 cells express minimal levels of programmed cell death 1 (PD1) and forkhead box P3 (FOXP3) suggesting they do not contribute to immune suppression in the tumour microenvironment14. Therefore, TH17 cells seem to be distinct from TReg cells and other effector T cells present in the tumour.
Cytokine profile
Tumour-infiltrating TH17 cells express other cytokines in addition to IL-17, and this might be functionally relevant in several physiological and pathological settings. Human tumour-infiltrating TH17 cells express negligible levels of the anti-inflammatory cytokine IL-10, but around 50–90% of TH17 cells produce high levels of effector cytokines such as IL-2, granulocyte–macrophage colony-stimulating factor (GM-CSF), interferon-γ (IFNγ) and tumour necrosis factor (TNF)14. Following culture under TH17-polarizing conditions, mouse CD4+ and CD8+ T cells have been shown to express IFNγ in vitro and also in vivo following transfer into irradiated mice15,23. Therefore, tumour-associated TH17 cells exhibit an effector T cell cytokine profile similar to that of effector T cells that have been described in infectious diseases24,25. A similar cytokine profile has been observed in TH17 cells associated with distinct human tumour types, including carcinomas of the skin, intestine, pancreas, liver and ovaries14 (TABLE 1). These data indicate that TH17 cells might have a protective role in tumour immunopathology by promoting antitumour immunity.
TH17 cell interactions in the tumour
TH17 cells and antigen-presenting cells
It is not known whether tumour-associated TH17 cells are induced in the tumour microenvironment itself or if they are recruited from distal sites. Antigen-presenting cells (APCs), such as dendritic cells, induce T cell polarization into different effector T cell subsets (FIG. 1). Although tumour-associated plasmacytoid dendritic cells isolated from ovarian cancers had minimal effect on TH17 cell induction, tumour-associated macrophages and myeloid dendritic cells stimulated IL-17 production from memory T cells but not from naive T cells14,16,26. In addition, tumour-associated macrophages were shown to be more efficient than normal macrophages in eliciting T cell IL-17 production14,26. As macrophages outnumber myeloid dendritic cells in many human cancers18,27 and are superior to myeloid 14,26, macrophages dendritic cells in inducing TH17 cells might be the main inducers of TH17 cells in the human tumour microenvironment.
Consistent with this possibility, tumour-associated macrophages expressed higher levels of IL-1β than normal tissue macrophages and normal monocyte-derived macrophages14,28. IL-1β, but not IL-1α, IL-6, IL-23 or transforming growth factor-β (TGFβ), is crucial for TH17 cell induction by human tumour-associated myeloid APCs14. Consistent with this observation, the levels of IL-1α and IL-23 are negligible in ovarian cancer ascites14. As IL-1α, IL-1β and IL-23 are involved in memory TH17 cell expansion in patients with psoriasis16,26, it is possible that the molecular mechanisms involved in inducing TH17 cells in patients with tumours are different from those in patients with autoimmune diseases. The involvement of IL-6 and TGFβ in human TH17 cell development remains controversial. It is suggested that TGFβ is essential for human TH17 cell development 29–31. However, high concentrations of TGFβ promote the development of TReg cells and suppress TH17 cell differentiation 29,30,32,33. High levels of IL-6 and TGFβ are often detected in the tumour microenvironment34. Therefore, if IL-6 and TGFβ have potent roles in promoting TH17 cell induction, one might expect substantial numbers of TH17 cells in human tumours. However, compared with the numbers of TReg cells and other T cell subsets, the numbers of TH17 cells present in both human and mouse tumours are limited13. Blockade of IL-1β, but not IL-6 or TGFβ, decreases TH17 cell induction by myeloid APCs isolated from patients with ovarian cancer14. Furthermore, the levels of IL-17 and numbers of TH17 cells do not correlate with IL-6 and TGFβ levels in patients with ovarian cancer14. Therefore, only IL-1β, but not IL-6 or TGFβ, seems to be crucial for TH17 cell development in the ovarian cancer microenvironment. Recent mouse studies also support a crucial role for IL-1β in promoting TH17 cell development5,35–37.
Thus, myeloid APCs can induce TH17 cells in the human tumour microenvironment through IL-1β production14, but TH17 cells in turn promote dendritic cell trafficking into tumour-draining lymph nodes and the tumour environment by producing CCL20. This chemokine can lead to the recruitment of dendritic cells to the tumour in a CCR6-dependent manner17. As human TH17 cells also express high levels of CCR6 (REFs 14,16) and efficiently migrate towards CCL20 (REF. 16), TH17 cells might increase their own frequency in the tumour by both direct and indirect mechanisms.
TH17 and TReg cells
As already mentioned, fewer TH17 cells are found in the tumour microenvironment than cells and other effector T cell subsets14,38. Interestingly, TReg the numbers of TReg and TH17 cells are inversely associated in the same tumours13,14. This suggests that there could be a dynamic interaction between TH17 and TReg cells in the tumour microenvironment. Consistent with this possibility, mouse peripheral mature TReg cells can be converted into TH17 cells; this event is favoured by inflammation and IL-6 production10,39,40. In addition, IL-17+FOXP3+ T cells can be detected in humans41,42. However, it is not known whether these IL-17+FOXP3+ T cells originate from TH17 cells or from TReg cells. These IL-17+FOXP3+ T cells also express CD25 and the TH17 lineage specific transcription factor retinoic acid receptor-related orphan receptor-γt (RORγt), and have suppressive functions. This indicates that IL-17+FOXP3+ cells have certain functional characteristics of TReg and TH17 cells. Notably, despite the high levels of IL-6 detected in some human epithelial cancers34,43, the number of TH17 cells is limited in the tumour microenvironment; therefore, the positive effect of IL-6 in inducing TH17 cells might be subverted by an unidentified mechanism. Interestingly, in the presence of retinoic acid, which enhances TGFβ signalling and inhibits IL-6 signalling, IL-6 could not induce IL-17 production from FOXP3+ T cells39. However, it is unknown whether retinoic acid affects the balance between TReg and TH17 cells in the tumour microenvironment. Nonetheless, the plasticity of the TReg cell lineage might allow the initial skewing of TReg cells towards an IL-17+FOXP3+ phenotype10 and eventually into potentially protective FOXP3− TH17 cells that can promote antitumour immunity.
Then why are there limited numbers of TH17 cells in tumours? Although tumour-associated macrophages are potent TH17 cell inducers in vitro, tumour-associated TReg cells express high levels of CD39 (also known as NTPDase 1), an ectonucleotidase that converts ATP into adenosine, which suppresses TH17 cell development through the adenosinergic pathway14. Supporting this hypothesis, it has been reported that mouse TReg cells can use this pathway to suppress T cell activation44,45 and T cell-mediated protective immunity46. It was also reported that mouse TReg cells inhibit TH17 cell responses in vivo in a signal transducer and activator of transcription 3 (STAT3)-dependent manner, and TReg cell-specific ablation of STAT3 leads to the loss of their suppressive functions47. If TH17 cells mediate protective immunity, inhibition of TH17 cell development could be a previously unappreciated mechanism by which tumours evade the immune system14.
TH17 and TH1 cells
Although there are no experiments directly demonstrating the lineage association between TH17 and TH1 cell development, there is evidence indicating that TH17 cells and TH1 cells might be phenotypically, developmentally and functionally linked in the tumour microenvironment48. IFNγ, a typical TH1-type cytokine, is expressed by primary TH17 cells in human tumours14 and TH17-polarized mouse cells 15. IFNγ+IL-17+ T cells are also found in patients with autoimmune diseases16. It is possible that IFNγ+IL-17+ T cells can develop from TH1 cells and/or TH17 cells 49. Consistent with this, adoptive transfer of antigen-specific IL-17+CD8+ T cells into antigen-bearing hosts results in their conversion to IFNγ+CD8+ T cells50. In mouse models, under lymphopenic conditions, TH17 cells can redifferentiate into TH1 cells 51–53. Given that after chemotherapy and radiotherapy, patients with cancer might mimic lymphopenic hosts, and given that there are substantial numbers of IFNγ+IL-17+ T cells14, TH17 cells could initially express low levels of IFNγ but gradually be converted into TH1 cells in vivo. However, although IFNγ inhibits TH17 cell differentiation from naive T cells in mice, TH1 cell-derived IFNγ might drive APCs to promote memory TH17 cell expansion through inducing the production of IL-1 and IL-23 by APCs16,26.
The functional interaction between TH1 cells and TH17 cells has been appreciated in human tumours 14, mouse immunotherapeutic settings15,17,23 and auto-immune disease models16,26,54,55. This interaction might contribute to antitumour immunity, and is functionally relevant and therapeutically meaningful.
TH17 cells and antitumour immunity
Evidence for antitumour activity
The relationship between TH17 cells and tumour immunopathology has been controversial56–58. However, there are several lines of evidence suggesting that TH17 cells can promote protective antitumour immune responses. Firstly, tumour-infiltrating TH17 cells express several effector cytokines, similar to that observed in patients with infectious diseases24,25. This suggests that tumour-associated TH17 cells might be functional effector T cells. Consistent with this possibility, TH17 cells are negatively correlated with the presence of TReg cells14,38 and are positively correlated with effector immune cells, including IFNγ+ effector T cells, cytotoxic CD8+ T cells and natural killer (NK) cells, in the same tumour microenvironment14. These observations are supported by data from both human and mouse tumours14,17,59.
Transgenic T cells polarized to a TH17 cell phenotype following treatment with TGFβ and IL-6 were shown to induce tumour eradication in mice15,17,23. In addition, IL-17-deficient mice show accelerated tumour growth and lung metastasis in many tumour models, and forced expression of IL-17 in tumour cells was shown to suppress tumour progression17,59–62. Furthermore, immunotherapies associated with enhanced TH17 cell activity, such as blocking indoleamine 2,3-dioxygenase (IDO)63, treatment with IL-7 (REF. 64) or vaccination with heat shock protein 70 (HSP70)65, resulted in improved antitumour immunity.
In patients with prostate cancer, a significant inverse correlation is found between TH17 cell differentiation and tumour progression66. Treatment with specific antibody against cytotoxic T lymphocyte antigen 4 (CTLA4)67 induces TH17 cells in patients with melanoma and the levels of IL-17 detected in tumour-associated ascites positively predicts patient survival. Taken together, these data provide strong evidence that TH17 cells can have protective roles in tumour immunity.
Mechanisms of antitumour activity
How do TH17 cells mediate antitumour immunity in patients with cancer? Human TH17 cells, including TH17 cells found in tumours, do not express granzyme B or perforin and have no direct effects on primary ovarian cancer cell proliferation and apoptosis. Therefore TH17 cells may not mediate direct cytotoxic activity against tumour cells14,50. Instead, TH17 cells might mediate their antitumour activity indirectly, by facilitating the recruitment of other effector immune cells68–70. Consistent with this hypothesis, IL-17 was positively associated with tumour-infiltrating IFNγ+ effector T cells14. Mechanistically, TH17 cell-derived IL-17 and IFNγ synergistically induced the production of the TH1-type chemokines CXCL9 and CXCL10 by tumour cells, which in turn promoted effector T cell migration towards tumours14. Levels of CXCL9 and CXCL10 were found to directly correlate with the number of tumour-infiltrating CD8+ T cells and NK cells. These data strongly suggest that TH17 cells have an indirect role in antitumour immunity by promoting effector T cell and NK cell trafficking to, and retention within, the tumour microenvironment (FIG. 2).
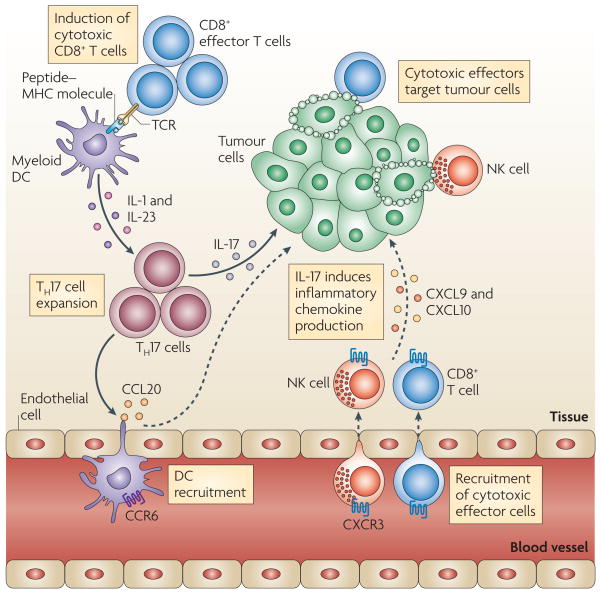
Figure 2. TH17 cells and antitumour immunity.
T helper 17 (TH17) cells traffic to the tumour microenvironment and are expanded by antigen-presenting cells, such as myeloid dendritic cells (DCs) through interleukin-1 (IL-1) and IL-23. TH17 cells promote the trafficking and retention of effector T cells and natural killer (NK) cells in the tumour environment, through inducing the production of the chemokines CXC-chemokine ligand 9 (CXCL9) and CXCL10 by primary tumour cells. In addition, TH17 cells induce the production of CC-chemokine ligand 20 (CCL20) by tumour cells and this leads to the recruitment of CC-chemokine receptor 6 (CCR6)+ DCs. Therefore TH17 cells can promote protective antitumour immunity by inducing the recruitment of pro-inflammatory immune effector cells; TCR, T cell receptor.
TH17 cell-mediated antitumour activity could also be linked to dendritic cell recruitment into the tumour microenvironment or into tumour-draining lymph nodes. As mentioned above, TH17 cells can stimulate CCL20 expression in tumour tissues and promote dendritic cell tumour trafficking in a CCL20–CCR6 dependent manner. In turn, CD8+ T cells are effectively primed and activated by dendritic cells and mediate potent antitumour immune responses. Altogether, these data suggest that TH17 cells might not mediate direct anti-tumour activity, but can promote antitumour immunity indirectly through the recruitment of dendritic cells and cytotoxic effector cells17 (FIG. 2).
Pro-tumour role of TH17 cell-associated cytokines
IL-17 and TH17 cells
Although IL-17 is the signature cytokine of TH17 cells, the production of IL-17 is not the sole function of TH17 cells. Thus, the biological activities of IL-17 should not be equated with the biological activities of TH17 cells (BOX 1). In addition to leukocytes of the immune system, the cellular targets of IL-17 in the tumour microenvironment can be vascular endothelial cells, stromal cells and cells of the tumour itself. Early studies showed that exogenous IL-17 could promote tumour growth by inducing tumour vascularization, particularly in immune-deficient nude mice and severe combined immunodeficient (SCID) mice71–73 (TABLE 2). However, the overall effect of IL-17 on tumour development and growth might be different in immune-competent hosts, as shown by the potent antitumour effects mediated by IL-17 in immune-competent mice60,61 (TABLE 2). Furthermore, as a result of differences in local concentrations, bioavailability and potential targets, the biological activities of endogenous IL-17 (such as IL-17 derived from TH17 cells) and exogenously administered IL-17 might differ. Two recent reports have shown that, in mice, endogenous IL-17 promotes Bacteroides fragilis-induced tumour formation74 and tumour growth in a transplanted tumour model75. IL-17 induces IL-6 production by tumour cells and tumour-associated stromal cells, which in turn activates STAT3, an oncogenic transcription factor that upregulates pro-survival and pro-angiogenic genes75. Furthermore, although IL-17 deficiency leads to increased numbers of IFNγ-producing NK cells in the tumour-draining lymph nodes of tumour-bearing mice59, it has also been reported that IL-17 can decrease NK cell activity in a mouse model of dermatitis76. Therefore IL-17 can promote tumour growth in certain tumour-bearing mouse models, and the effects of IL-17 on tumour growth might be highly context dependent (BOX 1; TABLE 2).
Box 1. TH17 cell biology and important research contexts.
The biological activities of T helper 17 (TH17) cells and the TH17 cell-associated cytokines (interleukin-17 (IL-17) and IL-23) may be highly context dependent. The following aspects could be crucial for our understanding of the antitumour versus pro-tumour activities of IL-17 and/or TH17 cells.
Exogenous versus endogenous Il-17
The findings that IL-17 can have pro-tumorigenic or antitumorigenic activity might be due, in part, to the source of IL-17 in each of the studies. Exogenously delivered IL-17 might differ in dose from endogenous IL-17 that is produced by TH17 cells and other IL-17-expressing cells. Therefore, the biological activities of exogenous versus endogenous IL-17 might not be identical (TABLE 2).
Il-17+ cell populations
IL-17 can be produced by TH17 cells, CD8+ T cells, natural killer T (NKT) cells and osteoclasts1–4,104. The biological activities of IL-17 made by different cell types may not be identical owing to the different levels of IL-17 and other contexts, including localization, cytokine profile and cellular environments.
TH17 cell subsets
Both IL-10+ and IL-10− TH17 cell subsets can be detected in mice. Although they both express IL-17, it has been reported that IL-10− but not IL-10+ TH17 cells are pathogenic in experimental autoimmune encephalomyelitis (EAE) models105–107. Furthermore, there are IL-17+CD8+ T cells. Current evidence indicates that IL-17+CD8+ T cells might be functionally similar to TH17 cells in certain settings17–23.
TH17 cell cytokine profile
The function of TH17 cells may not be solely determined by IL-17. In addition to IL-17, tumour TH17 cells can express many cytokines, including IL-2, IL-9, IL-22, granulocyte–macrophage colony-stimulating factor (GM-CSF), interferon-γ (IFNγ) and tumour necrosis factor (TNF)14,15,23. These cytokines may synergize with IL-17 to mediate biological activities. For example, IFNγ and IL-17 derived from TH17 cells synergistically induce β-defensin 2 and TH1-type chemokines14,16.
TH17 cells and associated cytokines
Several cytokines are associated with TH17 cell development. The role of IL-23 is controversial in the context of tumour pathology. Although IL-23 is involved in regulating TH17 cell development, the biological activities of IL-23 should not necessarily be associated with only TH17 cell activity77–82 (TABLE 2).
Cellular targets
IL-17 and TH17 cells can target tumour cells, stromal cells, vascular endothelial cells and immune cells 14–17,60,61,71–73. The functional activities of IL-17 may be determined by the net effects of IL-17 on different cellular targets.
Research models
The research models can be human subjects or animals, immune-competent or immune-deficient hosts, and subjects with or without chemical or infection-associated inflammation. The role of IL-17 in tumorigenesis might depend on the research model used. For example, IL-17 can be pro-tumorigenic in immune-deficient models71–73 but has been shown to have antitumorigenic properties in immune-competent models60,61.
Disease stages
Human tumorigenesis is often a slow process that occurs over many years. Immune responses are likely to be dynamically altered in the tumour microenvironment at different stages of tumour development14,66. Mouse tumour models may not represent a satisfactory model for dissecting the nature of human tumours at specific stages (TABLEs 1, 2). Presumably, the predominant role of IL-17 and TH17 cells can vary at different stages of tumorigenesis 14,66,74,75.
Table 2.
Antitumour and pro-tumour activities of IL-17 and IL-23
| Cytokine | model system | Antitumour or pro-tumour effects | Refs |
|---|---|---|---|
| IL-17 | Nude or SCID mice | Enhanced tumour vascularization and tumour growth | 71–73 |
| Immune-competent mice | Enhanced antitumour immunity | 60,61 | |
| IL-23 | IL-23-deficient mice | Reduced MMP9 expression, tumour angiogenesis and fewer chemically-induced tumours | 77 |
| IL-23-transfected B16F10 cells | Enhanced antitumour immunity; the effects are IFNγ and/or CD8+ T cell dependent | 78 | |
| IL-23-transduced DCs | 81 | ||
| IL-23-expressing bone marrow cells | 79 | ||
| Liver IL-23 overexpression and gp100-specific T cells | 80 | ||
| Systemic IL-23 treatment | 82 |
DC, dendritic cell; IFNγ, interferon-γ; IL, interleukin; MMP9, matrix metalloproteinase 9; SCID, severe combined immunodeficient.
IL-23 and TH17 cells
IL-23 is an IL-12 cytokine family member, which is produced by APCs and promotes the expansion and survival of TH17 cells. It has been reported that IL-23-deficient mice are resistant to chemically induced tumours77. This resistance is associated with decreased expression of matrix metal-loproteinase 9 (MMP9) in the skin, a decrease in the expression of angiogenic markers and high levels of CD8+ T cell infiltration. Given the close relationship between IL-23 and TH17 cells, it has been proposed that TH17 cells or IL-17 derived from TH17 cells can promote tumorigenesis in this model.
However, this hypothesis remains to be tested and antitumour effects of IL-23 have been observed in several mouse tumour models78–82. Vaccination with IL-23-transduced dendritic cells78 or overexpression of IL-23 at tumour sites has been shown to result in a robust infiltration of CD8+ T cells to the tumour and inhibition of tumour growth79–81. In addition, systemic administration of IL-23 suppressed the growth of a pre-existing fibrosarcoma in mice and resulted in increased survival82. Altogether, these data indicate that IL-23 might have distinct roles in antitumour immune responses and can either promote or inhibit tumorigenesis depending on the context of the experimental conditions (BOX 1; TABLE 2).
Targeting TH17 cells for cancer therapy
The involvement of TH17 cells in the pathogenesis of various autoimmune diseases has been reviewed elsewhere1–8,10. It has been argued that cancer rejection can be viewed as intentional induction of autoimmune disease83. The antitumour activities of CD8+ T cells have received the most attention in the field of tumour immunology; these cells produce IFNγ, GM-CSF and TNF and can specifically lyse antigen-presenting MHC class I+ tumours. The infusion of large numbers of tumour-specific CD8+ T cells, expanded in vitro with IL-2, can induce the regression of large tumour burdens in mice and in humans84. However, the use of CD4+ helper T cell subsets in tumour immunotherapy has been under-explored, especially in the clinic. This can be attributed partly to the massive diversity of the MHC class II loci and partly to the complexity of CD4+ helper T cell subtypes in mice and humans. Initial studies focused on the potential for helper CD4+ T cells to promote CD8+ T cell responses by enhancing their activation and persistence in the tumour environment. However, it soon became clear that suppressive immune networks, such as TReg cells, could suppress APCs and other tumour associated-immune responses and dramatically inhibit the antitumour response34,85–90.
In early experiments, both TH1 and TH2 cell subsets were shown to have some antitumour activity, but the IFNγ-secreting TH1 cells were thought to be the more efficacious at tumour destruction48. TH17 cells generated under the influence of TGFβ and IL-6 (and, more recently, expanded by cytokine cocktails) have been found to be highly effective in triggering the eradication of large, established tumours in mice15. Effective antitumour TH17 cells are less prone to apoptosis than their TH1 cell counterparts, although the reasons for this are not completely understood.
Although IL-17-secreting CD8+ T cells are not +CD8+ T cells can be polarized using TH17 cells, IL-17 a TH17-inducing cytokine cocktail, and their potential roles in cancer immunotherapy have been explored in two recent publications23,50. IL-17-producing CD8+ T cells have greatly enhanced expression of RORγt, decreased expression of eomesodermin and their canonical cytotoxic activity is diminished. Like their CD4+ counterparts, these CD8+ T cells can acquire the ability to produce IFNγ and mediate regression of large, established tumours. This enhanced antitumour activity is associated with increased in vivo expansion and persistence of the transferred cells. Type 17-skewed CD8+ T cells have decreased expression of killer cell lectin-like receptor G1 (KLRG1), a marker of CD8+ T cell senescence, and also show increased expression of IL-7Rα, a cytokine receptor associated with memory T cell formation. This evidence suggests that the skewing of both CD8+ T cells and CD4+ T cells towards an IL-17-producing phenotype improves their antitumour activity.
Efforts to use polarized helper T cell subsets in the clinic to induce tumour regression have been minimal. Although a single case study reported a dramatic antitumour response in a patient treated with ex vivo generated autologous CD4+ T cell clones, which recognized the tumour-associated antigen NY-ESO1 (REF. 91), these cells were not specifically polarized to any helper T cell subset. Based on the data from animal models, the treatment of patients with cancer with TH17-polarized T cells seems to be a promising approach; however, at the time of writing, there have been no published reports on the use of TH17 cells to treat any human cancer. Given our knowledge of human tumour systems, it seems plausible that a clinical trial involving the adoptive transfer of TH17-polarized, tumour-specific T cells could be carried out. One credible trial design involves the polarization of naive human T cells using a cocktail of cytokines, such as IL-1β, IL-6, IL-21, IL-23, TGFβ and TNF92. Recombinant gammaretroviruses or lenti-viruses that encode genes for tumour antigen-specific T cell receptors or chimeric antigen receptors could be used to confer antitumour activity onto autologous, TH17-polarized T cells. Similar to the strategy that has been used for genetically engineered CD8+ T cells, the resulting TH17 cells could be expanded to large numbers in vitro and then transferred back into patients with cancer93.
Concluding remarks
Despite the recent identification of TH17 cells, over the past few years we have made rapid and large advances in our understanding of the development, regulation and function of these cells. This has been particularly true in the context of autoimmune diseases, where the pathogenic role of TH17 cells has been well documented. However, the exact nature of TH17 cells in antitumour immunity is not a ‘black and white’ picture: the roles of TH17 cells in tumorigenesis are best represented by many colours. TH17 cells and TH17-associated cytokines have been shown to have both antitumorigenic and pro-tumorigenic functions. Therefore, the relationship between TH17 cells and tumour immunopathology are highly dependent on context (BOX 1), but a better understanding of these contexts could be used to develop and refine new cancer therapies.
Acknowledgments
We thank our former and current trainees and collaborators for their intellectual input and hard work. The work described in this Review was supported by the extramural (W.Z.) and intramural (N.P.R.) funds from the United States National Cancer Institute.
Glossary
- Regulatory T (TReg) cells
A specialized subset of CD4+ T cells that can suppress inflammation and the responses of other T cells. These cells provide a crucial mechanism for the maintenance of peripheral self tolerance. A subset of these cells is characterized by expression of CD25 and the transcription factor FOXP3
- Granzyme B
A secreted serine protease that enters target cells through perforin pores, it then cleaves and activates intracellular caspases, leading to target-cell apoptosis
- Plasmacytoid dendritic cells
A subset of dendritic cells that is described as plasmacytoid because their microscopic appearance resembles plasmablasts. In humans, these cells can be derived from lineage-negative stem cells in peripheral blood and are the main producers of type I IFN in response to virus infections
- Indoleamine 2,3-dioxygenase
(IDO). An intracellular haeme-containing enzyme that catalyses the oxidative catabolism of tryptophan. Insufficient availability of tryptophan can lead to T cell apoptosis and anergy
- Cytotoxic T lymphocyte antigen 4
(CTLA4). A T cell surface protein that, following its ligation by CD80 or CD86 on antigen-presenting cells, delivers a negative signal to activated T cells. This induces cell cycle arrest and inhibits cytokine production. CTLA4 is constitutively expressed by, and functionally associated with, regulatory T cells
Footnotes
Competing interests statement
The authors declare no competing financial interests.
DATABASES
UniProtKB: http://www.uniprot.org
CCR6 | CD39 | CD161 | CXCR4 | GM-CSF | IFNγ | IL-1β | IL-2 | IL-17 | RORγt | TNF
FURTHER INFORMATION
Weiping Zou’s homepage:
http://sitemaker.umich.edu/zou/home
Nicholas P. Restifo’s homepage:
http://ccr.nci.nih.gov/staff/staff.asp?profileid=5762
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
Contributor Information
Weiping Zou, Email: wzou@med.umich.edu.
Nicholas P. Restifo, Email: restifo@nih.gov.
References
- 1.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nature Rev Immunol. 2006;6:329–333. doi: 10.1038/nri1807. [DOI] [PubMed] [Google Scholar]
- 3.Wynn TA. TH-17: a giant step from TH1 and TH2. Nature Immunol. 2005;6:1069–1070. doi: 10.1038/ni1105-1069. [DOI] [PubMed] [Google Scholar]
- 4.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nature Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 5.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Komiyama Y, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 7.Tato CM, O’Shea JJ. Immunology: what does it mean to be just 17? Nature. 2006;441:166–168. doi: 10.1038/441166a. [DOI] [PubMed] [Google Scholar]
- 8.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Weaver CT, Hatton RD. Interplay between the TH17 and TReg cell lineages: a (co-)evolutionary perspective. Nature Rev Immunol. 2009;9:883–889. doi: 10.1038/nri2660. [DOI] [PubMed] [Google Scholar]
- 11.Dubin PJ, Kolls JK. Th17 cytokines and mucosal immunity. Immunol Rev. 2008;226:160–171. doi: 10.1111/j.1600-065X.2008.00703.x. [DOI] [PubMed] [Google Scholar]
- 12.O’Quinn DB, Palmer MT, Lee YK, Weaver CT. Emergence of the Th17 pathway and its role in host defense. Adv Immunol. 2008;99:115–163. doi: 10.1016/S0065-2776(08)00605-6. [DOI] [PubMed] [Google Scholar]
- 13.Kryczek I, et al. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumour microenvironment. J Immunol. 2007;178:6730–6733. doi: 10.4049/jimmunol.178.11.6730. This is the first study showing that TH17 cells are found in both mouse and human tumours and that cells IL-2 can oppositely regulate TH17 and TReg in the tumour microenvironment. [DOI] [PubMed] [Google Scholar]
- 14.Kryczek I, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumour environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. This study systemically and mechanistically investigates the phenotype, distribution, generation, and functional and clinical relevance of TH17 cells in the human tumour microenvironment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muranski P, et al. Tumour-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. This is the first functional study showing that TH17-polarized CD4+ T cells induce potent tumour eradication in mice, and it provides support for a clinical trial involving the adoptive transfer of TH17-polarized, tumour-specific CD4+ T cells to patients with cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kryczek I, et al. Induction of IL-17+ T cell trafficking and development by IFN-γ: mechanism and pathological relevance in psoriasis. J Immunol. 2008;181:4733–4741. doi: 10.4049/jimmunol.181.7.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin-Orozco N, et al. T helper 17 cells promote cytotoxic T cell activation in tumour immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. This study provides direct mechanistic and functional evidence that TH17 cells mediate antitumour immunity by promoting dendritic cell trafficking to tumour-draining lymph nodes and to the tumour itself. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou W, et al. Stromal-derived factor-1 in human tumours recruits and alters the function of plasmacytoid precursor dendritic cells. Nature Med. 2001;7:1339–1346. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- 19.Kryczek I, et al. CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Res. 2005;65:465–472. [PubMed] [Google Scholar]
- 20.Bell D, et al. In breast carcinoma tissue, immature dendritic cells reside within the tumour, whereas mature dendritic cells are located in peritumoral areas. J Exp Med. 1999;190:1417–1426. doi: 10.1084/jem.190.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acosta-Rodriguez EV, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nature Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 22.Annunziato F, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hinrichs CS, et al. Type 17 CD8+ T cells display enhanced anti-tumour immunity. Blood. 2009;114:596–599. doi: 10.1182/blood-2009-02-203935. This is the first functional study to show that TH17-polarized CD8+ T cells induce potent tumour eradication in mice, and it provides support for a clinical trial involving the adoptive transfer of TH17-polarized, tumour-specific CD8+ T cells to patients with cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Precopio ML, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8+T cell responses. J Exp Med. 2007;204:1405–1416. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Almeida JR, et al. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J Exp Med. 2007;204:2473–2485. doi: 10.1084/jem.20070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kryczek I, et al. Cutting edge: IFN-γ enables APC to promote memory Th17 and abate Th1 cell development. J Immunol. 2008;181:5842–5846. doi: 10.4049/jimmunol.181.9.5842. [DOI] [PubMed] [Google Scholar]
- 27.Kryczek I, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyahara Y, et al. Generation and regulation of human CD4+ IL-17-producing T cells in ovarian cancer. Proc Natl Acad Sci USA. 2008;105:15505–15510. doi: 10.1073/pnas.0710686105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang L, et al. IL-21 and TGF-β are required for differentiation of human TH17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manel N, Unutmaz D, Littman DR. The differentiation of human TH-17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nature Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volpe E, et al. A critical function for transforming growth factor-β, interleukin 23 and proinflammatory cytokines in driving and modulating human TH-17 responses. Nature Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 32.Wilson NJ, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nature Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 33.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17-producing human T helper cells. Nature Immunol. 2007;8:639–646. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 34.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nature Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 35.Kryczek I, et al. Cutting edge: opposite effects of IL-1 and IL-2 on the regulation of IL-17+ T cell pool IL-1 subverts IL-2-mediated suppression. J Immunol. 2007;179:1423–1426. doi: 10.4049/jimmunol.179.3.1423. [DOI] [PubMed] [Google Scholar]
- 36.Chung Y, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signalling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gulen MF, et al. The receptor SIGIRR suppresses Th17 cell proliferation via inhibition of the interleukin-1 receptor pathway and mTOR kinase activation. Immunity. 2010;32:54–66. doi: 10.1016/j.immuni.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curiel TJ, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 39.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 40.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 41.Beriou G, et al. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113:4240–4249. doi: 10.1182/blood-2008-10-183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Voo KS, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci USA. 2009;106:4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kryczek I, Grybos M, Karabon L, Klimczak A, Lange A. IL-6 production in ovarian carcinoma is associated with histiotype and biological characteristics of the tumour and influences local immunity. Br J Cancer. 2000;82:621–628. doi: 10.1054/bjoc.1999.0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deaglio S, et al. Adenosine generation catalysed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borsellino G, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 46.Kao JY, et al. Helicobacter pylori immune escape is mediated by dendritic cell-induced Treg skewing and Th17 suppression in mice. Gastroenterology. 2010;138:1046–1054. doi: 10.1053/j.gastro.2009.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chaudhry A, et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muranski P, Restifo NP. Adoptive immunotherapy of cancer using CD4+ T cells. Curr Opin Immunol. 2009;21:200–208. doi: 10.1016/j.coi.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee YK, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yen HR, et al. Tc17 CD8 T cells: functional plasticity and subset diversity. J Immunol. 2009;183:7161–7168. doi: 10.4049/jimmunol.0900368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bending D, et al. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009;119:565–572. doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol. 2009;39:216–224. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nurieva R, Yang XO, Chung Y, Dong C. Cutting edge: in vitro generated Th17 cells maintain their cytokine expression program in normal but not lymphopenic hosts. J Immunol. 2009;182:2565–2568. doi: 10.4049/jimmunol.0803931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luger D, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Connor W, Jr , et al. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nature Immunol. 2009;10:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muranski P, Restifo NP. Does IL-17 promote tumour growth? Blood. 2009;114:231–232. doi: 10.1182/blood-2009-04-215541. [DOI] [PubMed] [Google Scholar]
- 57.Munn DH. Th17 cells in ovarian cancer. Blood. 2009;114:1134–1135. doi: 10.1182/blood-2009-06-224246. [DOI] [PubMed] [Google Scholar]
- 58.Bronte V. Th17 and cancer: friends or foes? Blood. 2008;112:214. doi: 10.1182/blood-2008-04-149260. [DOI] [PubMed] [Google Scholar]
- 59.Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumour growth and metastasis. Blood. 2009;114:357–359. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hirahara N, et al. Inoculation of human interleukin-17 gene-transfected Meth-A fibrosarcoma cells induces T cell-dependent tumour-specific immunity in mice. Oncology. 2001;61:79–89. doi: 10.1159/000055357. [DOI] [PubMed] [Google Scholar]
- 61.Benchetrit F, et al. Interleukin-17 inhibits tumour cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99:2114–2121. doi: 10.1182/blood.v99.6.2114. [DOI] [PubMed] [Google Scholar]
- 62.Wei S, Kryczek I, Namm J, Szeliga W, Vatan L, Chang AE, Zou W. Endogenous IL-17, tumour growth and metastasis. Blood. in the press This study, along with references 17 and 59, reports increased tumour growth and metastasis in IL-17-deficient mice. [Google Scholar]
- 63.Sharma MD, et al. Indoleamine 2, 3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumour-draining lymph nodes. Blood. 2009;113:6102–6111. doi: 10.1182/blood-2008-12-195354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pellegrini M, et al. Adjuvant IL-7 antagonizes multiple cellular and molecular inhibitory networks to enhance immunotherapies. Nature Med. 2009;15:528–536. doi: 10.1038/nm.1953. [DOI] [PubMed] [Google Scholar]
- 65.Kottke T, et al. Induction of hsp70-mediated Th17 autoimmunity can be exploited as immunotherapy for metastatic prostate cancer. Cancer Res. 2007;67:11970–11979. doi: 10.1158/0008-5472.CAN-07-2259. [DOI] [PubMed] [Google Scholar]
- 66.Sfanos KS, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.von Euw E, et al. CTLA4 blockade increases Th17 cells in patients with metastatic melanoma. J Transl Med. 2009;7:35. doi: 10.1186/1479-5876-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Galon J, et al. Type, density, and location of immune cells within human colorectal tumours predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 69.Zhang L, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 70.Sato E, et al. Intraepithelial CD8+ tumour-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favourable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tartour E, et al. Interleukin 17, a T-cell-derived cytokine, promotes tumorigenicity of human cervical tumours in nude mice. Cancer Res. 1999;59:3698–3704. [PubMed] [Google Scholar]
- 72.Numasaki M, et al. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol. 2005;175:6177–6189. doi: 10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- 73.Numasaki M, et al. Interleukin-17 promotes angiogenesis and tumour growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 74.Wu S, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nature Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang L, et al. IL-17 can promote tumour growth through an IL-6–Stat3 signalling pathway. J Exp Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kawakami Y, et al. Inhibition of NK cell activity by IL-17 allows vaccinia virus to induce severe skin lesions in a mouse model of eczema vaccinatum. J Exp Med. 2009;206:1219–1225. doi: 10.1084/jem.20082835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Langowski JL, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 78.Hu J, et al. Induction of potent antitumour immunity by intratumoral injection of interleukin 23-transduced dendritic cells. Cancer Res. 2006;66:8887–8896. doi: 10.1158/0008-5472.CAN-05-3448. [DOI] [PubMed] [Google Scholar]
- 79.Yuan X, Hu J, Belladonna ML, Black KL, Yu JS. Interleukin-23-expressing bone marrow-derived neural stem-like cells exhibit antitumour activity against intracranial glioma. Cancer Res. 2006;66:2630–2638. doi: 10.1158/0008-5472.CAN-05-1682. [DOI] [PubMed] [Google Scholar]
- 80.Overwijk WW, et al. Immunological and antitumour effects of IL-23 as a cancer vaccine adjuvant. J Immunol. 2006;176:5213–5222. doi: 10.4049/jimmunol.176.9.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oniki S, et al. Interleukin-23 and interleukin-27 exert quite different antitumour and vaccine effects on poorly immunogenic melanoma. Cancer Res. 2006;66:6395–6404. doi: 10.1158/0008-5472.CAN-05-4087. [DOI] [PubMed] [Google Scholar]
- 82.Kaiga T, et al. Systemic administration of IL-23 induces potent antitumour immunity primarily mediated through Th1-type response in association with the endogenously expressed IL-12. J Immunol. 2007;178:7571–7580. doi: 10.4049/jimmunol.178.12.7571. [DOI] [PubMed] [Google Scholar]
- 83.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nature Rev Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hinrichs CS, Gattinoni L, Restifo NP. Programming CD8+ T cells for effective immunotherapy. Curr Opin Immunol. 2006;18:363–370. doi: 10.1016/j.coi.2006.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Antony PA, et al. CD8+ T cell immunity against a tumour/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nature Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 87.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nature Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 88.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nature Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 89.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nature Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 90.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nature Rev Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 91.Hunder NN, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 93.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nature Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM. Malignant B cells skew the balance of regulatory T cells and TH17 cells in B-cell non-Hodgkin’s lymphoma. Cancer Res. 2009;69:5522–5530. doi: 10.1158/0008-5472.CAN-09-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Horlock C, et al. The effects of trastuzumab on the CD4+CD25+FoxP3+ and CD4+IL17A+ T-cell axis in patients with breast cancer. Br J Cancer. 2009;100:1061–1067. doi: 10.1038/sj.bjc.6604963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang B, et al. The prevalence of Th17 cells in patients with gastric cancer. Biochem Biophys Res Commun. 2008;374:533–537. doi: 10.1016/j.bbrc.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 97.Zhang JP, et al. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50:980–989. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 98.Wang W, et al. Effects of high-dose IFNα2b on regional lymph node metastases of human melanoma: modulation of STAT5, FOXP3, and IL-17. Clin Cancer Res. 2008;14:8314–8320. doi: 10.1158/1078-0432.CCR-08-0705. [DOI] [PubMed] [Google Scholar]
- 99.Dhodapkar KM, et al. Dendritic cells mediate the induction of polyfunctional human IL17-producing cells (Th17-1 cells) enriched in the bone marrow of patients with myeloma. Blood. 2008;112:2878–2885. doi: 10.1182/blood-2008-03-143222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Charles KA, et al. The tumour-promoting actions of TNF-α involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J Clin Invest. 2009;119:3011–3023. doi: 10.1172/JCI39065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Derhovanessian E, et al. Pretreatment frequency of circulating IL-17+CD4+ T-cells, but not Tregs, correlates with clinical response to whole-cell vaccination in prostate cancer patients. Int J Cancer. 2009;125:1372–1379. doi: 10.1002/ijc.24497. [DOI] [PubMed] [Google Scholar]
- 102.Inozume T, Hanada K, Wang QJ, Yang JC. IL-17 secreted by tumour reactive T cells induces IL-8 release by human renal cancer cells. J Immunother. 2009;32:109–117. doi: 10.1097/CJI.0b013e31819302da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Koyama K, et al. Reciprocal CD4+ T-cell balance of effector CD62Llow CD4+ and CD62LhighCD25+ CD4+ regulatory T cells in small cell lung cancer reflects disease stage. Clin Cancer Res. 2008;14:6770–6779. doi: 10.1158/1078-0432.CCR-08-1156. [DOI] [PubMed] [Google Scholar]
- 104.Coury F, et al. Langerhans cell histiocytosis reveals a new IL-17A-dependent pathway of dendritic cell fusion. Nature Med. 2008;14:81–87. doi: 10.1038/nm1694. [DOI] [PubMed] [Google Scholar]
- 105.McGeachy MJ, et al. TGF-β and IL-6 drive the production of Il-17 and IL-10 by T cells and restrain TH-17 cell mediated pathology. Nature Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 106.Fitzgerald DC, et al. Suppression of autoimmune inflammation of central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nature Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 107.Stumhofer JS, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nature Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]